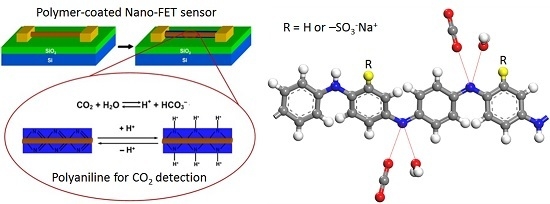
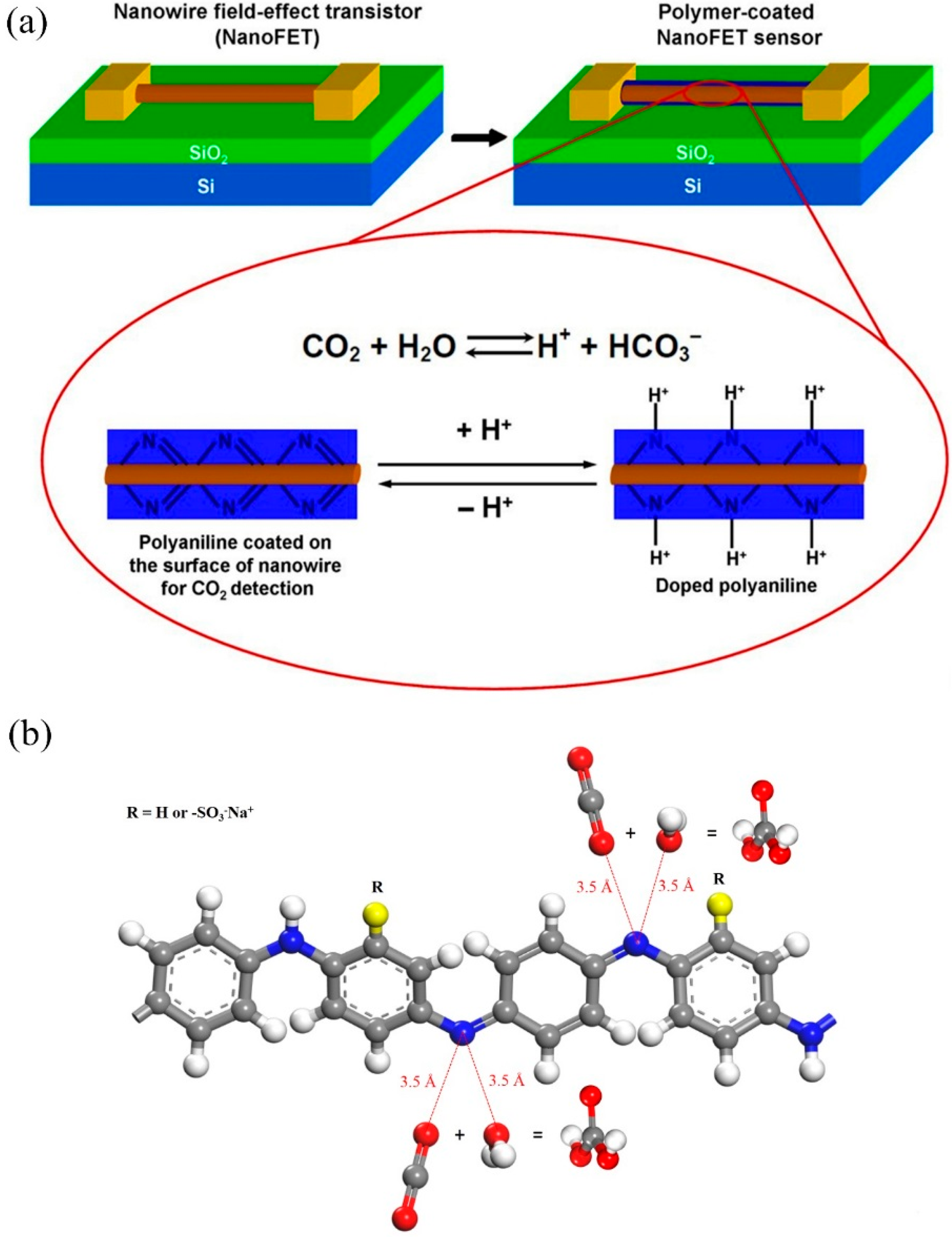
Sorption and Diffusion of Water Vapor and Carbon Dioxide in Sulfonated Polyaniline as Chemical Sensing Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atomistic Models
2.2. Simulation
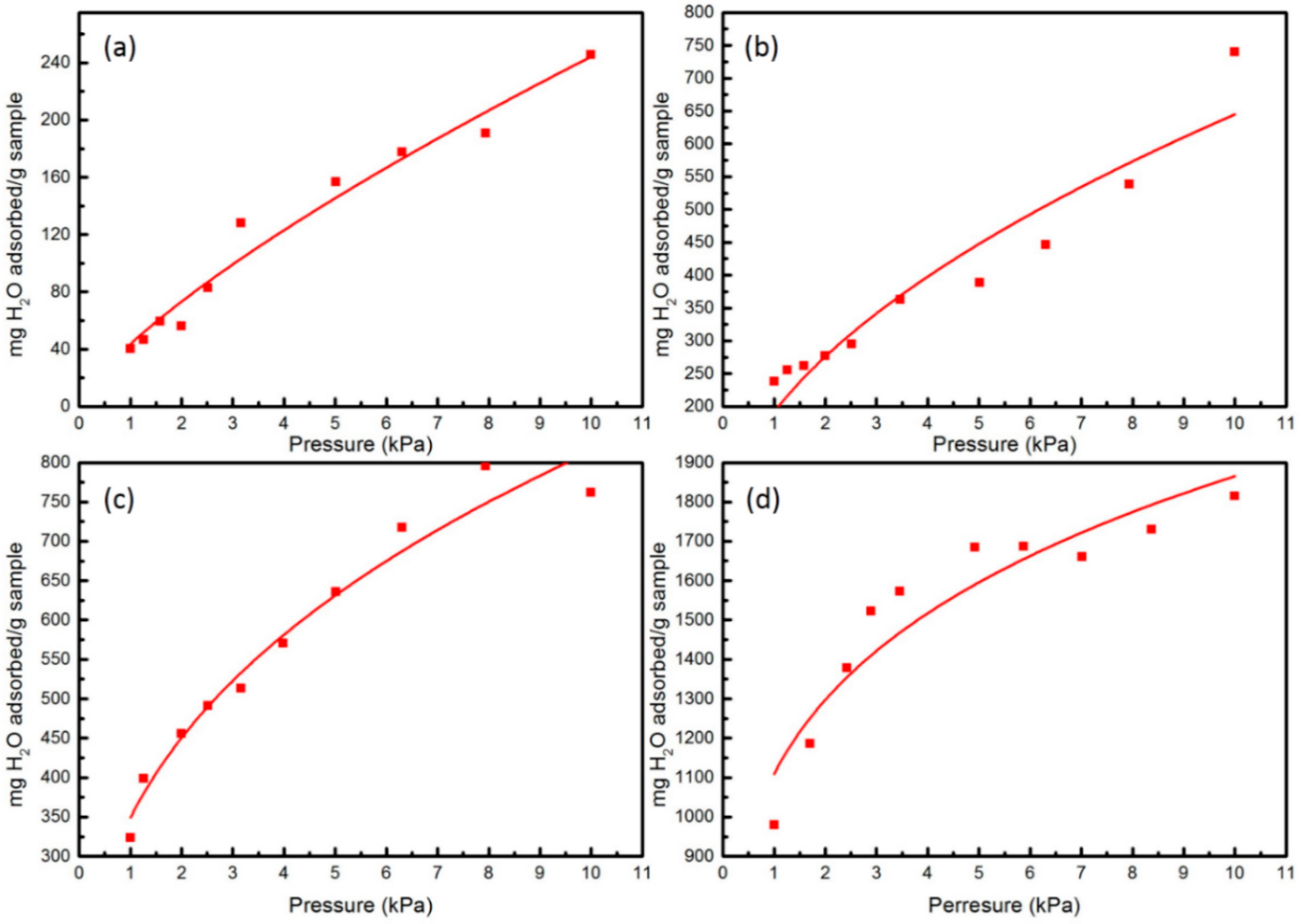
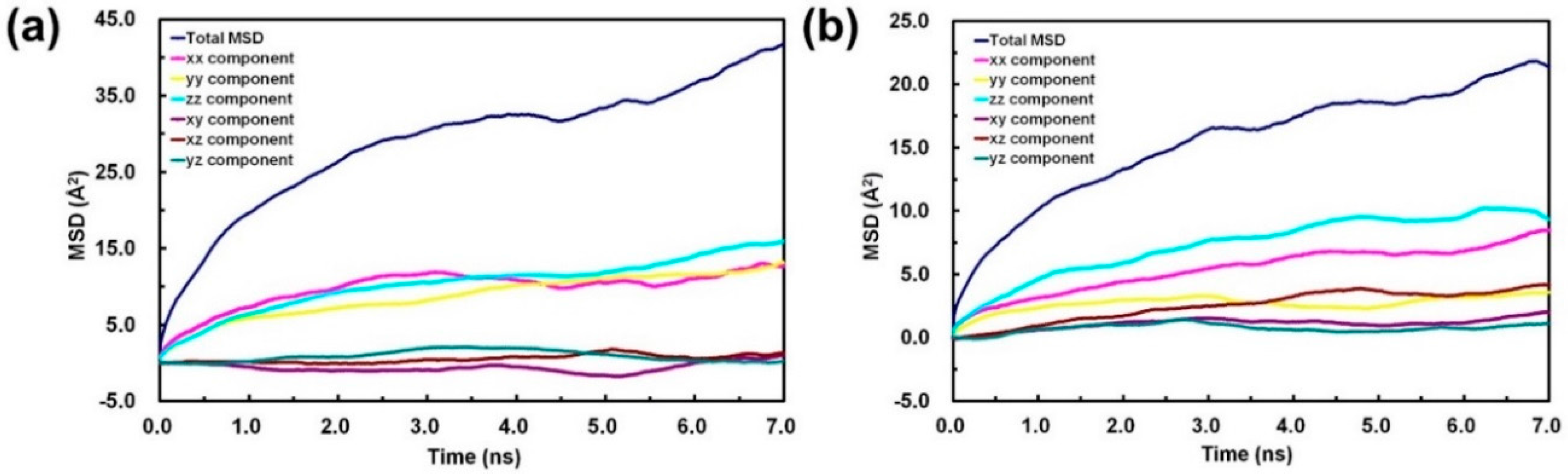
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- MacDiarmid, A.G. A novel role for organic polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Chen, X.P.; Shen, L.; Yuan, C.A.; Wong, C.K.Y.; Zhang, G.Q. Molecular model for the charge carrier density dependence of conductivity of polyaniline as chemical sensing materials. Sens. Actuators B Chem. 2013, 177, 856–861. [Google Scholar] [CrossRef]
- Freund, M.S.; Deore, B.A. Self-Doped Conducting Polymers; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Pinto, N.J.; Johnson, A.T., Jr.; MacDiarmid, A.G.; Mueller, C.H.; Theofylaktos, N.; Robinson, D.C.; Miranda, F.A. Electrospun polyaniline/polyethylene oxide nanofiber field-effect transistor. Appl. Phys. Lett. 2003, 83, 4244–4246. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.X.; Duan, Y.X. An improved optical pH sensor based on polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Gustafsson, G.; Cao, Y.; Treacy, G.M.; Klavetter, F.; Colaneri, N.; Heeger, A.J. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992, 357, 477–479. [Google Scholar] [CrossRef]
- Wang, K.; Meng, Q.H.; Zhang, Y.J.; Wei, Z.X.; Miao, M.H. High-performance two-ply yarn supercapacitors based on carbon nanotubes and polyaniline nanowire arrays. Adv. Mater. 2013, 25, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Liang, J.; Tao, Z.L.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tan, T.; Lee, J. Corrosion protection of mild steel by electroactive polyaniline coatings. Synthetic. Met. 1997, 88, 237–242. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X. Intrinsically conducting polymers for electromagnetic interference shielding. Polym. Adv. Technol. 2005, 16, 344–351. [Google Scholar] [CrossRef]
