Enhanced Tissue Ablation Efficiency with a Mid-Infrared Nonlinear Frequency Conversion Laser System and Tissue Interaction Monitoring Using Optical Coherence Tomography
Abstract
:1. Introduction
2. Materials and Methods
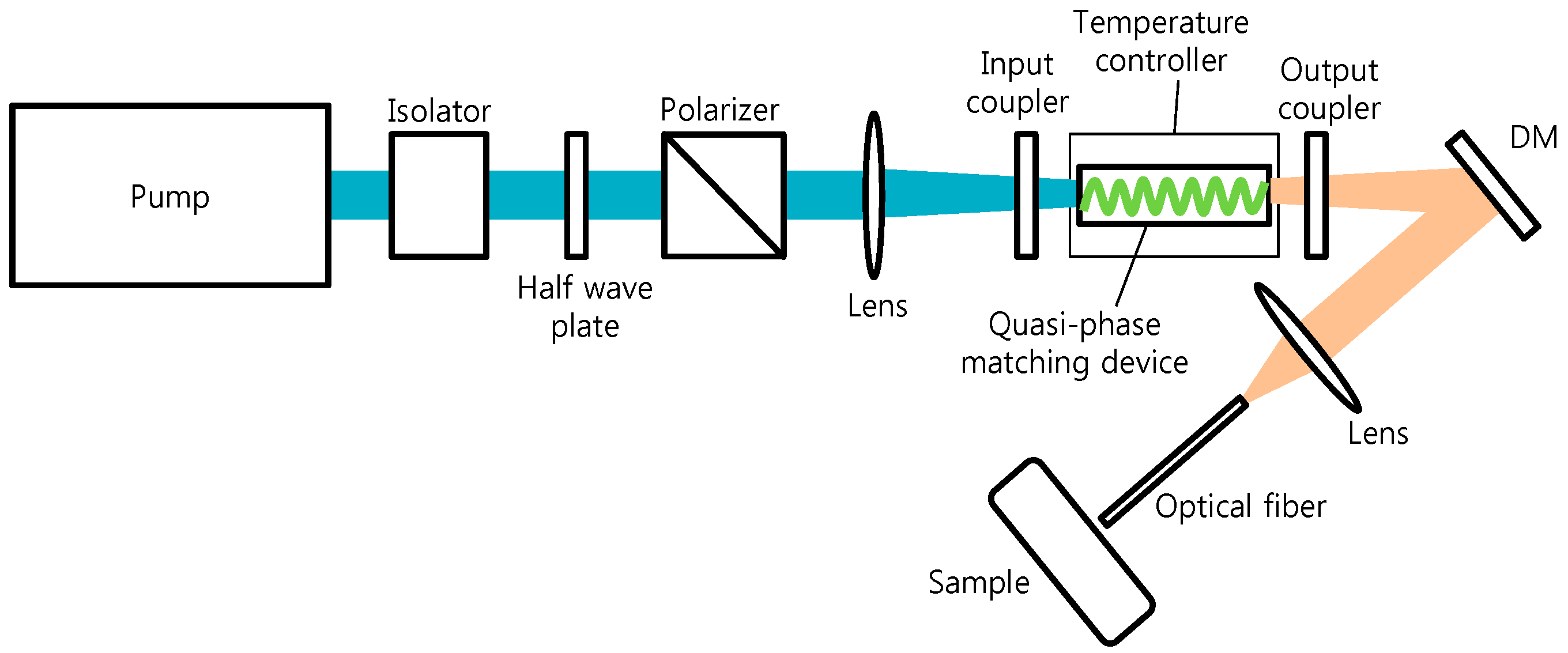
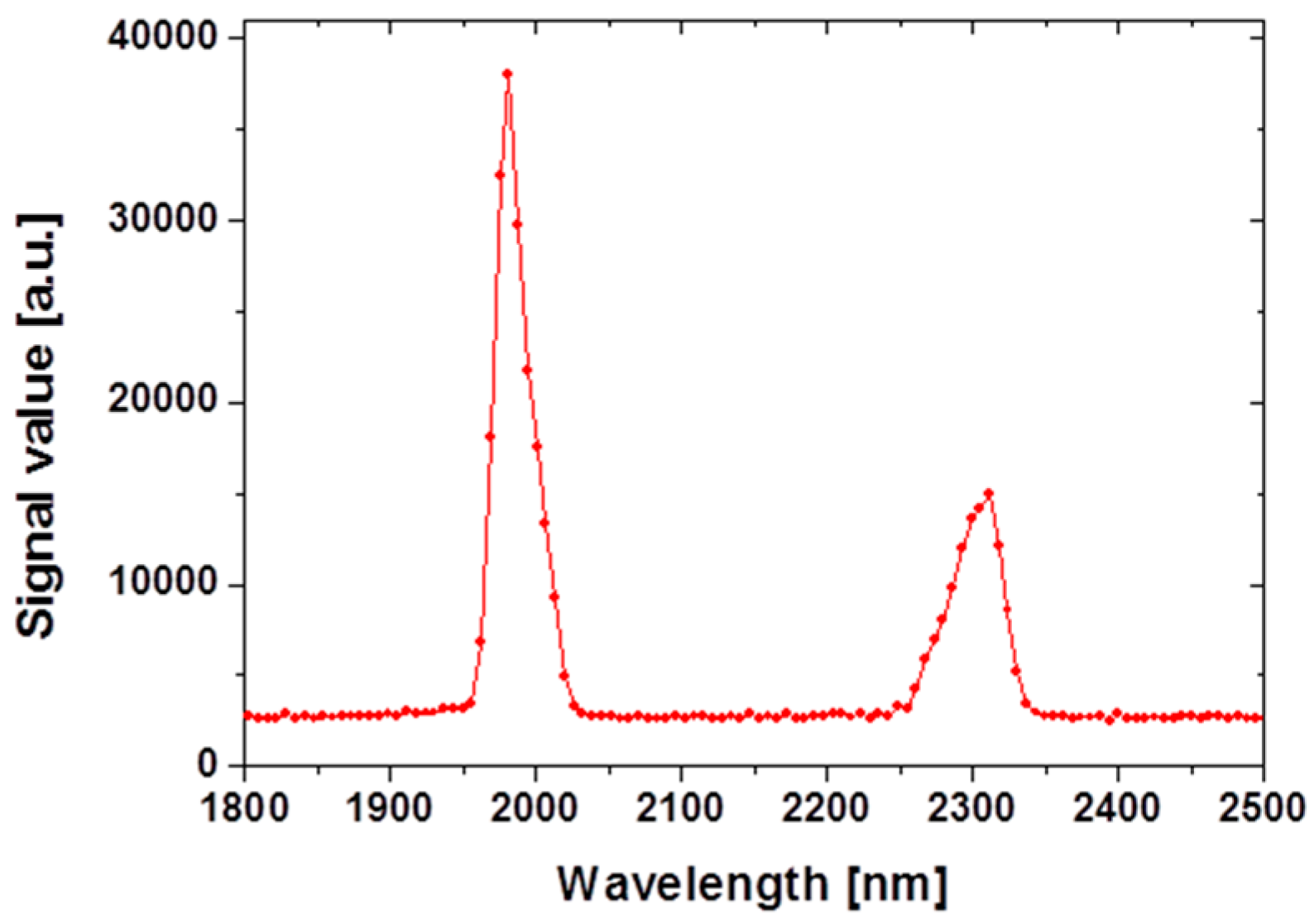
2.1. Mid-Infrared Generation Using Quasi-Phase Matched Nonlinear Frequency Conversion
2.2. Tissue Sample
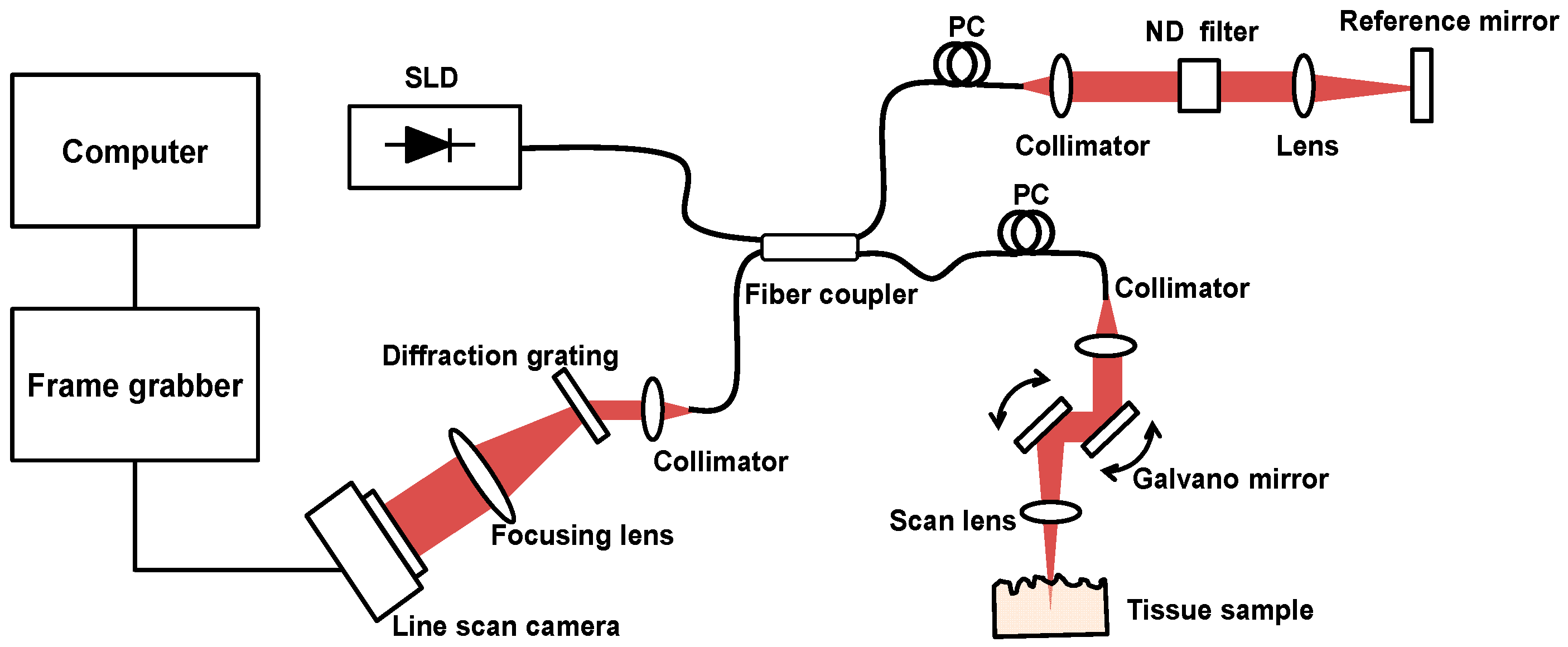
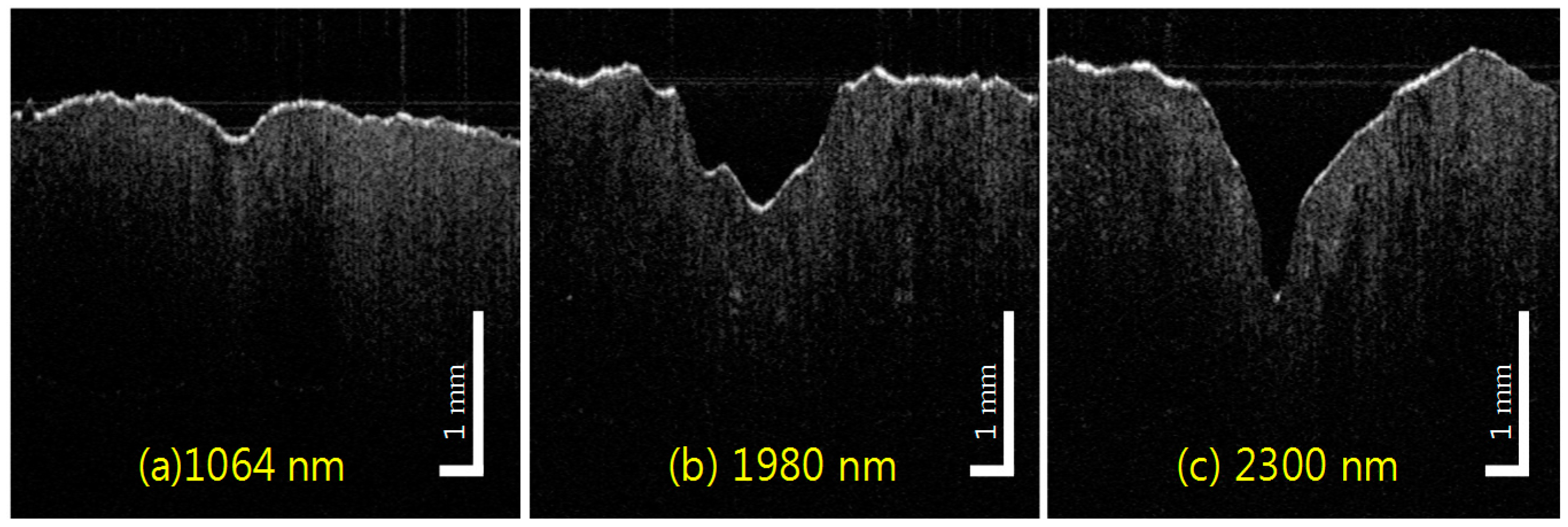
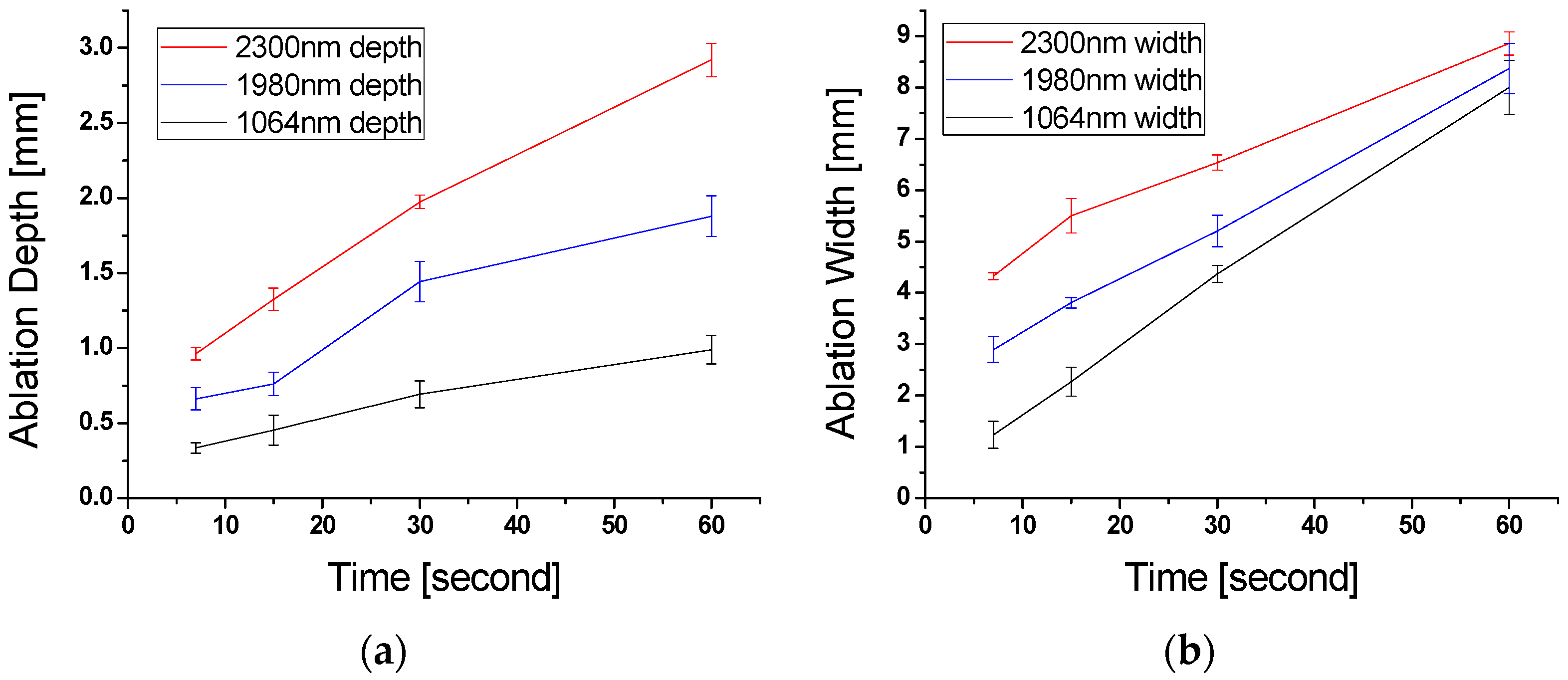
2.3. Tissue Ablation Measurements with OCT
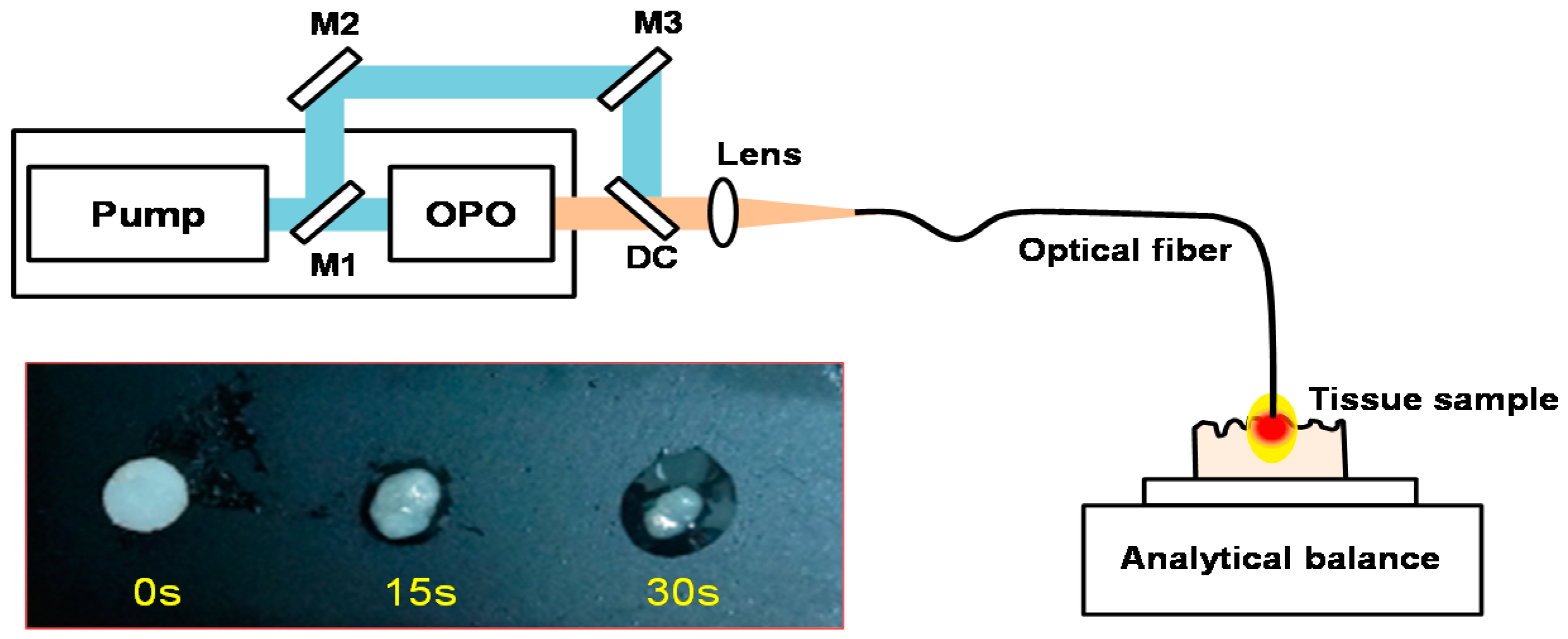
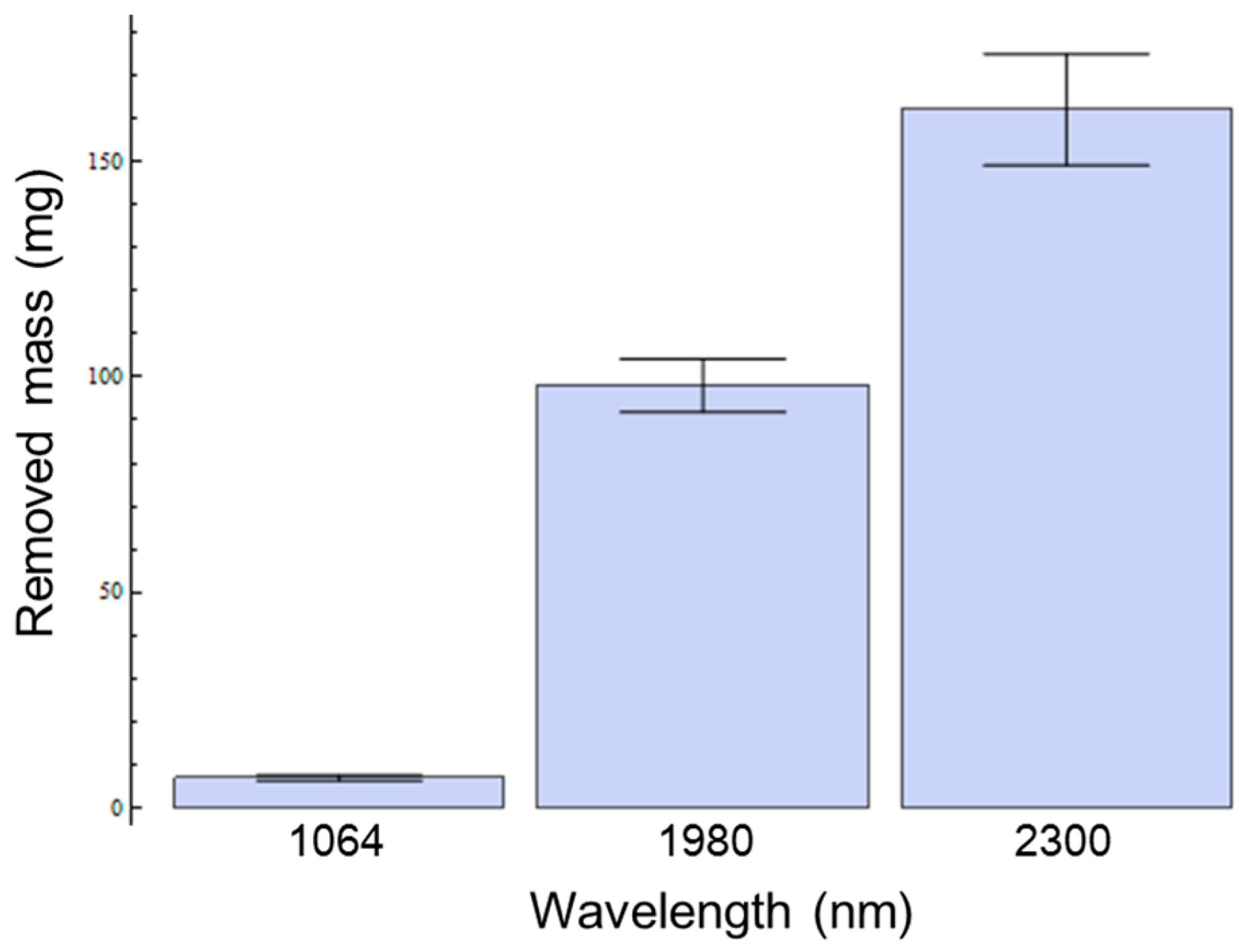
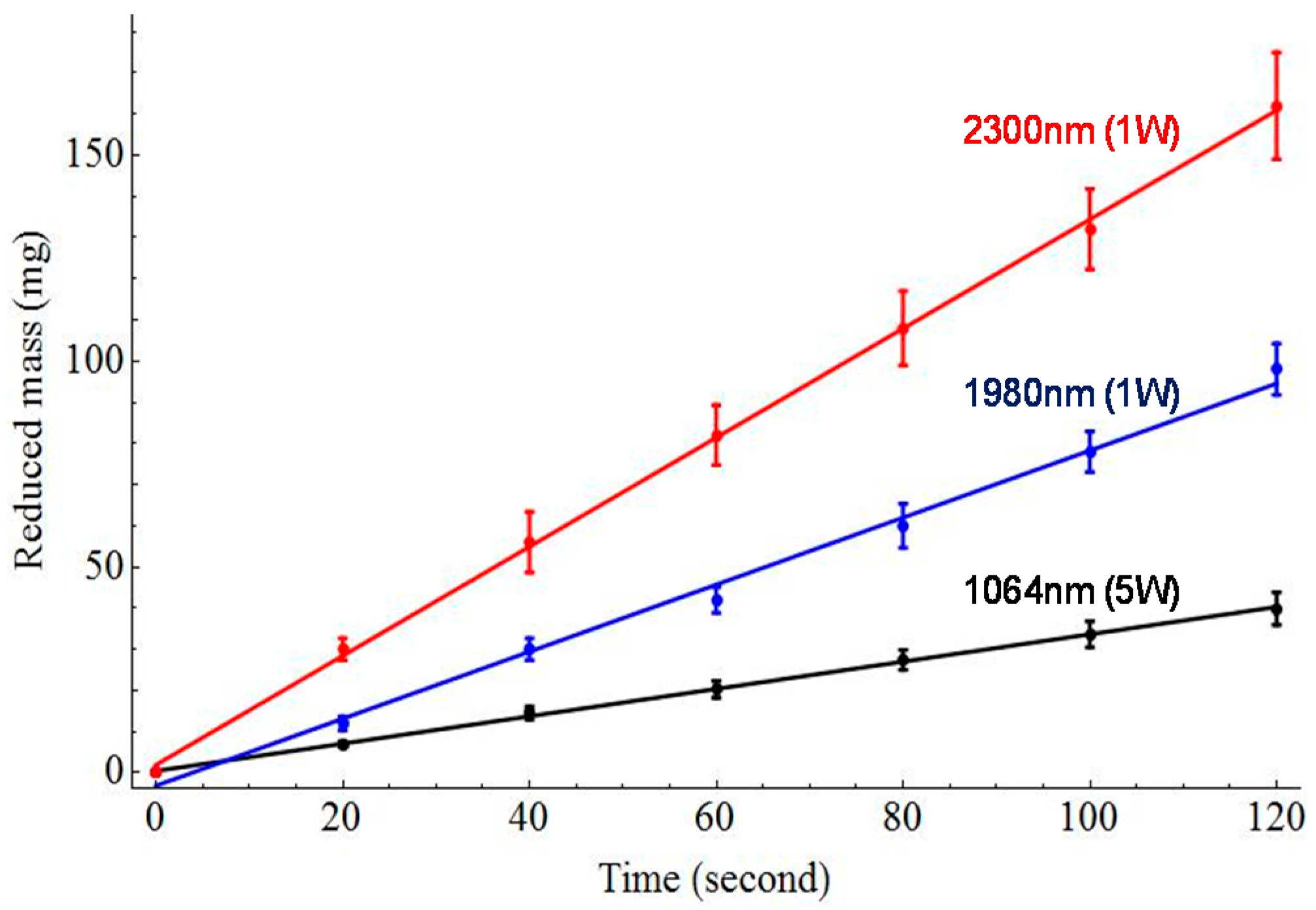
2.4. In Vitro Tissue Mass Loss Measurements
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Grégoire, J.-M. Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Blackwell, R.E.; Jemison, D.M.; Foy, B.D. The holmium: Yttrium-aluminum-garnet laser in wrist arthroscopy: A five-year experience in the treatment of central triangular fibrocartilage complex tears by partial excision. J. Hand Surg. 2001, 26A, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Logan, R.; Copeland, M.; Reinisch, L.; Davidson, J.; Johnson, B.; Maciunas, R.; Mendenhall, M.; Ossoff, R.; Tribble, J.; et al. Tissue ablation by a free-electron laser tuned to the amide II band. Nature 2004, 371, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Peavy, G.M.; Venugopalan, V. Free electron laser ablation of articular and fibro-cartilage at 2.79, 2.9, 6.1, and 6.45 μm: Mass removal studies. Lasers Surg. Med. 2005, 36, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Heya, M.; Fukami, Y.; Nagats, H.; Nishida, Y.; Awazu, K. Gelatin ablation wavelength dependency in the range of 5.6–6.7 μm using a mid-infrared free electron laser. Nucl. Instrum. Methods. Phys. Res. A 2003, 507, 564–568. [Google Scholar] [CrossRef]
- Mackanos, M.A.; Kozub, J.A.; Hachey, D.L.; Joos, K.M.; Ellis, D.L.; Jansen, E.D. The effect of free-electron laser pulse structure on mid-infrared soft tissue ablation: Biological effects. Phys. Med. Biol. 2005, 50, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Iwatsuki, K.; Ishii, K.; Suzuki, S.; Fujinaka, T.; Yoshimine, T.; Awazu, K. Medical application of an infrared free-electron laser: Selective removal of cholesterol ester in carotid artery atheromatous plaques. J. Neurosurg. 2006, 104, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.M.; Ionin, A.A.; Kinyaevsky, I.O.; Klimachev, Y.M.; Kozlov, Y.A.; Kotkov, A.A.; Lanskii, G.V.; Shaiduko, A.V. Broadband carbon monoxide laser system operating in the wavelength range of 2.5–8.3 μm. Quantum Electron. 2013, 43, 139–143. [Google Scholar] [CrossRef]
