Electronic Noses for Well-Being: Breath Analysis and Energy Expenditure
Abstract
:1. Introduction
2. Non-Invasive Measurements
3. Breath Contents
3.1. Exhaled Gas
3.2. Exhaled VOCs
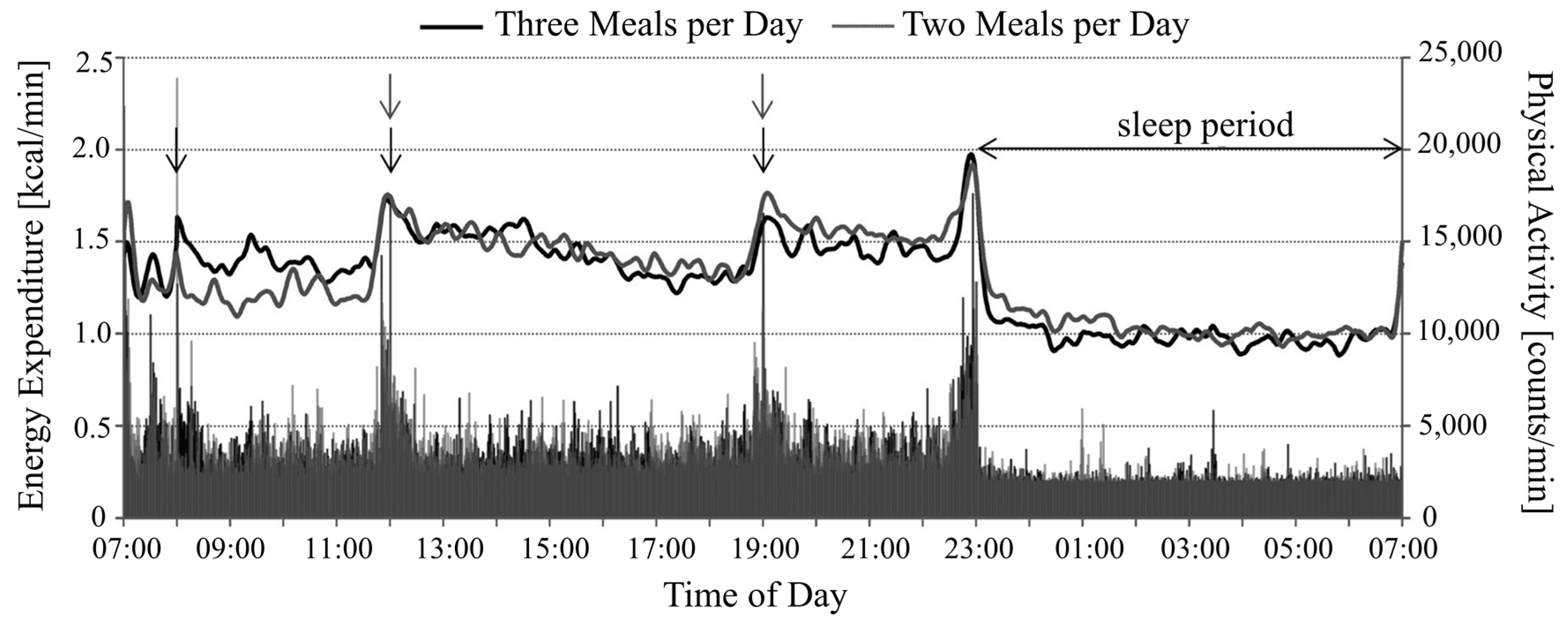
4. Energy Expenditure
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| CO2 | Carbon dioxide |
| CO | Carbon monoxide |
| CF | Cystic fibrosis |
| DLW | Doubly-labelled water |
| EE | Energy expenditure |
| E-nose | Electronic nose |
| FBAR | Film bulk acoustic resonator |
| HR | Heart rate |
| ICU | Intensive care unit |
| MR | Metabolic rate |
| MS | Mass spectrometer |
| NO | Nitric oxide |
| O2 | Oxygen |
| ppb | Parts per billion |
| ppm | Parts per million |
| SAW | Surface acoustic wave |
| SIT | Sprint interval training |
| VOC | Volatile organic compound |
References
- Verderber, S. Sprawling Cities and Our Endangered Public Health; Routledge: London, UK, 2012. [Google Scholar]
- Rodrigo, C.P. Current mapping of obesity. Nutr. Hosp. 2013, 28, 21–31. [Google Scholar]
- Collins, B.; Capewell, S.; O’Flaherty, M.; Timpson, H.; Razzaq, A.; Cheater, S.; Ireland, R.; Bromley, H. Modelling the health impact of an english sugary drinks duty at national and local levels. PLoS ONE 2015, 10, e0130770. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, R.; Sawers, C.; Thompson, F.; Manyika, J.; Woetzel, J.R.; Child, P.; McKenna, S.; Spatharou, A. Overcoming Obesity: An Initial Economic Analysis. Available online: http://www.mckinsey.com/insights/economic_studies/how_the_world_could_better_fight_obesity (accessed on 20 May 2015).