- Shen, P.; Huang, H.; Tseung, A. A Study of tungsten trioxide and polyaniline composite films I. Electrochemical and electrochromic behavior. J. Am. Chem. Soc. 1992, 139, 1840–1845. [Google Scholar]
- Ostwal, M.M.; Tsotsis, T.T.; Sahimi, M. Molecular dynamics simulation of diffusion and sorption of water in conducting polyaniline. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, M.M.; Sahimi, M.; Tsotsis, T.T. Water harvesting using a conducting polymer: A study by molecular dynamics simulation. Phys. Rev. E 2009, 79. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; MacDiarmid, A.G. Effect of sulfonic acid group on polyaniline backbone. J. Am. Chem. Soc. 1991, 113, 2665–2671. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Aqueous microwave chemistry: A clean and green synthetic tool for rapid drug discovery. Chem. Soc. Rev. 2008, 37, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens. Actuators B Chem. 2006, 114, 218–222. [Google Scholar] [CrossRef]
- Chen, X.P.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G.Q. Impact of the functional group on the working range of polyaniline as carbon dioxide sensors. Sens. Actuators B Chem. 2012, 175, 15–21. [Google Scholar] [CrossRef]
- Wei, X.L.; Wang, Y.Z.; Long, S.; Bobeczko, C.; Epstein, A.J. Synthesis and physical properties of highly sulfonated polyaniline. J. Am. Chem. Soc. 1996, 118, 2545–2555. [Google Scholar] [CrossRef]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; O’Keeffe, M.; Yaghi, O.M. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J. Am. Chem. Soc. 2009, 131, 3875–3877. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, X.; Ruan, L.-W.; Liu, B.; Wang, Y.-M.; Zhu, J.-F.; Qiu, L.-G. Post-combustion CO2 capture with the HKUST-1 and MIL-101(Cr) metal–organic frameworks: Adsorption, separation and regeneration investigations. Micropor. Mesopor. Mater. 2013, 179, 191–197. [Google Scholar] [CrossRef]
