Towards a Multifunctional Electrochemical Sensing and Niosome Generation Lab-on-Chip Platform Based on a Plug-and-Play Concept
Abstract
:1. Introduction
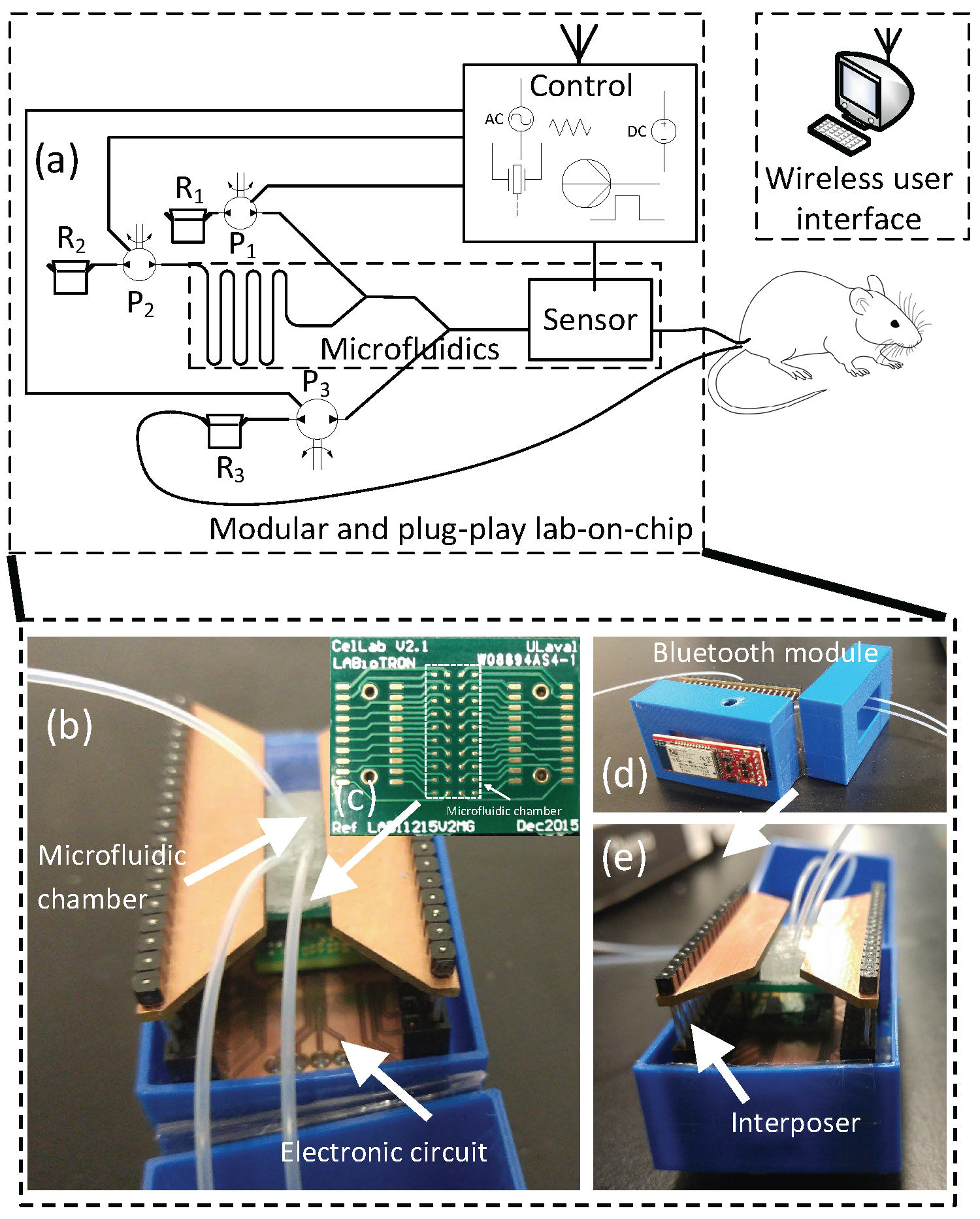
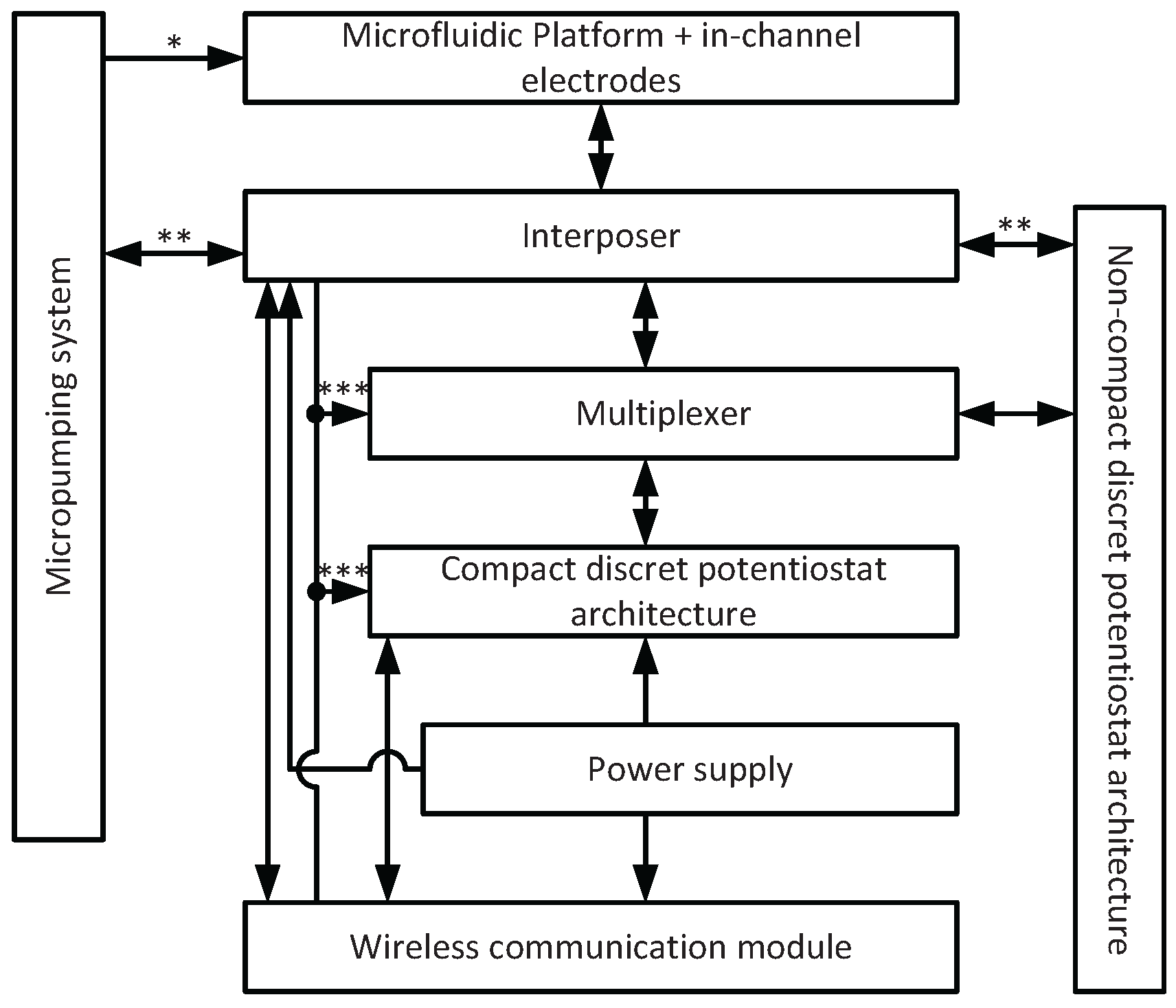
2. General System Description
3. Experimental Setup
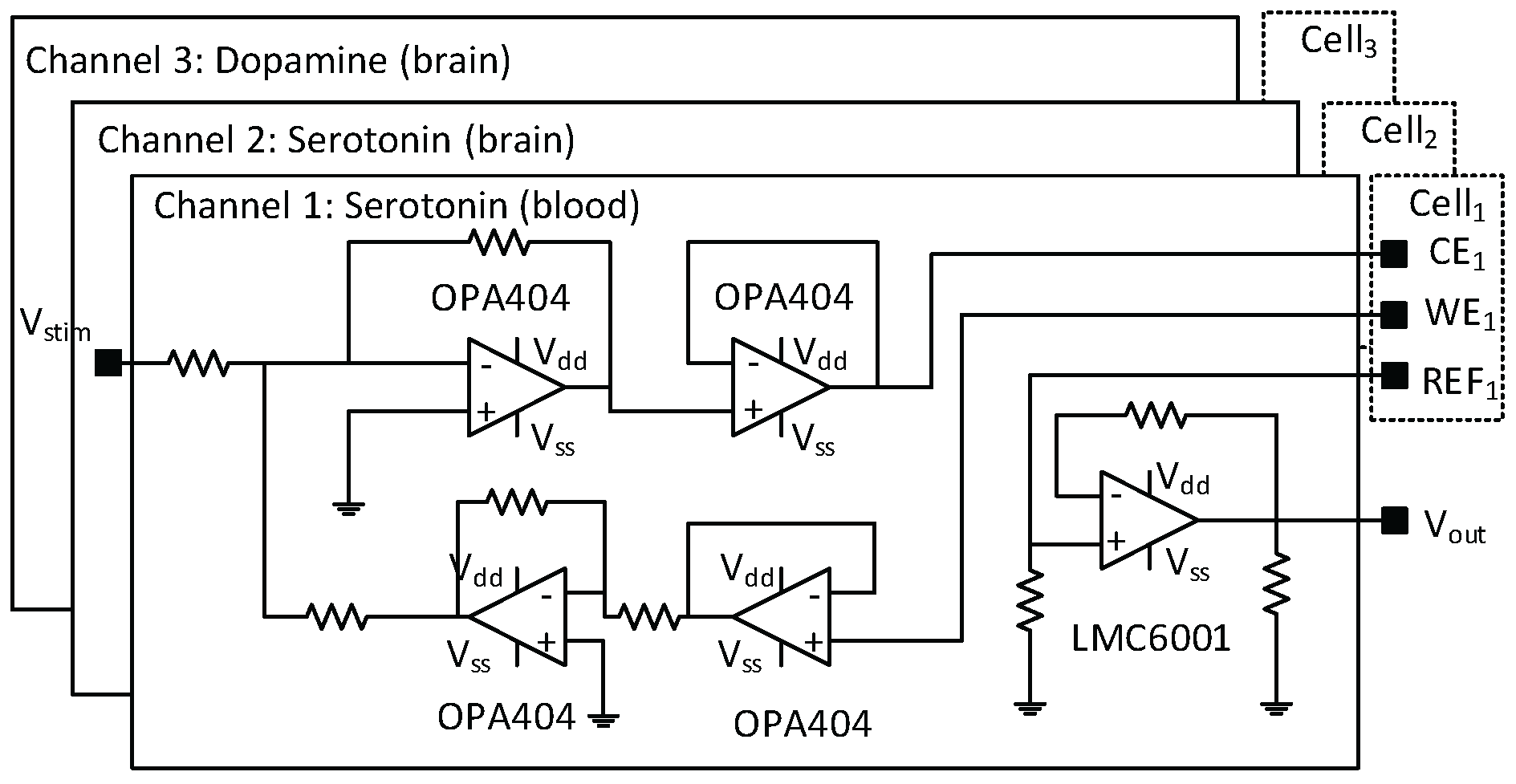
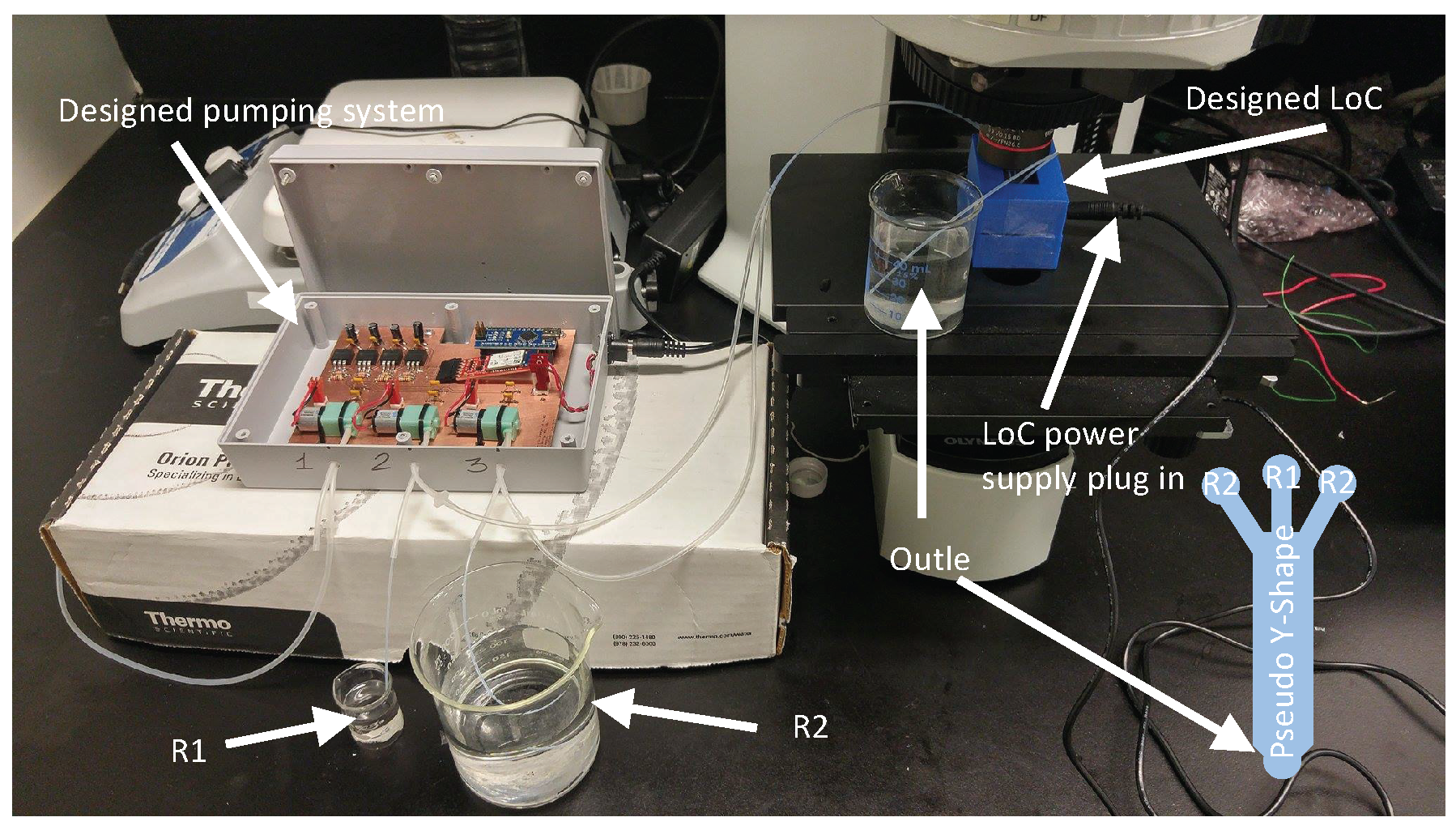
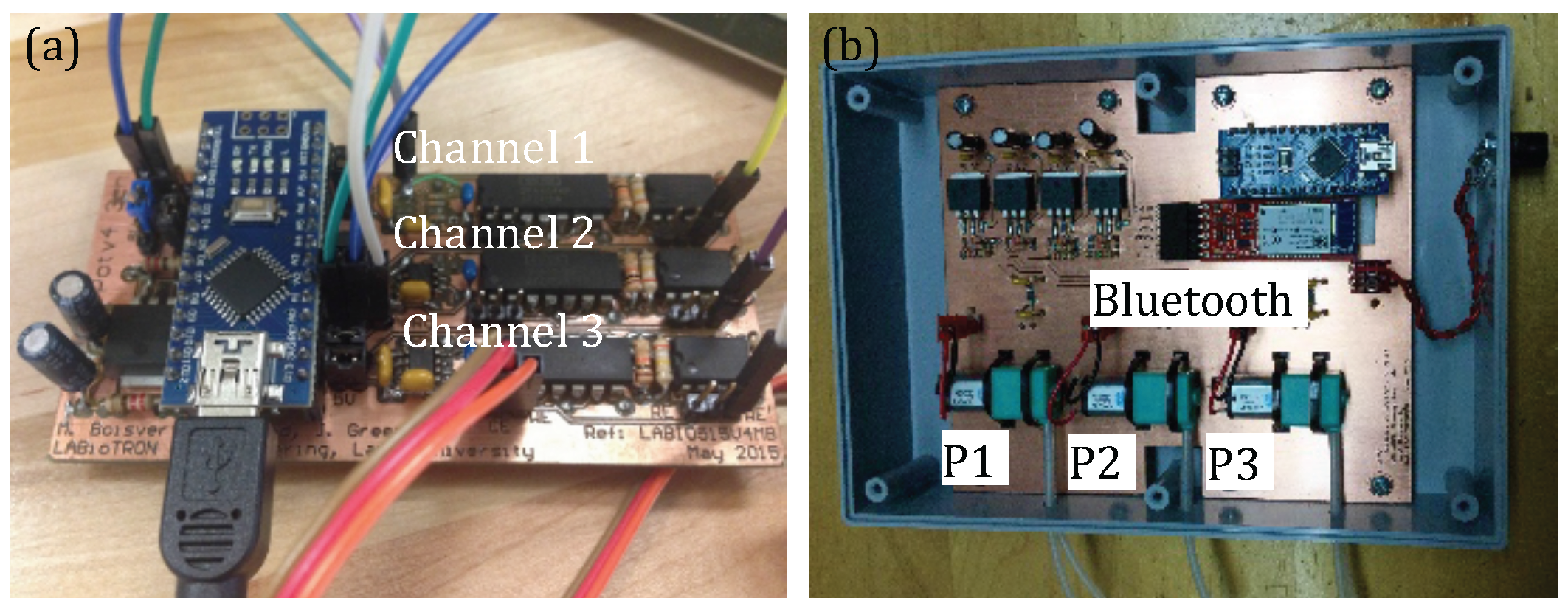
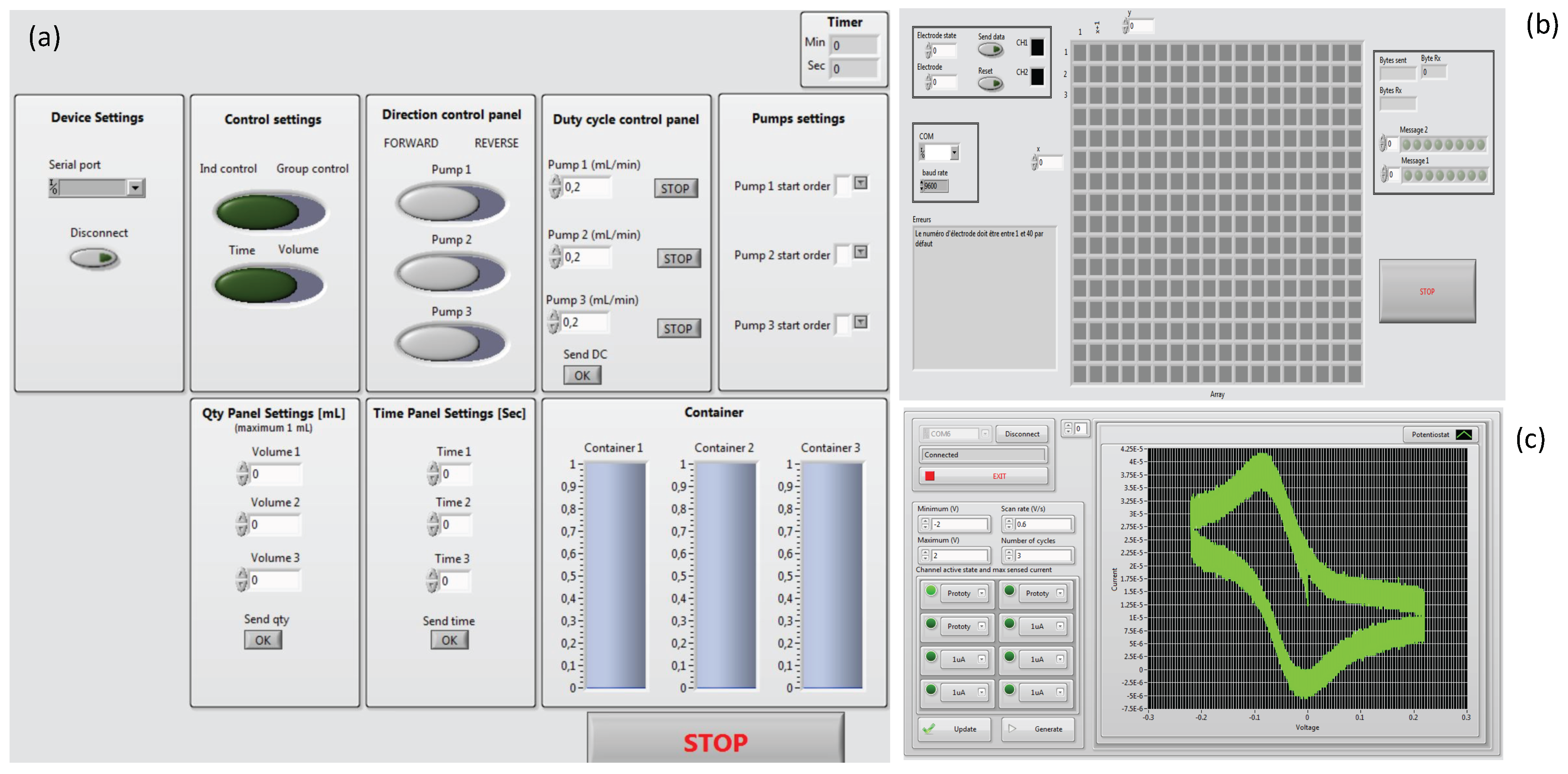
3.1. System Configuration
3.2. Niosome Generation System
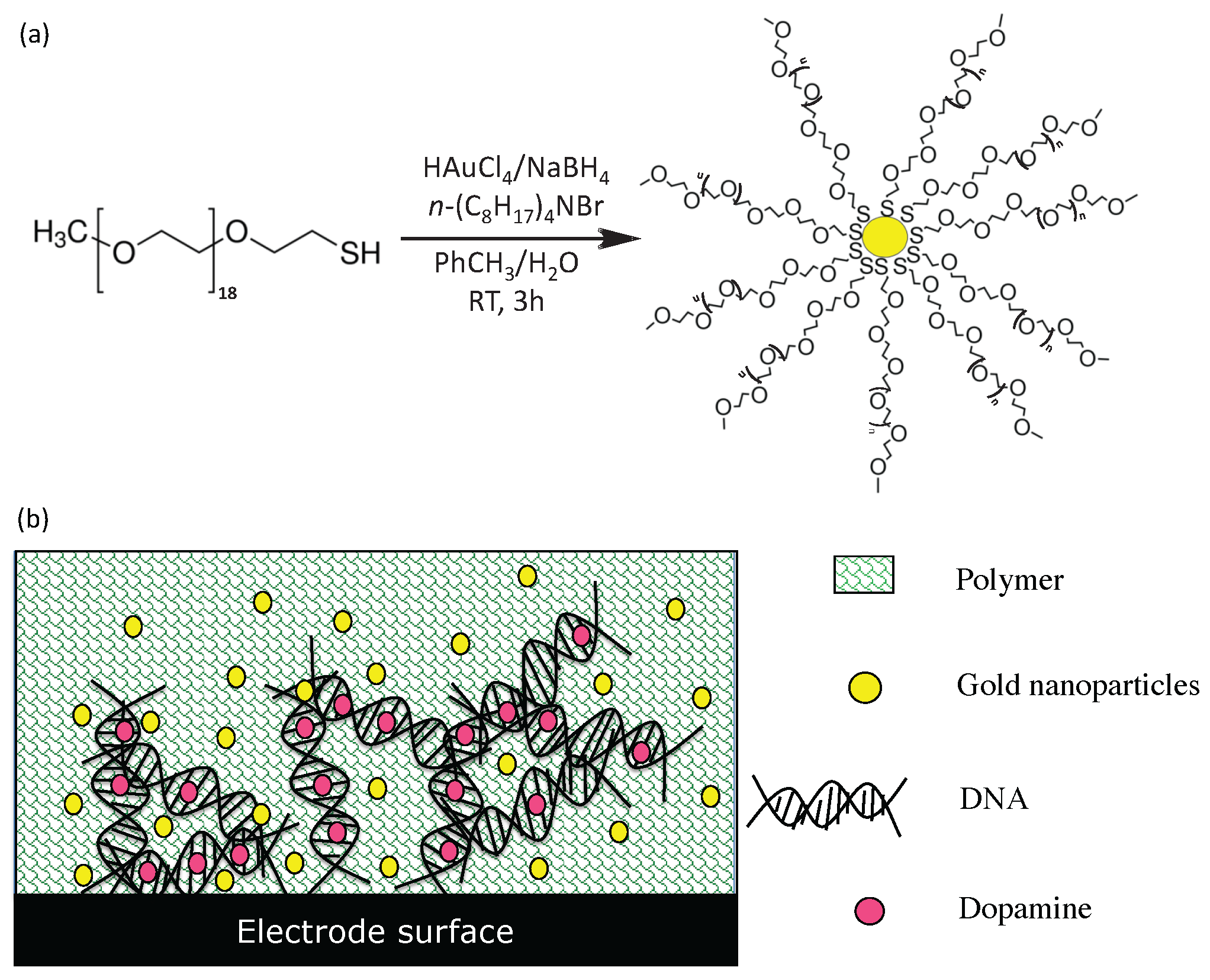
3.3. Fabrication of Functionalized Electrodes for Enhanced Sensitivity and Selectivity of Dopamine Detection
4. Results
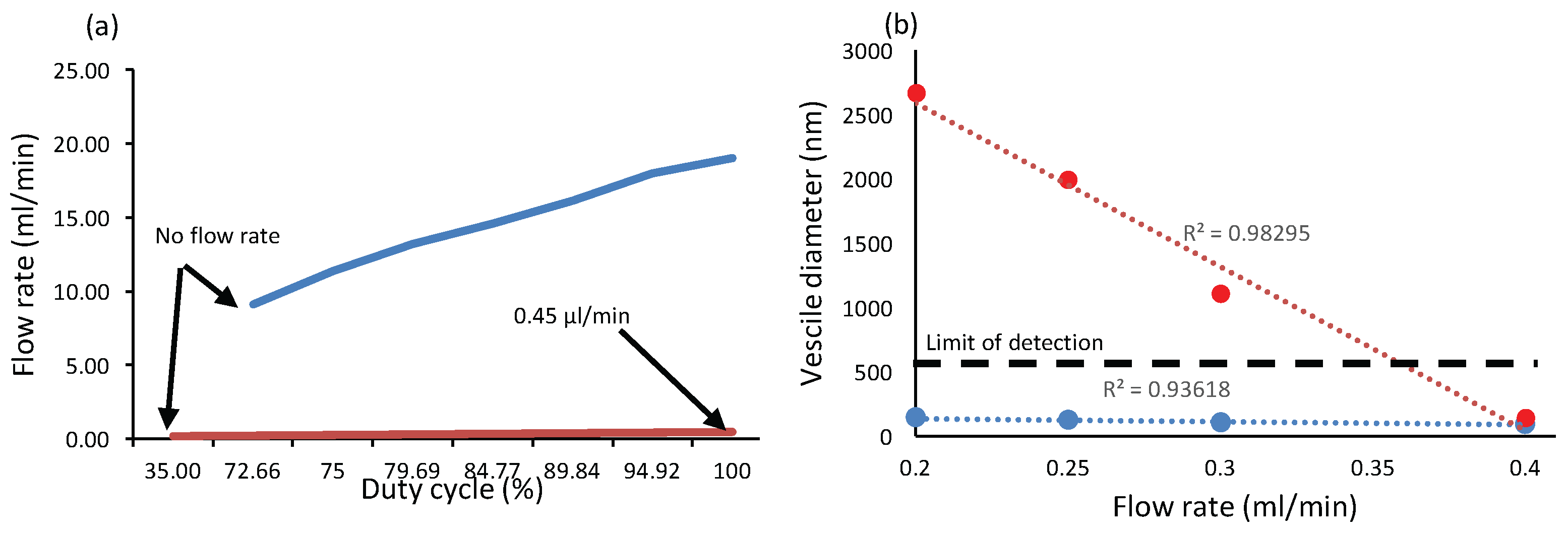
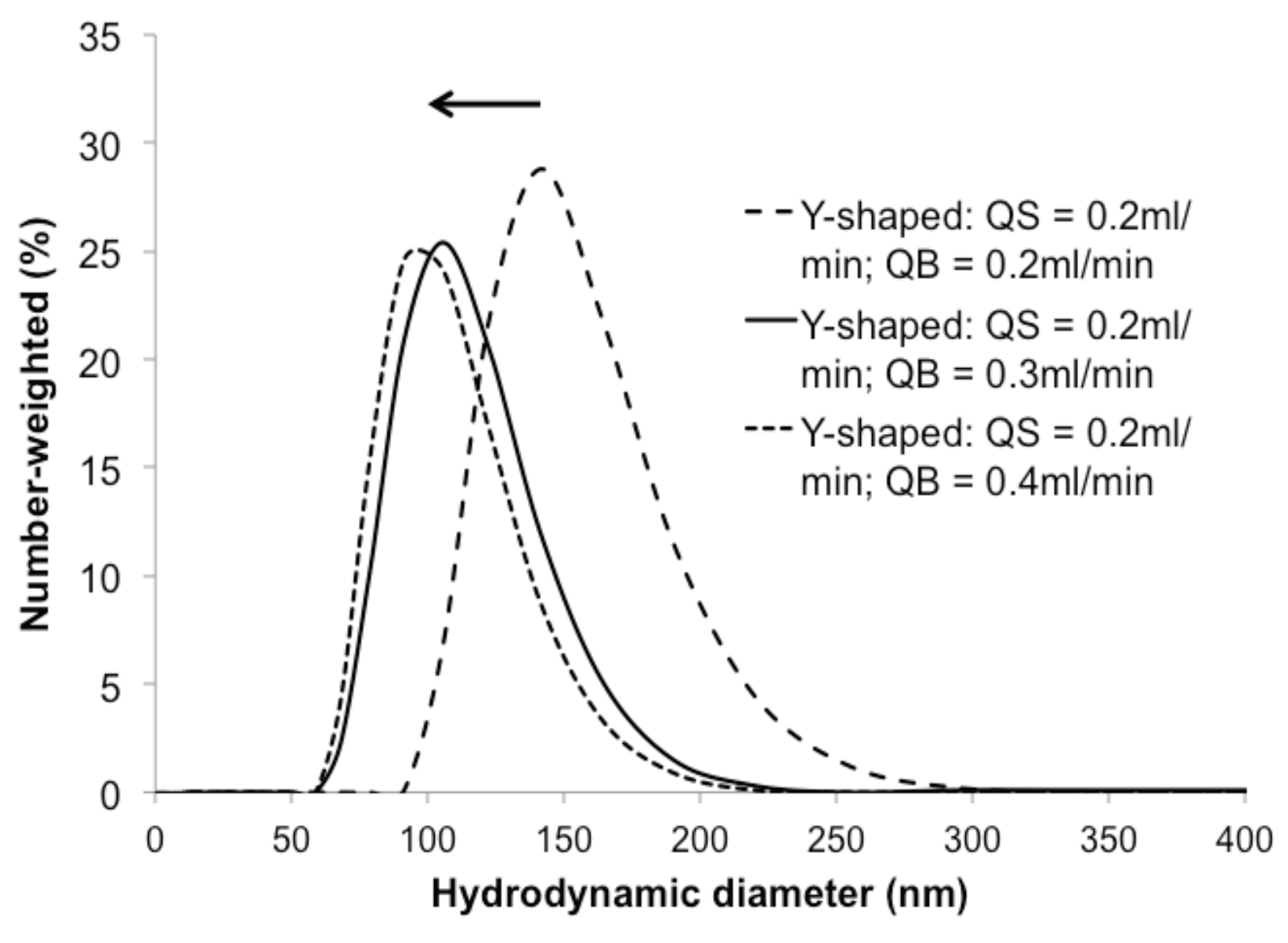
4.1. Niosome Generation Module
4.2. Electrochemical Detection of Serotonin
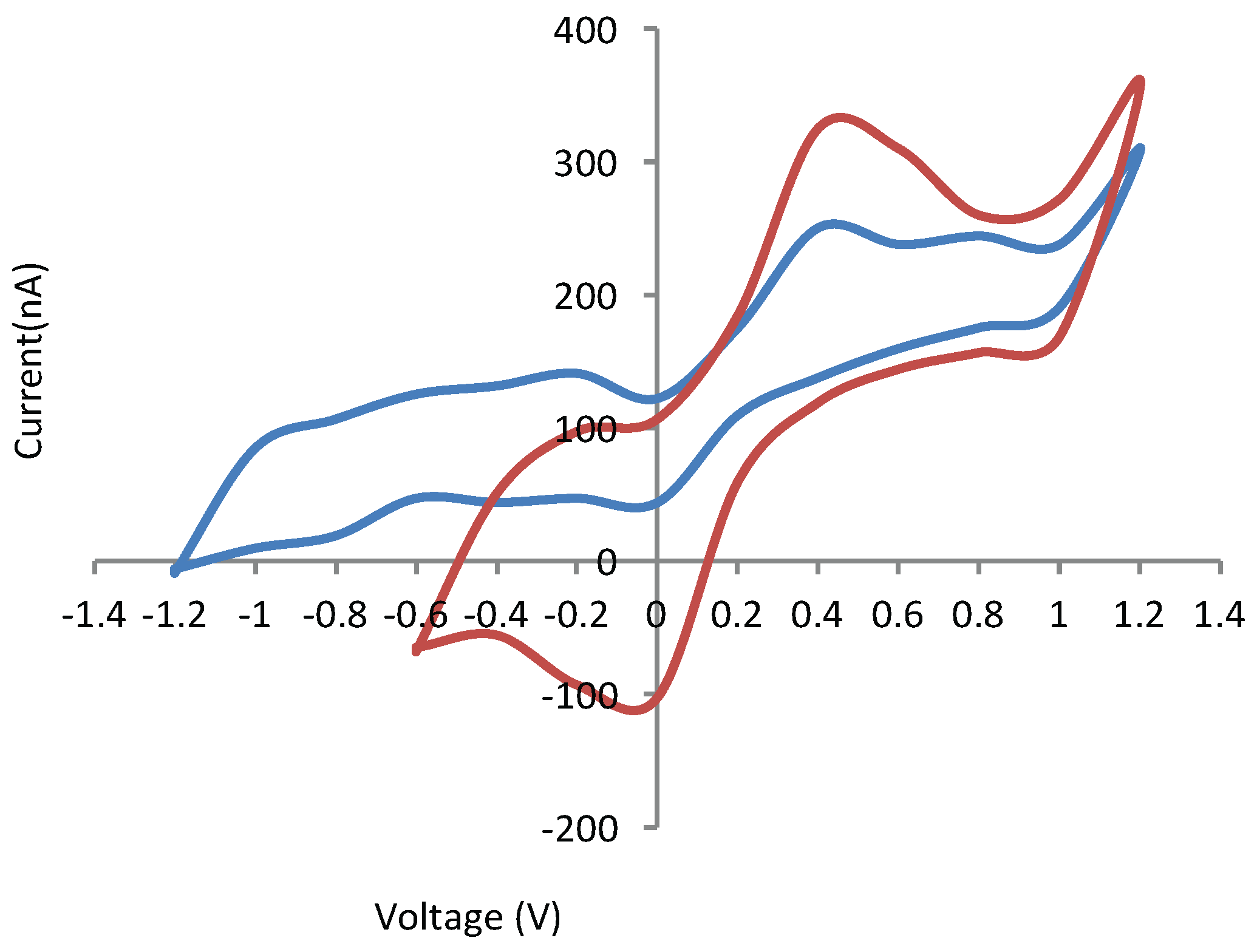
4.3. Electrochemical Detection of Dopamine Module
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Serotonin Detection
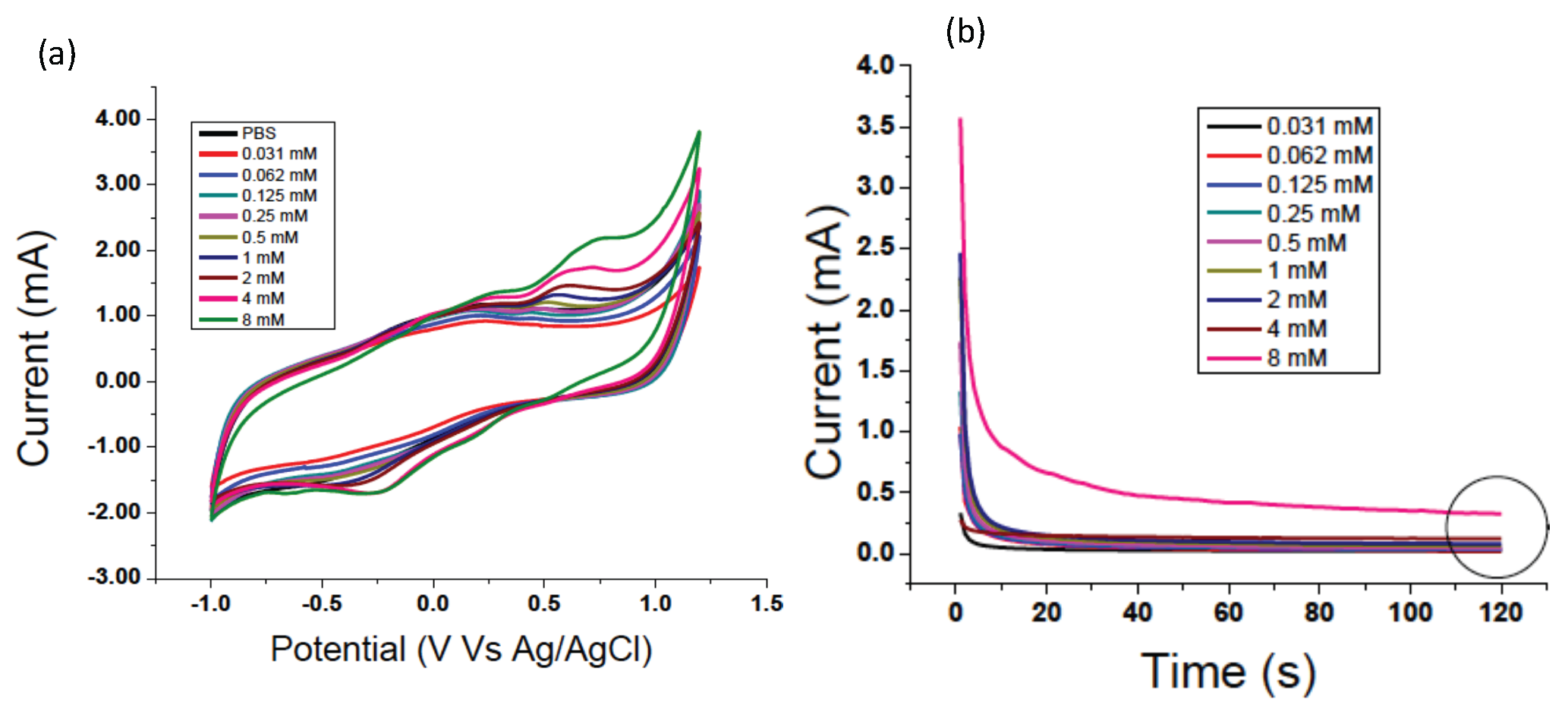
Appendix B. Gold-Based Electrode Experiments
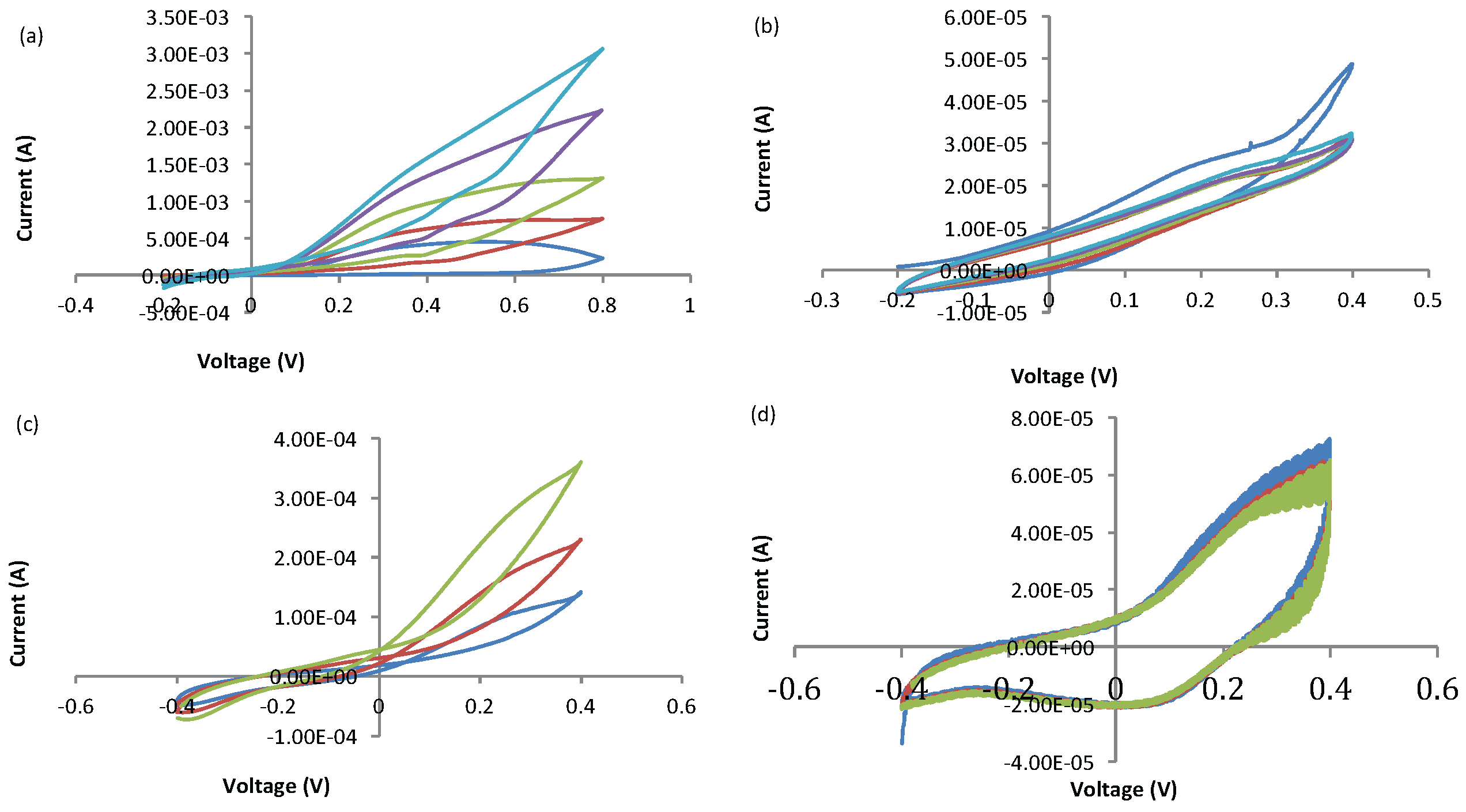
| (V) | (V) | (V) | (μA) | (μA) | |
|---|---|---|---|---|---|
| Electrode 1 | −0.07 | 0.16 | 0.23 | −1.50 | 2.09 |
| Electrode 2 | −0.07 | 0.16 | 0.23 | −1.16 | 1.70 |
| Electrode 3 | −0.07 | 0.14 | 0.21 | −1.47 | 2.05 |
| Electrode 4 | −0.07 | 0.14 | 0.21 | −1.26 | 1.78 |
| Electrode 5 | −0.08 | 0.15 | 0.23 | −1.90 | 2.30 |
| Electrode 6 | −0.07 | 0.13 | 0.20 | −1.35 | 1.80 |
