Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review
Abstract
:1. Introduction
2. Wearable Sensing Mechanisms
2.1. Inertial Motion Sensors
2.1.1. Accelerometers
2.1.2. Gyroscopes
2.1.3. Magnetometers
2.1.4. Integrated Sensing Mechanism
2.2. Plantar Force Sensors
3. Evaluation of Static and Dynamic Balance
3.1. Instrumented Tests
3.1.1. Measurement of Center of Pressure (COP) Displacement—Static and Dynamic Balance Assessments
3.1.2. Measurement of Center of Mass (COM) Displacement—Static and Dynamic Balance Assessments
3.1.3. Balance Perturbations
3.2. Non-Instrumented Tests
3.2.1. The Romberg Test—Static Balance Assessment
3.2.2. Tandem Standing—Static Balance Assessment
3.2.3. Limits of Stability (LOS) Balance Test—Static Balance Assessment
3.2.4. The Star Excursion Balance Test (SEBT)—Dynamic Balance Assessment
3.2.5. The Tandem Gait Performance—Dynamic Balance Assessment
3.2.6. The Berg Balance Scale (BBS)—Dynamic Balance Assessment
3.2.7. Timed Up and Go test (TUG)—Dynamic Balance Assessment
4. Review on Previous Studies
4.1. Inclusion Criteria
4.1.1. Types of Participants
4.1.2. Types of Sensors and Feedback
4.1.3. Types of Intervention Outcomes
4.1.4. Types of Studies
4.2. Searching Strategy
4.3. Assessment of Methodological Quality
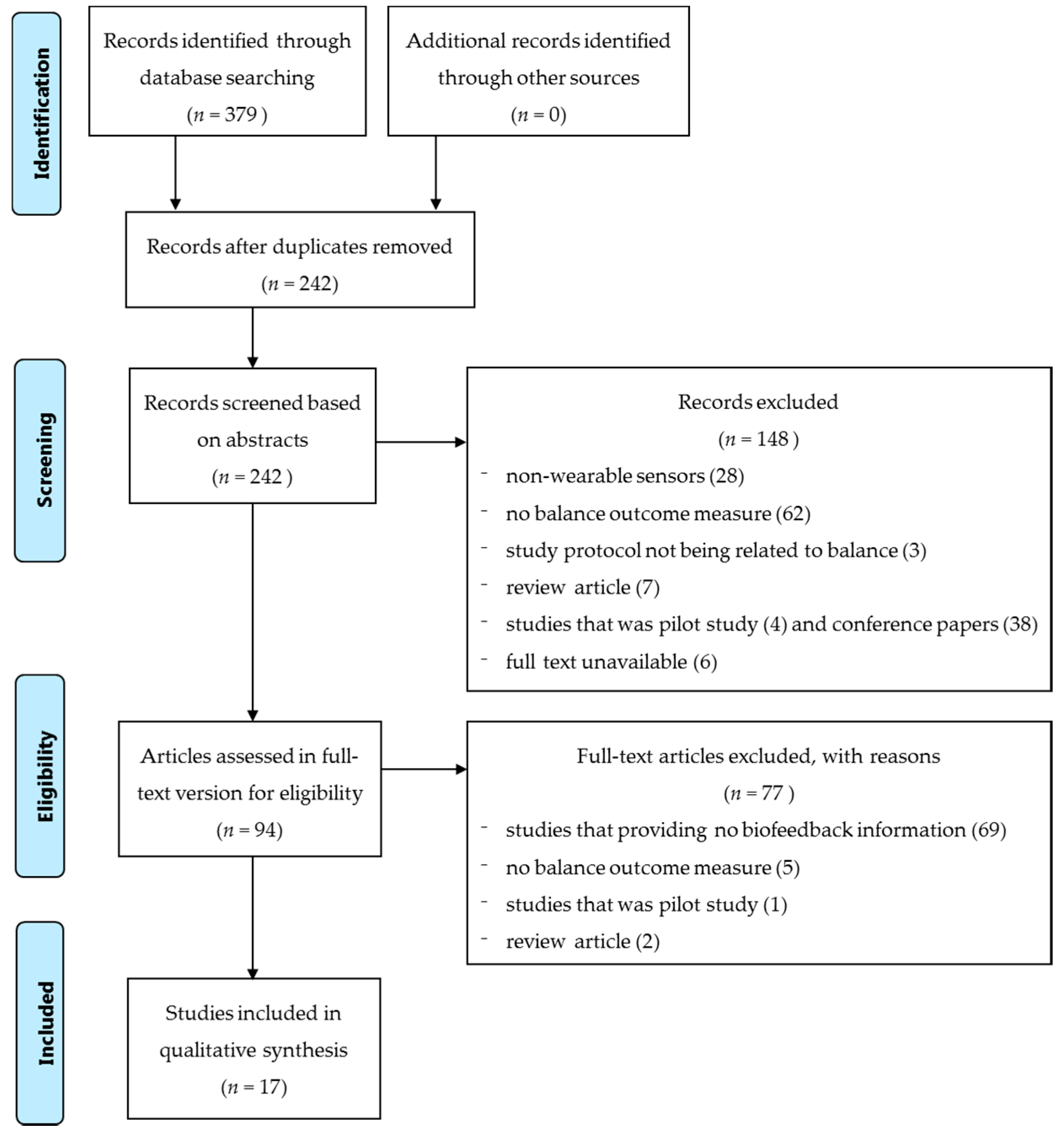
4.4. Searching Results and Screening Strategy
4.5. Data Collection and Data Synthesis
4.6. Methodological Quality and Level of Evidence
4.7. Sample Characteristics
4.8. Types of Sensors, Biofeedback, and Balance Outcome Measurement Methods
4.9. Summary on the Effectiveness of the Devices
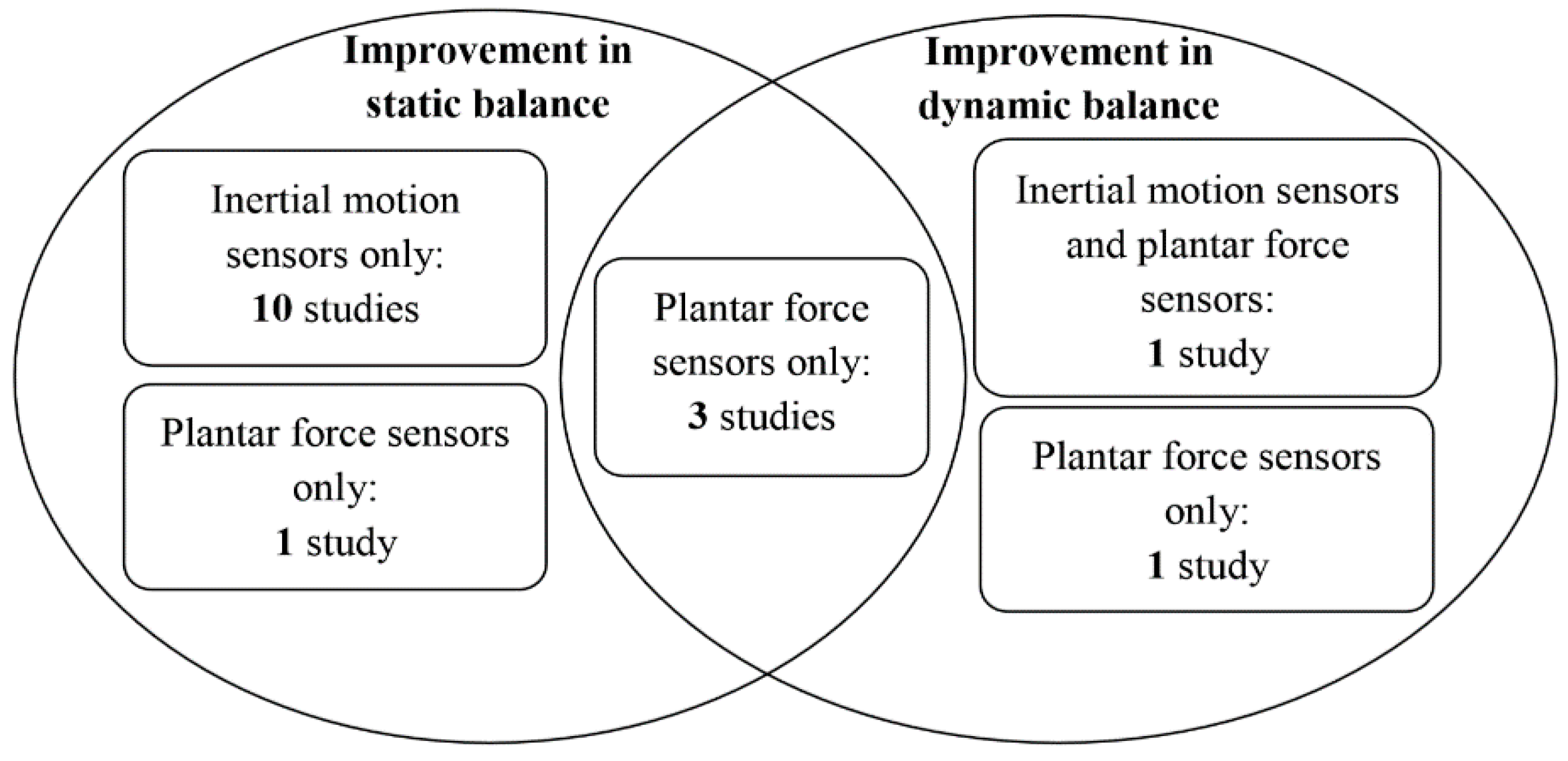
4.9.1. Effect of Inertial Motion Sensors on Static Balance
4.9.2. Effect of Inertial Motion Sensors on Dynamic Balance
4.9.3. Effect of Inertial Motion Sensors and Plantar Force Sensors on Dynamic Balance
4.9.4. Effect of Plantar Force Sensors on Static Balance
4.9.5. Effect of Plantar Force Sensors on Dynamic Balance
5. Discussion
5.1. Effectiveness
5.2. Wearable Sensors in Static and Dynamic Conditions
5.3. Biofeedback Information
5.4. Future Directions
5.5. Limitations
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Anterior-Posterior |
| BBS | Berg Balance Scale |
| COM | Center-of-Mass |
| COP | Center-of-Pressure |
| DPN | Diabetic Peripheral Neuropathy |
| IMU | Inertial Measurement Unit |
| LOS | Limits-of-Stability |
| MEMS | Micro-Electro-Mechanical Systems |
| ML | Medial-Lateral |
| PD | Parkinson’s Disease |
| PSIS | Posterior Superior Iliac Spine |
| TUG | Timed Up and Go test |
References
- Kannus, P.; Niemi, S.; Parkkari, J.; Palvanen, M.; Vuori, I.; Järvinen, M. Hip fractures in finland between 1970 and 1997 and predictions for the future. Lancet 1999, 353, 802–805. [Google Scholar] [CrossRef]
- Kannus, P.; Niemi, S.; Palvanen, M.; Parkkari, J. Continuously increasing number and incidence of fall-induced, fracture-associated, spinal cord injuries in elderly persons. Arch. Intern. Med. 2000, 160, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E. Preventing falls in elderly persons. N. Engl. J. Med. 2003, 348, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Sievänen, H.; Palvanen, M.; Järvinen, T.; Parkkari, J. Prevention of falls and consequent injuries in elderly people. Lancet 2005, 366, 1885–1893. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Baker, D.I.; McAvay, G.; Claus, E.B.; Garrett, P.; Gottschalk, M.; Koch, M.L.; Trainor, K.; Horwitz, R.I. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N. Engl. J. Med. 1994, 331, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L. Preventing falls in elderly people: We need to target interventions at people most likely to benefit from them. BMJ 2004, 328, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Bergland, A.; Wyller, T.B. Risk factors for serious fall related injury in elderly women living at home. Inj. Prev. 2004, 10, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.; Gillespie, W.; Robertson, M.; Lamb, S.; Cumming, R.; Rowe, B. Interventions for preventing falls in elderly people (review). Cochrane Libr. 2007, 11, 1–289. [Google Scholar]
- Trauma, A.C. Advanced Trauma Life Support Program for Doctors: Atls; American College of Surgeons: Chicago, IL, USA, 1997. [Google Scholar]
