Synthesis and Characterizations of Novel Ca-Mg-Ti-Fe-Oxides Based Ceramic Nanocrystals and Flexible Film of Polydimethylsiloxane Composite with Improved Mechanical and Dielectric Properties for Sensors
Abstract
:1. Introduction
2. Materials and Methods
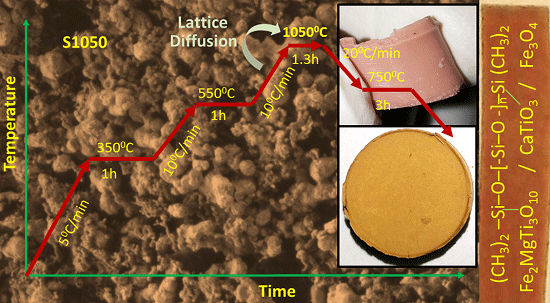
2.1. Preparation of Armalcolite and Perovskite Type Oxide Ceramics
2.2. Preparation of Flexible PDMS/S1050 Composite Film
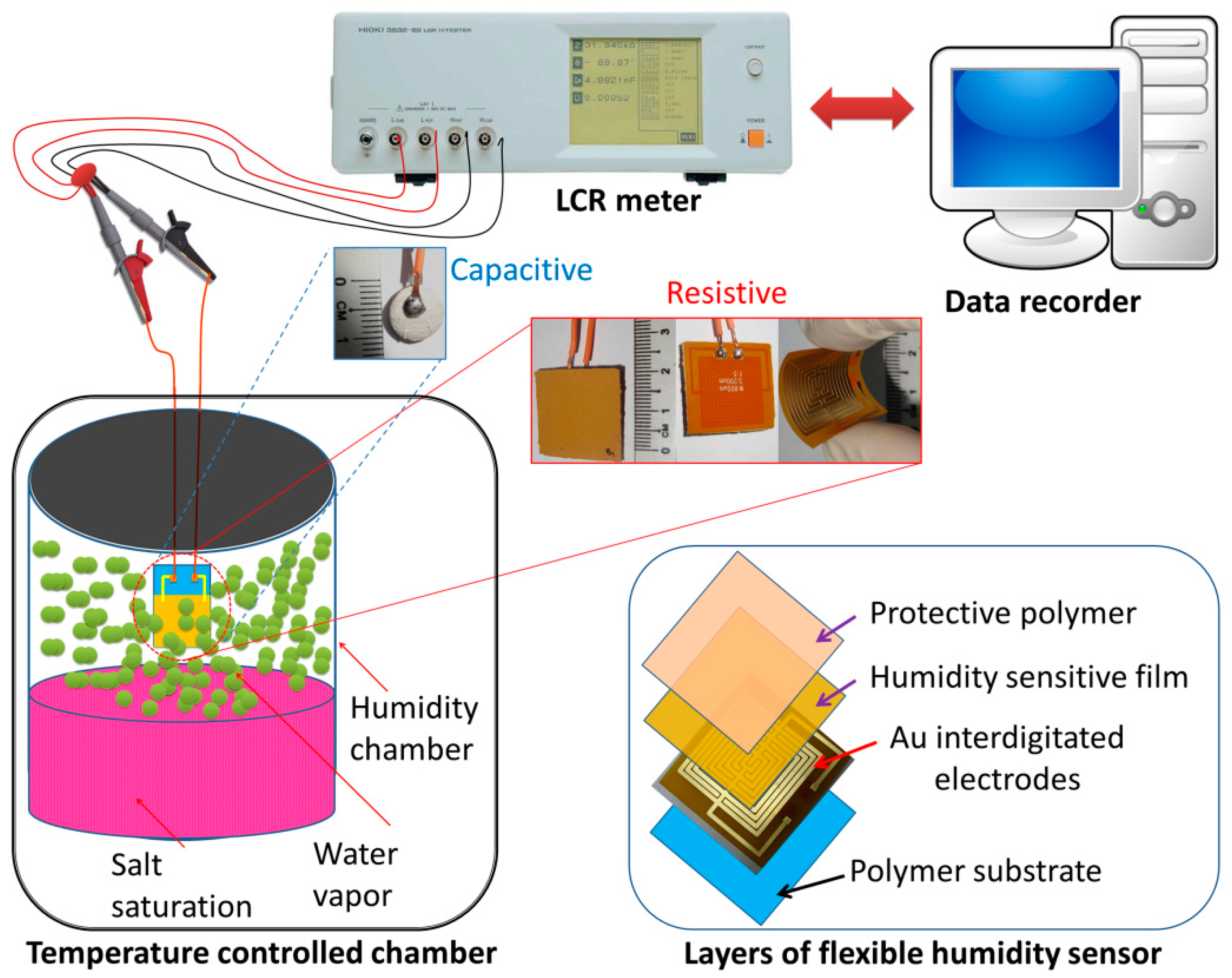
2.3. Preparation of Humidity Sensors
2.4. Characterizations
3. Results and Discussion
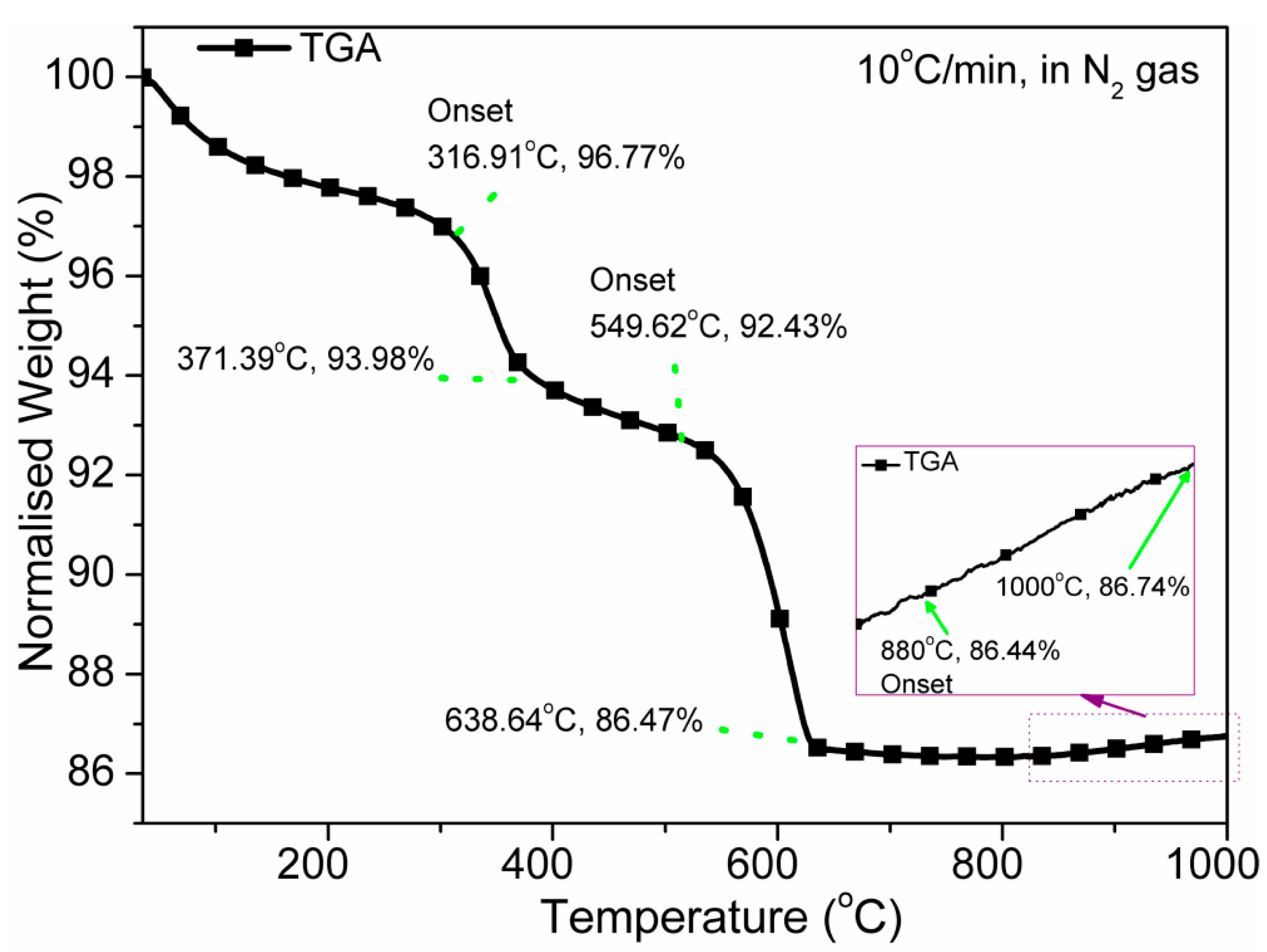
3.1. Thermal Analysis
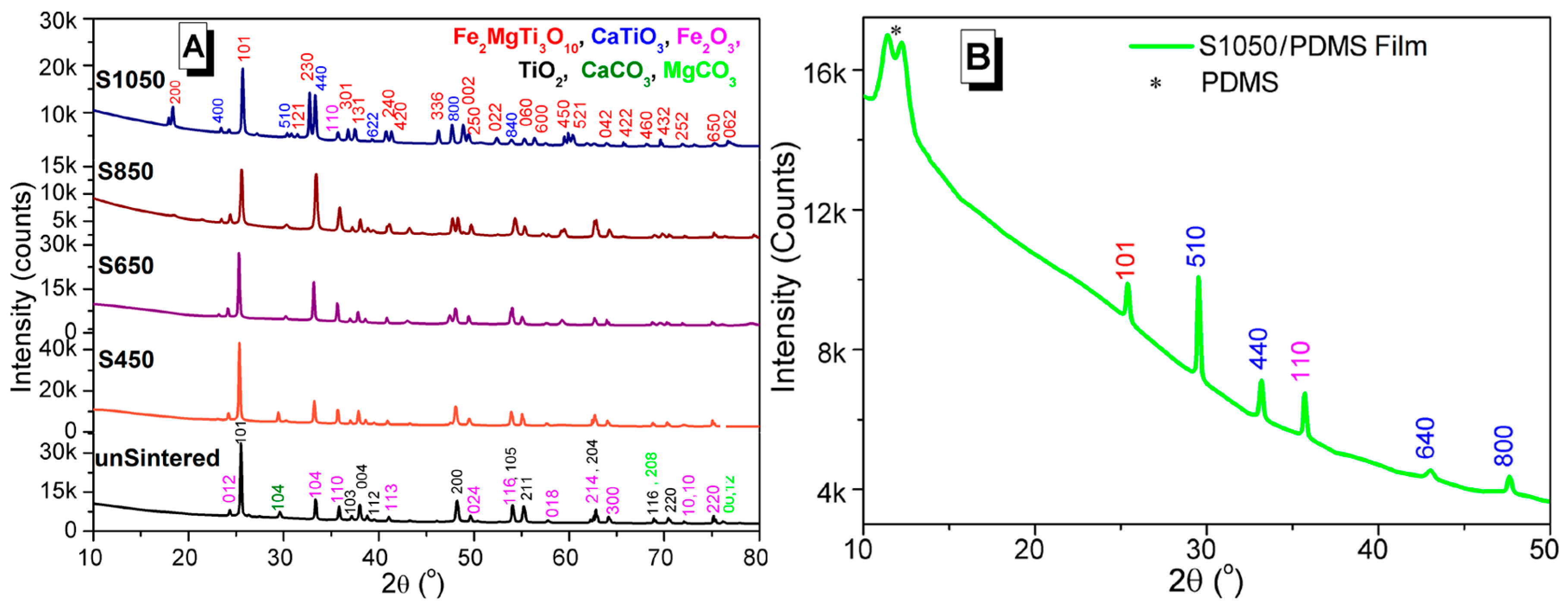
3.2. Crystal Structures Analysis
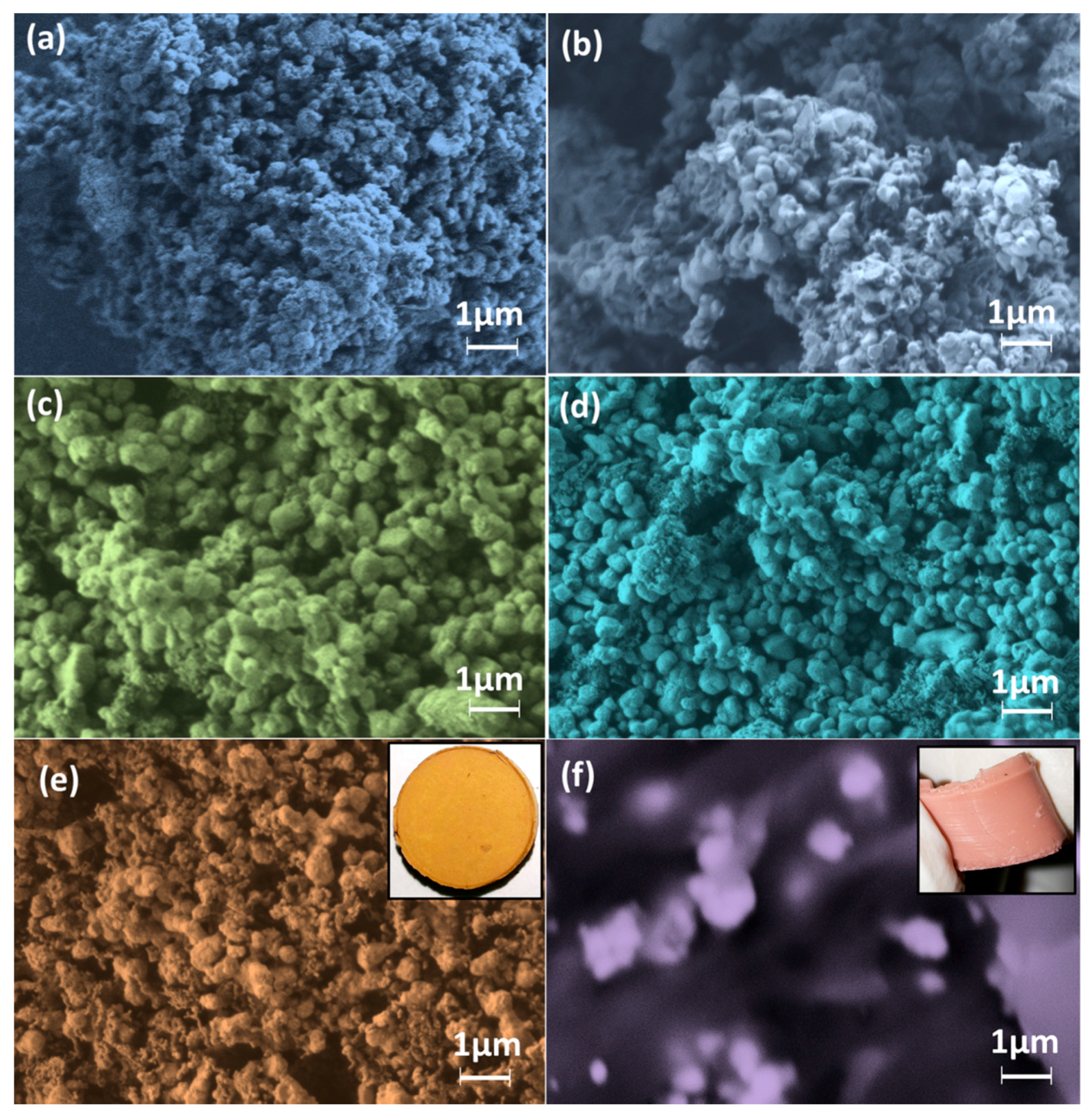
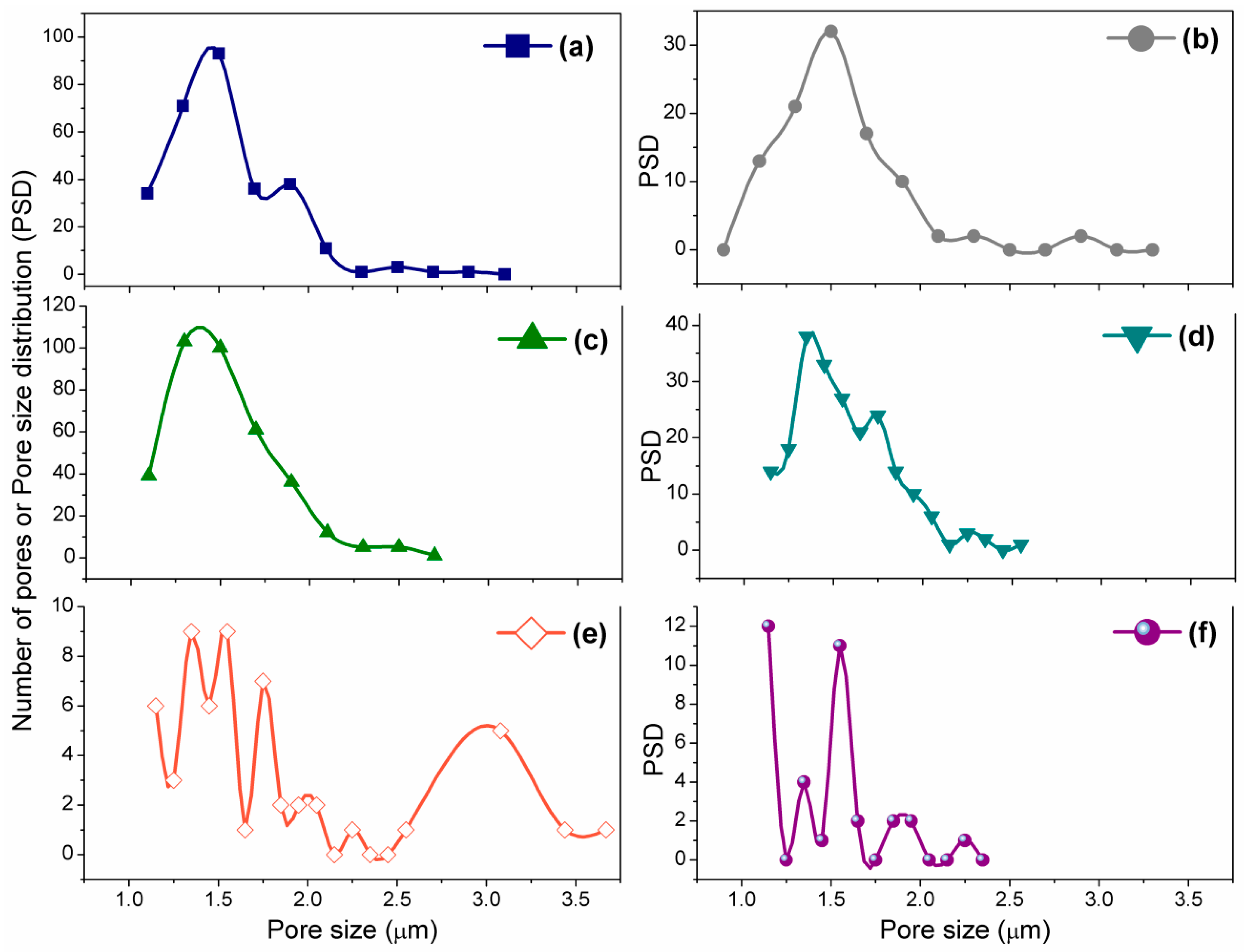
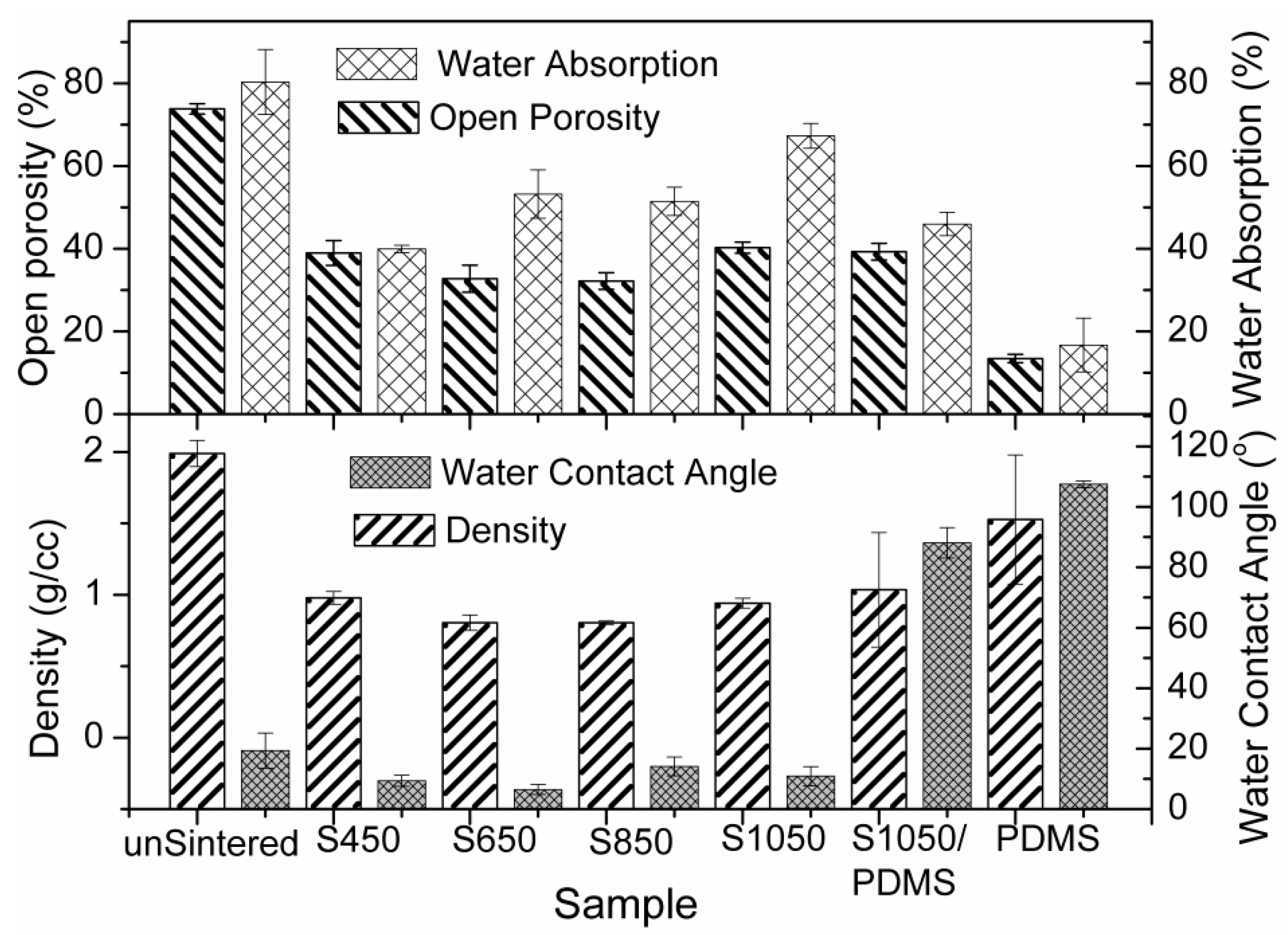
3.3. Morphological and Surface Analyses
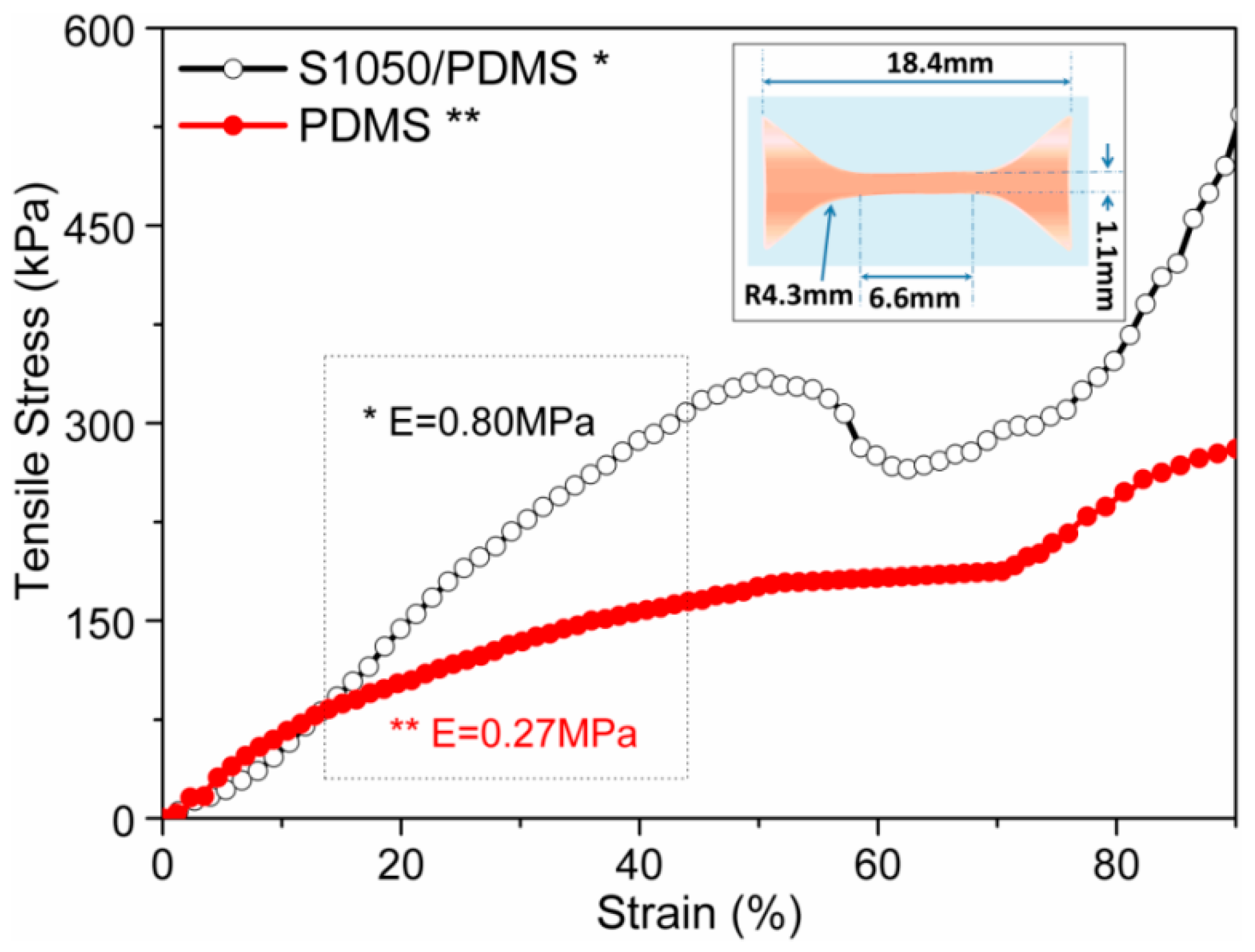
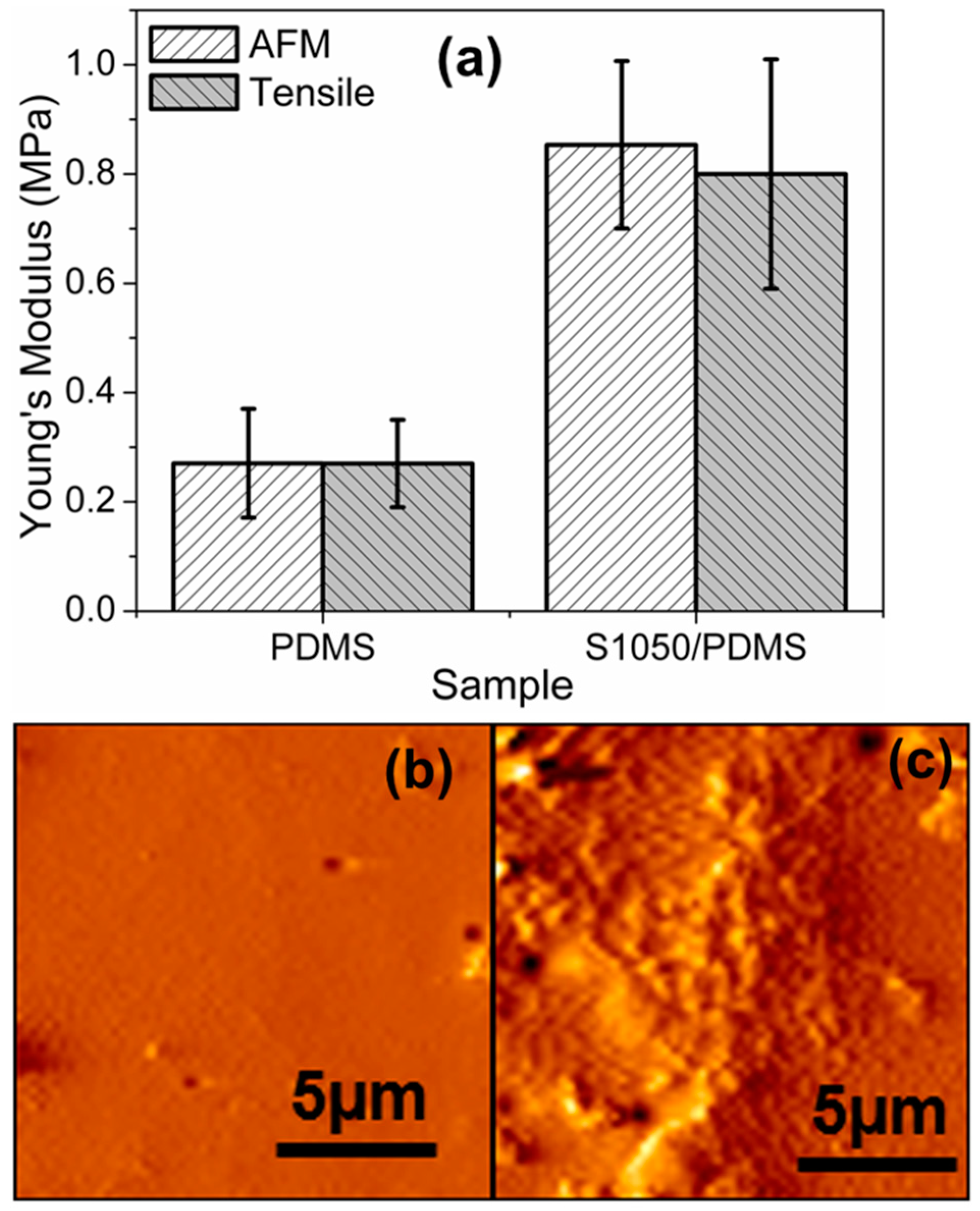
3.4. Static Mechanical Analysis
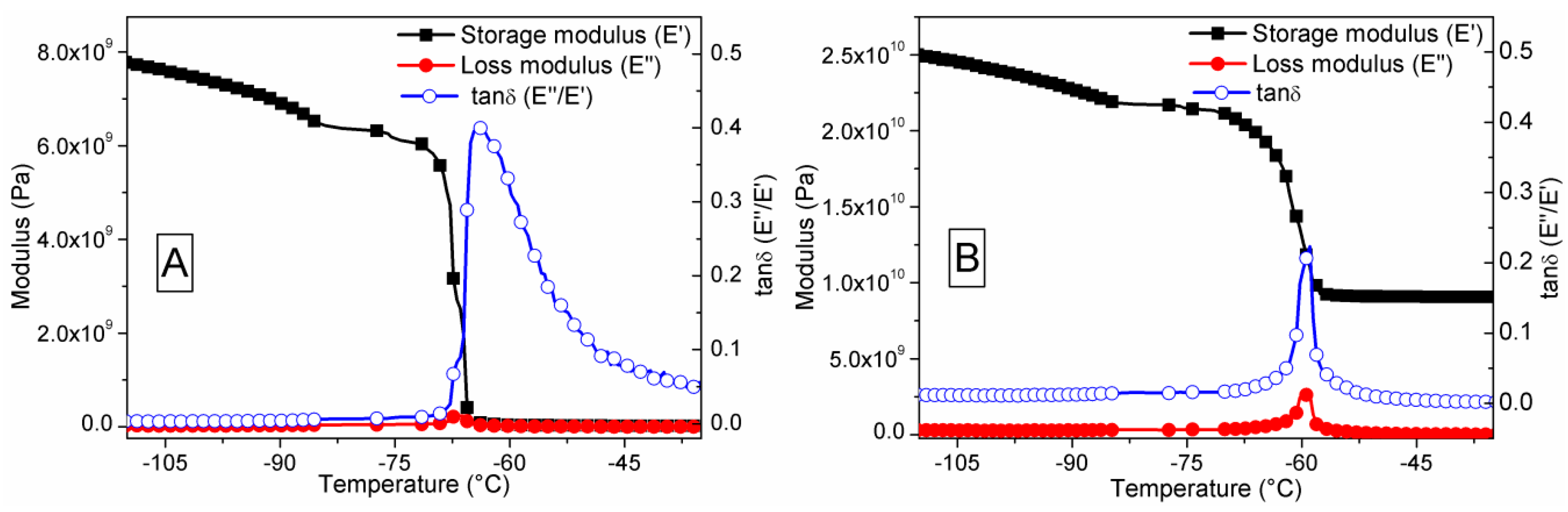
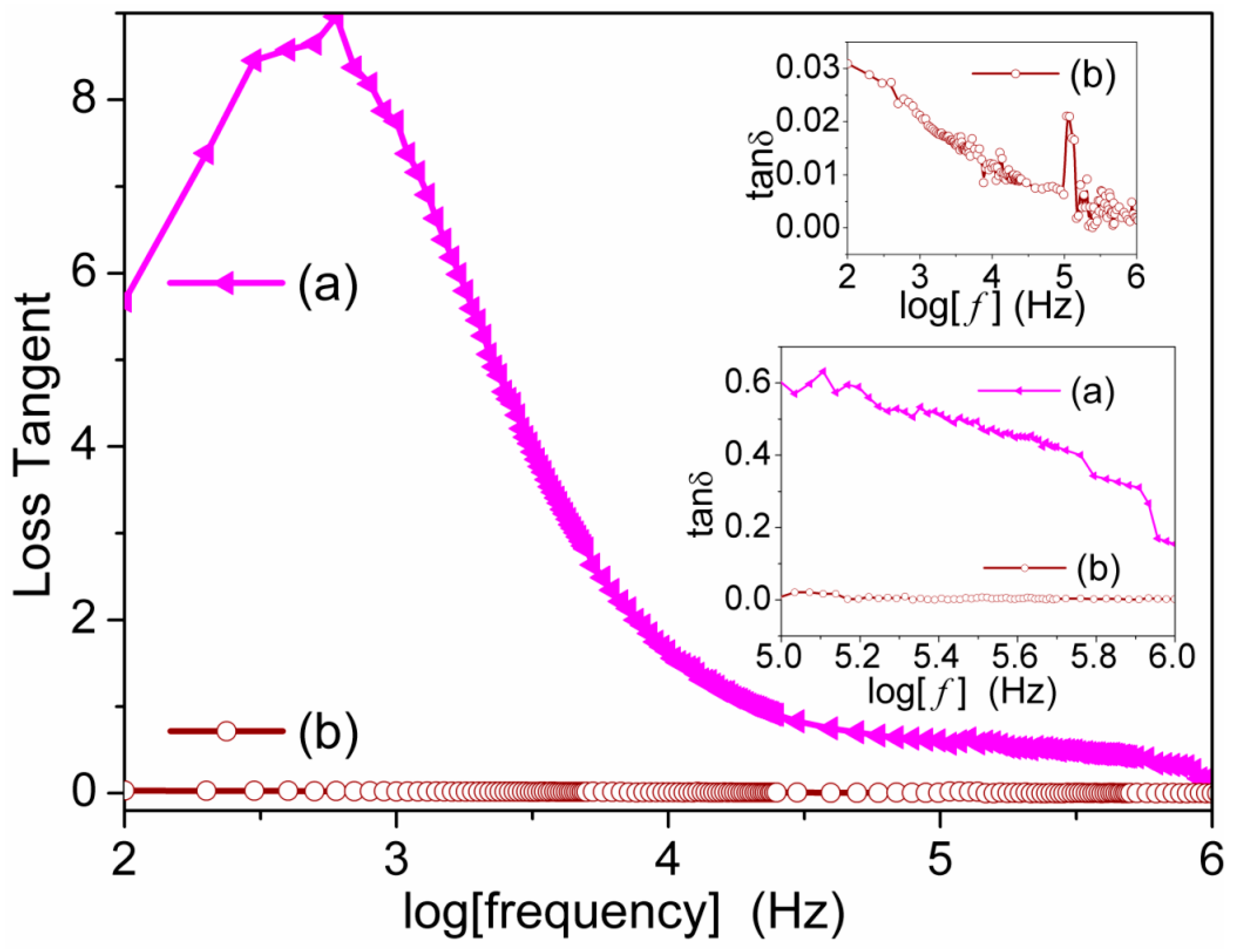
3.5. Dynamic Mechanical Analysis

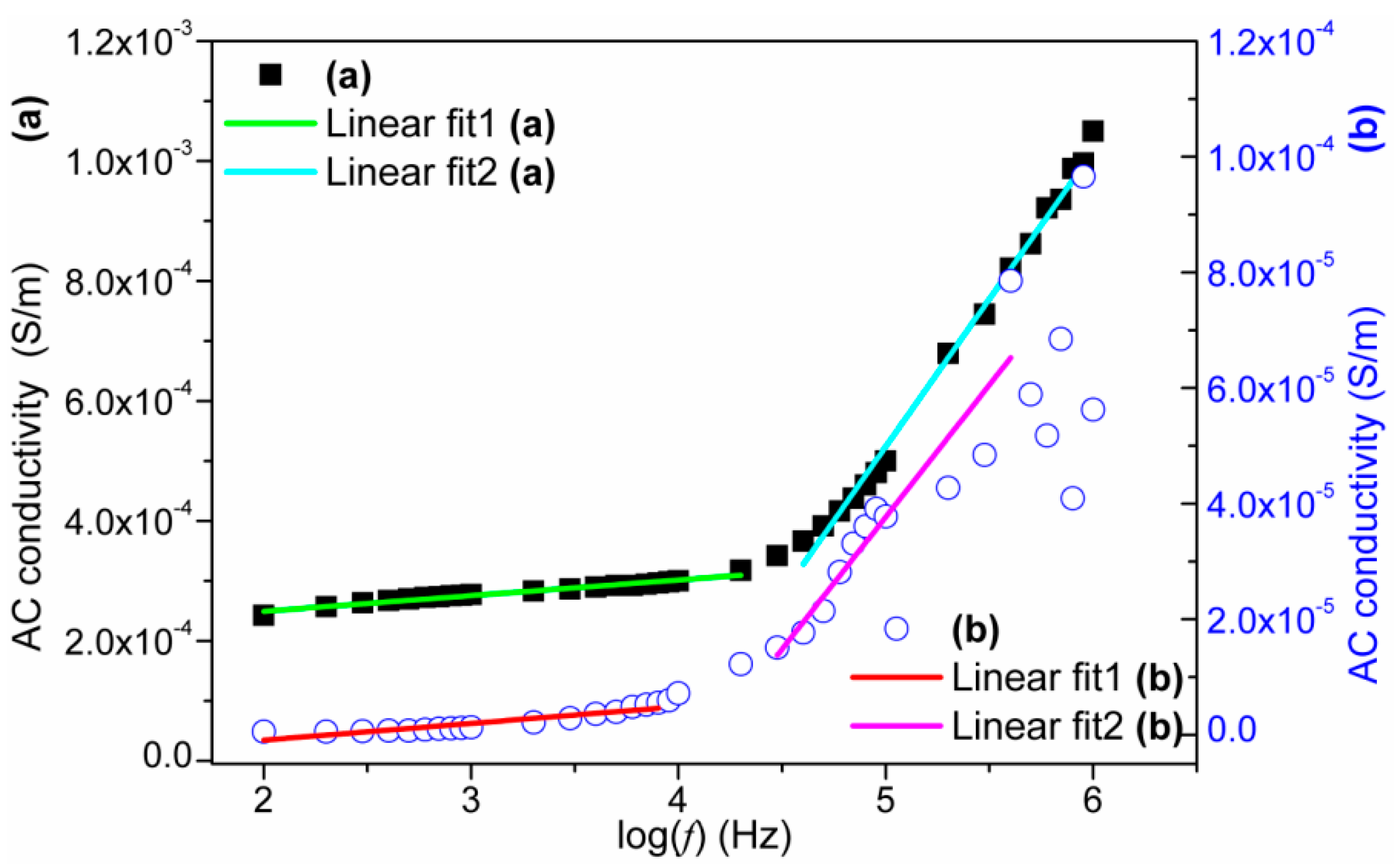
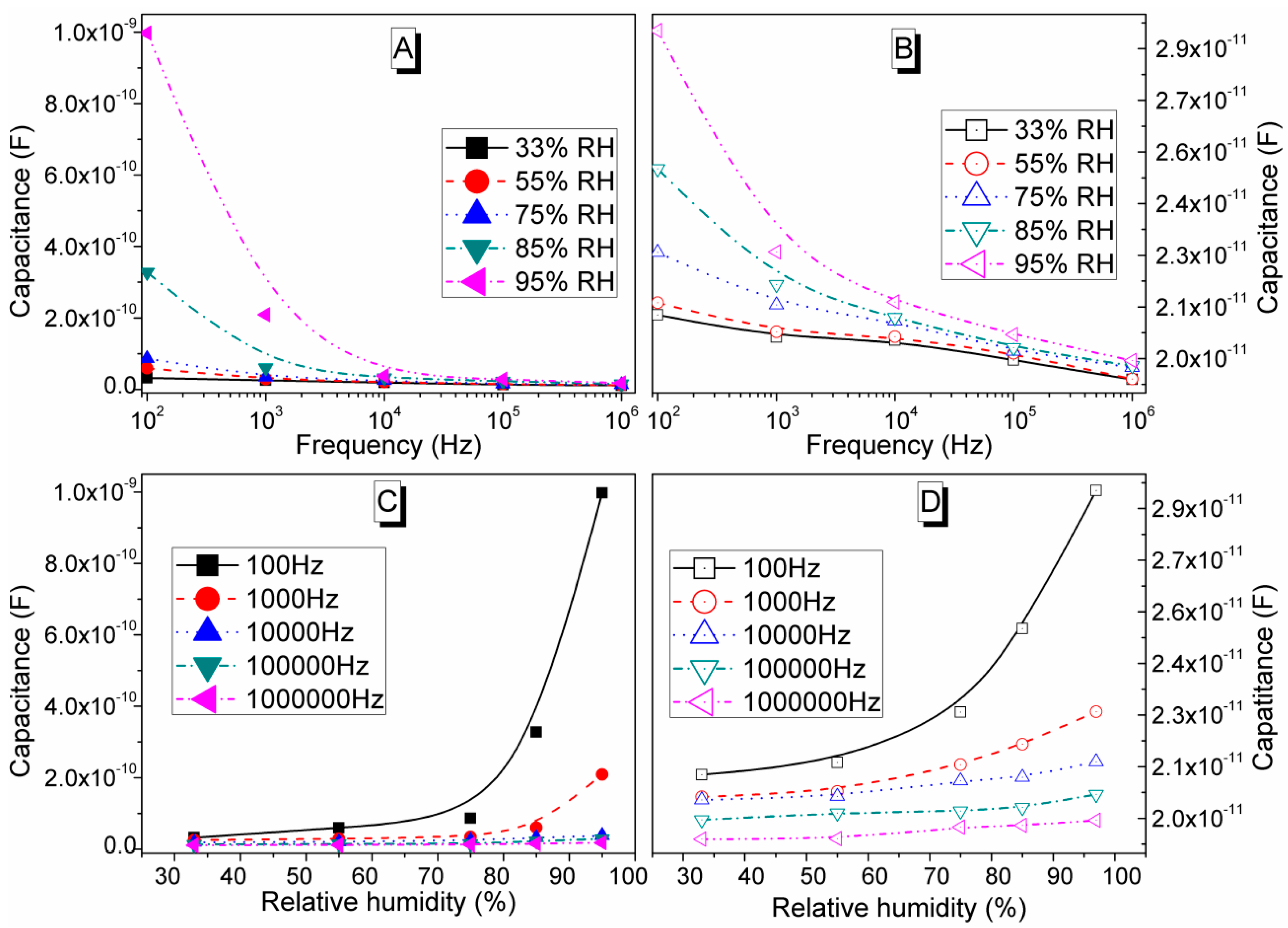
3.6. Dielectric Properties Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rittersma, Z. Recent achievements in miniaturised humidity sensors—A review of transduction techniques. Sens. Actuators A Phys. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Islam, T.; Nimal, A.; Mittal, U.; Sharma, M. A micro interdigitated thin film metal oxide capacitive sensor for measuring moisture in the range of 175–625 ppm. Sens. Actuators B Chem. 2015, 221, 357–364. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Cho, J.; Santhosh, J.; Abu-Osman, N.A. Role of morphological structure, doping, and coating of different materials in the sensing characteristics of humidity sensors. Sensors 2014, 14, 16343–16422. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Rai, U.; Mandal, K.; Rai, A.K. Effect of processing routes on microstructure, electrical and dielectric behavior of mg-doped CaCu3Ti4O12 electro-ceramic. Appl. Phys. A 2013, 112, 891–900. [Google Scholar] [CrossRef]
- Hu, Y.; Jeng, T.-S.; Liu, J.-S. Effect of the mgo substitution for cuo on the properties of CaCu3Ti4O12 ceramics. Ceram. Int. 2012, 38, 3459–3464. [Google Scholar] [CrossRef]
- Thomas, P.; Varughese, K.; Dwarakanath, K.; Varma, K. Dielectric properties of poly(vinylidene fluoride)/CaCu3Ti4O12 composites. Compos. Sci. Technol. 2010, 70, 539–545. [Google Scholar] [CrossRef]
- Matko, V.; Donlagic, D. Sensor for high-air-humidity measurement. IEEE Trans. Instrum. Meas. 1996, 45, 561–563. [Google Scholar] [CrossRef]
- Matko, V. Next generation at-cut quartz crystal sensing devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Matko, V.; Milanović, M. Temperature-compensated capacitance-frequency converter with high resolution. Sens. Actuators A Phys. 2014, 220, 262–269. [Google Scholar] [CrossRef]
