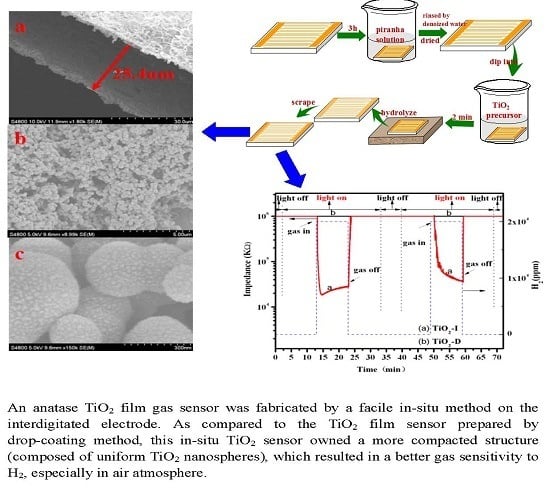
Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature
Abstract
:1. Introduction
2. Experiments
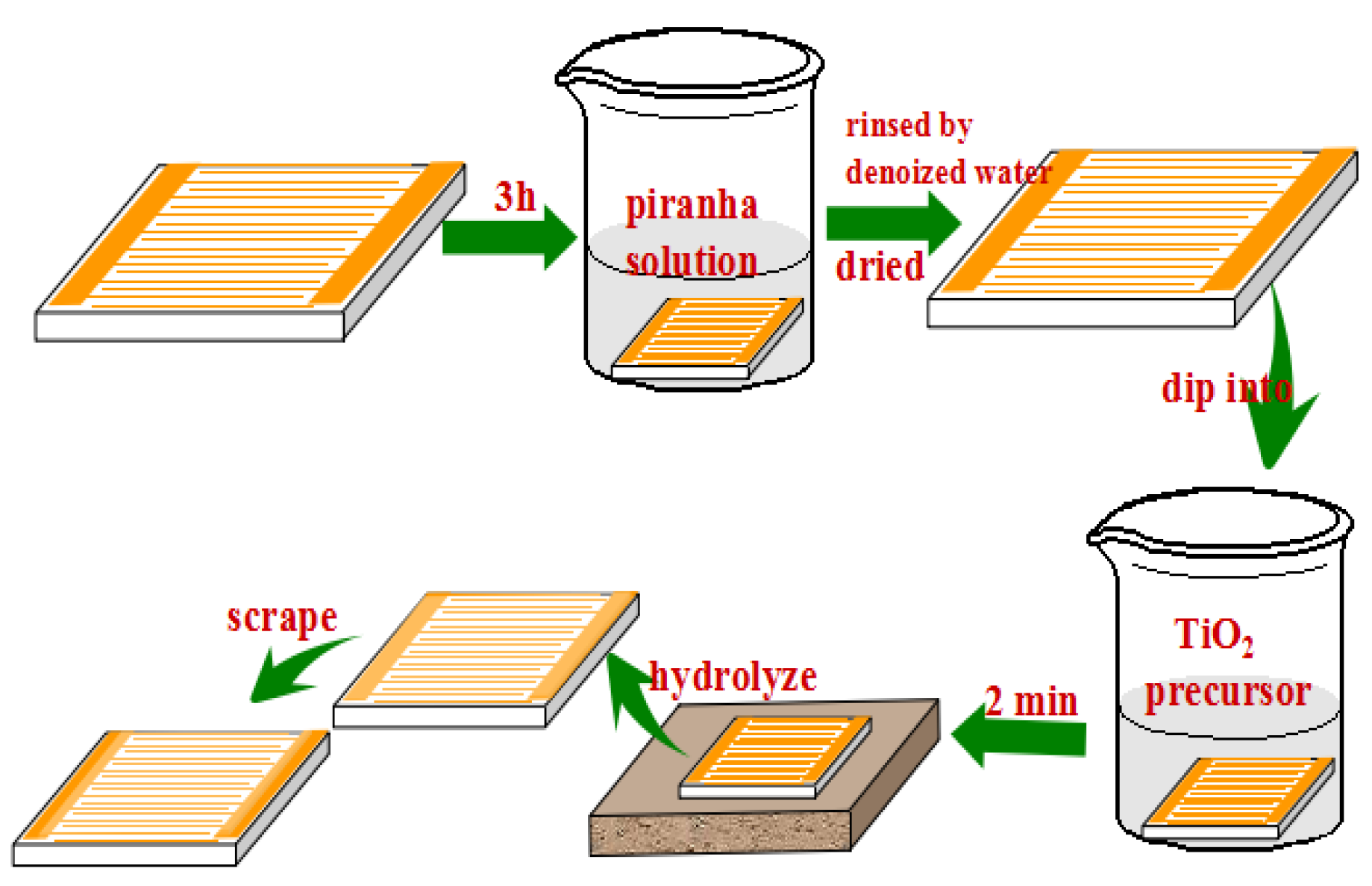
2.1. Fabrication of the Gas Sensor
2.2. Characterization
2.3. Photoelectrochemical Measurement
2.4. Testing of the Gas Sensors
3. Results and Discussion
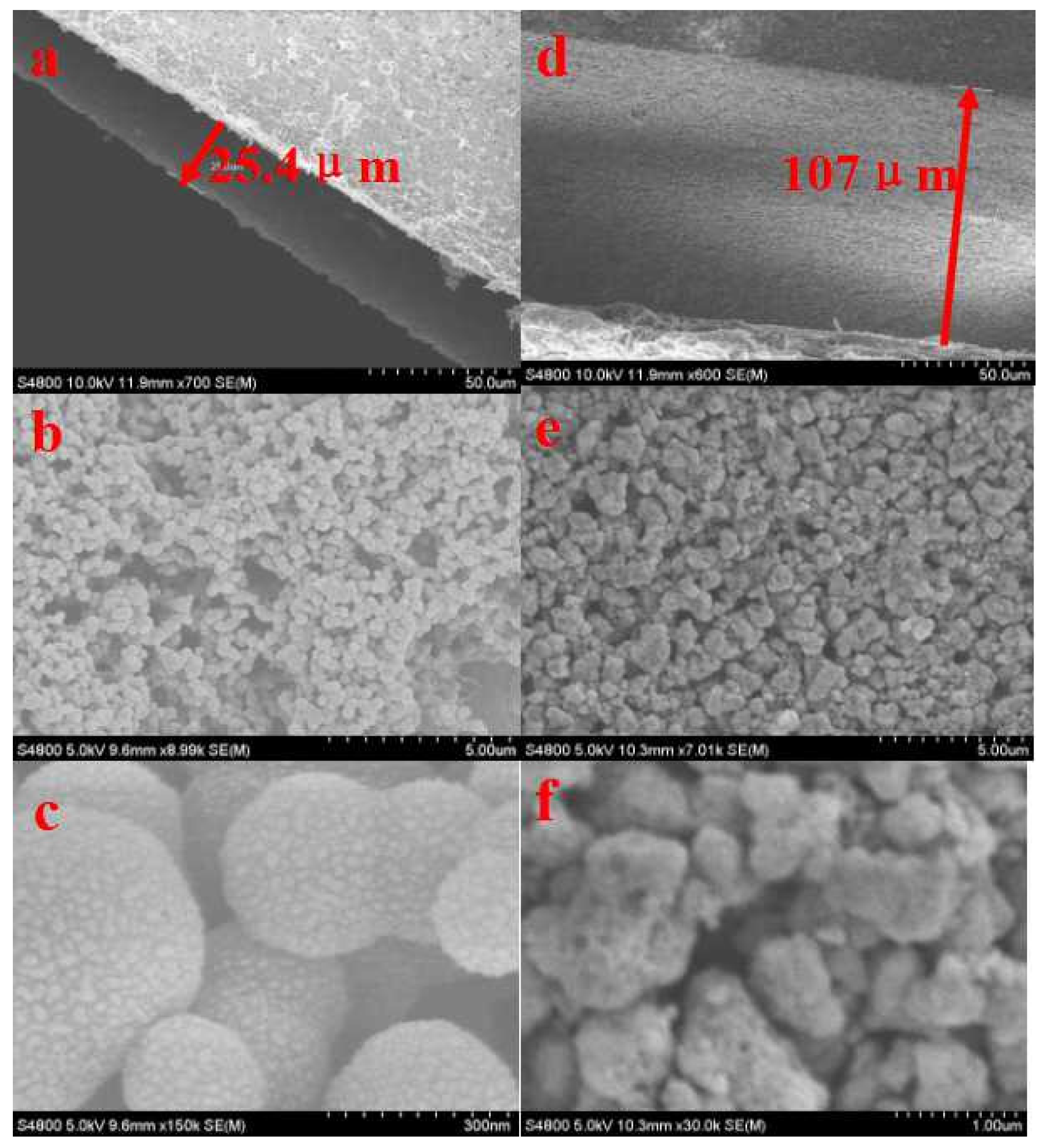
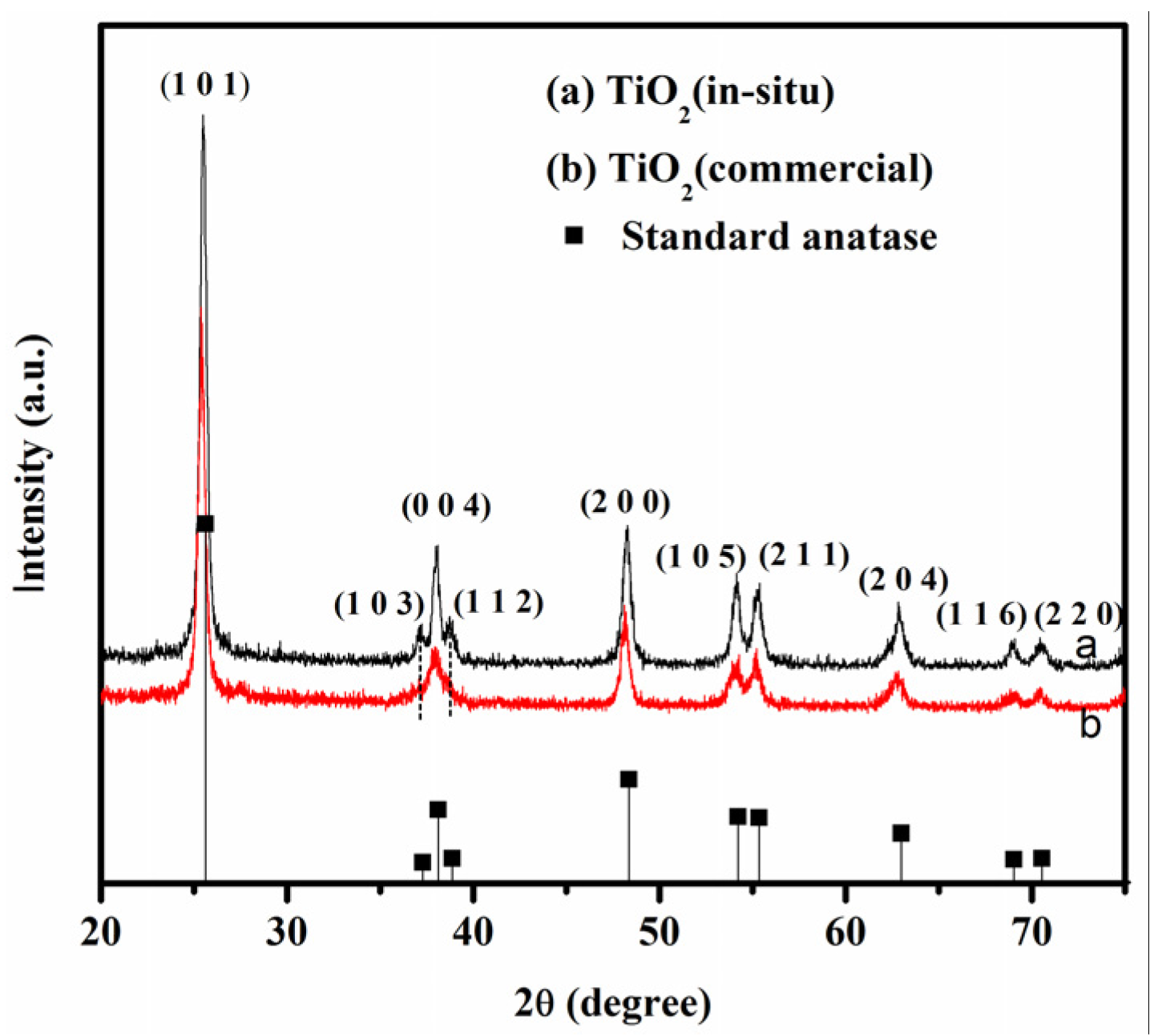