- Dallas, S.K.; Liu, P.J.; Ubel, P.A. Potential problems with increasing serving sizes on the nutrition facts label. Appetite 2015, 95, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St. Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [PubMed]
- WEIR, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Whybrow, S.; Ritz, P.; Horgan, G.W.; Stubbs, R.J. An evaluation of the IDEEA™ activity monitor for estimating energy expenditure. Br. J. Nutr. 2013, 109, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.A.; Gardner, J.W.; Hattersley, J.G.; Wilson, A.; Chappell, M.J. Environmental Monitoring of Human Body Odours. In COST Action TD1105—New Sensing Technologies for Air-Pollution Control and Environmental Sustainability; COST: Cambridge, UK, 2013; p. 71. [Google Scholar]
- Henderson, M.J.; Karge, B.A.; Wrenshall, G.A. Acetone in the breath: A study of acetone exhalation in diabetic and nondiabetic human subjects. Diabetes 1952, 1, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lord, H.; Yu, Y.; Segal, A.; Pawliszyn, J. Breath analysis and monitoring by membrane extraction with sorbent interface. Anal. Chem. 2002, 74, 5650–5657. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Spanĕl, P.; Smith, D. On-line measurement of the absolute humidity of air, breath and liquid headspace samples by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 563–569. [Google Scholar] [PubMed]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A. The diagnostic potential of breath analysis. Clin. Chem. 1983, 29, 5–15. [Google Scholar] [PubMed]
- Schlein, K.M.; Coulter, S.P. Best practices for determining resting energy expenditure in critically Ill adults. Nutr. Clin. Pract. 2013, 29. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.A.; Chan, L.-N.; Li, F. Indirect calorimetry: A practical guide for clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Plasqui, G.; Bonomi, A.G.; Westerterp, K.R. Daily physical activity assessment with accelerometers: New insights and validation studies. Obes. Rev. 2013, 14, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Kroke, A.; Klipstein-Grobusch, K.; Voss, S.; Moseneder, J.; Thielecke, F.; Noack, R.; Boeing, H.; Möseneder, J. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water. Am. J. Clin. Nutr. 1999, 70, 439–447. [Google Scholar] [PubMed]
- Hill, R.J.; Davies, P.S.W. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br. J. Nutr. 2001, 85, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F. Obesity and the Economics of Prevention—OECD. Available online: http://www.oecd-ilibrary.org/social-issues-migration-health/obesity-and-the-economics-of-prevention_9789264084865-en (accessed on 4 August 2014).