- Gross, M.T.; Mercer, V.S.; Lin, F.-C. Effects of foot orthoses on balance in older adults. J. Orthop. Sports Phys. Ther. 2012, 42, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Pereira, M.A.; Caspersen, C.J. Physical activity, falls, and fractures among older adults: A review of the epidemiologic evidence. J. Am. Geriatr. Soc. 2000, 48, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R. Falls in Older People: Risk Factors and Strategies for Prevention; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Stevens, J.A.; Corso, P.S.; Finkelstein, E.A.; Miller, T.R. The costs of fatal and non-fatal falls among older adults. Inj. Prev. 2006, 12, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Josephson, K.R. The epidemiology of falls and syncope. Clin. Geriatr. Med. 2002, 18, 141–158. [Google Scholar] [CrossRef]
- Salzman, B. Gait and balance disorders in older adults. Am. Fam. Phys. 2010, 82, 61–68. [Google Scholar]
- Vuillerme, N.; Chenu, O.; Demongeot, J.; Payan, Y. Controlling posture using a plantar pressure-based, tongue-placed tactile biofeedback system. Exp. Brain Res. 2007, 179, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, S.E.; Sipilä, S.; Era, P.A. Changes in postural balance in frail elderly women during a 4-week visual feedback training: A randomized controlled trial. Gerontology 2004, 50, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.; Danilov, Y.; Bach-y-Rita, P. Closing an open-loop control system: Vestibular substitution through the tongue. J. Integr. Neurosci. 2003, 2, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Vuillerme, N.; Pinsault, N.; Fleury, A.; Chenu, O.; Demongeot, J.; Payan, Y.; Pavan, P. Effectiveness of an electro-tactile vestibular substitution system in improving upright postural control in unilateral vestibular-defective patients. Gait Posture 2008, 28, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.A.; Allen, J.B.; O'Keefe, J.; Hicks, R.R. Balance and mobility following stroke: Effects of physical therapy interventions with and without biofeedback/forceplate training. Phys. Ther. 2001, 81, 995–1005. [Google Scholar] [PubMed]
- Bisson, E.; Contant, B.; Sveistrup, H.; Lajoie, Y. Functional balance and dual-task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychol. Behav. 2007, 10, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Dozza, M.; Conrad, R.J.P.; Chiari, L.; Horak, F.B. Effects of practicing tandem gait with and without vibrotactile biofeedback in subjects with unilateral vestibular loss. J. Vestib. Res. Equilib. Orientat. 2007, 17, 195–123. [Google Scholar]
- Liu-Ambrose, T.; Donaldson, M.G.; Ahamed, Y.; Graf, P.; Cook, W.L.; Close, J.; Lord, S.R.; Khan, K.M. Otago home-based strength and balance retraining improves executive functioning in older fallers: A randomized controlled trial. J. Am. Geriatr. Soc. 2008, 56, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Robertson, M.C.; Ashe, M.C.; Liu-Ambrose, T.; Khan, K.M.; Marra, C.A. Does a home based strength and balance programme in people aged ≥80 years provide the best value for money to prevent falls? A systematic review of economic analyses of falls prevention interventions. Br. J. Sports Med. 2010, 44, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H.; Dorsch, A. The promise of mhealth daily activity monitoring and outcome assessments by wearable sensors. Neurorehabilit. Neural Repair 2011, 25, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Putti, A.; Arnold, G.; Cochrane, L.; Abboud, R. The pedar® in-shoe system: Repeatability and normal pressure values. Gait Posture 2007, 25, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Kiran, P.; Arnold, G.; Wang, W.; Abboud, R. Repeatability of the pedar-x® in-shoe pressure measuring system. Foot Ankle Surg. 2010, 16, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.-H.; Wan, A.H.-P.; Wong, D.W.-C.; Zheng, Y.-P.; Lee, W.C.-C. A vibrotactile and plantar force measurement-based biofeedback system: Paving the way towards wearable balance-improving devices. Sensors 2015, 15, 31709–31722. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.; Wan, A.H.; Wong, D.W.; Zheng, Y.-P.; Lee, W.C. Improving postural control using a portable plantar pressure-based vibrotactile biofeedback system. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 855–860.
