Reverse-Bumpy-Ball-Type-Nanoreactor-Loaded Nylon Membranes as Peroxidase-Mimic Membrane Reactors for a Colorimetric Assay for H2O2
Abstract
:1. Introduction
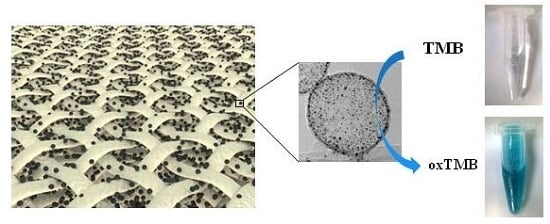
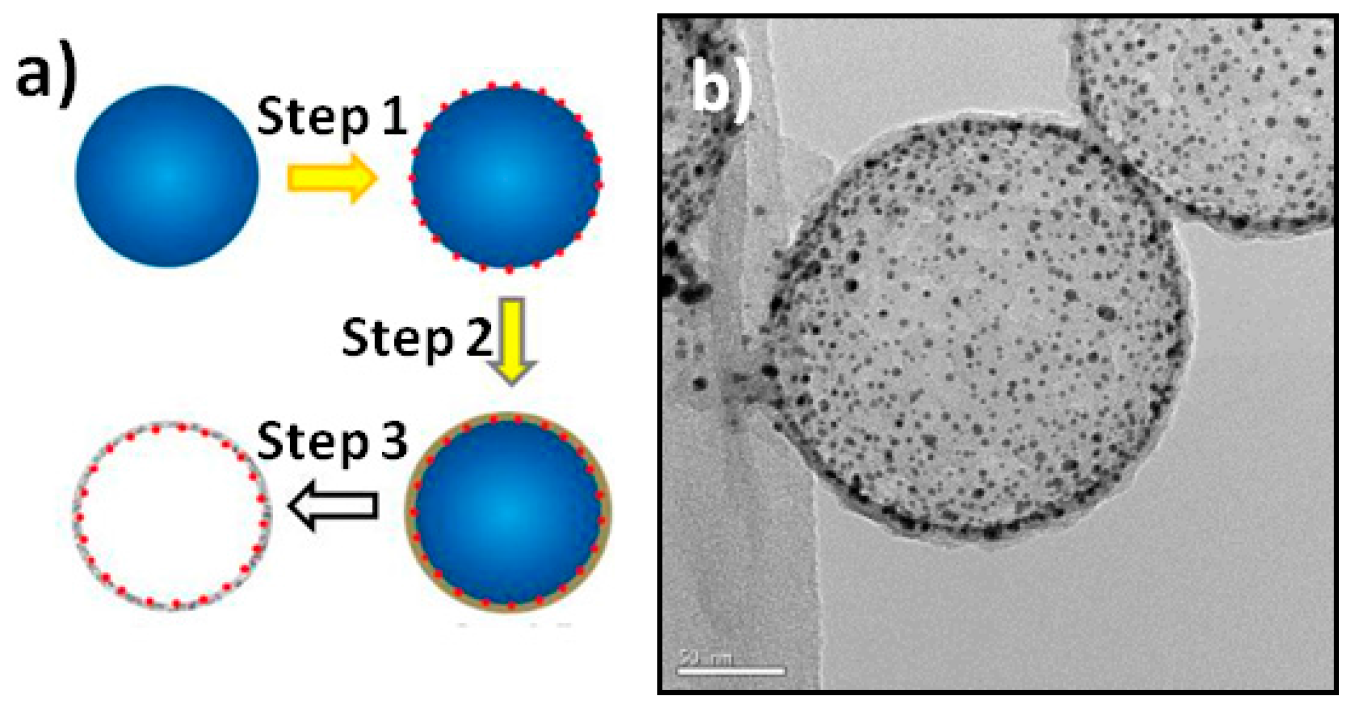
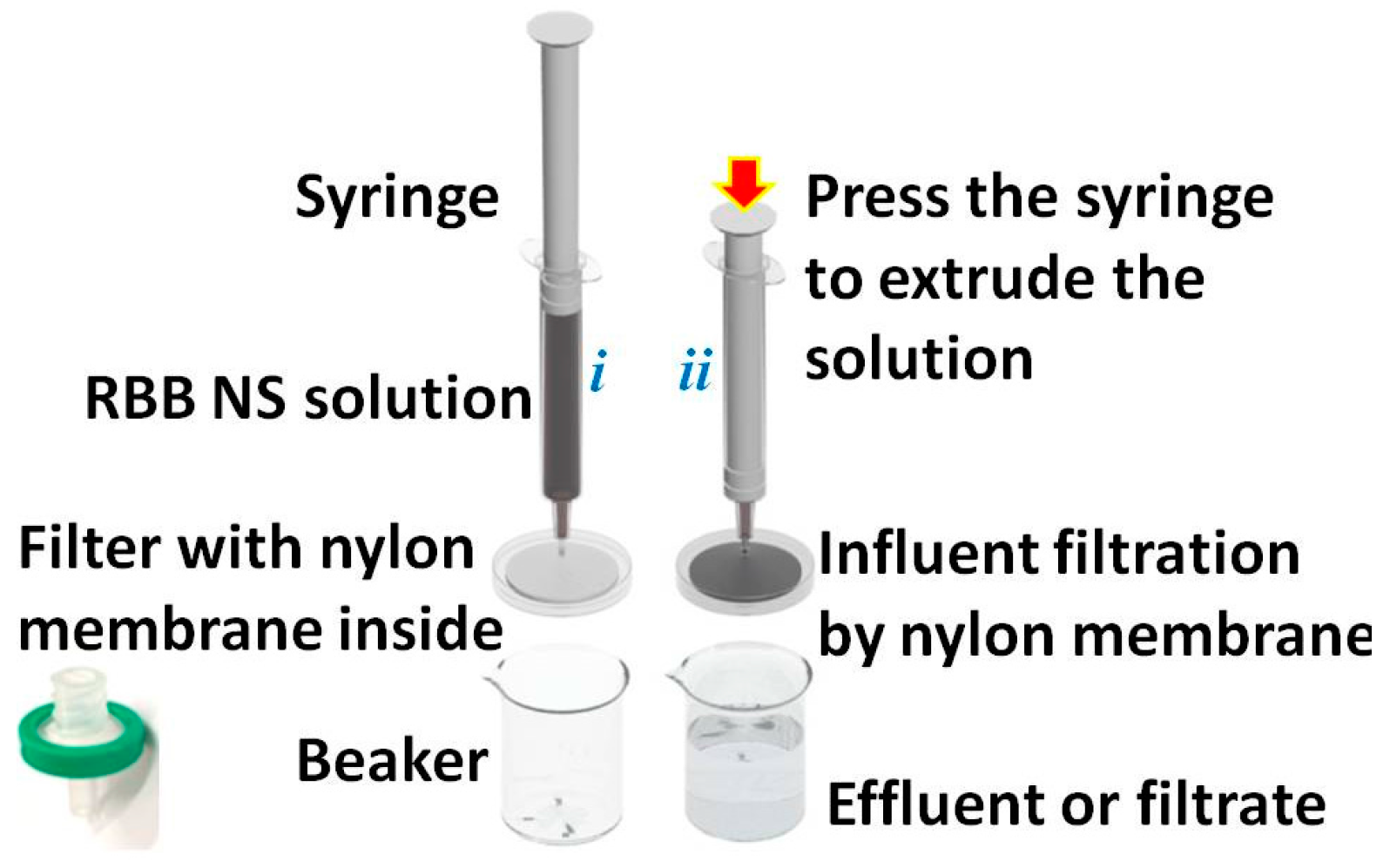
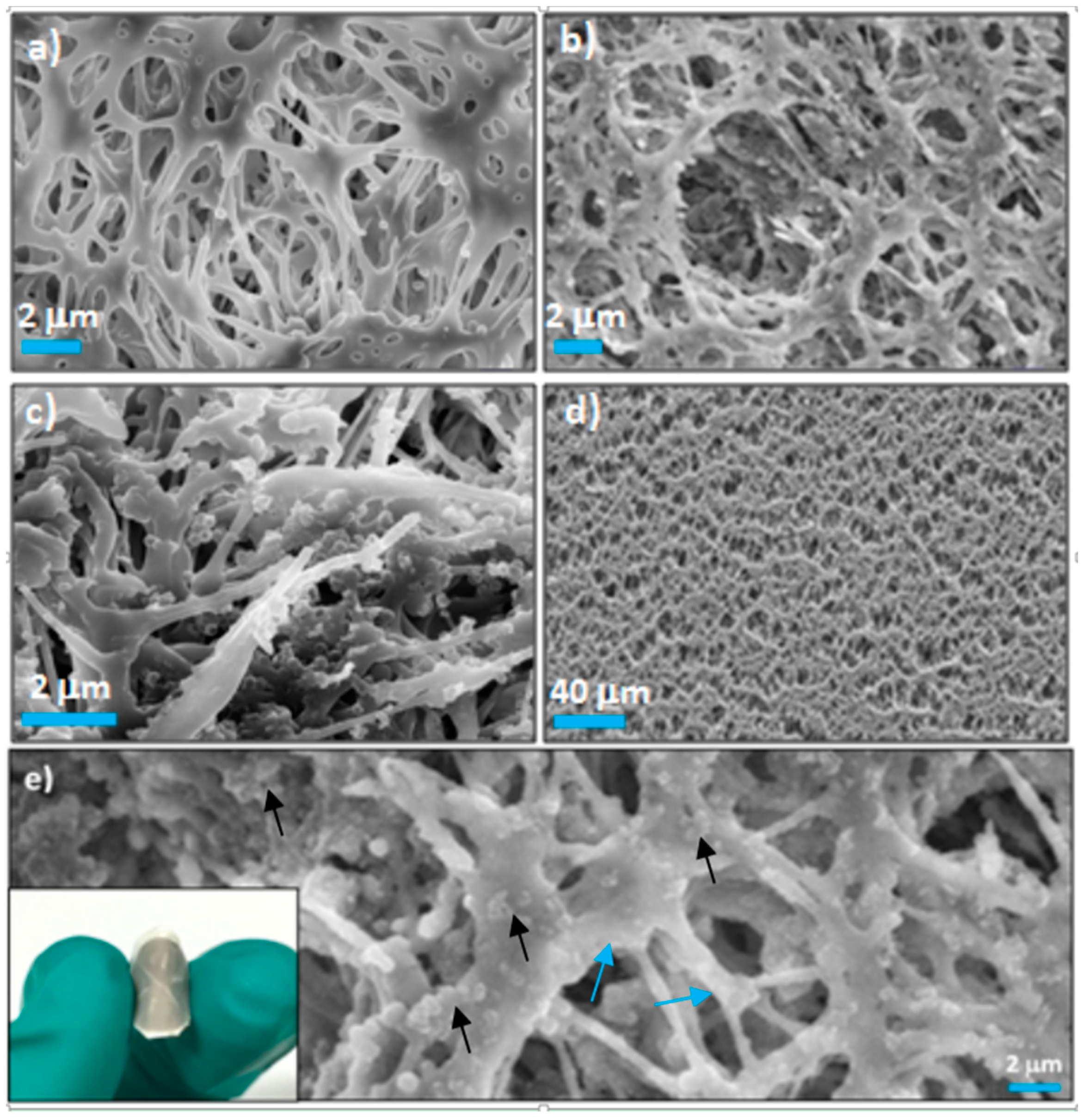
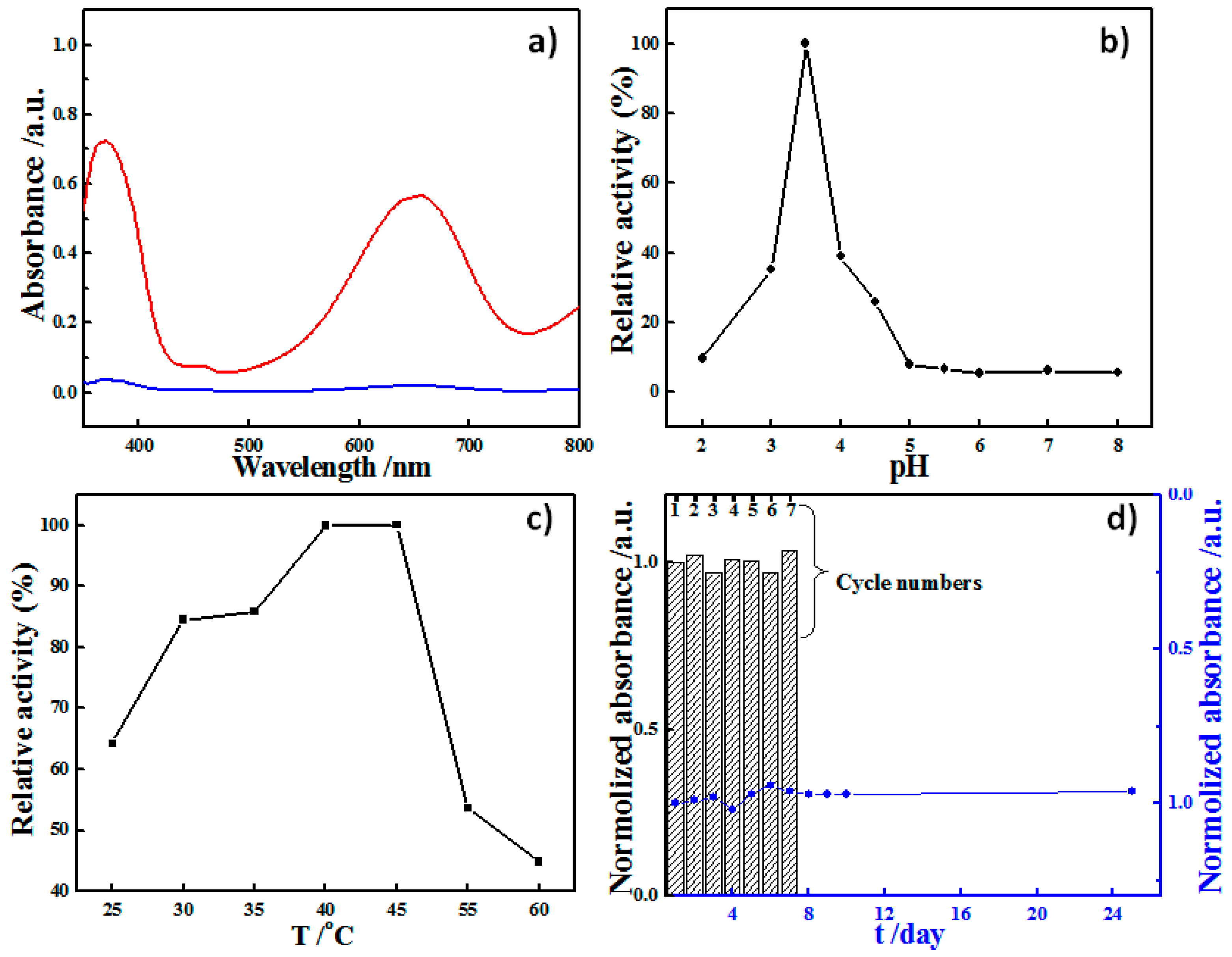
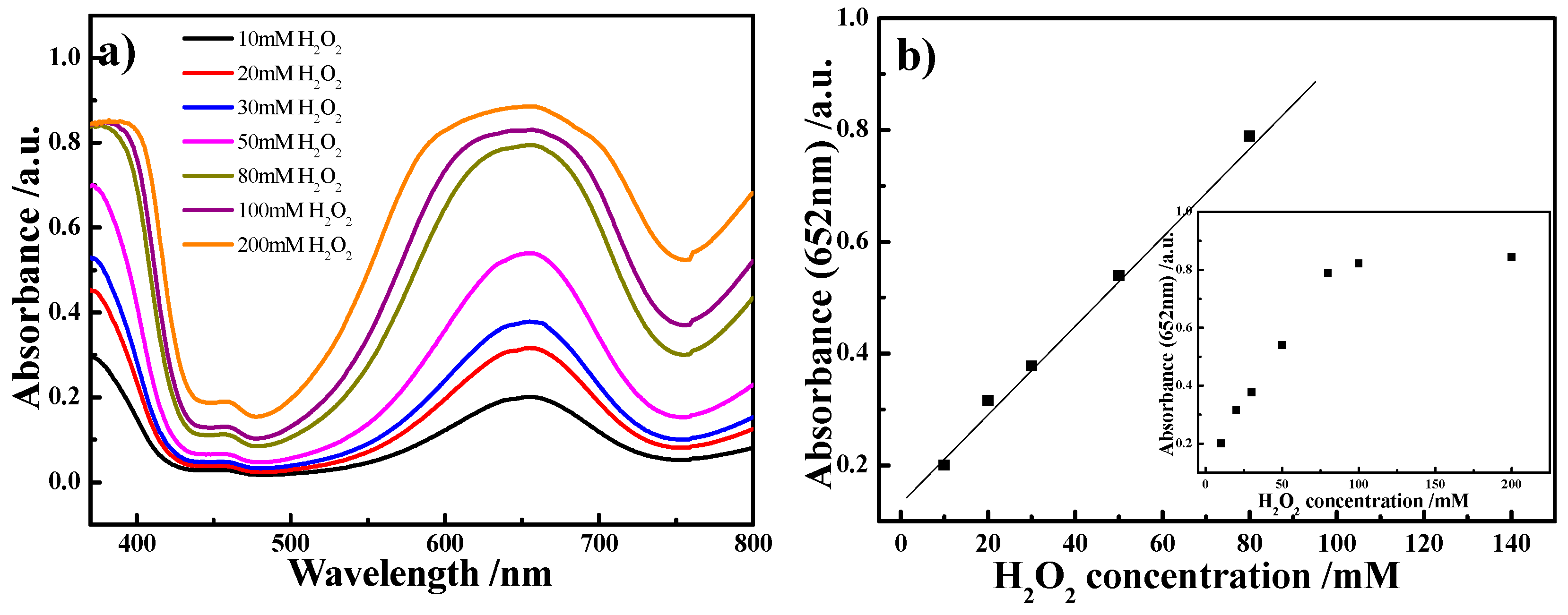
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of the RBB-Structured NSs
3.3. Preparation of the Flexible Catalytic Membranes
3.4. Instruments and Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perez-Lorenzo, M.; Vaz, B.; Salgueirino, V.; Correa-Duarte, M.A. Hollow-Shelled Nanoreactors Endowed with High Catalytic Activity. Chem. Eur. J. 2013, 19, 12196–12211. [Google Scholar] [CrossRef] [PubMed]
- Vaz, B.; Salgueirino, V.; Perez-Lorenzo, M.; Correa-Duarte, M.A. Enhancing the Exploitation of Functional Nanomaterials through Spatial Confinement: The Case of Inorganic Submicrometer Capsules. Langmuir 2015, 31, 8745–8755. [Google Scholar] [CrossRef] [PubMed]