3.1. Properties of TiO2 Sensor Films
3.2. Performance of TiO2 Gas Sensor
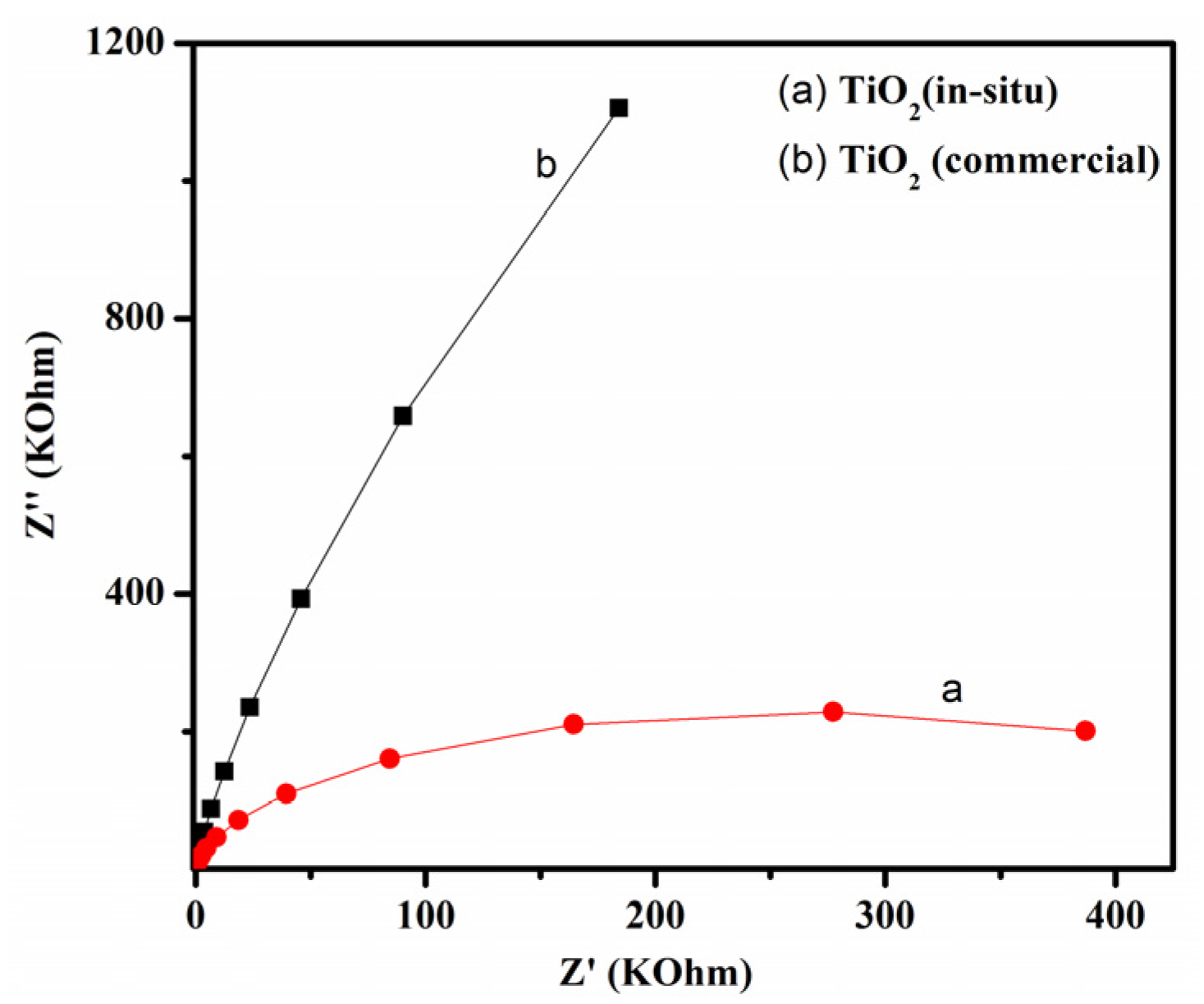
3.3. Photoelectrochemical Properties of TiO2
4. Conclusions
- A TiO2-I film sensor formed by uniform TiO2 microspheres, fabricated by a facile in-situ method, which exhibited an effective sensitivity towards H2 in both N2 atmosphere and synthetic air at the room temperature under UV irradiation.
- A TiO2-D film sensor, prepared by drop-coating method, exhibited an apparent sensitivity towards H2 only in N2 atmosphere but not in synthetic air under UV irradiation.
- The better photo-assisted sensitivity of TiO2-I could be attributed to the more effective charge transfer at TiO2 interface, which can be evaluated by the photoelectrochemical measurement.
- The effective charge transfer behaviors in TiO2-I could be originated from the uniform TiO2 nanosphere structure.