- Verhoeff, L.L.; Horlings, C.G.; Janssen, L.J.; Bridenbaugh, S.A.; Allum, J.H. Effects of biofeedback on trunk sway during dual tasking in the healthy young and elderly. Gait Posture 2009, 30, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Nitz, J.; Kuys, S.; Isles, R.; Fu, S. Is the wii fit™ a new-generation tool for improving balance, health and well-being? A pilot study. Climacteric 2010, 13, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.; Weinberg, M.S.; Schmidt, P.B.; Krebs, D.E. Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans. Biomed. Eng. 2001, 48, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Sienko, K.H.; Balkwill, M.D.; Wall, C. Biofeedback improves postural control recovery from multi-axis discrete perturbations. J. Neuroeng. Rehabil. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Goebel, J.A.; Sinks, B.C.; Parker, B.E., Jr.; Richardson, N.T.; Olowin, A.B.; Cholewiak, R.W. Effectiveness of head-mounted vibrotactile stimulation in subjects with bilateral vestibular loss: A phase 1 clinical trial. Otol. Neurotol. 2009, 30, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Kim, J.; Chen, S.; Sienko, K.H. Cell phone based balance trainer. J. Neuroeng. Rehabil. 2012, 9, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nanhoe-Mahabier, W.; Allum, J.; Pasman, E.; Overeem, S.; Bloem, B. The effects of vibrotactile biofeedback training on trunk sway in parkinson's disease patients. Parkinsonism Relat. Disord. 2012, 18, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Sienko, K.H.; Balkwill, M.D.; Oddsson, L.; Wall, C. Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations. J. Vestib. Res. 2008, 18, 273–285. [Google Scholar] [PubMed]
- Rossi-Izquierdo, M.; Ernst, A.; Soto-Varela, A.; Santos-Pérez, S.; Faraldo-García, A.; Sesar-Ignacio, Á.; Basta, D. Vibrotactile neurofeedback balance training in patients with parkinson’s disease: Reducing the number of falls. Gait Posture 2013, 37, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Sienko, K.H.; Balkwill, M.D.; Oddsson, L.I.; Wall, C. The effect of vibrotactile feedback on postural sway during locomotor activities. J. Neuroeng. Rehabil. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.; Wrisley, D.M.; Statler, K.D. Vibrotactile tilt feedback improves dynamic gait index: A fall risk indicator in older adults. Gait Posture 2009, 30, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.; Weinberg, M.S. Balance prostheses for postural control. IEEE Eng. Med. Biol. Mag. 2003, 22, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.; King, L.; Mancini, M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys. Ther. 2015, 95, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: A randomized controlled trial. Gerontology 2015. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. Neuroeng. Rehabil. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Leardini, A.; Lullini, G.; Giannini, S.; Berti, L.; Ortolani, M.; Caravaggi, P. Validation of the angular measurements of a new inertial-measurement-unit based rehabilitation system: Comparison with state-of-the-art gait analysis. J. Neuroeng. Rehabil. 2014, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hatton, A.L.; Rome, K.; Dixon, J.; Martin, D.J.; McKeon, P.O. Footwear interventions a review of their sensorimotor and mechanical effects on balance performance and gait in older adults. J. Am. Podiatr. Med. Assoc. 2013, 103, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Byl, N.; Zhang, W.; Coo, S.; Tomizuka, M. Clinical impact of gait training enhanced with visual kinematic biofeedback: Patients with parkinson’s disease and patients stable post stroke. Neuropsychologia 2015. [Google Scholar] [CrossRef] [PubMed]
- Crea, S.; Cipriani, C.; Donati, M.; Carrozza, M.C.; Vitiello, N. Providing time-discrete gait information by wearable feedback apparatus for lower-limb amputees: Usability and functional validation. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Oh, M.-K.; Lee, C.-H.; Park, Y.S.; Yoon, J. A portable gait asymmetry rehabilitation system for individuals with stroke using a vibrotactile feedback. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sungkarat, S.; Fisher, B.E.; Kovindha, A. Efficacy of an insole shoe wedge and augmented pressure sensor for gait training in individuals with stroke: A randomized controlled trial. Clin. Rehabil. 2011, 25, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-Y.; Lin, C.-F.; Soon, K.-S. Balance control enhancement using sub-sensory stimulation and visual-auditory biofeedback strategies for amputee subjects. Prosthet. Orthot. Int. 2007, 31, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, J.M.; Geertzen, J.; Zijlstra, W.; Hof, A.L.; Postema, K. Effects of vibrating insoles on standing balance in diabetic neuropathy. J. Rehabil. Res. Dev. 2008, 45, 1442–1450. [Google Scholar] [CrossRef]
- Hijmans, J.M.; Geertzen, J.H.; Schokker, B.; Postema, K. Development of vibrating insoles. Int. J. Rehabil. Res. 2007, 30, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Priplata, A.A.; Niemi, J.B.; Harry, J.D.; Lipsitz, L.A.; Collins, J.J. Vibrating insoles and balance control in elderly people. Lancet 2003, 362, 1123–1124. [Google Scholar] [CrossRef]
- Priplata, A.A.; Niemi, J.B.; Veves, A.; Lipsitz, L.A.; Collins, J.J. Vibrating insoles improve balance in diabetic patients with peripheral neuropathy. Med. Sci. Sports Exerc. 2004, 36. [Google Scholar] [CrossRef]
- Barkovich, A.; Szefler, S.; Olson, E.; Rymer, W. White paper: Scientific vision workshop on diagnostics and therapeutics. Bethesda MD NICHHD 2011, 1–13. [Google Scholar]
- Esculier, J.-F.; Vaudrin, J.; Beriault, P.; Gagnon, K.; Tremblay, L.E. Home-based balance training programme using wii fit with balance board for parkinson's disease: A pilot study. J. Rehabil. Med. 2012, 44, 144–150. [Google Scholar] [PubMed]
- Koslucher, F.; Wade, M.G.; Nelson, B.; Lim, K.; Chen, F.C.; Stoffregen, T.A. Nintendo wii balance board is sensitive to effects of visual tasks on standing sway in healthy elderly adults. Gait Posture 2012, 36, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.D.; Chang, W.Y.; Lee, C.L.; Feng, C.Y. Validity and reliability of wii fit balance board for the assessment of balance of healthy young adults and the elderly. J. Phys. Ther. Sci. 2013, 25, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Dozza, M.; Chiari, L.; Horak, F.B. Audio-biofeedback improves balance in patients with bilateral vestibular loss. Arch. Phys. Med. Rehabil. 2005, 86, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kojima, S.; Takeda, H.; Ino, S.; Ifukube, T. The influence of moving auditory stimuli on standing balance in healthy young adults and the elderly. Ergonomics 2001, 44, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Dozza, M.; Horak, F.B.; Chiari, L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp. Brain Res. 2007, 178, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, R.; Audu, M.L.; Triolo, R.J. Center of mass acceleration feedback control of functional neuromuscular stimulation for standing in the presence of internal postural perturbations. J. Rehabil. Res. Dev. 2012, 49, 889–912. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, D.; Dozza, M.; Chiari, L.; Maccioni, G.; Cappello, A. Energetic assessment of trunk postural modifications induced by a wearable audio-biofeedback system. Med. Eng. Phys. 2009, 31, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chiari, L.; Dozza, M.; Cappello, A.; Horak, F.B.; Macellari, V.; Giansanti, D. Audio-biofeedback for balance improvement: An accelerometry-based system. IEEE Trans. Biomed. Eng. 2005, 52, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Sayeed, R.; Schwenk, M.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Balance rehabilitation: Promoting the role of virtual reality in patients with diabetic peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Caudron, S.; Guerraz, M.; Eusebio, A.; Gros, J.-P.; Azulay, J.-P.; Vaugoyeau, M. Evaluation of a visual biofeedback on the postural control in parkinson's disease. Neurophys. Clin. Neurophysiol. 2014, 44, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Stokroos, R.; Aarts, J.; van Lummel, R.; Kingma, H. Salient and placebo vibrotactile feedback are equally effective in reducing sway in bilateral vestibular loss patients. Gait Posture 2010, 31, 213–217. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, K.J.; Kamnik, R.; O’Keeffe, D.T.; Lyons, G.M. An inertial and magnetic sensor based technique for joint angle measurement. J. Biomech. 2007, 40, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Abdul Razak, A.H.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot plantar pressure measurement system: A review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef] [PubMed]
- Baram, Y. Virtual sensory feedback for gait improvement in neurological patients. Front. Neurol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Mohktar, M.S.; Kamaruzzaman, S.B.; Lim, K.S.; Pin, T.M.; Ibrahim, F. Smartphone-based solutions for fall detection and prevention: Challenges and open issues. Sensors 2014, 14, 7181–7208. [Google Scholar] [CrossRef] [PubMed]
- Prieto, T.E.; Myklebust, J.; Hoffmann, R.; Lovett, E.; Myklebust, B. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Bebek, Ö.; Suster, M.; Rajgopal, S.; Fu, M.J.; Huang, X.; Çavuşoğlu, M.C.; Young, D.J.; Mehregany, M.; Van den Bogert, A.J.; Mastrangelo, C.H. Personal navigation via high-resolution gait-corrected inertial measurement units. IEEE Trans. Instrum. Meas. 2010, 59, 3018–3027. [Google Scholar] [CrossRef]
- Halická, Z.; Lobotková, J.; Bučková, K.; Hlavačka, F. Effectiveness of different visual biofeedback signals for human balance improvement. Gait Posture 2014, 39, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhou, Z. A real-time articulated human motion tracking using tri-axis inertial/magnetic sensors package. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.J. An Introduction to Inertial Navigation; Technical Report for University of Cambridge Computer Labruary (UCAMCL-TR-696): Cambridge, UK, 2007. [Google Scholar]
- Luinge, H.J.; Veltink, P.H. Measuring orientation of human body segments using miniature gyroscopes and accelerometers. Med. Biol. Eng. Comput. 2005, 43, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, A.; Fejer, R.; Walker, B. The test–retest reliability of centre of pressure measures in bipedal static task conditions—A systematic review of the literature. Gait Posture 2010, 32, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Hass, C.J.; Gregor, R.J.; Waddell, D.E.; Oliver, A.; Smith, D.W.; Fleming, R.P.; Wolf, S.L. The influence of tai chi training on the center of pressure trajectory during gait initiation in older adults. Arch. Phys. Med. Rehabil. 2004, 85, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Ashayeri, H.; Salavati, M.; Sarafzadeh, J.; Taghipoor, K.D.; Saeedi, A.; Salehi, R. Reliability of center of pressure measures of postural stability in healthy older adults: Effects of postural task difficulty and cognitive load. Gait Posture 2011, 33, 651–655. [Google Scholar] [CrossRef] [PubMed]
- O'Connor, S.M.; Kuo, A.D. Direction-dependent control of balance during walking and standing. J. Neurophysiol. 2009, 102, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Frank, J.S. Assessment of balance control in humans. Med. Prog. Technol. 1990, 16, 31–51. [Google Scholar] [PubMed]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the star excursion balance test to assess dynamic postural-control deficits and outcomes in lower extremity injury: A literature and systematic review. J. Athl. Train. 2012, 47, 339–357. [Google Scholar] [PubMed]
- Hernandez, M.E.; Ashton-Miller, J.A.; Alexander, N.B. Age-related changes in speed and accuracy during rapid targeted center of pressure movements near the posterior limit of the base of support. Clin. Biomech. 2012, 27, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Palmieri, R.; Ingersoll, C.; Stone, M.; Krause, B. Center-of-pressure parameters used in the assessment of postural control. J. Sport Rehabil. 2002, 11, 51–66. [Google Scholar]
- Chaudhry, H.; Bukiet, B.; Ji, Z.; Findley, T. Measurement of balance in computer posturography: Comparison of methods—A brief review. J. Bodyw. Mov. Ther. 2011, 15, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Fleury, A.; Guméry, P.-Y.; Diot, B.; Demongeot, J.; Vuillerme, N. Ibalance-abf: A smartphone-based audio-biofeedback balance system. IEEE Trans. Biomed. Eng. 2013, 60, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Lafond, D.; Duarte, M.; Prince, F. Comparison of three methods to estimate the center of mass during balance assessment. J. Biomech. 2004, 37, 1421–1426. [Google Scholar] [CrossRef]
- Hof, A.; Gazendam, M.; Sinke, W. The condition for dynamic stability. J. Biomech. 2005, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.L. The extrapolated center of mass’ concept suggests a simple control of balance in walking. Hum. Mov. Sci. 2008, 27, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Fransson, P.; Lush, D.; Gomez, S. The effect of foam surface properties on postural stability assessment while standing. Gait Posture 2008, 28, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Fransson, P.-A.; Lush, D.; Petersen, H.; Magnusson, M.; Johansson, R.; Gomez, S. The effects of foam surface properties on standing body movement. Acta Otolaryngol. 2008, 128, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Duckrow, R.; Abu-Hasaballah, K.; Whipple, R.; Wolfson, L. Stance perturbation-evoked potentials in old people with poor gait and balance. Clin. Neurophysiol. 1999, 110, 2026–2032. [Google Scholar] [CrossRef]
- Matjacic, Z.; Sok, D.; Jakovljevic, M.; Cikajlo, I. Organization of functional postural responses following perturbations in multiple directions in elderly fallers standing quietly. Int. J. Rehabil. Res. 2013, 36, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J. Romberg and his test. J. Laryngol. Otol. 1980, 94, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.; Shepard, N. Balance Function Assessment and Management; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Furman, J.; Cass, S. Vestibular disorders: A case study approach. J. Neurol. 2003, 250, 1392–1393. [Google Scholar]
- Juras, G.; Słomka, K.; Fredyk, A.; Sobota, G.; Bacik, B. Evaluation of the limits of stability (los) balance test. J. Hum. Kinet. 2008, 19, 39–52. [Google Scholar] [CrossRef]