- Liu, Q.; Ning, L.; Zheng, S.; Tao, M.; Shi, Y.; He, Y. Adsorption of carbon dioxide by MIL-101 (Cr): Regeneration conditions and influence of flue gas contaminants. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Freund, M.S.; Jayas, D.S.; Shafai, C.; Thomson, D.J.; White, N.D.G. Development of carbon dioxide (CO2) sensor for grain quality monitoring. Biosyst. Eng. 2010, 106, 395–404. [Google Scholar] [CrossRef]
- Ostwal, M.M.; Pellegrino, J.; Norris, I.; Tsotsis, T.T.; Sahimi, M.; Mattes, B.R. Water sorption of acid-doped polyaniline solid fibers: Equilibrium and kinetic response. Ind. Eng. Chem. Res. 2005, 44, 7860–7867. [Google Scholar] [CrossRef]
- Zhang, T.; Nix, M.B.; Yoo, B.-Y.; Deshusses, M.A.; Myung, N.V. Electrochemically functionalized single-walled carbon nanotube gas sensor. Electroanalysis 2006, 18, 1153–1158. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, Y.; Pan, W.; Li, D.; Wu, Z.; Li, Y. Fabrication and characterization of polyaniline-based gas sensor by ultra-thin film technology. Sens. Actuators B Chem. 2002, 81, 158–164. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Baker, C.O.; Powell, D.A.; Wlodarski, W.; Kaner, R.B.; Kalantar-zadeh, K. Polyaniline nanofiber based surface acoustic wave gas sensors—Effect of nanofiber diameter on H2 response. IEEE Sens. J. 2007, 7, 213–218. [Google Scholar] [CrossRef]
- Arsat, R.; Yu, X.; Li, Y.; Wlodarski, W.; Kalantar-Zadeh, K. Hydrogen gas sensor based on highly ordered polyaniline nanofibers. Sens. Actuators B Chem. 2009, 137, 529–532. [Google Scholar] [CrossRef]
- Atashbar, M.; Sadek, A.; Wlodarski, W.; Sriram, S.; Bhaskaran, M.; Cheng, C.; Kaner, R.; Kalantar-Zadeh, K. Layered SAW gas sensor based on CSA synthesized polyaniline nanofiber on AlN on 64° YX LiNbO3 for H2 sensing. Sens. Actuators B Chem. 2009, 138, 85–89. [Google Scholar] [CrossRef]
- Li, G.; Martinez, C.; Semancik, S. Controlled electrophoretic patterning of polyaniline from a colloidal suspension. J. Am. Chem. Soc. 2005, 127, 4903–4909. [Google Scholar] [CrossRef] [PubMed]
- Athawale, A.A.; Bhagwat, S.; Katre, P.P. Nanocomposite of Pd-polyaniline as a selective methanol sensor. Sens. Actuators B Chem. 2006, 114, 263–267. [Google Scholar] [CrossRef]