- This study shows that changing the structure and morphology of film sensor (e.g., preparing a uniform nanostructure) maybe improve the photo-assisted sensing properties of TiO2 and other semi-conductor sensors by enhancing electron transfer efficiency.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Usha, S.P.; Mishra, S.K.; Gupta, B.D. Fiber optic hydrogen sulfide gas sensors utilizing ZnO thin film/ZnO nanoparticles: A comparison of surface plasmon resonance and lossy mode resonance. Sens. Actuators B Chem. 2015, 218, 196–204. [Google Scholar] [CrossRef]
- Cuong, N.D.; Khieu, D.Q.; Hoa, T.T.; Quang, D.T.; Viet, P.H.; Lam, T.D.; Hoa, N.D.; Hieu, N.V. Facile synthesis of α-Fe2O3 nanoparticles for high-performance CO gas sensor. Mater. Res. Bull. 2015, 68, 302–307. [Google Scholar] [CrossRef]
- Blais, F. Review of 20 years of range sensor development. J. Electron. Imaging 2004, 13, 231–243. [Google Scholar] [CrossRef]
- VinothKumar, J.; Maldonado, A.; Olvera, M. A simple and cost-effective zinc oxide thin film sensor for propane gas detection. Mater. Lett. 2015, 157, 169–171. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–41. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Millis, A.; Hunte, S.L. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Hazra, A.; Das, S.; Kanungo, J.; Sarkar, C.K.; Basu, S. Studies on a resistive gas sensor based on sol–gel grown nanocrystalline p-TiO2 thin film for fast hydrogen detection. Sens. Actuators B Chem. 2013, 183, 87–95. [Google Scholar] [CrossRef]
- Lim, S.K.; Hong, S.H.; Hwang, S.H.; Choi, W.M.; Kim, S.H.; Park, H.W.; Jeong, M.G. Synthesis of Al-doped ZnO Nanorods via Microemulsion Method and Their Application as a CO Gas Sensor. J. Mater. Sci. Tech. 2015, 1, 639–644. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Park, J.A.; Lee, S.J.; Zyung, T.; Kim, I.D. Pd-doped TiO2 nanofiber networks for gas sensor applications. Sens. Actuators B Chem. 2010, 149, 301–305. [Google Scholar] [CrossRef]
- Sharma, K.R.; Bhatnagar, M.C.; Sharma, G.L. Mechanism of highly sensitive and fast response Cr doped TiO2 oxygen gas sensor. Sens. Actuators B Chem. 1997, 45, 209–215. [Google Scholar] [CrossRef]
- Comini, E.; Sberveglieri, G.; Ferroni, M.; Guidi, V.; Martinelli, G. Response to ethanol of thin films based on Mo and Ti oxides deposited by sputtering. Sens. Actuators B Chem. 2003, 93, 409–415. [Google Scholar] [CrossRef]
- Ruiz, A.M.; Cornet, A.; Morante, J.R. Study of La and Cu influence on the growth inhibition and phase transformation of nano-TiO2 used for gas sensors. Sens. Actuators B Chem. 2004, 100, 256–260. [Google Scholar] [CrossRef]
