Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of PANI-SSA and PANI-HCl/SSA
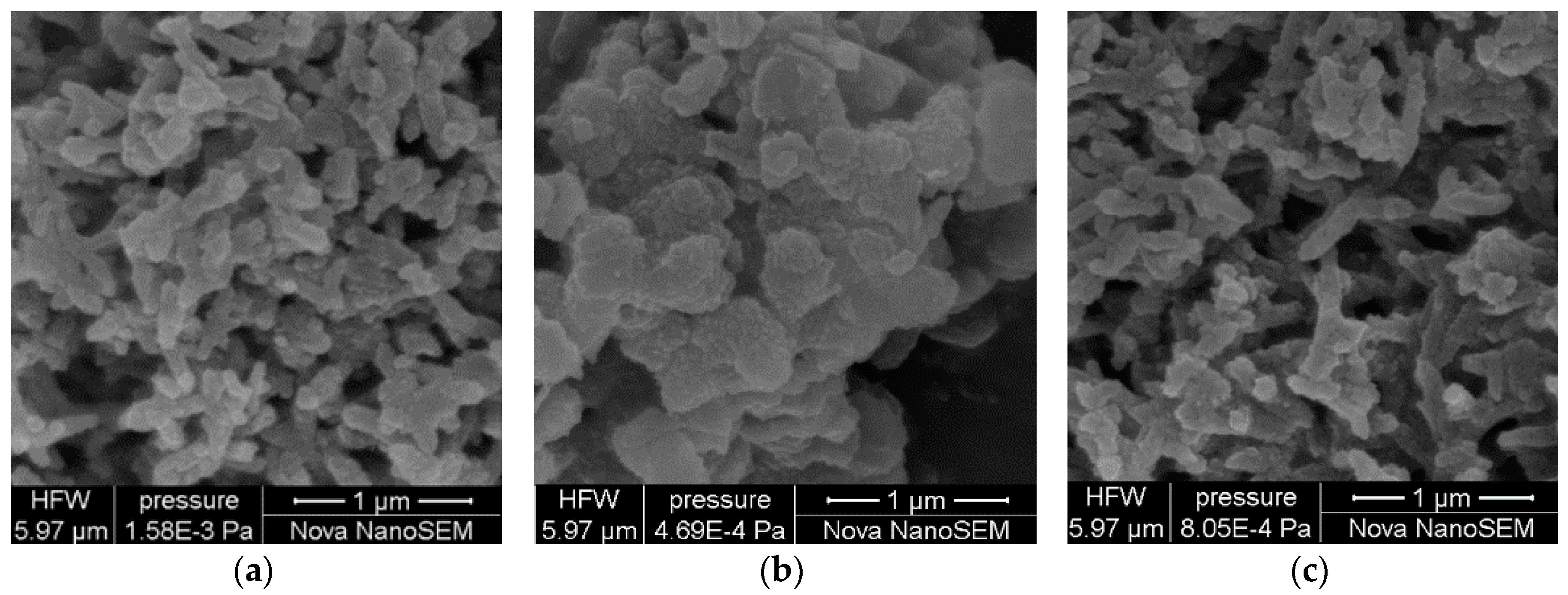
2.2. Characterization of PANI-SSA and PANI-HCl/SSA
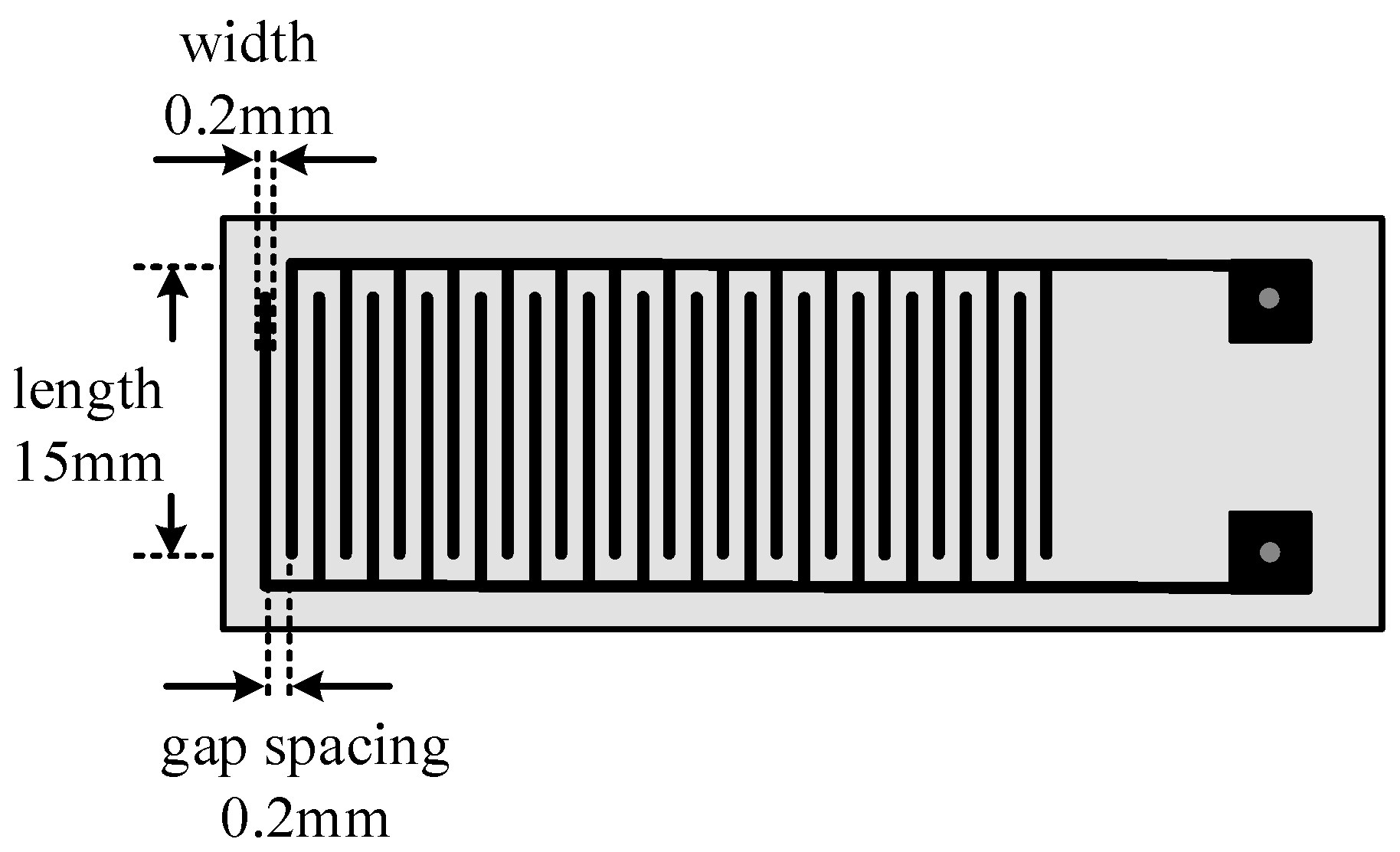
2.3. Schematic Diagram of PANI-SSA and PANI-HCl/SSA Sensors
3. Results and Discussion
3.1. Gas-Sensing Property Test of PANI-SSA
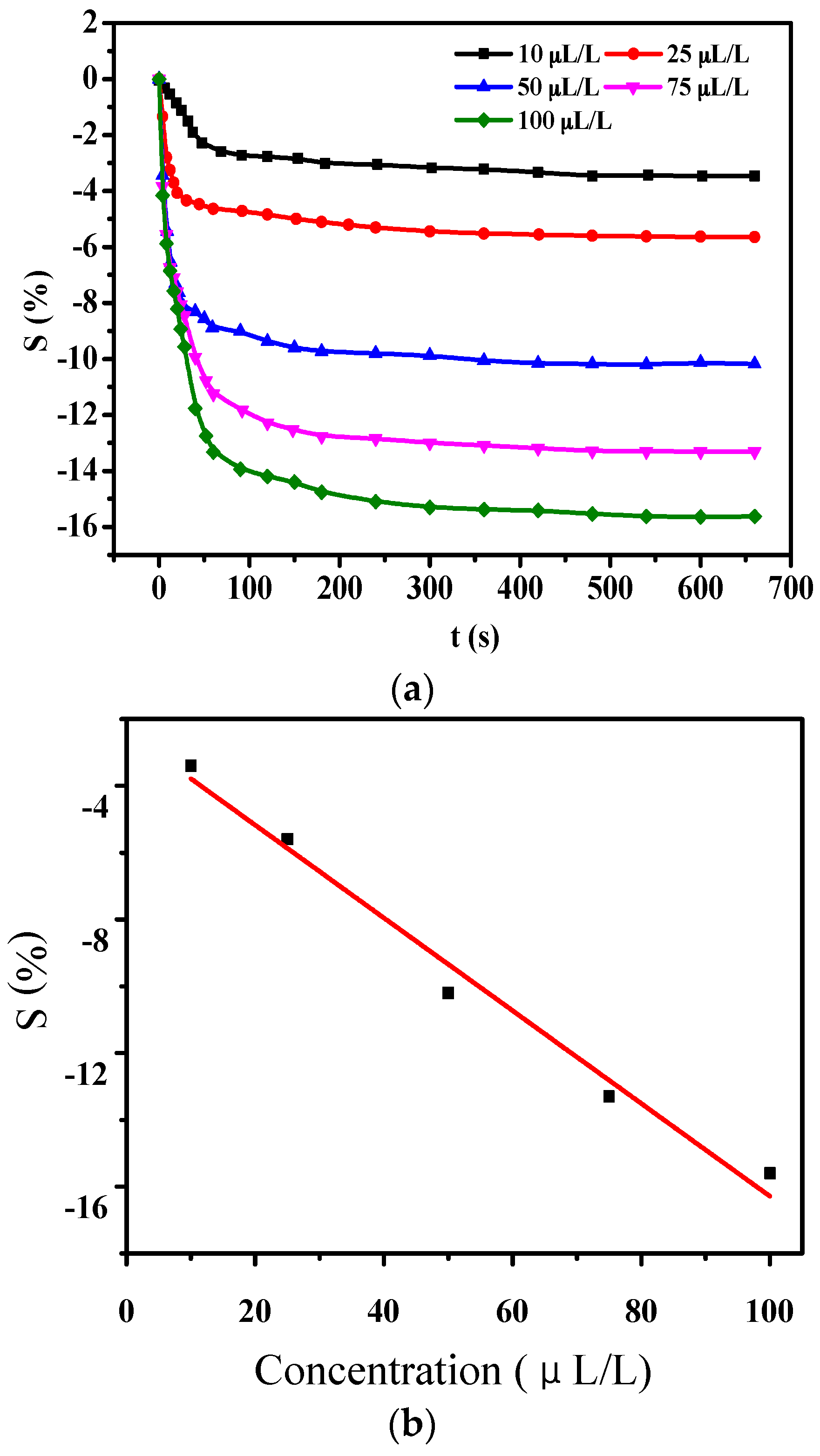
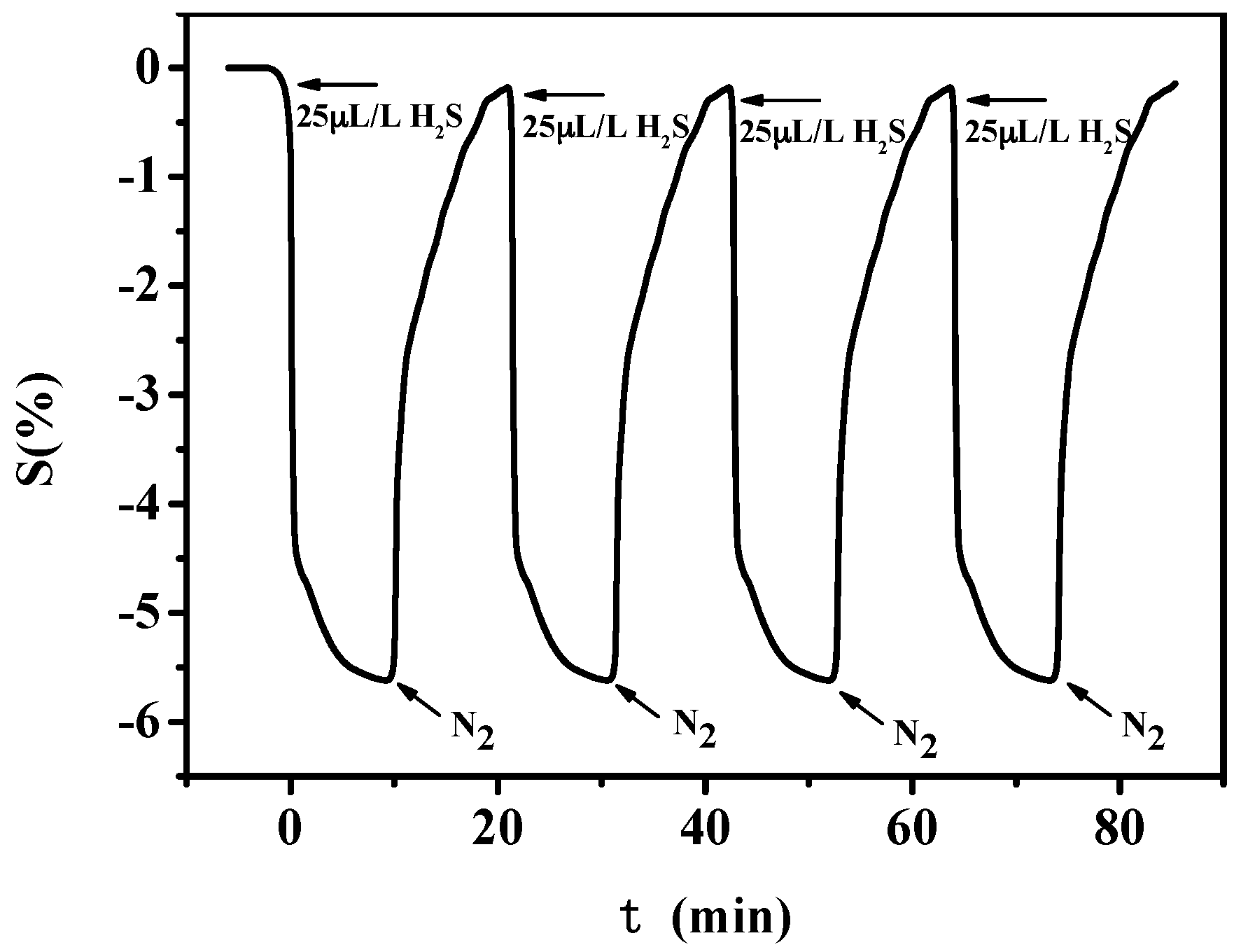
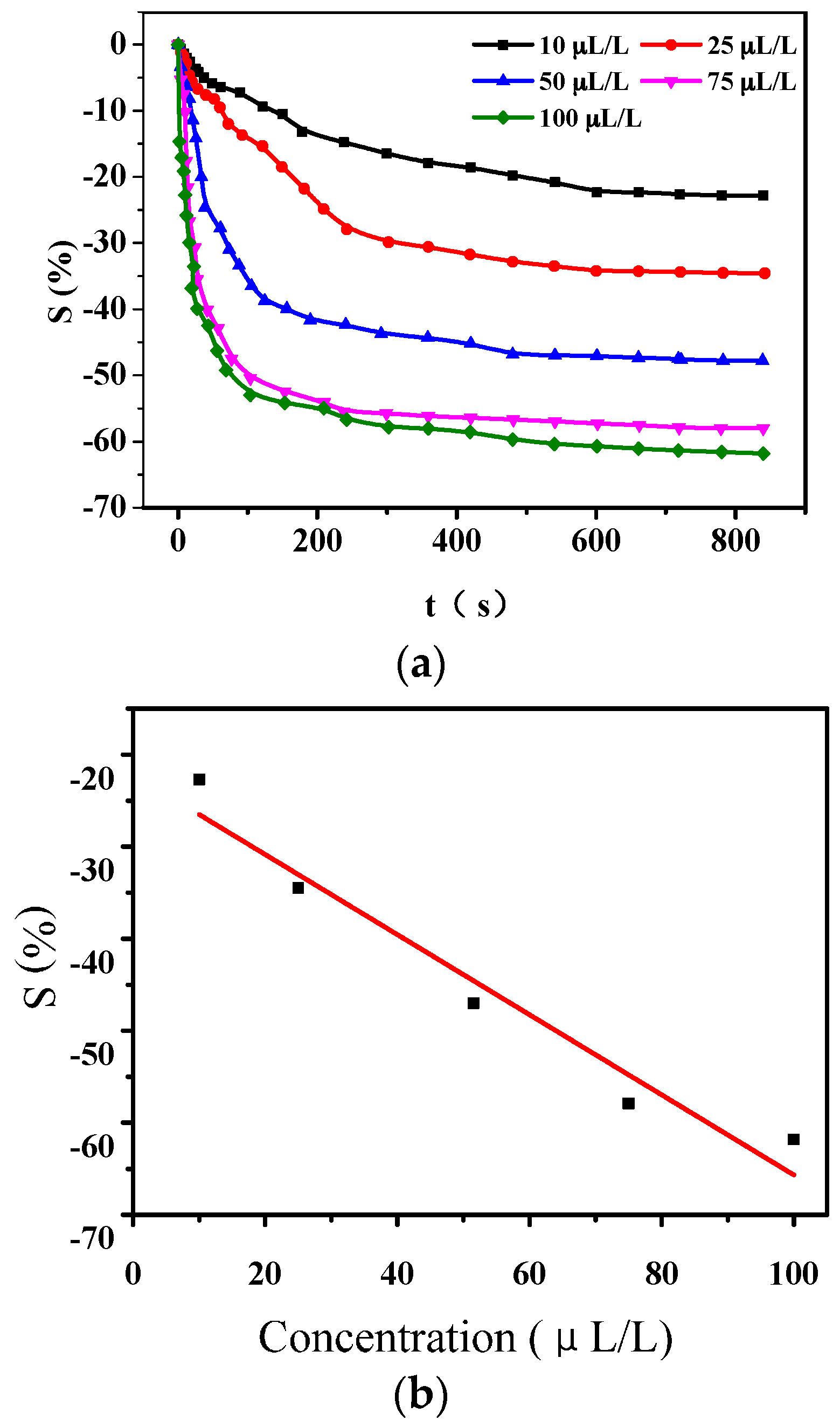