- Booyens, J.; Hervey, G.R. The pulse rate as a means of measuring metabolic rate in man. Biochem. Cell Biol. 1960, 38, 1301–1309. [Google Scholar] [CrossRef]
- Spurr, G.B.; Prentice, A.M.; Murgatroyd, P.; Goldberg, G.; Reina, J.C.; Christman, N. Energy expenditure from minute-by-minute heart-rate recording: Comparison with indirect calorimetry. Am. J. Clin. Nutr. 1988, 48, 552–559. [Google Scholar] [PubMed]
- Driskell, J.; Wolinsky, I. Sports Nutrition: Energy Metabolism and Exercise, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ceesay, S.M.; Prentice, A.M.; Day, K.C.; Murgatroyd, P.R.; Goldberg, G.R.; Scott, W.; Spurr, G.B. The use of heart rate monitoring in the estimation of energy expenditure: A validation study using indirect whole-body calorimetry. Br. J. Nutr. 1989, 61, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Rennie, K.; Rowsell, T.; Jebb, S.A.; Holburn, D.; Wareham, N.J. A combined heart rate and movement sensor: Proof of concept and preliminary testing study. Eur. J. Clin. Nutr. 2000, 54, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Bitar, A.; Vermorel, M.; Fellmann, N.; Bedu, M.; Chamoux, A.; Coudert, J. Heart rate recording method validated by whole body indirect calorimetry in 10-year-old children. J. Appl. Physiol. 1996, 81, 1169–1173. [Google Scholar] [PubMed]
- Lovelady, C.A.; Meredith, C.N.; McCrory, M.A.; Nommsen, L.A.; Joseph, L.J.; Dewey, K.G.; Bash, E. Energy expenditure in lactating women: A comparison of doubly labeled water and heart-rate-monitoring methods. Am. J. Clin. Nutr. 1993, 57, 512–518. [Google Scholar] [PubMed]
- Rothenberg, E.; Bosaeus, I.; Lernfelt, B.; Landahl, S.; Steen, B. Energy intake and expenditure: Validation of a diet history by heart rate monitoring, activity diary and doubly labeled water. Eur. J. Clin. Nutr. 1998, 52, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Paschalaki, K.; Logan-Sinclair, R.; Horváth, I.; Kharitonov, S.A.; Barnes, P.J.; Usmani, O.S.; Paredi, P. Standardised exhaled breath collection for the measurement of exhaled volatile organic compounds by proton transfer reaction mass spectrometry. BMC Pulm. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-H.; Lee, W.-J. Technology development in breath microanalysis for clinical diagnosis. J. Lab. Clin. Med. 1999, 133, 218–228. [Google Scholar] [CrossRef]
- Schoene, R.B. Limits of human lung function at high altitude. J. Exp. Biol. 2001, 204, 3121–3127. [Google Scholar] [PubMed]
- Zhang, X.; Wargocki, P.; Lian, Z.; Thyregod, C. Effects of exposure to carbon dioxide and bioeffluents on perceived air quality, self-assessed acute health symptoms and cognitive performance. Indoor Air 2016. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Strzoda, R.; Schrobenhauser, R.; Weigel, R. CO2 sensor for mainstream capnography based on TDLAS. Appl. Phys. B 2014, 116, 1023–1026. [Google Scholar] [CrossRef]
- Walsh, B.K.; Crotwell, D.N.; Restrepo, R.D. Capnography/Capnometry during mechanical ventilation: 2011. Respir. Care 2011, 56, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Deveci, S.E.; Deveci, F.; Açik, Y.; Ozan, A.T. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir. Med. 2004, 98, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.T.; Morice, A.H. Breath carbon monoxide as an indication of smoking habit. Chest 2000, 117, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.E.; Robinson, A.; Erb, P.; Spieler, C.A.; Klugman, N.; Dutta, P.; Dallery, J. A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine Tob. Res. 2014, 16, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zegdi, R.; Perrin, D.; Burdin, M.; Boiteau, R.; Tenaillon, A. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Med. 2002, 28, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Zayasu, K.; Sekizawa, K.; Okinaga, S.; Yamaya, M.; Ohrui, T.; Sasaki, H. Increased carbon monoxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1997, 156, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Patterson, L.; Goldrich, A.; Marsh, A. Point-of-care end-tidal carbon monoxide reflects severity of hemolysis in sickle cell anemia. Pediatr. Blood Cancer 2015, 62, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Nordvall, S.L.; Kuylenstierna, R.; Lundberg, J.M.; Alving, K. Primarily nasal origin of exhaled nitric oxide and absence in Kartagener’s syndrome. Eur. Respir. J. 1994, 7, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Berkman, N.; Avital, A.; Breuer, R.; Bardach, E.; Springer, C.; Godfrey, S. Exhaled nitric oxide in the diagnosis of asthma: Comparison with bronchial provocation tests. Thorax 2005, 60, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Stirling, R.G.; Kharitonov, S.A.; Campbell, D.; Robinson, D.S.; Durham, S.R.; Chung, K.F.; Barnes, P.J. Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Thorax 1998, 53, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Cancelliere, N.; Barranco, P.; López-Carrasco, V.; Quirce, S.Q.M. Usefulness of exhaled nitric oxide for diagnosing asthma. J. Asthma 2010, 47, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Martin, U.; Howarth, P.; Bryden, K.; Devoy, M.; Howarth, P. Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J. Allergy Clin. Immunol. 1996, 97, 768–772. [Google Scholar] [CrossRef]