- Hashimura, K.; Ishii, K.; Akikusa, N.; Edamura, T.; Yoshida, H.; Awazu, K. Coagulation and ablation of biological soft tissue by quantum cascade laser with peak wavelength of 5.7 μm. J. Innov. Opt. Health Sci. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Kozub, J.; Ivanov, B.; Jayasinghe, A.; Prasad, R.; Shen, J.; Klosner, M.; Heller, D.; Mendenhall, M.; Piston, D.W.; Joos, K.; et al. Raman-shifted alexandrite laser for soft tissue ablation in the 6 to 7 μm wavelength range. Biomed. Opt. Express 2011, 2, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.S.; Pearlstein, R.D.; Copeland, M.L.; Hutson, M.S.; Latone, K.; Spiro, A.; Pasmanik, G. 6450 nm wavelength tissue ablation using a nanosecond laser based on difference frequency mixing and stimulated Raman scattering. Opt. Lett. 2007, 32, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Mackanos, M.A.; Simanovskii, D.M.; Contag, C.H.; Kozub, J.A.; Jansen, E.D. Comparing an optical parametric oscillator (OPO) as a viable alternative for mid-infrared tissue ablation with a free electron laser (FEL). Lasers Med. Sci. 2012, 27, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Mackanos, M.A.; Simanovskii, D.M.; Schriver, K.E.; Hutson, M.S.; Contag, C.H.; Kozub, J.A.; Jansen, E.D. Pulse-duration-dependent mid-infrared laser ablation for biological applications. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1514–1522. [Google Scholar] [CrossRef]
- Zhang, F.; Shao, Q.; Herrmann, T.R.W.; Tian, Y.; Zhang, Y. Thulium laser versus holmium laser transurethral enucleation of the prostate: 18-Month follow-up data of a single center. Urology 2012, 79, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Fried, N.M.; Murray, K.E. High-power thulium fiber laser ablation of urinary tissues at 1.94 micron. J. Endourol. 2005, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Greene, T.D.; Gupta, M. Treatment of proximal ureteral calculi: Holmium: YAG laser ureterolithotripsy versus extracorporeal shock wave lithotripsy. J. Urol. 2002, 167, 1972–1976. [Google Scholar] [CrossRef]