- Kinzey, S.J.; Armstrong, C.W. The reliability of the star-excursion test in assessing dynamic balance. J. Orthop. Sports Phys. Ther. 1998, 27, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Dozza, M.; Peterka, R.; Chiari, L.; Wall, C. Vibrotactile biofeedback improves tandem gait in patients with unilateral vestibular loss. Ann. N. Y. Acad. Sci. 2009, 1164, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Wood-Dauphinee, S.; Williams, J. The balance scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar] [PubMed]
- Berg, K. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reference values for the timed up and go test: A descriptive meta-analysis. J. Geriatr. Phys. Ther. 2006, 29, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.M.; Hacker, T.A.; Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-minute walk test, berg balance scale, timed up & go test, and gait speeds. Phys. Ther. 2002, 82, 128–137. [Google Scholar] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The 2011 Oxford CEBM Level of Evidence. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 2 March 2016).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Jaeschke, R.; Cook, D.J.; Bria, W.F.; El-Solh, A.A.; Ernst, A.; Fahy, B.F.; Gould, M.K.; Horan, K.L.; Krishnan, J.A. An official ats statement: Grading the quality of evidence and strength of recommendations in ats guidelines and recommendations. Am. J. Respir. Crit. Care Med. 2006, 174, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Alahakone, A.U.; Senanayake, S.; Arosha, M. A real-time system with assistive feedback for postural control in rehabilitation. IEEE/ASME Trans. Mechatron. 2010, 15, 226–233. [Google Scholar] [CrossRef]
- Mulavara, A.P.; Fiedler, M.J.; Kofman, I.S.; Wood, S.J.; Serrador, J.M.; Peters, B.; Cohen, H.S.; Reschke, M.F.; Bloomberg, J.J. Improving balance function using vestibular stochastic resonance: Optimizing stimulus characteristics. Exp. Brain Res. 2011, 210, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mayagoitia, R.E.; Lötters, J.C.; Veltink, P.H.; Hermens, H. Standing balance evaluation using a triaxial accelerometer. Gait Posture 2002, 16, 55–59. [Google Scholar] [CrossRef]
- Zhang, W.; Tomizuka, M.; Byl, N. A Wireless Human Motion Monitoring System Based on Joint Angle Sensors and Smart Shoes. In Proceedings of the ASME 2014 Dynamic Systems and Control Conference, Washington, DC, USA, 22–24 October 2014; p. V003T046A002.
- Liu, T.; Inoue, Y.; Shibata, K.; Shiojima, K. A mobile force plate and three-dimensional motion analysis system for three-dimensional gait assessment. IEEE Sens. J. 2012, 12, 1461–1467. [Google Scholar] [CrossRef]
- Wall, C. Application of vibrotactile feedback of body motion to improve rehabilitation in individuals with imbalance. J. Neurol. Phys. Ther. JNPT 2010, 34, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.F.; Oddsson, L.I.; De Luca, C.J. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 2004, 156, 505–512. [Google Scholar] [PubMed]
- Cruz-Almeida, Y.; Black, M.L.; Christou, E.A.; Clark, D.J. Site-specific differences in the association between plantar tactile perception and mobility function in older adults. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Burcham, J.; Cheng, H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1157–1162. [Google Scholar] [CrossRef]
- Witana, C.P.; Goonetilleke, R.S.; Xiong, S.; Au, E.Y. Effects of surface characteristics on the plantar shape of feet and subjects’ perceived sensations. Appl. Ergon. 2009, 40, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Kavounoudias, A.; Roll, R.; Roll, J.-P. The plantar sole is a ‘dynamometric map’for human balance control. Neuroreport 1998, 9, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Lin, S.-I. Sensitivity of plantar cutaneous sensation and postural stability. Clin. Biomech. 2008, 23, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hennig, E.M.; Sterzing, T. Sensitivity mapping of the human foot: Thresholds at 30 skin locations. Foot Ankle Int. 2009, 30, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.H.; Wong, D.W.; Ma, C.Z.; Zhang, M.; Lee, W.C. Wearable vibrotactile biofeedback device allowing identification of different floor conditions for lower-limb amputees. Arch. Phys. Med. Rehabil. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.; Lam, W.K.; Yeung, L.F.; Lee, W.C. Does long-distance-walking improve or deteriorate walking stability of transtibial amputees? Clin. Biomech. 2015, 30, 867–873. [Google Scholar] [CrossRef] [PubMed]
| Type of Wearable Sensor | Outcome Measurement | Location of Sensor | |
|---|---|---|---|
| Inertial motion sensor | Accelerometer | Linear acceleration of X, Y, and Z movements in a three dimensional space | Body segment |
| Gyroscope | Angular velocity: extent and rate of rotation in a three dimensional space (roll, pitch, and yaw) | Body segment | |
| Magnetometer | Direction: absolute angular movements relative to the Earth’s magnetic field | Body segment | |
| Planter force sensor | Plantar force/pressure information | Plantar surface of foot | |
| Level | Therapy/Prevention, Aetiology/Harm |
|---|---|
| 1a | Systematic Review (with homogeneity) of Randomized Controlled Trials |
| 1b | Individual Randomized Controlled Trial (with narrow Confidence Interval) |
| 1c | All or none |
| 2a | Systematic Review (with homogeneity) of cohort studies |
| 2b | Individual cohort study (including low quality Randomized Controlled Trial; e.g., <80% follow-up) |
| 2c | “Outcomes” Research; Ecological studies |
| 3a | Systematic Review (with homogeneity) of case-control studies |
| 3b | Individual Case-Control Study |
| 4 | Case-series (and poor quality cohort and case-control studies) |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
| Grade | Contents |
|---|---|
| A | consistent level 1 studies |
| B | consistent level 2 or 3 studies or extrapolations from level 1 studies |
| C | level 4 studies or extrapolations from level 2 or 3 studies |
| D | level 5 evidence or troublingly inconsistent or inconclusive studies of any level |
| Subscale | Item | Index | Score | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | 0 | |||
| Reporting | 1 | Is the hypothesis/aim/objective of the study clearly described? | - | - | - | - | Y | N |
| 2 | Are the main outcomes to be measured clearly described in the Introduction or Methods section? | - | - | - | - | Y | N | |
| 3 | Are the characteristics of the patients included in the study clearly described? | - | - | - | - | Y | N | |
| 4 | Are the interventions of interest clearly described? | - | - | - | - | Y | N | |
| 5 | Are the distributions of principal confounders in each group of subjects to be compared clearly described? | - | - | - | Y | P | N | |
| 6 | Are the main findings of the study clearly described? | - | - | - | - | Y | N | |
| 7 | Does the study provide estimates of the random variability in the data for the main outcomes? | - | - | - | - | Y | N | |
| 8 | Have all important adverse events that may be a consequence of the intervention been reported? | - | - | - | - | Y | N | |
| 9 | Have the characteristics of patients lost to follow-up been described? | - | - | - | - | Y | N | |