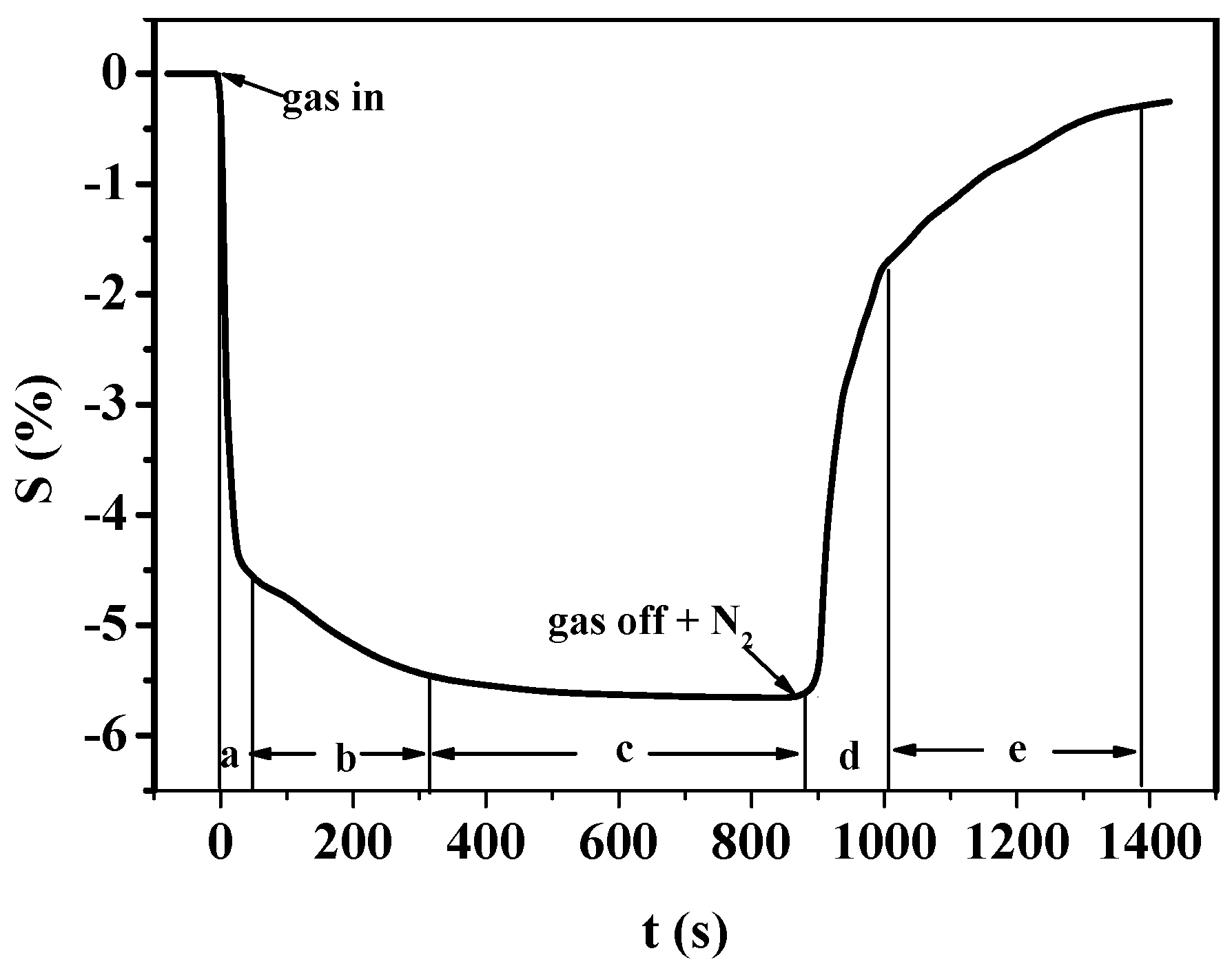
3.1.1. Gas-Sensing Response of PANI-SSA to H2S
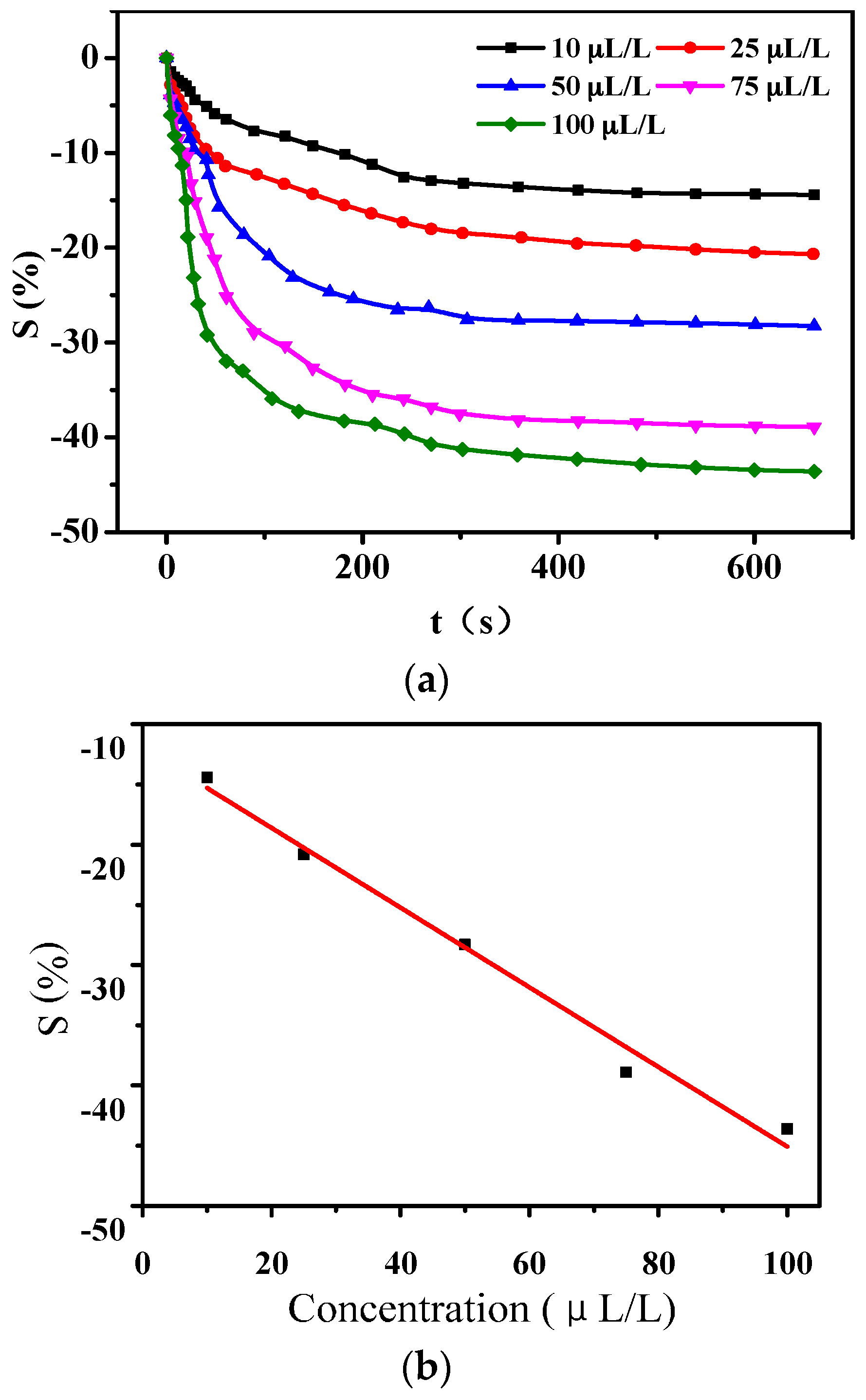
3.1.2. Gas-Sensing Response of PANI-SSA to SO2
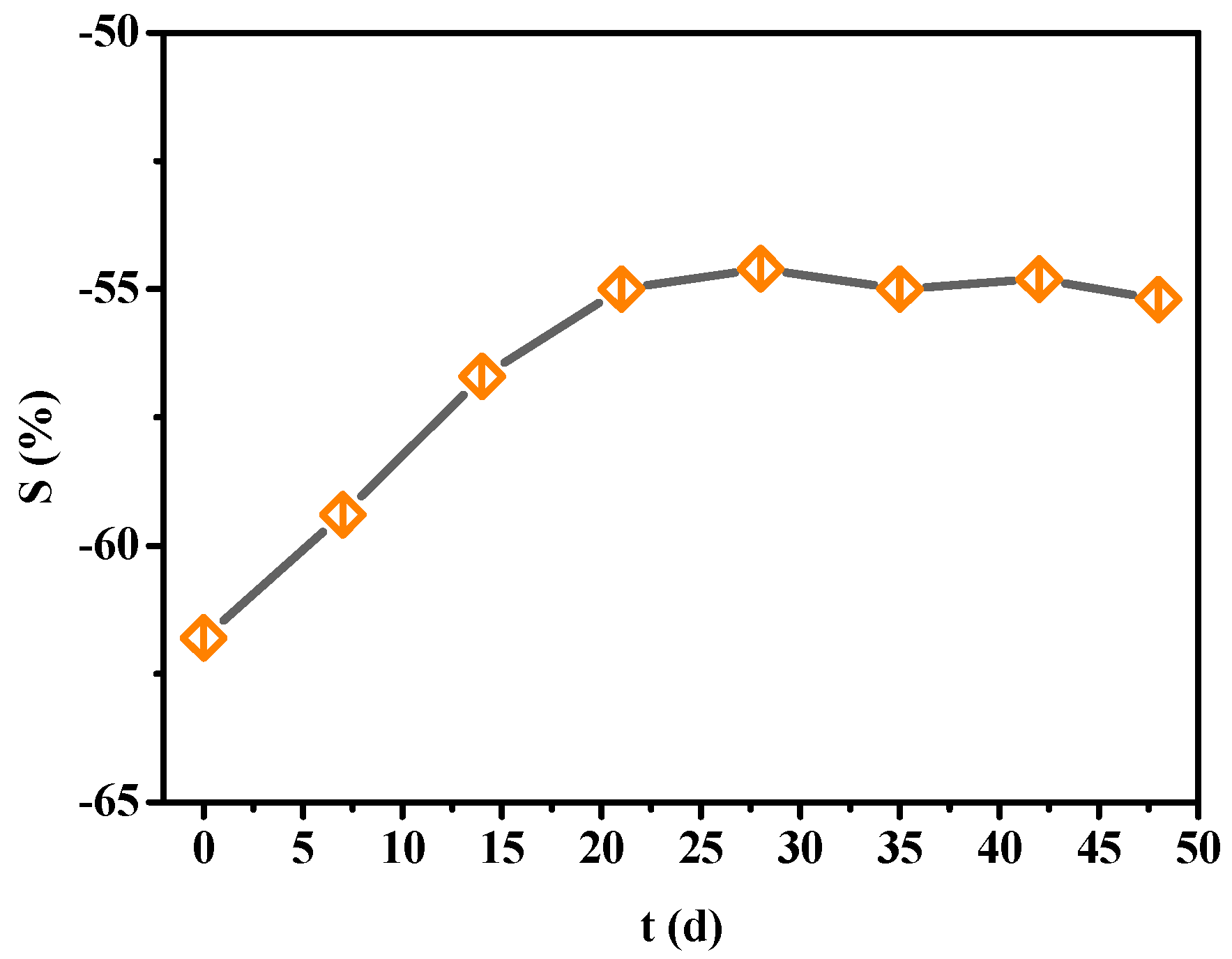
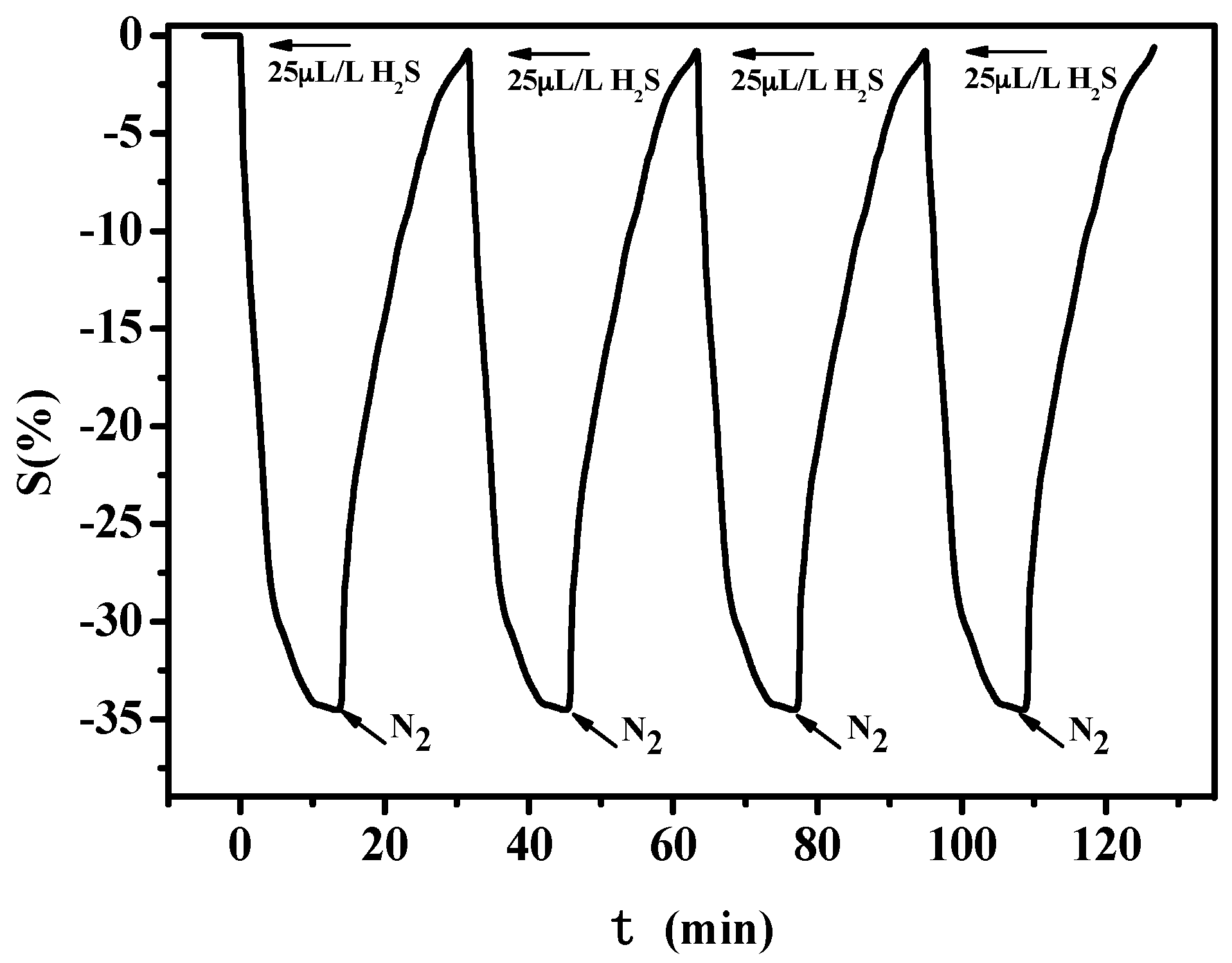
3.1.3. Stability Test of PANI-SSA Sensors
3.2. Gas-Sensing Property Test of PANI-HCl/SSA
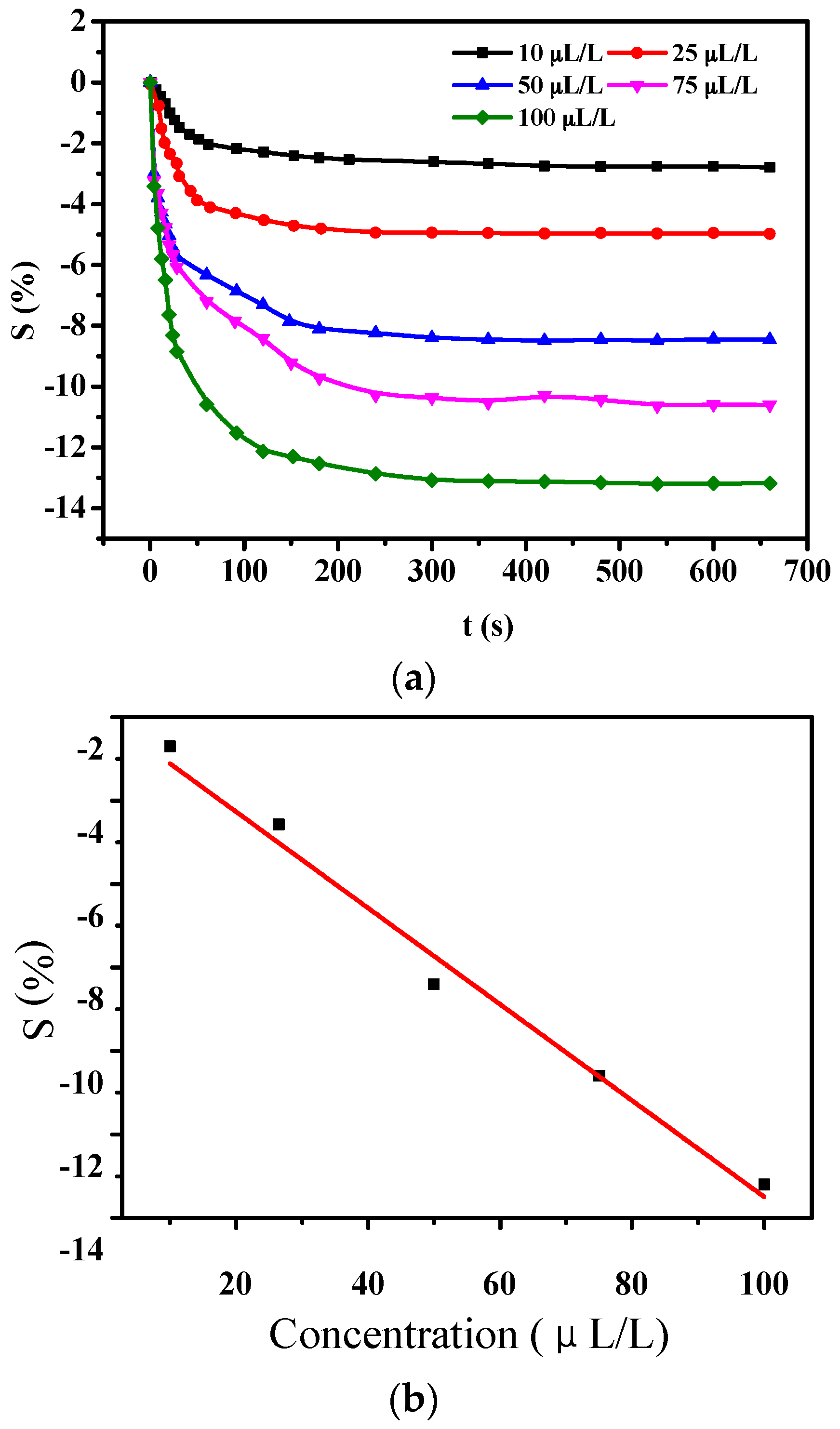
3.2.1. Gas-Sensing Response of PANI-HCl/SSA to H2S
3.2.2. Gas-Sensing Response of PANI-HCl/SSA to SO2