| Average | −0.07 | 0.15 | 0.22 | −1.44 | 1.95 |
| Standard deviation | 0.003 | 0.011 | 0.012 | 0.259 | 0.231 |
References
- Van Reenen, A.; de Jong, A.M.; den Toonder, J.M.J.; Prins, M.W.J. Integrated lab-on-chip biosensing systems based on magnetic particle actuation—A comprehensive review. Lab Chip 2014, 14, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Sharma, S.; Srisa-Art, M.; Hollfelder, F.; Edel, J.B.; Demello, A.J. Microdroplets: A sea of applications? Lab Chip 2008, 8, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Abgrall, P.; Gue, A.M. Lab-on-chip technologies: making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15–R49. [Google Scholar] [CrossRef]
- Schwarz, B.; Reininger, P.; Ristanic, D.; Detz, H.; Andrews, A.M.; Schrenk, W.; Strasser, G. Monolithically integrated mid-infrared lab-on-a-chip using plasmonics and quantum cascade structures. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lei, N.; Sheadel, D.A.; Xu, J.; Xue, W. Integration of nanosensors into a sealed microchannel in a hybrid lab-on-a-chip device. Sens. Actuators B Chem. 2012, 166–167, 870–877. [Google Scholar] [CrossRef]
- Zuo, P.; Li, X.; Dominguez, D.C.; Ye, B.C. A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 2013, 13, 3921–3928. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, J.; Niu, L.G.; Wu, S.Z.; Midorikawa, K.; Sugioka, K. In-channel integration of designable microoptical devices using flat scaffold-supported femtosecond-laser microfabrication for coupling-free optofluidic cell counting. Light Sci. Appl. 2015, 4, e228. [Google Scholar] [CrossRef]