- Murphy, A.W.; Platts-Mills, T.A.E.; Lobo, M.; Hayden, F. Respiratory nitric oxide levels in experimental human influenza. Chest 1998, 114, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, H.; Knauer, N.; Büscher, R.; Hübner, K.; Drazen, J.M.M.; Ratjen, F.; Buscher, R.; Hubner, K.; Drazen, J.M.M.; Ratjen, F. Airway nitric oxide levels in cystic fibrosis patients are related to a polymorphism in the neuronal nitric oxide synthase gene. Am. J. Respir. Crit Care Med. 2000, 162, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Balfour-Lynn, I.M.; Laverty, A.; Dinwiddie, R. Reduced upper airway nitric oxide in cystic fibrosis. Arch. Dis. Child. 1996, 75, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Tromp, T.K.; Shia, R.-L.; Allen, M.; Eiler, J.M.; Yung, Y.L. Potential environmental impact of a hydrogen economy on the stratosphere. Science 2003, 300, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, A.; Amann, A.; Said, M.; Datta, B.; Ledochowski, M. Implementation and interpretation of hydrogen breath tests. J. Breath Res. 2008, 2, 046002. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Stotzer, P.-O. Use and abuse of hydrogen breath tests. Gut 2006, 55, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C. How to interpret hydrogen breath tests. J. Neurogastroenterol. Motil. 2011, 17, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.G.; Binfield, P.; Belder, A.D.E.; Brien, J.O.; Warren, S.; Wilson, M.; De Belder, A.; O’Brien, J.; Warren, S.; Wilson, M. Extra intestinal influences. Gut 1985, 26, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.P.J.; Ledochowski, M.; Ratcliffe, N.M. The importance of methane breath testing: A review. J. Breath Res. 2013, 7, 024001. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.D.; Furne, J.K.; Kuskowski, M.; Ruddy, J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin. Gastroenterol. Hepatol. 2006, 4, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pimental, M.; Mayer, A.G.; Park, S.; Chow, E.J.; Hasan, A.; Kong, Y.; Pimentel, M.; Mayer, A.G.; Park, S.; Chow, E.J.; et al. Methane Production During lactulose breath test is associated with gastrointestinal disease presentation. Dig. Dis. Sci. 2003, 48, 86–92. [Google Scholar] [CrossRef]
- Repetto, J.E.; Donohue, P.A.-C.P.K.; Baker, S.F.; Kelly, L.; Nogee, L.M.; Seki, Y.; Sato, K.; Kono, T.; Akiba, Y. Use of capnography in the delivery room for assessment of endotracheal tube placement. Anim. Sci. J. 2005, 76, 491. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Sakamoto, O.; Kiyofuji, C.; Ito, K. A comparison of the end-tidal CO2 measured by portable capnometer and the arterial PCO2 in spontaneously breathing patients. Respir. Med. 2003, 97, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, C.B.F.; Mendes, R.G.; Di Thommazo, L.; Catai, A.M.; Sampaio, L.M.M.; Borghi-Silva, A. Acute application of bilevel positive airway pressure influences the cardiac autonomic nervous system. Clinics 2009, 64, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Kacmarek, R.M.; Stoller, J.K.; Heuer, A. Egan’s Fundamentals of Respiratory Care, 11th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2016. [Google Scholar]
- Carpagnano, G.E.; Kharitonov, S.A.; Foschino-Barbaro, M.P.; Resta, O.; Gramiccioni, E.; Barnes, P.J. Increased inflammatory markers in the exhaled breath condensate of cigarette smokers. Eur. Respir. J. 2003, 21, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Dupont, L.J.; Demedts, M.G.; Verleden, G.M. Prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma prospective evaluation of the validity of exhaled nitric oxide for the diagnosis of asthma. Chest 2007, 123, 751–756. [Google Scholar] [CrossRef]
- Anton, A.; Agapiou, A. Breath and sweat analysis as a tool for medical diagnostics. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication Healthcare (Mobihealth), Athens, Greece, 3–5 November 2014; pp. 250–253.