- Sanles-Sobrido, M.; Perez-Lorenzo, M.; Rodriguez-Gonzalez, B.; Salgueirino, V.; Correa-Duarte, M.A. Highly Active Nanoreactors: Nanomaterial Encapsulation Based on Confined Catalysis. Angew. Chem. Int. Ed. 2012, 51, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, Z.M.; Niu, F.; Jiang, L.; Song, W.G. Pd nanoparticles in silica hollow spheres with mesoporous walls: a nanoreactor with extremely high activity. Chem. Commun. 2010, 46, 6524–6526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; Pan, X.L.; Guo, S.J.; Ren, P.J.; Bao, X.H. Toward Fundamentals of Confined Catalysis in Carbon Nanotubes. J. Am. Chem. Soc. 2015, 137, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sanles-Sobrido, M.; Exner, W.; Rodriguez-Lorenzo, L.; Rodriguez-Gonzalez, B.; Correa-Duarte, M.A.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Design of SERS-Encoded, Submicron, Hollow Particles Through Confined Growth of Encapsulated Metal Nanoparticles. J. Am. Chem. Soc. 2009, 131, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.M.; Zhao, Y.W.; Wang, J.C.; Yang, X.L. Polymer shell as a protective layer for the sandwiched gold nanoparticles and their recyclable catalytic property. J. Colloid Interface Sci. 2013, 395, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Xiao, F.; Xi, J.B.; Sun, T.; Xiao, S.; Wang, H.R.; Wang, S.; Liu, Y.Q. Encapsulating Pd nanoparticles in double-shelled graphene@ carbon hollow spheres for excellent chemical catalytic property. Sci. Rep. UK 2014. [Google Scholar] [CrossRef] [PubMed]