- Bonfig, K.; Denker, M.; Kuipers, U. Das direkte digitale messverfahren (DDM) als grundlage einfacher und dennoch genauer und störsicherer sensoren. Sensor 1988, 4, 223–228. [Google Scholar]
- Neufuss, K.; Rudajevová, A. Thermal properties of the plasma-sprayed MgTiO3-CaTiO3 and CaTiO3. Ceram. Int. 2002, 28, 93–97. [Google Scholar] [CrossRef]
- Pramanik, S.; Pingguan-Murphy, B.; Abu-Osman, N.A. Developments of immobilized surface modified piezoelectric crystal biosensors for advanced applications. Int. J. Electrochem. Sci. 2013, 8, 8863–8892. [Google Scholar]
- Shen, Z.; Wang, X.; Luo, B.; Li, L. Batio 3-biybo 3 perovskite materials for energy storage applications. J. Mater. Chem. A 2015, 3, 18146–18153. [Google Scholar] [CrossRef]
- Zhou, Z.; Pang, S.; Liu, Z.; Xu, H.; Cui, G. Interface engineering for high-performance perovskite hybrid solar cells. J. Mater. Chem. A 2015, 3, 19205–19217. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Structure of calcium titanate/titania bioceramic composite coatings on titanium alloy and apatite deposition on their surfaces in a simulated body fluid. Surf. Coat. Technol. 2007, 201, 8715–8722. [Google Scholar] [CrossRef]
- Zabicky, J.; Frage, N.; Kimmel, G.; Hazan, N.; El-fahel, H.; Goncharov, E.; Manor, E.; Shneck, R. Metastable magnesium titanate phases synthesized in nanometric systems. Philos. Mag. B 1997, 76, 605–614. [Google Scholar] [CrossRef]
- Ang, B.; Yaacob, I.; Wong, Y. Phase and surface area studies of maghemite nanoparticles dispersed in silica gel. Mater. Res. Innov. 2014, 18, 32–35. [Google Scholar] [CrossRef]