| 10 | Have actual probability values been reported (e.g., 0.035 rather than <0.05) for the main outcomes except where the probability value is less than 0.001? | - | - | - | - | Y | N | |
| External Validity | 11 | Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | - | - | - | - | Y | N/UD |
| 12 | Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | - | - | - | - | Y | N/UD | |
| 13 | Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive? | - | - | - | - | Y | N/UD | |
| Internal Validity-Bias | 14 | Was an attempt made to blind study subjects to the intervention they have received? | - | - | - | - | Y | N/UD |
| 15 | Was an attempt made to blind those measuring the main outcomes of the intervention? | - | - | - | - | Y | N/UD | |
| 16 | If any of the results of the study were based on “data dredging”, was this made clear? | - | - | - | - | Y | N/UD | |
| 17 | In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls? | - | - | - | - | Y | N/UD | |
| 18 | Were the statistical tests used to assess the main outcomes appropriate? | - | - | - | - | Y | N/UD | |
| 19 | Was compliance with the intervention/s reliable? | - | - | - | - | Y | N/UD | |
| 20 | Were the main outcome measures used accurate (valid and reliable)? | - | - | - | - | Y | N/UD | |
| Internal Validity-Confounding (Selection Bias) | 21 | Were the patients in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited from the same population? | - | - | - | - | Y | N/UD |
| 22 | Were study subjects in different intervention groups (trials and cohort studies) or were the cases and controls (case-control studies) recruited over the same period of time? | - | - | - | - | Y | N/UD | |
| 23 | Were study subjects randomised to intervention groups? | - | - | - | - | Y | N/UD | |
| 24 | Was the randomised intervention assignment concealed from both patients and health care staff until recruitment was complete and irrevocable? | - | - | - | - | Y | N/UD | |
| 25 | Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? | - | - | - | - | Y | N/UD | |
| 26 | Were losses of patients to follow-up taken into account? | - | - | - | - | Y | N/UD | |
| Power | 27 | Did the study have sufficient power to detect a clinically important effect where the probability value for a difference being due to chance is less than 5%? | Size of smallest intervention group | |||||
| > n8 | n7 − n8 | n5 − n6 | n3 − n4 | n1 − n2 | < n1 | |||
| Study | Level of Evidence | Design | Level of Recommendation |
|---|---|---|---|
| Afzal et al. 2015 [49] | 3B | Individual Case-Control Study | B |
| Byl et al. 2015 [47] | 1B | Individual Randomized Controlled Trial | A |
| Crea et al. 2015 [48] | 3B | Individual Case-Control Study | B |
| Grewal et al. 2015 [43] | 1B | Individual Randomized Controlled Trial | A |
| Ma et al. 2015 [28] | 3B | Individual Case-Control Study | B |
| Caudron et al. 2014 [67] | 3B | Individual Case-Control Study | B |
| Halicka et al. 2014 [75] | 3B | Individual Case-Control Study | B |
| Franco et al. 2013 [89] | 3B | Individual Case-Control Study | B |
| Nanhoe-Mahabier et al. 2012 [36] | 3B | Individual Case-Control Study | B |
| Nataraj et al. 2012 [63] | 3B | Individual Case-Control Study | B |
| Sungkarat et al. 2011 [50] | 1B | Individual Randomized Controlled Trial | A |
| Alahakone et al. 2010 [110] | 3B | Individual Case-Control Study | B |
| Janssen et al. 2010 [68] | 3B | Individual Case-Control Study | B |
| Giansanti et al. 2009 [64] | 3B | Individual Case-Control Study | B |
| Lee et al. 2007 [51] | 3B | Individual Case-Control Study | B |
| Chiari et al. 2005 [65] | 3B | Individual Case-Control Study | B |
| Wall et al. 2001 [32] | 3B | Individual Case-Control Study | B |
| Study | Score of Subscale and Index | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reporting | External Validity | Internal Validity-Bias | Internal Validity-Confounding (Selection Bias) | Power | Total | |||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | ||
| Afzal et al. 2015 [49] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 17 |
| Byl et al. 2015 [47] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 19 |
| Crea et al. 2015 [48] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 15 |
| Grewal et al. 2015 [43] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 25 |
| Ma et al. 2015 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 19 |
| Caudron et al. 2014 [67] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 17 |
| Halicka et al. 2014 [75] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 17 |
| Franco et al. 2013 [89] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 18 |
| Nanhoe-Mahabier et al. 2012 [36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 20 |
| Nataraj et al. 2012 [63] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 15 |
| Sungkarat et al. 2011 [50] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 23 |
| Alahakone et al. 2010 [110] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 15 |
| Janssen et al. 2010 [68] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 17 |
| Giansanti et al. 2009 [64] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 16 |
| Lee et al. 2007 [51] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 18 |
| Chiari et al. 2005 [65] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 15 |
| Wall et al. 2001 [32] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 15 |
| Study | Sample Size (Gender, F/M) | Group (Sample Size, n): Mean (SD) Age, Years | Sample Characteristics | ||
|---|---|---|---|---|---|
| Physical | Cognitive | Fall History | |||
| Afzal et al. 2015 [49] | 9 (1/8) | Intervention (9): | -Stroke: clear symptoms of lower-limb weakness at the paretic side. -Young healthy: no history of musculoskeletal or neurological disorders. | Not specified | Not specified |
| -Stroke (4): 64.8 (9.5) | |||||
| -Healthy (5): 26.2 (3.3) | |||||
| Byl et al. 2015 [47] | 24 (8/16) | Intervention (12): | -With gait impairments and one year or more post stroke or diagnosis of PD. -Independent in self-care, could rise from a chair, and walk without personal assistance for a minimum of 100 feet. -PD: scored I to III on the Hoehn and Yahr Scale -Stroke: a minimum score of 10 of the lower-limb evaluated by the Cafe 40-Functional Independence Scale | -Able to follow instructions -No severe depression. | Not specified |
| -PD (7): 68.5 (3.6) | |||||
| -Stroke (5): 66.2 (5.0) | |||||
| Control (12): | |||||
| -PD (5): 70 (2.9) | |||||
| -Stroke (7): 60.8 (5.4) | |||||
| Crea et al. 2015 [48] | 10 (6/4) | Intervention (10): 27.0 (1.8) | -Able-bodied | Not specified | Not specified |
| Grewal et al. 2015 [43] | 39 (20/19) | Intervention (19): 62.6 (8.0) | -Type 2 diabetes with peripheral neuropathy; able to independently walk for 2m. -No vestibular or central neurological dysfunction, musculoskeletal abnormality, active foot ulcers, Charcot’s joints or a history of balance disorder unrelated to DPN. | No cognitive dysfunction | Not specified |
| Control (20): 64.9 (8.5) | |||||
| Ma et al. 2015 [28] | 30 (13/17) | Intervention (30): | -Fully independent, living in a community-based setting, and were capable of ambulation without assistive devices. -No neurological or vestibular disorders, diabetes, severe cardiovascular or pulmonary diseases, previous history of foot injury, foot deformity, amputation of the lower limbs, inability to attend the necessary re-evaluations, or inability to follow the instructions and procedures | Able to follow instructions | Not specified |