3.2.3. Stability Test of PANI-HCl/SSA Sensors
3.3. Gas-Sensing Property Comparison of PANI-SSA, PANI-HCl/SSA, and PANI-HCl
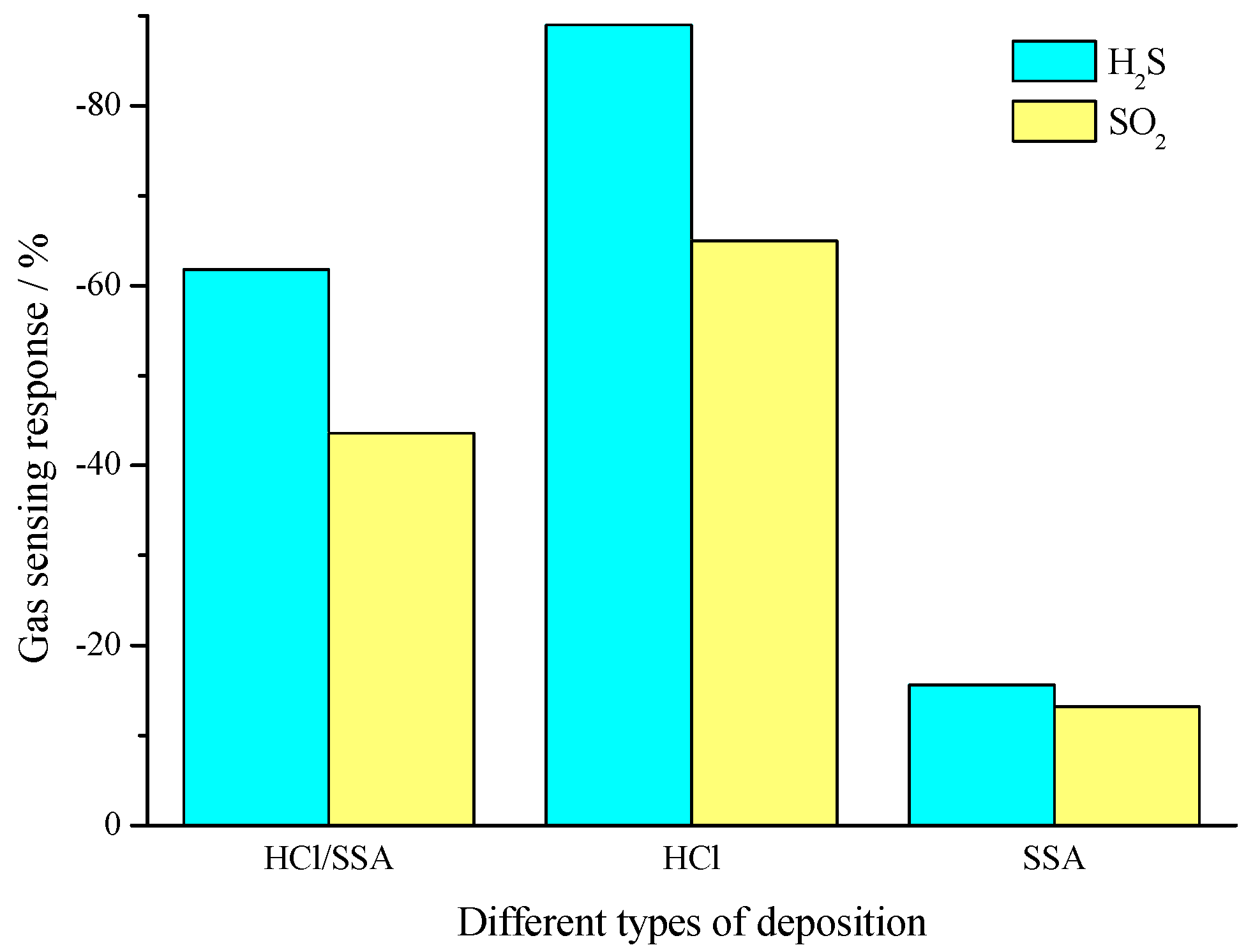
3.3.1. Comparison of Gas-Sensing Response Characteristics and Mechanism Analysis
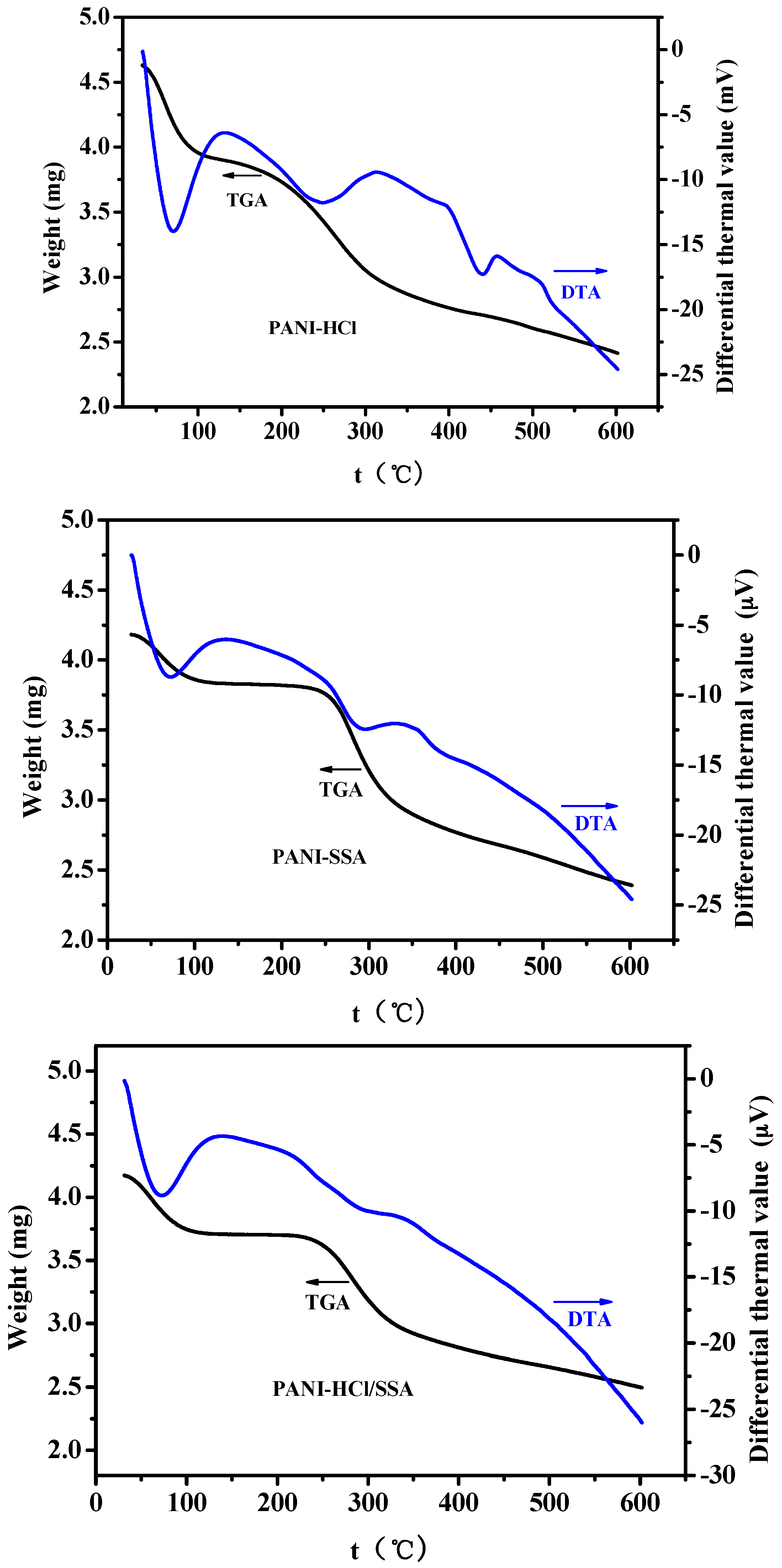
3.3.2. Comparison of Stability and Mechanism Analysis
4. Conclusions
- (1)
- The results of gas-sensing tests show that, when exposed to H2S and SO2 of various concentrations, PANI-HCl/SSA