- Bonini, N.; Carotta, M.C.; Chiorino, A.; Guidi, V.; Malagù, C.; Martinelli, G.; Paglialonga, L.; Sacerdoti, M. Doping of a nanostructured titania thick film: Structural and electrical investigations. Sens. Actuators B Chem. 2000, 68, 274–280. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, J.L.; Wu, R.J. Fast ozone detection by using a core–shell Au@TiO2 sensor at room temperature. Sens. Actuators B Chem. 2015, 214, 56–62. [Google Scholar] [CrossRef]
- Plecenik, T.; Moško, M.; Haidry, A.A.; Ďurina, P.; Truchlý, M.; Grančič, B.; Gregor, M.; Roch, T.; Satrapinskyy, L.; Mošková, A.; et al. Fast highly-sensitive room-temperature semiconductor gas sensor based on the nanoscale Pt–TiO2–Pt sandwich. Sens. Actuators B Chem. 2015, 207, 351–361. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kishimoto, K.; Nakato, Y.; Tsubomura, H. Mechanism of hydrogen sensing by Pd/TiO2 Schottky diodes. Sens. Actuators B Chem. 1993, 13, 125–127. [Google Scholar] [CrossRef]
- Walton, R.M.; Liu, H.; Gland, J.L.; Schwank, J.W. Resistance measurements of platinum-titania thin film gas detectors in ultra-high vacuum (UHV) and reactive ion etcher (RIE) systems. Sens. Actuators B Chem. 1997, 41, 143–151. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Ong, K.G.; Grimes, C.A. Fabrication of hydrogen sensors with transparent titanium oxide nanotube-array thin films as sensing elements. Thin Solids Films 2006, 496, 42–48. [Google Scholar] [CrossRef]
- Gao, M.; Ito, A.; Goto, T. Preparation of γ-Al2O3 films by laser chemical vapor deposition. Appl. Surf. Sci. 2015, 340, 160–165. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sabhajeet, S.R.; Singh, S.; Yadav, B.C. Synthesis of ZnO nanopetals and its application as NO2 gas sensor. Mater. Lett. 2015, 152, 189–191. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Salehifar, N. Improvement in gas-sensing properties of TiO2 nanofiber sensor by UV irradiation. Sens. Actuators B Chem. 2015, 211, 146–156. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.H.; Chai, X.S.; Hu, Z.S.; Deng, Y.L. UV-light-activated ZnO fibers for organic gas sensing at room temperature. J. Phys. Chem. C 2010, 114, 1293–1298. [Google Scholar] [CrossRef]
- Camagni, P.; Fanglia, G.; Galinetto, P.; Perego, C.; Samoggia, G.; Sberveglieri, G. Photosensitivity activation of SnO2 thin film gas sensors at room temperature. Sens. Actuators B Chem. 1996, 31, 99–103. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.H.; Wang, X.; Wang, S.X.; Tian, Y.S.; Zhang, H.L. UV sensor based on TiO2 nanorod arrays on FTO thin film. Sens. Actuators B Chem. 2011, 156, 114–119. [Google Scholar] [CrossRef]