- Vishinkin, R.; Haick, H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015, 11, 6142–6164. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, J.J.B.N.; Dallinga, J.W.; Möller, G.M.; Godschalk, R.W.L.; Moonen, E.J.; Wouters, E.F.M.; van Schooten, F.J. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir. Med. 2010, 104, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.O.; Syed, Y.; Parthaláin, N.M.; Zwiggelaar, R.; Claypole, T.C.; Lewis, K.E. Machine learning methods on exhaled volatile organic compounds for distinguishing COPD patients from healthy controls. J. Breath Res. 2012, 6, 036003. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.; Litterst, P.; Maddula, S.; Bödeker, B.; Rahmann, S.; Davies, A.N.; Baumbach, J.I. Differentiation of chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control group by breath analysis using ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2010, 13, 131–139. [Google Scholar] [CrossRef]
- Cazzola, M.; Segreti, A.; Capuano, R.; Bergamini, A.; Martinelli, E.; Calzetta, L.; Rogliani, P.; Ciaprini, C.; Ora, J.; Paolesse, R.; et al. Analysis of exhaled breath fingerprints and volatile organic compounds in COPD. COPD Res. Pract. 2015, 1, 7. [Google Scholar] [CrossRef]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T.; Jezierski, T.; Buszewski, B. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography–mass spectrometry versus trained dogs. Sens. Actuators B Chem. 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Corradi, M.; Poli, D.; Banda, I.; Bonini, S.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Andreoli, R.; Ampollini, L.; Casalini, A.; et al. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: A cross-sectional study. J. Breath Res. 2015, 9, 027101. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.F.; Laskowski, D.; Deffenderfer, O.; Burch, T.; Zheng, S.; Mazzone, P.J.; Mekhail, T.; Jennings, C.; Stoller, J.K.; Pyle, J.; et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Respir. Crit. Care Med. 2005, 171, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas. Sci. Technol. 2005, 16, 1535–1546. [Google Scholar] [CrossRef]
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Salehi, L.B.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs associated with different characteristics of breast cancer cells. Sci. Rep. 2015, 5, 13246. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile biomarkers in the breath of women with breast cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef] [PubMed]
- Mangler, M.; Cornelia, F.; Malgorzata, L.; Oliver, S.; Achim, S.; Dorothee, S. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Polish Gynaecol. 2014, 83, 730–736. [Google Scholar]
- Xu, Y.; Lee, H.; Hu, Y.; Huang, J.; Kim, S.; Yun, M. Detection and identification of breast cancer volatile organic compounds biomarkers using highly-sensitive single nanowire array on a chip. J. Biomed. Nanotechnol. 2013, 9, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.-W.A.; Cheng, C.-J.; Gwo, S.; Yeh, J.A. A semiconductor gas system of healthcare for liver disease detection using ultrathin InN-based sensor. ECS Trans. 2015, 66, 151–157. [Google Scholar] [CrossRef]