- Medvedev, A.Y. Synthetic armalcolite and pseudobrookite. Mineral. Mag. 1996, 60, 347–354. [Google Scholar] [CrossRef]
- Thacker, C.; Liang, Y.; Peng, Q.; Hess, P. The stability and major element partitioning of ilmenite and armalcolite during lunar cumulate mantle overturn. Geochim. Cosmochim. Acta 2009, 73, 820–836. [Google Scholar] [CrossRef]
- Khan, A.; Pommier, A.; Neumann, G.A.; Mosegaard, K. The lunar moho and the internal structure of the moon: A geophysical perspective. Tectonophysics 2013, 609, 331–352. [Google Scholar] [CrossRef]
- Hayob, J.L.; Essene, E.J. Armalcolite in crustal paragneiss xenoliths, central mexico. Am. Mineral. 1995, 80, 810–822. [Google Scholar] [CrossRef]
- Chuang, S.-H.; Gao, R.-H.; Wang, D.-Y.; Liu, H.-P.; Chen, L.-M.; Chiang, M.Y. Synthesis and characterization of ilmenite-type cobalt titanate powder. J. Chin. Chem. Soc. 2010, 57, 932–937. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yamaguchi, A.; Takeda, H. Lunar minerals and their resource utilization with particular reference to solar power satellites and potential roles for humic substances for lunar agriculture. In Moon; Springer: Berlin/Heidelberg, Germany, 2012; pp. 105–138. [Google Scholar]
- Kar, K.K.; Pramanik, S. Hydroxyapatite Poly(etheretherketone) Nanocomposites and Method of Manufacturing Same. U.S. Patent 8652373 B2, 18 February 2014. [Google Scholar]