- Du, C.H.; Guo, Y.; Guo, Y.L.; Gong, X.Q.; Lu, G.Z. Polymer-templated synthesis of hollow Pd-CeO2 nanocomposite spheres and their catalytic activity and thermal stability. J. Mater. Chem. A 2015, 3, 23230–23239. [Google Scholar] [CrossRef]
- Yin, Y.Y.; Chen, M.; Zhou, S.X.; Wu, L.M. A general and feasible method for the fabrication of functional nanoparticles in mesoporous silica hollow composite spheres. J. Mater. Chem. 2012, 22, 11245–11251. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.P.; Song, S.Y.; Zhang, H.J. Pt@CeO2 Multicore@Shell Self-Assembled Nanospheres: Clean Synthesis, Structure Optimization, and Catalytic Applications. J. Am. Chem. Soc. 2013, 135, 15864–15872. [Google Scholar] [CrossRef] [PubMed]
- Anisur, R.M.; Shin, J.; Choi, H.H.; Yeo, K.M.; Kang, E.J.; Lee, I.S. Hollow silica nanosphere having functionalized interior surface with thin manganese oxide layer: nanoreactor framework for size-selective Lewis acid catalysis. J. Mater. Chem. 2010, 20, 10615–10621. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, L.P.; Yu, L.H.; Kong, J.H.; Yao, X.Y.; Liu, W.S.; Xu, Z.C.; Lu, X.H. Fe/N/C hollow nanospheres by Fe(III)-dopamine complexation-assisted one-pot doping as nonprecious-metal electrocatalysts for oxygen reduction. Nanoscale 2015, 7, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, W.; Feng, H.L.; Yang, X.L. Rattle-type microspheres as a support of tiny gold nanoparticles for highly efficient catalysis. Chem. Commun. 2011, 47, 11727–11729. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Xue, Z.T.; Feng, S.S.; Tu, B.; Zhao, D.Y. Synthesis of mesoporous silica hollow nanospheres with multiple gold cores and catalytic activity. J. Colloid Interface Sci. 2014, 429, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, E.; Radhakrishnan, T.P. A Highly Efficient and Extensively Reusable “Dip Catalyst” Based on a Silver-Nanoparticle-Embedded Polymer Thin Film. Chem. Eur. J. 2010, 16, 14378–14384. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Su, L.; Zhang, X.J. Preparation of catalytic films of the Au nanoparticle–carbon composite tubular arrays. Chem. Commun. 2015, 51, 6333–6336. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, E.; Radhakrishnan, T.P. Palladium Nanoparticle-Embedded Polymer Thin Film “Dip Catalyst” for Suzuki-Miyaura Reaction. ACS Catal. 2012, 2, 1179–1186. [Google Scholar] [CrossRef]