- Eng, K.; Alkhouri, N.; Cikach, F.; Patel, N.; Yan, C.; Grove, D.; Lopez, R.; Rome, E.; Dweik, R.A. Analysis of breath volatile organic compounds in children with chronic liver disease compared to healthy controls. J. Breath Res. 2015, 9, 026002. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Singh, T.; Alsabbagh, E.; Guirguis, J.; Chami, T.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Isoprene in the exhaled breath is a novel biomarker for advanced fibrosis in patients with chronic liver disease: A pilot study. Clin. Transl. Gastroenterol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; de Palma, G.D.; et al. Rapid “breath-print” of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Pijls, K.E.; Smolinska, A.; Jonkers, D.M.A.E.; Dallinga, J.W.; Masclee, A.A.M.; Koek, G.H.; van Schooten, F.-J. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci. Rep. 2016, 6, 19903. [Google Scholar] [CrossRef] [PubMed]
- Dadamio, J.; van den Velde, S.; Laleman, W.; van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 905, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fernández Del Río, R.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile biomarkers in breath associated with liver cirrhosis—Comparisons of pre- and post-liver transplant breath samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Kahn, N.; Lavie, O.; Paz, M.; Segev, Y.; Haick, H. Dynamic nanoparticle-based flexible sensors: Diagnosis of ovarian carcinoma from exhaled breath. Nano Lett. 2015, 15, 7023–7028. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Shi, D.-Y.; Ionescu, R.; Zhang, W.; Hua, Q.-L.; Pan, Y.-Y.; Tao, L.; Liu, H.; Haick, H. Assessment of ovarian cancer conditions from exhaled breath. Int. J. Cancer 2015, 136, E614–E622. [Google Scholar] [CrossRef] [PubMed]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: A review of array-based sensors. J. Breath Res. 2014, 8, 027112. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Di Natale, C.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016, 10, 016007. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M.; Vernile, C.; Incalzi, R.A.; de Vincentis, A.; Picardi, A. Breathprinting of liver diseases. In Proceedings of the IEEE 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 Febuary 2015; pp. 1–3.
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- De Meij, T.G.; Larbi, I.B.; van der Schee, M.P.; Lentferink, Y.E.; Paff, T.; Terhaar Sive Droste, J.S.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: Proof of principle study. Int. J. Cancer 2014, 134, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Malagù, C.; Fabbri, B.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Zonta, G. Chemoresistive gas sensors for the detection of colorectal cancer biomarkers. Sensors 2014, 14, 18982–18992. [Google Scholar] [CrossRef] [PubMed]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Ravussin, E.; Lillioja, S.; Anderson, T.E.; Christin, L.; Bogardus, C. Determinants of 24-h energy expenditure in man. Methods and results using a respiratory chamber. J. Clin. Invest. 1986, 78, 1568–1578. [Google Scholar] [PubMed]
- Gottschlich, M.M. The Science and Practice of Nutrition Support: A Case-based Core Curriculum; Kendall Hunt: Dubuque, IA, USA, 2001. [Google Scholar]
- Insel, P.; Ross, D.; McMahon, K.; Bernstein, M. Nutrition; Jones & Bartlett Learning: Sudbury, MA, USA, 2010. [Google Scholar]
- Tarnopolsky, M. Gender Differences in Metabolism: Practical and Nutritional Implications; CRC Press: Boca Raton, FL, USA, 1998; Volume 1998. [Google Scholar]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.; Schwartz, M.; Rozin, R.; Lister, J. Relationships between oxygen consumption and body composition of obese patients. Metabolism 1970, 19, 418–429. [Google Scholar] [CrossRef]