- Geng, Q.; He, Z.J.; Chen, X.; Dai, W.X.; Wang, X.X. Gas sensing property of ZnO under visible light irradiation at room temperature. Sens. Actuators B Chem. 2013, 18, 293–297. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xie, C.S.; Wu, J.; Zeng, D.W.; Liao, Y.C. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Su, P.G.; Shiu, C.C. Flexible H2 sensor fabricated by layer-by-layer self-assembly of thin films of polypyrrole and modified in situ with Pt nanoparticles. Sens. Actuators B Chem. 2011, 157, 275–281. [Google Scholar] [CrossRef]
- Rella, R.; Spadavecchia, J.; Manera, M.G.; Capone, S.; Taurino, A.; Martino, M.; Caricato, A.P.; Tunno, T. Acetone and ethanol solid-state gas sensors based on TiO2 nanoparticles thin film deposited by matrix assisted pulsed laser evaporation. Sens. Actuators B Chem. 2007, 127, 426–431. [Google Scholar] [CrossRef]
- Kawano, T.; Chiamori, H.C.; Suter, M.; Zhou, Q.; Sosnowchik, B.D.; Lin, L.W. An Electrothermal Carbon Nanotube Gas Sensor. Nano Lett. 2007, 7, 3686–3690. [Google Scholar] [CrossRef] [PubMed]
- Vanaraja, M; Muthukrishnan, K.; Boomadevi, S.; Karn, R.K.; Singh, V.; Singh, P.K.; Pandiyana, K. Dip coated nanostructured ZnO thin film: Synthesis and application. Ceram. Int. 2016, 42, 4413–4420. [Google Scholar]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Fadeyev, G.; Kalyakin, A.; Gorbova, E.; Brouzgou, A.; Demin, A.; Volkov, A.; Tsiakaras, P. A simple and low-cost amperometric sensor for measuring H2, CO, and CH4. Sens. Actuators B Chem. 2015, 221, 879–883. [Google Scholar] [CrossRef]
- Lee, E.B.; Hwang, I.S.; Cha, J.H.; Lee, H.J.; Lee, W.B.; Pak, J.J.; Lee, J.H.; Ju, B.K. Micromachined catalytic combustible hydrogen gas sensor. Sens. Actuators B Chem. 2011, 153, 392–397. [Google Scholar] [CrossRef]
- Kalyakin, A.; Fadeyev, G.; Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Application of Solid oxide proton-conducting electrolytes for amperometric analysis of hydrogen in H2+N2+H2O gas mixtures. Electrochim. Acta 2014, 141, 120–125. [Google Scholar] [CrossRef]
- Iwahara, H.; Asakura, Y.; Katahira, K.; Tanaka, M. Prospect of hydrogen technology using proton-conducting ceramics. Solids State Ion. 2004, 168, 299–310. [Google Scholar] [CrossRef]
- Schwandt, C.; Fray, D.J. The titanium/hydrogen system as the solid-state reference in high-temperature proton conductor-based hydrogen sensors. J. Appl. Electrochem. 2006, 36, 557–565. [Google Scholar] [CrossRef]