- Ataollahi, F.; Pramanik, S.; Moradi, A.; Dalilottojari, A.; Pingguan-Murphy, B.; Wan-Abas, W.A.B.; Abu-Osman, N.A. Endothelial cell responses in terms of adhesion, proliferation, and morphology to stiffness of polydimethylsiloxane elastomer substrates. J. Biomed. Mater. Res. A 2015, 103A, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-G.; Wang, C.-P. Flexible humidity sensor based on TiO2 nanoparticles-polypyrrole-poly-[3-(methacrylamino)propyl] trimethyl ammonium chloride composite materials. Sens. Actuators B Chem. 2008, 129, 538–543. [Google Scholar] [CrossRef]
- Nayak, S.; Sahoo, B.; Kumar Chaki, T.; Khastgir, D. Development of polyurethane-titania nanocomposites as dielectric and piezoelectric material. RSC Adv. 2013, 3, 2620–2631. [Google Scholar] [CrossRef]
- Tiercelin, N.; Coquet, P.; Sauleau, R.; Senez, V.; Fujita, H. Polydimethylsiloxane membranes for millimeter-wave planar ultra flexible antennas. J. Micromech. Microeng. 2006, 16, 2389. [Google Scholar] [CrossRef]
- Lim, W.H.; Yap, Y.K.; Chong, W.Y.; Ahmad, H. All-optical graphene oxide humidity sensors. Sensors 2014, 14, 24329–24337. [Google Scholar] [CrossRef] [PubMed]
- Matko, V. Porosity determination by using two stochastic signals. Sens. Actuators A Phys. 2004, 112, 320–327. [Google Scholar] [CrossRef]
- Pramanik, S.; Ataollahi, F.; Pingguan-Murphy, B.; Oshkour, A.A.; Osman, N.A.A. In vitro study of surface modified poly(ethylene glycol)-impregnated sintered bovine bone scaffolds on human fibroblast cells. Sci. Rep. 2015, 5, 9806. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Pingguan-Murphy, B.; Cho, J.; Abu Osman, N.A. Design and development of potential tissue engineering scaffolds from structurally different longitudinal parts of a bovine-femur. Sci. Rep. 2014, 4, 5843. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Mohd Hanif, A.S.; Pingguan-Murphy, B.; Abu Osman, N.A. Morphological change of heat treated bovine bone: A comparative study. Materials 2013, 6, 65–75. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Chen, Q. Influences of heating temperature on mechanical properties of polydimethylsiloxane. Sens. Actuators A Phys. 2009, 151, 42–45. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Z.; Zhang, Y.; Yu, Z.; Shi, J.; Xiong, R. Low temperature fabrication of the giant dielectric material CaCu3Ti4O12 by oxalate coprecipitation method. Mater. Chem. Phys. 2009, 113, 746–748. [Google Scholar] [CrossRef]
- Said, M.; Velayutham, T.S.; Gan, W.C.; Abd Majid, W.H. The structural and electrical properties of SrxBa(1−x)Nb2o6 (SBN) ceramic with varied composition. Ceram. Int. 2015, 41, 7119–7124. [Google Scholar] [CrossRef]
- Morris, R.V.; Lauer, H.V.; Lawson, C.A.; Gibson, E.K.; Nace, G.A.; Stewart, C. Spectral and other physicochemical properties of submicron powders of hematite (α-Fe2O3), maghemite (γ-Fe2O3), magnetite (Fe3O4), goethite (α-Feooh), and lepidocrocite (γ-Feooh). J. Geophys. Res. Solid Earth 1985, 90, 3126–3144. [Google Scholar] [CrossRef]