- Ogata, H.; Kobayashi, F.; Hibi, M.; Tanaka, S.; Tokuyama, K. A novel approach to calculating the thermic effect of food in a metabolic chamber. Physiol. Rep. 2016, 4, e12717. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, P.A.; Larson, D.E.; Snitker, S.; Ravussin, E. Thermic effect of food in humans: Methods and results from use of a respiratory chamber. Am. J. Clin. Nutr. 1995, 61, 1013–1019. [Google Scholar] [PubMed]
- Ravussin, E.; Burnand, B.; Schutz, Y.; Jéquier, E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am. J. Clin. Nutr. 1982, 35, 566–573. [Google Scholar] [PubMed]
- Brehm, B. Elevation of metabolic rate following exercise. Sports Med. 1988, 6, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Shanely, R.A.; Corbin, K.D.; Jin, F.; Sha, W.; Nieman, D.C. A 45-min vigorous exercise bout increases metabolic rate for 14 h. Med. Sci. Sports Exerc. 2011, 43, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Sevits, K.J.; Melanson, E.L.; Swibas, T.; Binns, S.E.; Klochak, A.L.; Lonac, M.C.; Peltonen, G.L.; Scalzo, R.L.; Schweder, M.M.; Smith, A.M.; et al. Total daily energy expenditure is increased following a single bout of sprint interval training. Physiol. Rep. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.L. Nutritional requirements of surgical and critically-ill patients: Do we really know what they need? J. Parenter. Enter. Nutr. 2004, 63, 467–472. [Google Scholar] [CrossRef]
- De Waele, E.; Spapen, H.; Honoré, P.M.; Mattens, S.; Rose, T.; Huyghens, L. Bedside calculation of energy expenditure does not guarantee adequate caloric prescription in long-term mechanically ventilated critically ill patients: a quality control study. Sci. World J. 2012, 2012, 909564. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Sylvia, L.G.; Lynch, M.; Sonnenberg, L.; Lee, H.; Nathan, D.M. Comparison of energy assessment methods in overweight individuals. J. Acad. Nutr. Diet. 2014, 114, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.M.; Parker, L.J.F.; Amirabdollahian, F. Accuracy and preference of measuring resting energy expenditure using a handheld calorimeter in healthy adults. J. Hum. Nutr. Diet. 2013, 26, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Quach, A.; Bridgeman, D.; Tsow, F.; Forzani, E.; Tao, N. Personalized indirect calorimeter for energy expenditure (EE) measurement. Glob. J. Obes. Diabetes Metab. Syndr. 2015, 2, 4–8. [Google Scholar]
- Vincent, T.A.; Wilson, A.; Hattersley, J.G.; Chappell, M.J.; Gardner, J.W. Design and modelling of a portable breath analyser for metabolic rate measurement. Procedia Eng. 2014, 87, 668–671. [Google Scholar] [CrossRef]
- Vincent, T.A.; Urasinska-Wojcik, B.; Gardner, J.W. Development of a low-cost NDIR system for ppm detection of carbon dioxide in exhaled breath analysis. Procedia Eng. 2015, 120, 388–391. [Google Scholar] [CrossRef]
- Vincent, T.A.; Wilson, A.; Hattersley, J.G.; Chappell, M.J.; Gardner, J.W. Design and modelling of a handheld side-stream breath sampling system for metabolic rate analyis. In Proceedings of the 16th International Symposium On Olfaction And Electronic Nose, Dijon, France, 28 June–1 July 2015; p. 198.


| Gas | Inhaled | Normal Exhaled | Abnormal Exhaled | Healthcare Application/Diagnosis | Reference/s |
|---|---|---|---|---|---|
| Carbon dioxide | 0.04% | 3%–4% | - | Respiration monitoring, control of mechanical ventilator, capnography | [34,35,36] |
| Carbon Monoxide | 0.25 ppm | 0.5–2.1 ppm | 16.7–29.3 ppm | Identification as smoker, chronic obstructive pulmonary disease (COPD) | [37,38,39] |
| 1.13 ppm | Severe sepsis | [40] | |||
| 5.6 ppm | Asthmatic patients | [41] | |||