| -Elderly (15): 70.1 (3.7) | |||||
| -Young (15): 26.7 (2.9) | |||||
| Caudron et al. 2014 [67] | 17 (7/10) | Intervention (17): 61.9 (8.2) | -Patients with idiopathic PD. -Scored II to III on the Hoehn and Yahr Scale and UPDRS motor score | Not specified | With or without |
| Halicka et al. 2014 [75] | 20 (11/9) | Intervention (20): 22.6 (nil) | Healthy young subjects did not report any neurological, orthopaedic, or balance impairments. | Not specified | Not specified |
| Franco et al. 2013 [89] | 20 (11/9) | Intervention (20): 26.5 (3.7) | Healthy young subjects with no history of sensory or motor problems, neurological diseases, or disorders. | Not specified | Not specified |
| Nanhoe-Mahabier et al. 2012 [36] | 20 (4/16) | Intervention (10): 59.3 (2.0) | -Patients with PD. -With no causes of balance impairment other than PD, able to walk without walking aids, and no severe co-morbidity. | No cognitive dysfunction | Not specified |
| Control (10): 58.6 (2.5) | |||||
| Nataraj et al. 2012 [63] | 1 (1/0) | Intervention (1): nil (nil) | Patient with thoracic-4 level complete paraplegia. | Not specified | Not specified |
| Sungkarat et al. 2011 [50] | 35 (11/24) | Intervention (17): 52.1 (7.2) | -Patients with first episode of unilateral stroke with hemiparesis; orpington prognostic score at initial assessment between 3.2 and 5.2 (moderately severe); able to walk at least 10 m with or without assistance; stable medical condition; and to participate. -Patients without any comorbidity or complication that would preclude gait training, severe leg spasticity (Modified Ashworth Scale ≥3 [50]), neglect, or missed more than three training sessions. | No impaired cognition and/or communication | Not specified |
| Control (18): 53.8 (11.2) | |||||
| Alahakone et al. 2010 [110] | 6 (3/3) | Intervention (6): 23.2 (nil) | Healthy young subjects | Not specified | Not specified |
| Janssen et al. 2010 [68] | 20 (8/12) | Intervention (10): 63.1 (9.3) | Patients with severe bilateral vestibular losses (flexia or hyporeflexia). With severe balance problems | Not specified | >5 times falls per year |
| Control (10): 40-65 | |||||
| Giansanti et al. 2009 [64] | 9 (nil) | Intervention (9): 55.0 (33-71) | Healthy subjects | Not specified | Not specified |
| Lee et al. 2007 [51] | 7 (2/5) | Intervention (7): 38.9 (14.1) | Lower-limb amputees with no orthopaedic or neurological conditions, disabling arthritis, uncorrected visual problems, dizziness or vertigo, use of assistive walking devices, joint injury, or joint implants | Not specified | Not specified |
| Chiari et al. 2005 [65] | 9 (nil) | Intervention (9): 55.0 (33-71) | Healthy subjects | Not specified | Not specified |
| Wall et al. 2001 [32] | 6 (4/2) | Intervention (6): 24.8 (22-29) | Healthy subjects | Not specified | Not specified |
| Study | Type of Sensors | Location of Sensors | Type of Biofeedback | Function of Device |
|---|---|---|---|---|
| Afzal et al. 2015 [49] | Plantar force sensors | Heel, toe, 1st and 5th MT heads | Vibrotactile | Diagnose gait abnormalities and provide vibration feedback to help compensate for the asymmetric gait. |
| Byl et al. 2015 [47] | Plantar force sensors | Toe, 1st and 2nd MTP, 4th and 5th MTP, and heel | Visual | Gait training with visual kinematic feedback on iPad. |
| Accelerometer | Shank and thigh | |||
| Magnetometer | ||||
| Gyroscope | ||||
| Crea et al. 2015 [48] | Plantar force sensors (64 at each insole) | Plantar surface of the foot | Vibrotactile | Provide simultaneous vibration based on the detected gait phase transitions. |
| Grewal et al. 2015 [43] | Accelerometer | Shank, thigh and lower back | Auditory & Visual | Provide audio-visual feedback on a display of the sway of COM and ankle joints |
| Magnetometer | ||||
| Gyroscope | ||||
| Ma et al. 2015 [28] | Plantar force sensors | Heel, 1st and 5th MT heads | Vibrotactile | Provide vibrotactile feedback of postural sway. |
| Caudron et al. 2014 [67] | Accelerometer | Cranial vertex and the spine processes of the T7-T8 | Visual | Real time biofeedback of anterior-posterior trunk and head tilts |
| Magnetometer | ||||
| Halicka et al. 2014 [75] | Accelerometer | Posterior side of T4, L5 | Visual | Capture body sway and provide visual biofeedback |
| Force plate | ||||
| Franco et al. 2013 [89] | Accelerometer | Posterior side of L5 | Auditory | Monitor the trunk angular evolution during bipedal stance and improve user’s balance through auditory biofeedback by earphone |
| Magnetometer | ||||
| Gyroscope of a smartphone | ||||
| Nanhoe-Mahabier et al. 2012 [36] | Angular velocity sensors | Lower back at level L1-L3 | Vibrotactile | Deliver vibrotactile feedback of trunk sway to head |
| Nataraj et al. 2012 [63] | Accelerometer | Pelvis and torso | Electrotactile | Estimate COM acceleration using inputs from body-mounted accelerometer measurements. |
| Deliver stimulation via surgically implanted intramuscular electrodes to bilateral muscle groups of trunk and lower limb | ||||
| Sungkarat et al. 2011 [50] | Plantar force sensors | Heel of the paretic foot | Auditory | Rehabilitation and gait training based on footswitch and the amount of weight bearing at the paretic limb |
| Alahakone et al. 2010 [110] | Accelerometer | Lower back | Vibrotactile & Visual | Measure the ML trunk tilt angles |
| Gyroscope | Custom-developed software for data processing, data display and feedback generation | |||
| Temperature sensor | ||||
| Janssen et al. 2010 [68] | Accelerometer | Head or upper trunk | Vibrotactile | Detect head or body tilt |
| Deliver vibrotactile biofeedback to the waist. | ||||
| Giansanti et al. 2009 [64] | Accelerometer | Body centre of mass (COM). | Auditory | Assess the trunk sway and provide biofeedback information |
| Gyroscope | ||||
| Lee et al. 2007 [51] | Plantar force sensors | Heel and the 3rd MT head of the prosthetic foot | Electrical & Visual-auditory | Detect heel strike and toe off. |
| Provide sub-threshold low-level electrical stimulation to the quadriceps, and visual-auditory biofeedback on a screen | ||||
| Chiari et al. 2005 [65] | Accelerometer | Trunk | Auditory | Measure the linear accelerations of the trunk in anteroposterior and mediolateral directions |
| Provide audio-biofeedback via headphones | ||||
| Wall et al. 2001 [32] | Accelerometer | Head | Vibrotactile | Measure lateral head tilt and mount vibrotactile elements on the body to display head tilt |
| Gyroscope |
| Study | Assessment Point | Outcome Measures | Measurement Tool | Results | Balance Improvement |
|---|---|---|---|---|---|
| Afzal et al. 2015 [49] | (1) Pre-test | -Postural stability during standing;
-Gait asymmetry | Smartphone with inertial sensors | (1) Vibration cue based on temporal information was more effective than intensity information | Yes, static and dynamic balance |
| (2) Post-test | (2) Individuals with stroke revealed significant improvement in gait symmetry with minimal disturbance caused to the balance and gait speed as an effect of the biofeedback. | ||||
| Byl et al. 2015 [47] | (1) Pre-test | -Mobility (gait speed, step length, endurance, and quality)