- Lozano-Sánchez, L.; Lee, S.-W.; Sekino, T.; Rodríguez-González, V. Practical microwave-induced hydrothermal synthesis of rectangular prism-like CaTiO3. CrystEngComm 2013, 15, 2359–2362. [Google Scholar] [CrossRef]
- Bosq, N.; Guigo, N.; Persello, J.; Sbirrazzuoli, N. Melt and glass crystallization of pdms and pdms silica nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 7830–7840. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Chu, J.; Xue, J.; Liu, C.; Wang, Z.; Zhang, L. Design and synthesis of non-crystallizable, low-t g polysiloxane elastomers with functional epoxy groups through anionic copolymerization and subsequent epoxidation. RSC Adv. 2014, 4, 31249–31260. [Google Scholar] [CrossRef]
- Maex, K.; Baklanov, M.; Shamiryan, D.; Brongersma, S.; Yanovitskaya, Z. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Vayunandana Reddy, Y.K.; Mergel, D. Frequency and temperature-dependent dielectric properties of batio3 thin film capacitors studied by complex impedance spectroscopy. Phys. B Condens. Matter 2007, 391, 212–221. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Ibrahim, M.; Al-Ghamdi, A.; Dishovsky, N.; Ivanov, M.; Mihaylov, M.; El-Tantawy, F. Dynamic mechanical thermal analysis and dielectric thermal analysis of siloxane rubber-based composites filled with carbon black. J. Compos. Mater. 2012, 46, 1765–1770. [Google Scholar] [CrossRef]
- Du, P.; Lin, X.; Zhang, X. Dielectric constants of pdms nanocomposites using conducting polymer nanowires. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 645–648.