- Lee, J.; Shim, W.; Lee, E.; Noh, J.; Lee, W. Highly mobile palladium thin films on an elastomeric substrate: Nanogap-based hydrogen gas sensors. Angew. Chem. Int. Ed. 2011, 50, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Lee, K.; Noh, J.; Lee, W. Nanogap-based electrical hydrogen sensors fabricated from Pd-PMMA hybrid thin films. Sens. Actuators B Chem. 2014, 193, 530–535. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, R.; Srivastava, S.K. Sensing properties of palladium-gate MOS (Pd-MOS) hydrogen sensor-based on plasma grown silicon dioxide. Sens. Actuators B Chem. 2000, 71, 161–168. [Google Scholar] [CrossRef]
- Aroutiounian, V. Metal oxide hydrogen, oxygen and carbon monoxide sensorsfor hydrogen setups and cells. Int. J. Hydrog. Energy 2007, 32, 1145–1158. [Google Scholar] [CrossRef]
- Heszler, P.; Ionescu, R.; Llobet, E.; Reyes, L.F.; Smulko, J.M.; Kish, L.B.; Granqvist, C.G. On the selectivity of nanostructured semiconductor gas sensors. Phys. Status Solids B 2007, 244, 4331–4335. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A.; Ong, K.G. Unprecedented ultra-high hydrogen gas sensitivity in undoped titania nanotubes. Nanotechnology 2006, 17, 398–402. [Google Scholar] [CrossRef]
- Moon, J.; Hedman, H.; Kemell, M.; Tuominen, A.; Punkkinen, R. Hydrogen sensor of Pd-decorated tubular TiO2 layer prepared by anodization with patterned electrodes on SiO2/Si substrate. Sens. Actuators B Chem. 2016, 222, 190–197. [Google Scholar] [CrossRef]
- Cui, C.; Qiu, Y.W.; Zhao, J.H.; Lu, B.Q.; Hu, H.H.; Yang, Y.N.; Ma, N.; Xu, S.; Xu, L.B.; Li, X.Y. A comparative study on the quantum-dot-sensitized. dye-sensitized and co-sensitized solar cells based on hollow spheres embedded porous TiO2 photoanodes. Electrochim. Acta 2015, 173, 551–558. [Google Scholar] [CrossRef]
- Li, B.; Shao, L.L. The identifying of Al2O3 and Al(OH)3 by XRD. Inorg. Chem. Ind. 2008, 40, 54–57. (In Chinese) [Google Scholar]
- Pal, S.; Laera, A.M.; Licciulli, A.; Catalano, M.; Taurino, A. Biphase TiO2 Microspheres with Enhanced Photocatalytic Activity. Ind. Eng. Chem. Res. 2014, 53, 7931–7938. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Meng, S.G.; Chen, J.; Wang, J.J.; Xian, J.J.; Shao, Y.; Fu, X.Z.; Li, D.Z. Titanium dioxide photonic crystals with enhanced photocatalytic activity: Matching photonic band gaps of TiO2 to the absorption peaks of dyes. J. Phys. Chem. C 2013, 117, 21263–21273. [Google Scholar] [CrossRef]
- Dai, J.; Yang, J.; Wang, X.H.; Zhang, L.; Li, Y.J. Enhanced visible-light photocatalytic activity for selective oxidation of amines into imines over TiO2(B)/anatase mixed-phase nanowires. Appl. Surf. Sci. 2015, 349, 343–352. [Google Scholar] [CrossRef]