- Sharma, S.; Nirkhe, C.; Pethkar, S.; Athawale, A.A. Chloroform vapour sensor based on copper/polyaniline nanocomposite. Sens. Actuators B Chem. 2002, 85, 131–136. [Google Scholar] [CrossRef]
- Doan, T.C.; Ramaneti, R.; Baggerman, J.; van der Bent, J.F.; Marcelis, A.T.; Tong, H.D.; van Rijn, C.J. Carbon dioxide sensing with sulfonated polyaniline. Sens. Actuators B Chem. 2012, 168, 123–130. [Google Scholar] [CrossRef]
- Chen, X.P.; Jiang, J.K.; Liang, Q.H.; Yang, N.; Ye, H.Y.; Cai, M.; Shen, L.; Yang, D.G.; Ren, T.L. First-principles study of the effect of functional groups on polyaniline backbone. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Shah, A.-H.A.; Bilal, S.; Ayub, K. DFT Study of Polyaniline NH3, CO2, and CO Gas Sensors: Comparison with Recent Experimental Data. J. Phys. Chem. C 2013, 117, 23701–23711. [Google Scholar] [CrossRef]
- Chen, X.P.; Yang, N.; Jiang, J.K.; Liang, Q.H.; Yang, D.G.; Zhang, G.Q.; Ren, T.L. Ab initio Study of Temperature, Humidity and Covalent Functionalization Induced Band Gap Change of Single-Walled Carbon Nanotubes. IEEE Electr. Device Lett. 2015, 36, 606–608. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Athawale, A.A.; Kulkarni, M.V. Polyaniline and its substituted derivatives as sensor for aliphatic alcohols. Sens. Actuators B Chem. 2000, 67, 173–177. [Google Scholar] [CrossRef]
- Chen, X.P.; Yuan, C.A.; Wong, C.K.Y.; Koh, S.W.; Zhang, G.Q. Validation of forcefields in predicting the physical and thermophysical properties of emeraldine base polyaniline. Mol. Simulat. 2011, 37, 990–996. [Google Scholar] [CrossRef]
- Chen, X.P.; Yuan, C.A.; Wong, C.K.Y.; Zhang, G.Q. Molecular modeling of temperature dependence of solubility parameters for amorphous polymers. J. Mol. Model. 2012, 18, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Berashevich, J.; Chakraborty, T. Tunable band gap and magnetic ordering by adsorption of molecules on graphene. Phys. Rev. B 2009, 80. [Google Scholar] [CrossRef]