- Sivakumar, N.; Narayanasamy, A.; Greneche, J.-M.; Murugaraj, R.; Lee, Y. Electrical and magnetic behaviour of nanostructured MgFe2O4 spinel ferrite. J. Alloys Compd. 2010, 504, 395–402. [Google Scholar] [CrossRef]
- Rai, A.K.; Mandal, K.D.; Kumar, D.; Parkash, O. Dielectric properties of CaCu3Ti4−xCoxO12 (x = 0.10, 0.20, and 0.30) synthesized by semi-wet route. Mater. Chem. Phys. 2010, 122, 217–223. [Google Scholar] [CrossRef]
- Dyre, J.C.; Schrøder, T.B. Universality of ac conduction in disordered solids. Rev. Mod. Phys. 2000, 72, 873. [Google Scholar] [CrossRef]
- George, M.; Nair, S.S.; John, A.M.; Joy, P.; Anantharaman, M. Structural, magnetic and electrical properties of the sol-gel prepared Li0.5Fe2.5O4 fine particles. J. Phys. D Appl. Phys. 2006, 39, 900. [Google Scholar] [CrossRef]
- Wang, C.-T.; Wu, C.-L.; Chen, I.-C.; Huang, Y.-H. Humidity sensors based on silica nanoparticle aerogel thin films. Sens. Actuators B Chem. 2005, 107, 402–410. [Google Scholar] [CrossRef]
- Imran, Z.; Batool, S.; Jamil, H.; Usman, M.; Israr-Qadir, M.; Shah, S.; Jamil-Rana, S.; Rafiq, M.; Hasan, M.; Willander, M. Excellent humidity sensing properties of cadmium titanate nanofibers. Ceram. Int. 2013, 39, 457–462. [Google Scholar] [CrossRef]
- Bai, Y.; Jin, L. Characterization of frequency-dependent glass transition temperature by vogel-fulcher relationship. J. Phys. D Appl. Phys. 2008, 41, 152008. [Google Scholar] [CrossRef]
- Bondarenka, V.; Grebinskij, S.; Mickevičius, S.; Volkov, V.; Zacharova, G. Thin films of poly-vanadium-molybdenum acid as starting materials for humidity sensors. Sens. Actuators B Chem. 1995, 28, 227–231. [Google Scholar] [CrossRef]
- Wang, J.; Wan, H.; Lin, Q. Properties of a nanocrystalline barium titanate on silicon humidity sensor. Meas. Sci. Technol. 2003, 14, 172. [Google Scholar] [CrossRef]
- Wang, W.C.; Tian, Y.T.; Li, K.; Lu, E.Y.; Gong, D.S.; Li, X.J. Capacitive humidity-sensing properties of Zn2 SiO4 film grown on silicon nanoporous pillar array. Appl. Surf. Sci. 2013, 273, 372–376. [Google Scholar] [CrossRef]
- Pandey, N.; Tiwari, K. Morphological and relative humidity sensing properties of pure zno nanomaterial. Sens. Transducers 2010, 122, 9–19. [Google Scholar]
- Björkqvist, M.; Salonen, J.; Paski, J.; Laine, E. Characterization of thermally carbonized porous silicon humidity sensor. Sens. Actuators A Phys. 2004, 112, 244–247. [Google Scholar] [CrossRef]
- Yuk, J.; Troczynski, T. Sol-gel batio 3 thin film for humidity sensors. Sens. Actuators B Chem. 2003, 94, 290–293. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Wang, Z.; Yan, Q.; Hui, S. Humidity sensing behavior of silicon nanowires with hexamethyldisilazane modification. Sens. Actuators B Chem. 2011, 156, 631–636. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, B.; Lee, H.; Kim, H.; Lee, K.; Park, H. Capacitive humidity sensor design based on anodic aluminum oxide. Sens. Actuators B Chem. 2009, 141, 441–446. [Google Scholar] [CrossRef]





| Sample | Step-I | Step-II | Step-III | Step-IV | ||||
|---|---|---|---|---|---|---|---|---|
| Temperature for Time | Rate (°C/min) | Temperature for Time | Rate (°C/min) | Temperature for Time | Rate (°C/min) | Temperature for Time | Rate (°C/min) | |
| S450 | 450 °C/3.5 h | 5 | ||||||
| S650 | 250 °C/1 h | 5 | 650 °C/3.5 h | 10 | ||||
| S850 | 350 °C/1 h | 5 | 550 °C/3.5 h | 10 | 850 °C/1.3 h | 10 | 750 °C/3 h | 20 |
| S1050 | 350 °C/1 h | 5 | 550 °C/3.5 h | 10 | 1050 °C/1.3 h | 10 | 750 °C/3 h | 20 |
| Material | Maximum E′ (Pa) at 110 °C | Temperature (°C) at Maximum Loss of E′ | Temperature (°C) at E″ Peak | Temperature (°C) at tanδ Peak | tanδ Value (E″/E′) |
|---|---|---|---|---|---|
| PDMS | 7.7 × 109 | −68 | −67.5 | −63.9 | 0.404 |
| S1050/PDMS | 2.5 × 1010 | −60 | −59.2 | −58.8 | 0.225 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, A.; Pramanik, S.; Manna, A.; Azrin Shah, N.F.; Shasmin, H.N.; Radzi, Z.; Abu Osman, N.A. Synthesis and Characterizations of Novel Ca-Mg-Ti-Fe-Oxides Based Ceramic Nanocrystals and Flexible Film of Polydimethylsiloxane Composite with Improved Mechanical and Dielectric Properties for Sensors. Sensors 2016, 16, 292. https://doi.org/10.3390/s16030292
Tripathy A, Pramanik S, Manna A, Azrin Shah NF, Shasmin HN, Radzi Z, Abu Osman NA. Synthesis and Characterizations of Novel Ca-Mg-Ti-Fe-Oxides Based Ceramic Nanocrystals and Flexible Film of Polydimethylsiloxane Composite with Improved Mechanical and Dielectric Properties for Sensors. Sensors. 2016; 16(3):292. https://doi.org/10.3390/s16030292
Chicago/Turabian StyleTripathy, Ashis, Sumit Pramanik, Ayan Manna, Nabila Farhana Azrin Shah, Hanie Nadia Shasmin, Zamri Radzi, and Noor Azuan Abu Osman. 2016. "Synthesis and Characterizations of Novel Ca-Mg-Ti-Fe-Oxides Based Ceramic Nanocrystals and Flexible Film of Polydimethylsiloxane Composite with Improved Mechanical and Dielectric Properties for Sensors" Sensors 16, no. 3: 292. https://doi.org/10.3390/s16030292
APA StyleTripathy, A., Pramanik, S., Manna, A., Azrin Shah, N. F., Shasmin, H. N., Radzi, Z., & Abu Osman, N. A. (2016). Synthesis and Characterizations of Novel Ca-Mg-Ti-Fe-Oxides Based Ceramic Nanocrystals and Flexible Film of Polydimethylsiloxane Composite with Improved Mechanical and Dielectric Properties for Sensors. Sensors, 16(3), 292. https://doi.org/10.3390/s16030292