-Balance (Berg Balance) -Range of motion and strength of joints. | -Force sensors -Inertial motion sensors -Clinical tests | (1) All subjects revealed significant gains in mobility, balance, range of motion and strength. | Yes, dynamic balance |
| (2) Post-test | (2) Subjects with chronic post stroke achieved greater strength gains on the affected side than subjects with PD. | ||||
| (3) 6 weeks | (3) Dynamic visual kinematic feedback from wireless pressure and motion sensors had similar positive effects as verbal and therapist feedback. | ||||
| Crea et al. 2015 [48] | (1) Pre-test | -No. of correct/wrong detection of gait phrases
-Temporal gait phrases | -Questionnaire -Force sensors | (1) High recognition of feedback information | Yes, dynamic balance |
| (2) Post-test | (2) Time-discrete low-intensity feedback was readily perceived by humans and potentially can assist gait control | ||||
| Grewal et al. 2015 [43] | (1) Pre-test | Postural stability during standing | Inertial motion sensors | (1) Significant reduction in COM sway after training. | Yes, static balance |
| (2) A higher postural stability deficit (high body sway) at baseline was associated with higher training gains in postural balance (reduction in COM sway). | |||||
| (2) Post-test | (3) Significant improvement in postural coordination between the ankle and hip joints. | ||||
| Ma et al. 2015 [28] | (1) Pre-test | Postural stability during standing; | Force plate | Significant reduction in COP sway after training. | Yes, static balance |
| (2) Post-test | |||||
| Caudron et al. 2014 [67] | (1) Pre-test | -Postural stability
-Postural orientation during standing | Motion capture system using reflective markers | Visual biofeedback improved PD patients’ postural orientation and postural stability | Yes, static balance |
| (2) Post-test | |||||
| Halicka et al. 2014 [75] | (1) Pre-test | -COP
-Postural stability during standing | -Force plate -Inertial motion sensors | (1) Reduction of body sway was the most significant in the body segment upon receiving the visual biofeedback. | Yes, static balance |
| (2) Post-test | (2) The COP position and L5 position provided the best signals for visual biofeedback. | ||||
| Franco et al. 2013 [89] | (1) Pre-test | Postural stability during standing | Inertial motion sensors | Young healthy individuals were able to efficiently use auditory biofeedback on sagittal trunk tilt to improve their balance in the medial-lateral direction. | Yes, static balance |
| (2) Post-test | |||||
| Nanhoe-Mahabier et al. 2012 [36] | (1) Pre-test | Postural stability during standing | Angular velocity sensors | (1) Patients in the feedback group had a significantly greater reduction in ML and AP postural sway. | Yes, static balance |
| (2) Post-test | (2) Greater ML sway angle in controls after training suggested better training effects in the feedback group | ||||
| Nataraj et al. 2012 [63] | (1) Pre-test | Postural stability during standing | Motion capture system using reflective markers | Compared with constant muscle stimulation employed clinically, controlled stimulations based on COM acceleration improved standing performance more and reduced the upper limb loading required to resist internal postural disturbances by 27% | Yes, static balance |
| (2) Post-test | |||||
| Sungkarat et al. 2011 [50] | (1) Pre-test | Gait speed, step length and single support time asymmetry ratio, balance and amount of load on paretic leg during stance | -Motion capture system -Clinical tests: BBS, TUG | (1) The experimental group demonstrated significant increase in standing and gait symmetry compared with the control group. | Yes, static and dynamic balance |
| (2) The experimental group demonstrated three times greater improvement in gait speed than the control group. | |||||
| (2) 3 weeks (60 min × 5 days/week) | (3) Balance improvement was significantly greater for the experimental than the control group | ||||
| Alahakone et al. 2010 [110] | (1) Pre-test | ML trunk sway during tandem Romberg standing tests | -Inertial motion sensors -Web camera for sighted tests | (1) Feedback was triggered 100% of the time when trunk tilt exceeded the defined threshold. | Yes, static balance |
| (2) Post-test | (2) Significant reduction in trunk tilt angle. | ||||
| Janssen et al. 2010 [68] | (1) Pre-test | Body sway during standing (COP) | Force plate | (1) No significant change in body sway path was observed using biofeedback in six subjects. | Partially yes, static balance |
| (2) In four patients, body sway path decreased significantly using biofeedback and sensor on the head in all three activation modes, whereas with sensor on the trunk only one patient showed a significant improvement in sway path in all three activation modes. | |||||
| (2) Post-test | (3) However, the improvement with true biofeedback was only observed in those subjects where an improvement was present in placebo mode as well. | ||||
| Giansanti et al. 2009 [64] | (1) Pre-test | Changes in angular sway and kinetic energy variables | Inertial motion sensors | Using auditory biofeedback, all subjects significantly reduced pitch, roll and angular velocity with eyes open or closed while standing on a foam surface | Yes, static balance |
| (2) Post-test | |||||
| Lee et al. 2007 [51] | (1) Pre-test | -Single leg quiet standing balance -Dynamic treadmill ambulatory gait performance | Motion capture system | (1) Improvement in balance performance during single leg quiet standing by applying sub-sensory stimulation. | Yes, static and dynamic balance |
| (2) Post-test | (2) With visual-auditory biofeedback as a cue for heel contact and toe push-off condition during treadmill ambulation, the dynamic gait performance of amputees was improved. | ||||
| Chiari et al. 2005 [65] | (1) Pre-test | Postural stability during standing | Force plate | (1) Improved balance upon using the audio-biofeedback system and this improvement was greater when the subject’s balance was challenged by absent or unreliable sensory cues. | Yes, static balance |
| (2) Post-test | (2) High correlations were found between the COP displacement and trunk acceleration | ||||
| Wall et al. 2001 [32] | (1) Pre-test | -Lateral head sway -Postural stability during standing | -Inertial motion sensors -Force plate | Reduced lateral postural sway upon using the head tilt information. | Yes, static balance |
| (2) Post-test |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.Z.-H.; Wong, D.W.-C.; Lam, W.K.; Wan, A.H.-P.; Lee, W.C.-C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors 2016, 16, 434. https://doi.org/10.3390/s16040434
Ma CZ-H, Wong DW-C, Lam WK, Wan AH-P, Lee WC-C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors. 2016; 16(4):434. https://doi.org/10.3390/s16040434
Chicago/Turabian StyleMa, Christina Zong-Hao, Duo Wai-Chi Wong, Wing Kai Lam, Anson Hong-Ping Wan, and Winson Chiu-Chun Lee. 2016. "Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review" Sensors 16, no. 4: 434. https://doi.org/10.3390/s16040434
APA StyleMa, C. Z.-H., Wong, D. W.-C., Lam, W. K., Wan, A. H.-P., & Lee, W. C.-C. (2016). Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors, 16(4), 434. https://doi.org/10.3390/s16040434