- Shih, Y.C.; Chen, C.S.; Wu, K.C. First-principles surface stress calculations and multiscale deformation analysis of a self-assembled monolayer adsorbed on a micro-cantilever. Sensors 2014, 14, 7435–7450. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Liang, Q.H.; Jiang, J.K.; Wong, C.K.Y.; Leung, S.Y.Y.; Ye, H.Y.; Yang, D.G.; Ren, T.L. Functionalization-induced changes in the structural and physical properties of amorphous polyaniline: A first-principles and molecular dynamics study. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, M.M.; Qi, B.; Pellegrino, J.; Fadeev, A.G.; Norris, I.D.; Tsotsis, T.T.; Sahimi, M.; Mattes, B.R. Water sorption of acid-doped polyaniline powders and hollow fibers: Equilibrium and kinetic response. Ind. Eng. Chem. Res. 2006, 45, 6021–6031. [Google Scholar] [CrossRef]
- Meunier, M. Diffusion coefficients of small gas molecules in amorphous cis-1,4-polybutadiene estimated by molecular dynamics simulations. J. Chem. Phys. 2005, 123, 134906. [Google Scholar] [CrossRef] [PubMed]




| Geometry Optimization | Equilibration Process of Structure | ||
|---|---|---|---|
| Forefield: COMPASS | Step | Simulation conditions | Time (ps) |
| Quality: Fine | |||
| Summation method: Ewald for electrostatic and atom base for van der Waals (vdW) | 1 | NPT, 1 GPa, 298 K | 20 |
| Cutoff distance: 10.5 Å for sorption and 15.5 Å for diffusion | 2 | NPT, 0.5 GPa, 298 K | 50 |
| Algorithm: smart | 3 | NPT, 0.0001 GPa, 298 K | 200 |
| ‘Fine’ convergence tolerance | 4 | A stepwise procedure of NVT of heating from 298 K to 698 K and cooling from 698 K down to 298 K by a step of 50 K | 50 ps/stepwise |
| 5 cycles | |||
| Energy (kcal/mol): 1 × 10−4 | 5 | NPT, 1 atm, 298 K | 1000 |
| Buffer width: 0.5 Å | The total simulation time for the equilibration process is 5.27 ns | ||
| Spline width: 1 Å | MD simulation for CO2 diffusion in the polymer system | ||
| Displacement (Å): 5 × 10−5 | NVT, 298 K | 7000 | |
| Max. iterations: 50,000 | |||
| Species | ΔEad (eV) | Q (|e|) | B (eV) |
|---|---|---|---|