- Petralia, S.; Verardo, R.; Klaric, E.; Cavallaro, S.; Alessi, E.; Schneider, C. In-Check system: A highly integrated silicon Lab-on-Chip for sample preparation, PCR amplification and microarray detection of nucleic acids directly from biological samples. Sens. Actuators B Chem. 2013, 187, 99–105. [Google Scholar] [CrossRef]
- Miled, A.; Sawan, M. Dielectrophoresis-Based Integrated Lab-on-Chip for Nano and Micro-Particles Manipulation and Capacitive Detection. IEEE Trans. Biomed. Circuits. Syst. 2012, 6, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Schmalz, K.; Wenger, C.; Herzel, F. Self-calibrating highly sensitive dynamic capacitance sensor: towards rapid sensing and counting of particles in laminar flow systems. Analyst 2015, 140, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Shendruk, T.N.; Tahvildari, R.; Catafard, N.M.; Andrzejewski, L.; Gigault, C.; Todd, A.; Gagne-Dumais, L.; Slater, G.W.; Godin, M. Field-flow fractionation and hydrodynamic chromatography on a microfluidic chip. Anal. Chem. 2013, 85, 5981–5988. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kilpatrick, P.K.; Melvin, E.; Velev, O.D. On-chip latex agglutination immunoassay readout by electrochemical impedance spectroscopy. Lab Chip 2012, 12, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Picher, M.M.; Kupcu, S.; Huang, C.J.; Dostalek, J.; Pum, D.; Sleytr, U.B.; Ertl, P. Nanobiotechnology