- Faria, V.W.; Oliveira, D.G.M.; Kurz, M.H.S.; Goncalves, F.F.; Scheeren, C.W.; Rosa, G.R. Palladium nanoparticles supported in a polymeric membrane: an efficient phosphine-free “green” catalyst for Suzuki–Miyaura reactions in water. RSC Adv. 2014, 4, 13446–13452. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, X.T.; Zheng, Z.P.; Ma, Y.Y.; Qu, Y.Q. 3D graphene/nylon rope as a skeleton for noble metal nanocatalysts for highly efficient heterogeneous continuous-flow reactions. J. Mater. Chem. A 2015, 3, 10504–10511. [Google Scholar] [CrossRef]
- Yao, J.F.; Dong, D.H.; Li, D.; He, L.; Xu, G.S.; Wang, H.T. Contra-diffusion synthesis of ZIF-8 films on a polymer substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Mayer-Gall, T.; Opwis, K.; Song, C.E.; Gutmann, J.S.; List, B. Organotextile Catalysis. Science 2013, 341, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Mahurin, S.M.; Li, C.; Unocic, R.R.; Idrobo, J.C.; Gao, H.J.; Pennycook, S.J.; Dai, S. Dopamine as a Carbon Source: The Controlled Synthesis of Hollow Carbon Spheres and Yolk-Structured Carbon Nanocomposites. Angew. Chem. Int. Ed. 2011, 30, 6931–6934. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kasi, R.M. Nanocomposite hydrogels based on liquid crystalline brush-like block copolymer—Au nanorods and their application in H2O2 detection. Chem. Commun. 2015, 51, 12174–12177. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, S.; Pumera, M.; Cabruja, E.; Fàbregas, E. Carbon nanotube/polysulfone composite screen-printed electrochemical enzyme biosensors. Analyst 2007, 132, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, N.A.; Komkova, M.A.; Khomyalova, I.V.; Karyakina, E.E.; Karyakin, A.A. Transition Metal Hexacyanoferrates in Electrocatalysis of H2O2 Reduction: An Exclusive Property of Prussian Blue. Anal. Chem. 2014, 86, 4131–4134. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet Chemical Synthesis of High Aspect Ratio Cylindrical Gold Nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Bernsmann, F.; Voegel, J.C.; Ball, V. Different synthesis methods allow to tune the permeability and permselectivity of dopamine-melanin films to electrochemical probes. Electrochim. Acta 2011, 56, 3914–3919. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Hu, L.L.; Yin, L.; Guo, L. Electrochemical glucose biosensor with improved performance based on the use of glucose oxidase and Prussian Blue incorporated into a thin film of self-polymerized dopamine. Sens. Actuators B Chem. 2015, 210, 513–518. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Jiao, X.; Yang, H.; Wen, Y.; Su, L.; Zhang, X. Reverse-Bumpy-Ball-Type-Nanoreactor-Loaded Nylon Membranes as Peroxidase-Mimic Membrane Reactors for a Colorimetric Assay for H2O2. Sensors 2016, 16, 465. https://doi.org/10.3390/s16040465
Tong Y, Jiao X, Yang H, Wen Y, Su L, Zhang X. Reverse-Bumpy-Ball-Type-Nanoreactor-Loaded Nylon Membranes as Peroxidase-Mimic Membrane Reactors for a Colorimetric Assay for H2O2. Sensors. 2016; 16(4):465. https://doi.org/10.3390/s16040465
Chicago/Turabian StyleTong, Ying, Xiangyu Jiao, Hankun Yang, Yongqiang Wen, Lei Su, and Xueji Zhang. 2016. "Reverse-Bumpy-Ball-Type-Nanoreactor-Loaded Nylon Membranes as Peroxidase-Mimic Membrane Reactors for a Colorimetric Assay for H2O2" Sensors 16, no. 4: 465. https://doi.org/10.3390/s16040465
APA StyleTong, Y., Jiao, X., Yang, H., Wen, Y., Su, L., & Zhang, X. (2016). Reverse-Bumpy-Ball-Type-Nanoreactor-Loaded Nylon Membranes as Peroxidase-Mimic Membrane Reactors for a Colorimetric Assay for H2O2. Sensors, 16(4), 465. https://doi.org/10.3390/s16040465