| EB-PANI | 1.44 | ||
| Na-SPANI (I) | 1.44 | ||
| Na-SPANI (II) | 1.30 | ||
| Na-SPANI (III) | 1.29 | ||
| EB-PANI CO2_1 | −0.054 | −0.001 | 1.43 |
| EB-PANI CO2_2 | −0.082 | −0.001 | 1.45 |
| Na-SPANI (I) CO2_1 | −0.115 | −0.006 | 1.42 |
| Na-SPANI (I) CO2_2 | −0.111 | −0.005 | 1.42 |
| Na-SPANI (II) CO2_1 | −0.112 | −0.005 | 1.33 |
| Na-SPANI (II) CO2_2 | −0.32 | 0.013 | 1.33 |
| Na-SPANI (III) CO2_1 | −0.189 | −0.006 | 1.34 |
| Na-SPANI (III) CO2_2 | −0.564 | 0.014 | 1.34 |
| EB-PANI H2O_1 | −0.435 | −0.017 | 1.46 |
| EB-PANI H2O_2 | −0.463 | −0.014 | 1.42 |
| Na-SPANI (I) H2O_1 | −0.501 | −0.015 | 1.42 |
| Na-SPANI (I) H2O_2 | −0.587 | −0.014 | 1.42 |
| Na-SPANI (II) H2O_1 | −0.491 | −0.011 | 1.33 |
| Na-SPANI (II) H2O_2 | −0.899 | 0.021 | 1.32 |
| Na-SPANI (III) H2O_1 | −1.06 | −0.013 | 1.24 |
| Na-SPANI (III) H2O_2 | −1.188 | 0.014 | 1.36 |
| Polymers | Sincreasing |
|---|---|
| Na-SPANI (I)/EB-PANI | 41.4 |
| Na-SPANI (II)/EB-PANI | 171.3 |
| Na-SPANI (III)/EB-PANI | 355.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Jiang, J.; Ye, H.; Yang, N.; Cai, M.; Xiao, J.; Chen, X. Sorption and Diffusion of Water Vapor and Carbon Dioxide in Sulfonated Polyaniline as Chemical Sensing Materials. Sensors 2016, 16, 606. https://doi.org/10.3390/s16050606
Liang Q, Jiang J, Ye H, Yang N, Cai M, Xiao J, Chen X. Sorption and Diffusion of Water Vapor and Carbon Dioxide in Sulfonated Polyaniline as Chemical Sensing Materials. Sensors. 2016; 16(5):606. https://doi.org/10.3390/s16050606
Chicago/Turabian StyleLiang, Qiuhua, Junke Jiang, Huaiyu Ye, Ning Yang, Miao Cai, Jing Xiao, and Xianping Chen. 2016. "Sorption and Diffusion of Water Vapor and Carbon Dioxide in Sulfonated Polyaniline as Chemical Sensing Materials" Sensors 16, no. 5: 606. https://doi.org/10.3390/s16050606
APA StyleLiang, Q., Jiang, J., Ye, H., Yang, N., Cai, M., Xiao, J., & Chen, X. (2016). Sorption and Diffusion of Water Vapor and Carbon Dioxide in Sulfonated Polyaniline as Chemical Sensing Materials. Sensors, 16(5), 606. https://doi.org/10.3390/s16050606