advanced antifouling surfaces for the continuous electrochemical monitoring of glucose in whole blood using a lab-on-a-chip. Lab Chip 2013, 13, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, H.J.; Kang, M.W.; Jeon, N.L. Advances in microfluidics-based experimental methods for neuroscience research. Lab Chip 2013, 13, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Fukuda, M.; Shirakawa, K.; Jiko, H.; Gotoh, M. Carbon nanotube multi-electrode array chips for noninvasive real-time measurement of dopamine, action potentials, and postsynaptic potentials. Biosens. Bioelectron. 2013, 49, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Reitz, A.; Mathault, J.; Mehou-Loko, S.; Amirdehi, M.A.; Miled, A.; Greener, J. Electrochemical imaging for microfluidics: A full-system approach. Lab Chip 2016, 16, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Miled, A.; Sawan, M. Dielectrophoresis-Based Integrated Lab-on-Chip for Nano and Micro-Particles Manipulation and Capacitive Detection. IEEE Trans. Biomed. Circuits. Syst. 2012, 2, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Yuen, P.K. SmartBuild-A truly plug-n-play modular microfluidic system. Lab Chip 2008, 8, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Greener, J.; Tumarkin, E.; Debono, M.; Kwan, C.H.; Abolhasani, M.; Guenther, A.; Kumacheva, E. Development and applications of a microfluidic reactor with multiple analytical probes. Analyst 2012, 137, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Renaudot, R.; Agache, V.; Fouillet, Y.; Laffite, G.; Bisceglia, E.; Jalabert, L.; Kumemura, M.; Collard, D.; Fujita, H. A programmable and reconfigurable microfluidic chip. Lab Chip 2013, 13, 4517–4524. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kreit, E.; Liu, Y.; Heikenfeld, J.; Papautsky, I. Reconfigurable virtual electrowetting channels. Lab Chip 2012, 12, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.; Taylor, J.M.; Stoller, M.A.; Morin, S.A. Reconfigurable microfluidic systems with reversible seals compatible with 2D and 3D surfaces of arbitrary chemical composition. Lab Chip 2015, 15, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, F.; Harnois, M.; Coffinier, Y.; Boukherroub, R.; Thomy, V. Split and flow: Reconfigurable capillary connection for digital microfluidic devices. Lab Chip 2014, 14, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Yee, H.J.; Lee, K.S.; Lee, W.Y.; Shin, M.C.; Kim, T.H.; Kim, S.R. Determination of breath alcohol using a differential-type amperometric biosensor based on alcohol dehydrogenase. Anal. Chim. Acta 1999, 390, 83–91. [Google Scholar] [CrossRef]
- Tortora, G.J.; Grabowski, S.R. Principles of Anatomy and Physiology; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.D.; Tolliver, T.J.; Huang, S.J.; Li, Q.; Bengel, D.; Murphy, D.L. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am. J. Med. Genet. 1999, 88, 83–87. [Google Scholar] [CrossRef]