- Anderson, R.R.; Farinelli, W.; Laubach, H.; Manstein, D.; Yaroslavsky, A.N.; Gubeli, J.; Jordan, K.; Neil, G.R.; Shinn, M.; Chandler, W.; et al. Selective photothermolysis of lipid-rich tissues: A free electron laser study. Lasers Surg. Med. 2006, 38, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Fingler, J.; Zawadzki, R.J.; Park, S.; Morse, L.; Schwartz, D.M.; Fraser, S.E.; Werner, J.S. Optical Imaging of the Chorioretinal Vasculature in the Living Human Eye. Proc. Natl. Acad. Sci. USA 2013, 110, 14354–14359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Fingler, J.; Werner, J.S.; Schwartz, D.M.; Fraser, S.E.; Zawadzki, R.J. In vivo volumetric imaging of human retinal circulation with phase-variance optical coherence tomography. Biomed. Opt. Express. 2011, 2, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Park, K.; Wijesinghe, R.E.; Shin, Y.S.; Jung, W.; Kim, J. Development of Real-Time Dual-Display Handheld and Bench-Top Hybrid-Mode SD-OCTs. Sensors 2014, 14, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W. Optical Coherence Tomography: Technology and Applications; Springer Verlag: Berlin, Germany, 2008. [Google Scholar]
- Kim, B.; Ahn, J.-C.; Chung, P.-S.; Kim, D.Y. Development of 2-micron nonlinear frequency conversion laser system and tissue interaction monitoring using optical coherence tomography. Proc. SPIE 2016. [Google Scholar] [CrossRef]
- Youn, J.-I.; Holcomb, J.D. Ablation efficiency and relative thermal confinement measurements using wavelengths 1064, 1320, and 1444 nm for laser-assisted lipolysis. Lasers Med. Sci. 2013, 28, 519–527. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Kim, D.Y. Enhanced Tissue Ablation Efficiency with a Mid-Infrared Nonlinear Frequency Conversion Laser System and Tissue Interaction Monitoring Using Optical Coherence Tomography. Sensors 2016, 16, 598. https://doi.org/10.3390/s16050598
Kim B, Kim DY. Enhanced Tissue Ablation Efficiency with a Mid-Infrared Nonlinear Frequency Conversion Laser System and Tissue Interaction Monitoring Using Optical Coherence Tomography. Sensors. 2016; 16(5):598. https://doi.org/10.3390/s16050598
Chicago/Turabian StyleKim, Bongkyun, and Dae Yu Kim. 2016. "Enhanced Tissue Ablation Efficiency with a Mid-Infrared Nonlinear Frequency Conversion Laser System and Tissue Interaction Monitoring Using Optical Coherence Tomography" Sensors 16, no. 5: 598. https://doi.org/10.3390/s16050598
APA StyleKim, B., & Kim, D. Y. (2016). Enhanced Tissue Ablation Efficiency with a Mid-Infrared Nonlinear Frequency Conversion Laser System and Tissue Interaction Monitoring Using Optical Coherence Tomography. Sensors, 16(5), 598. https://doi.org/10.3390/s16050598