| 4.4 ppm | Haemolysis | [42] | |||
| Nitric Oxide | <2 ppb | 5.3–7.4 ppb | 19.2–30 ppb | Asthma diagnosis | [43,44,45,46] |
| 16.3 ppb | Seasonal rhinitis | [47] | |||
| 12.9 ppb | Experimental influenza | [48] | |||
| 2.3–4.7 ppb | Cystic fibrosis | [49,50] | |||
| Hydrogen | 0.5 ppm | <5 ppm | >16–20 ppm increase | Gastrointestinal diseases (small intestinal bacteria overgrowth, carbohydrate malabsorption), diarrhoea | [51,52,53,54,55] |
| Methane | 1.7 ppm | <1 ppm increase | >16 ppm increase | Diverticulitis, constipation, irritable bowel syndrome | [56,57,58] |
| Test Substance | Indications from the Test |
|---|---|
| Fructose |
|
| Lactose |
|
| Glucose |
|
| Disease | VOCs | Reference |
|---|---|---|
| COPD | Isoprene, C 16 hydrocarbon, 4,7-dimethyl-undecane, 2,6-dimethyl-heptane, 4-methyl-octane, hexadecane | [67] |
| Isoprene, acetic acid, benzaldehyde, benzene, nonadecane, toluene | [68] | |
| Benzaldehyde, limonene, 2-ethyl-1-hexanol, nonanal, menthone, menthol, decanal | [69] | |
| Decane, 6-ethyl-2-methyl-decane, benzene, butylated hydroxytoluene, limonene, 2-propanol | [70] | |
| Lung Cancer | Acetone, acetonitrile, 2-3-dimethylbutane, hexane, limonene, pentane | [71] |
| Ethyl benzene, hexane, trans-2-nonenal, pentane, 2-methyl pentane, heptanal | [72] | |
| Isobutane, methanol, ethanol, acetone, pentane, isoprene, benzene, toluene | [73] | |
| Toluene, o-xylene, styrene, 1-methyl-3-benzene, 2,3-dimethyl-hexane, 3-ethyl-3-methyl-2-pentanone | [74] | |
| Styrene, decane, isoprene, benzene, undecane, hexanol, heptanal | [75] | |
| Breast Cancer | 4-Methyl-2-heptanone, 2-nonanone, 2-dodecanone, 2,4-dimethyl-1-heptene, 2-xylene | [76] |
| Cyclopropane, ethylidene, 1,4-pentadiene, 1,3-butadiene, 2-methyl | [77] | |
| 3-methylhexane, decene, caryophyllene, naphthalene, trichloroethylene | [78] | |
| Heptanal, acetophenone, isopropyl myristate, 2-propanol | [79] | |
| Chronic Liver Disease | Ammonia | [80] |
| 1-Decene, 1-octene, €-2-nonene | [81] | |
| Isoprene, carbon disulphide, dimethyl sulphide, pentane, ethane, acetone | [82] | |
| Liver Cirrhosis | Methanol, 2-butanone, 2-pentanone, C8-kentone, sulfoxide-compound | [83] |
| 3-methylbutanal, propionic acid, octane, terpene, 3-carene, 1-hexadecanol | [84] | |
| Acetone, styrene, dimethyl sulphide, dimethylsilane, phenol, tetradecane | [85] | |
| Limonene, methanol, 2-pentanone | [86] | |
| Colorectal Cancer | Ethanol, acetone, ethyl acetate, 4-methyl octane | [87] |
| Nonanal, 4-methyl-2-pentanone, decanal, cyclohexane, 1,3-dimethylbenzene | [88] | |
| Cyclohexanone, 2,21-dimethyldecane, ethylaniline, cyclo-octylmethanol | [89] | |
| Ovarian Cancer | Styrene, nonanal, 2-ethylhexanol, 3-heptanone, decanal, hexadecane | [90] |
| Decanal, nonanal, styrene, 2-butanone, hexadecane | [91] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardner, J.W.; Vincent, T.A. Electronic Noses for Well-Being: Breath Analysis and Energy Expenditure. Sensors 2016, 16, 947. https://doi.org/10.3390/s16070947
Gardner JW, Vincent TA. Electronic Noses for Well-Being: Breath Analysis and Energy Expenditure. Sensors. 2016; 16(7):947. https://doi.org/10.3390/s16070947
Chicago/Turabian StyleGardner, Julian W., and Timothy A. Vincent. 2016. "Electronic Noses for Well-Being: Breath Analysis and Energy Expenditure" Sensors 16, no. 7: 947. https://doi.org/10.3390/s16070947
APA StyleGardner, J. W., & Vincent, T. A. (2016). Electronic Noses for Well-Being: Breath Analysis and Energy Expenditure. Sensors, 16(7), 947. https://doi.org/10.3390/s16070947