- Golino, P.; Piscione, F.; Willerson, J.T.; Cappelli-Bigazzi, M.; Focaccio, A.; Villari, B.; Indolfi, C.; Russolillo, E.; Condorelli, M.; Chiariello, M. Divergent effects of serotonin on coronary-artery dimensions and blood flow in patients with coronary atherosclerosis and control patients. N. Engl. J. Med. 1991, 324, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, E.R.; Yuwiler, A.; Geller, E.; Ornitz, E.M.; Saeger, K.; Plotkin, S. Increased blood serotonin and platelets in early infantile autism. Arch. Gen. Psychiatry 1970, 23, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Blanco, P.; Nigrovic, P.A. Platelets: Active players in the pathogenesis of arthritis and SLE. Nat. Rev. Rheumatol 2012, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Paré, A.; Farndale, R.W.; Schumacher, H.R.; Nigrovic, P.A.; Lacroix, S.; Boilard, E. Platelets can enhance vascular permeability. Blood 2012, 120, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.A.; Lindon, J.C.; Cloarec, O.; Antti, H.; Charuel, C.; Hanton, G.; Provost, J.P.; Le Net, J.L.; Baker, D.; Walley, R.J.; et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 2006, 440, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; DeVoe, D.L. High-hroughput Continuous Flow Production of Nanoscale Liposomes by Microfluidic Vertical Flow Focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; de Vos, P.; Niclou, S.P. Drug and cell encapsulation: Alternative delivery options for the treatment of malignant brain tumors. Advan. Drug Deliv. Rev. 2014, 67–68, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid-Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosens. Bioelectron. 