- Peng, X.Y.; He, Z.J.; Yang, K.; Chen, X.; Wang, X.X.; Dai, W.X.; Fu, X.Z. Correlation between donating or accepting electron behavior of the adsorbed CO or H2 and its oxidation over TiO2 under ultraviolet light irradiation. Appl. Surf. Sci. 2016, 360, 698–706. [Google Scholar] [CrossRef]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Ye, A.H.; Fan, W.Q.; Zhang, Q.H.; Deng, W.P.; Wang, Y. CdS−Graphene and CdS−CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal. Sci. Tech. 2012, 2, 969–978. [Google Scholar] [CrossRef]
- Yang, K.; Huang, K.; He, Z.J.; Chen, X.; Fu, X.Z.; Dai, W.X. Promoted effect of PANI as electron transfer promoter on CO oxidation over Au/TiO2. Appl. Catal. B Environ. 2014, 158, 250–257. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Wang, F.C.; Fu, X.Z.; Zheng, Y. A green and facile self-assembly preparation of gold nanoparticles/ZnO nanocomposite for photocatalytic and photoelectrochemical applications. J. Mater. Chem. 2012, 22, 2868–2877. [Google Scholar] [CrossRef]
- Ding, Y.; Mo, L.E.; Tao, L.; Ma, Y.M.; Hu, L.H.; Huang, Y. TiO2 nanocrystalline layer as a bridge linking TiO2 sub-microspheres layer and substrates for high-efficiency dye-sensitized solar cells. J. Power Sour. 2014, 272, 1046–1052. [Google Scholar] [CrossRef]

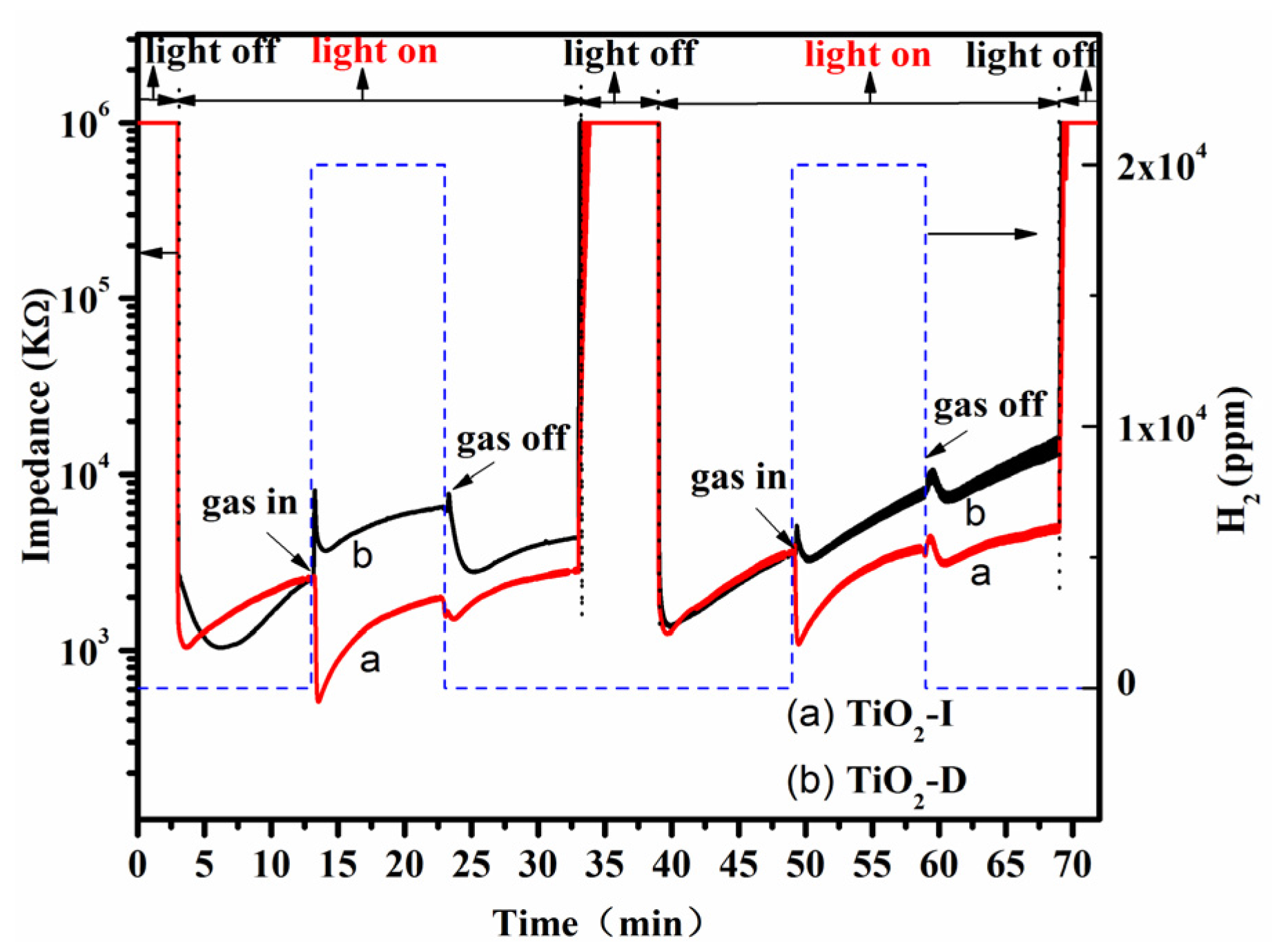
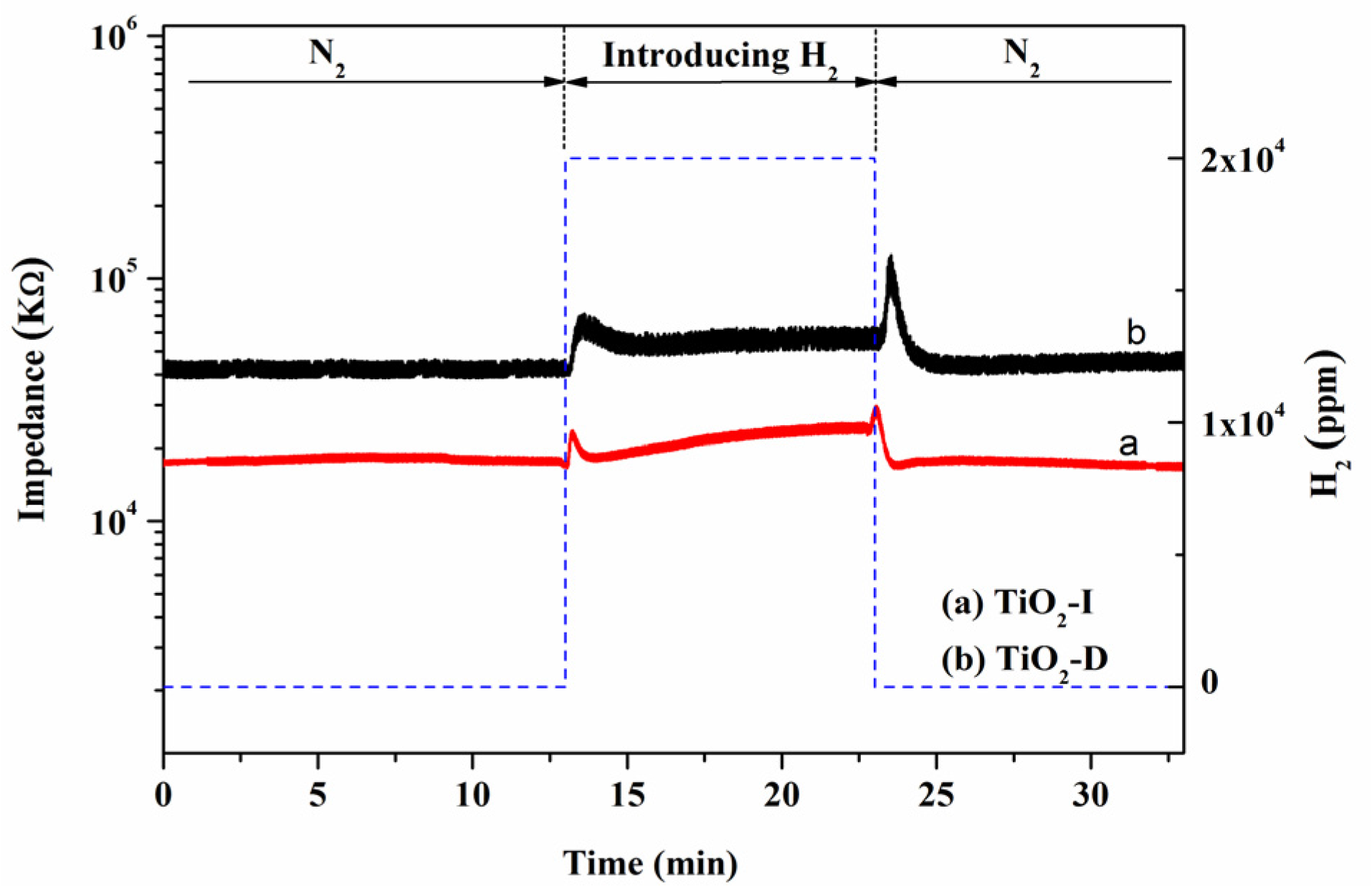
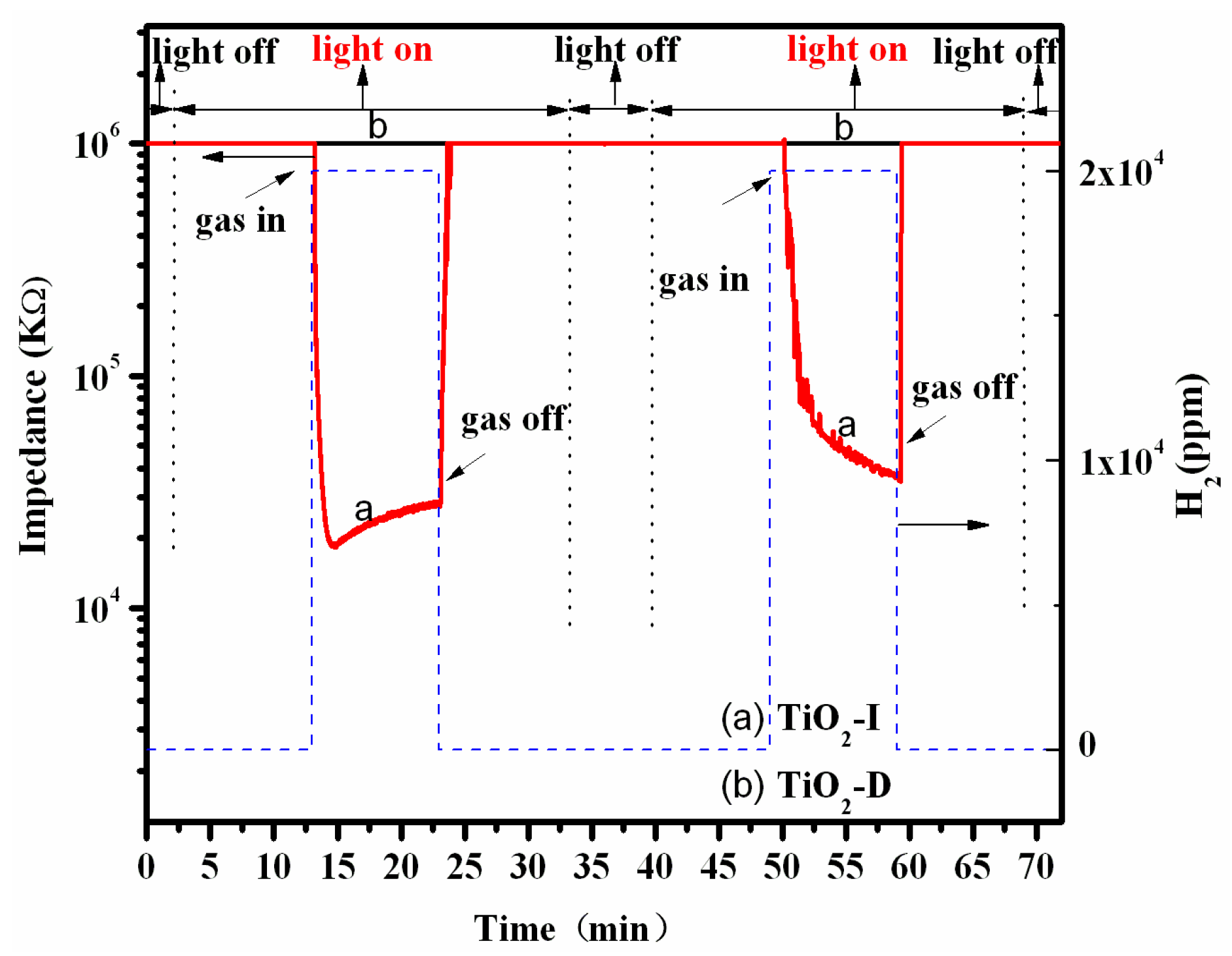
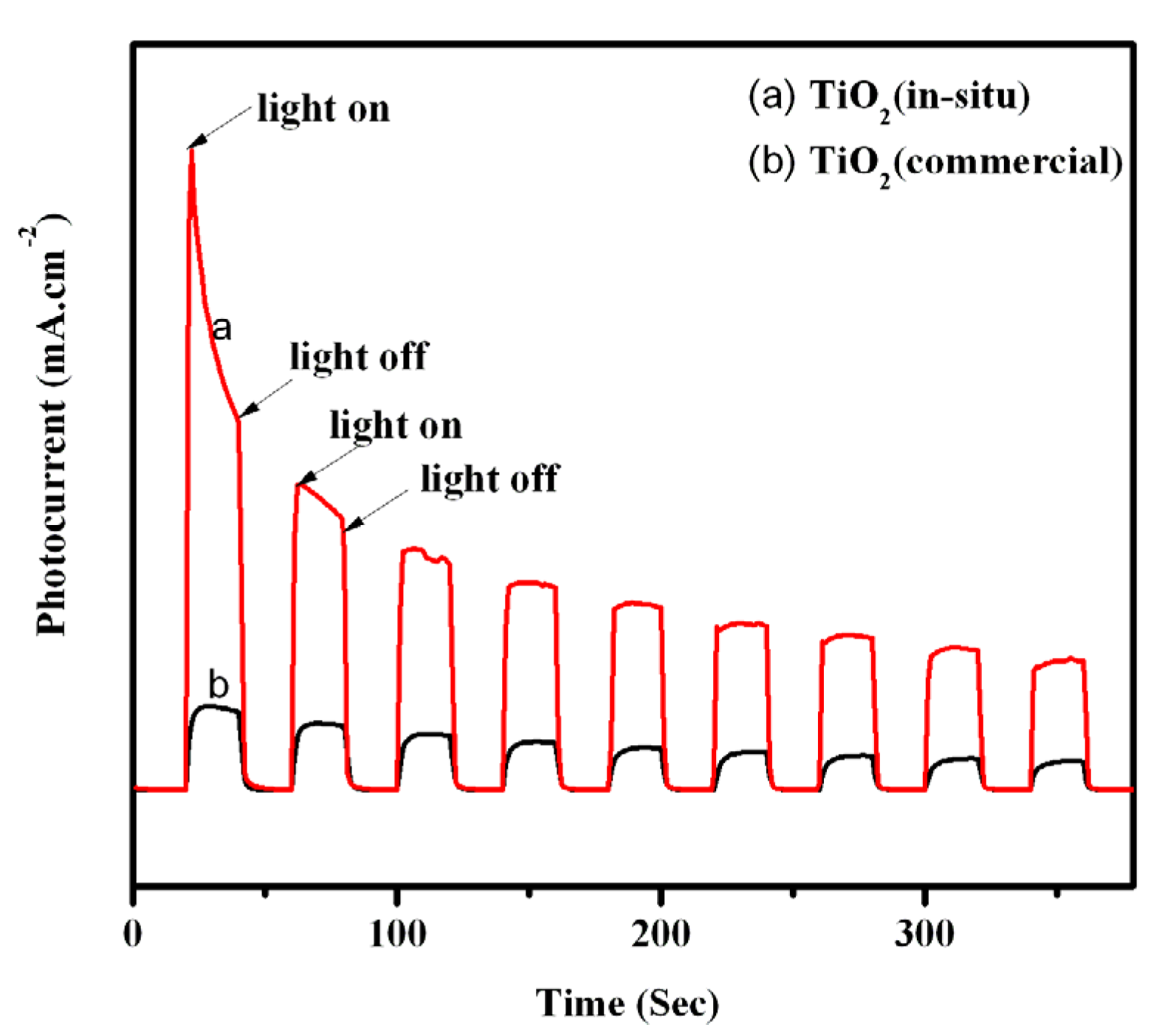
| Samples | R0 (kΩ) | Rgas (kΩ) | Sr = R0/Rgas | Response Time (s) | Recovery Time (s) |
|---|---|---|---|---|---|
| TiO2-I (First cycle) | 2564 | 528 | 4.856 | 18 | 56 |
| TiO2-I (Second cycle) | 3636 | 1100 | 3.305 | 18 | 113 |
| TiO2-D (First cycle) | 2698 | 3658 | 0.738 | 56 | 90 |
| TiO2-D (Second cycle) | 3403 | 3147 | 1.081 | 56 | 102 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Wang, Z.; Huang, P.; Chen, X.; Fu, X.; Dai, W. Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature. Sensors 2016, 16, 1249. https://doi.org/10.3390/s16081249
Peng X, Wang Z, Huang P, Chen X, Fu X, Dai W. Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature. Sensors. 2016; 16(8):1249. https://doi.org/10.3390/s16081249
Chicago/Turabian StylePeng, Xiaoying, Zhongming Wang, Pan Huang, Xun Chen, Xianzhi Fu, and Wenxin Dai. 2016. "Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature" Sensors 16, no. 8: 1249. https://doi.org/10.3390/s16081249
APA StylePeng, X., Wang, Z., Huang, P., Chen, X., Fu, X., & Dai, W. (2016). Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature. Sensors, 16(8), 1249. https://doi.org/10.3390/s16081249