2015, 66, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; Marrazza, G. Gold and Magnetic Nanoparticles-Based Electrochemical Biosensors for Cancer Biomarker Determination. J. Nanosci. Nanotechnol. 2015, 15, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, E. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.T.; Jahn, A.; Locascio, L.E.; Vreeland, W.N. Controlled self-assembly of monodisperse niosomes by microfluidic hydrodynamic focusing. Langmuir 2010, 26, 8559–8566. [Google Scholar] [CrossRef] [PubMed]



| Application Specifications | |||
|---|---|---|---|
| Serotonin | Dopamine | Niosome | |
| Liquid actuation | Pumping system: withdraw/infusion | Pumping system: withdraw/infusion | Pumping system: infusion |
| Sensing | Electrochemical | Electrochemical | No sensing |
| Data acquisition | Yes | Yes | No |
| LoC specifications | |||
| Pumping | Infusion/Withdraw | ||
| Sensing | Electrochemical | ||
| # channels | 3 independent potentiostat channels | ||
| # sensing site | 40 sites. Each site corresponds to one electrode | ||
| Voltage range | −1.5 V to 1.5 V (stimulation) | ||
| Maximum Frequency | 165 kHz (stimulation) | ||
| Wave shape | Sine, triangular or square (stimulation) | ||
| Microfluidics | Pseudo-Y-shape chamber, consumable | ||
| Interface | USB or Wireless | ||
| Software | LabVIEW | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, A.; Rouillard, C.; Mathault, J.; Boisvert, M.; Tessier, F.; Landari, H.; Melki, I.; Laprise-Pelletier, M.; Boisselier, E.; Fortin, M.-A.; et al. Towards a Multifunctional Electrochemical Sensing and Niosome Generation Lab-on-Chip Platform Based on a Plug-and-Play Concept. Sensors 2016, 16, 778. https://doi.org/10.3390/s16060778
Kara A, Rouillard C, Mathault J, Boisvert M, Tessier F, Landari H, Melki I, Laprise-Pelletier M, Boisselier E, Fortin M-A, et al. Towards a Multifunctional Electrochemical Sensing and Niosome Generation Lab-on-Chip Platform Based on a Plug-and-Play Concept. Sensors. 2016; 16(6):778. https://doi.org/10.3390/s16060778
Chicago/Turabian StyleKara, Adnane, Camille Rouillard, Jessy Mathault, Martin Boisvert, Frédéric Tessier, Hamza Landari, Imene Melki, Myriam Laprise-Pelletier, Elodie Boisselier, Marc-André Fortin, and et al. 2016. "Towards a Multifunctional Electrochemical Sensing and Niosome Generation Lab-on-Chip Platform Based on a Plug-and-Play Concept" Sensors 16, no. 6: 778. https://doi.org/10.3390/s16060778
APA StyleKara, A., Rouillard, C., Mathault, J., Boisvert, M., Tessier, F., Landari, H., Melki, I., Laprise-Pelletier, M., Boisselier, E., Fortin, M.-A., Boilard, E., Greener, J., & Miled, A. (2016). Towards a Multifunctional Electrochemical Sensing and Niosome Generation Lab-on-Chip Platform Based on a Plug-and-Play Concept. Sensors, 16(6), 778. https://doi.org/10.3390/s16060778