sensors show significant response compared with PANI-SSA, a little less than the response of PANI-HCl; PANI-SSA sensors respond most quickly, and PANI-HCl/SSA response is slightly slower, with PANI-HCl showing the longest response time.
- (2)
- Thermal stability tests of TGA-DTA to these three types of sensors show that composite acids deposited PANI sensors possess better stability than single acid deposited PANI sensors, including PANI-HCl and PANI-SSA sensors. Long-term stability tests also show that PANI-HCl/SSA sensors have the best stability.
- (3)
- The two aimed gases, that is, H2S and SO2, are generated in different stages with different production rates, which means that cross interference does not happen easily in the detection of these two gases. Considering the overall gas-sensing performances of PANI-HCl, PANI-SSA, and PANI-HCl/SSA sensors, PANI-HCl/SSA sensors are most likely to be utilized for PD detection inside GIS.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, F.; Tang, J.; Fan, Q.; Pan, J.; Zhang, X.; Yao, Q.; He, J. Decomposition characteristics of SF6 under thermal fault for temperatures below 400 °C. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 995–1004. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.; Pan, J.; Zhang, X.; Yao, Q.; He, J.; Hou, X. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Casanovas, A.; Casanovas, J.; Lagarde, F.; Belarbi, A. Study of the decomposition of SF6 under DC negative polarity corona discharges (point to plane geometry): Influence of the metal constituting the plane electrode. J. Appl. Phys. 1992, 72, 3344–3354. [Google Scholar] [CrossRef]
- Derdouri, A.; Casanovas, J.; Hergli, R.; Grob, R.; Mathieu, J. Study of the decomposition of wet SF6, subjected to 50 Hz AC corona discharges. J. Appl. Phys. 1989, 65, 1852–1857. [Google Scholar] [CrossRef]
- Zhang, X.; Gui, Y.; Dong, X. Preparation and application of TiO2 nanotube array gas sensor for SF6-insulated equipment detection: A review. Nanosc. Res. Lett. 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, X.; Gui, Y. Theoretical and experimental study on competitive adsorption of SF6, decomposed components on Au-modified anatase (101) surface. Appl. Surf. Sci. 2016, 387, 437–445. [Google Scholar] [CrossRef]
- Ding, W.; Hayashi, R.; Ochi, K.; Suehiro, J.; Imasaka, K.; Hara, M.; Sano, N.; Nagao, E.; Minagawa, T. Analysis of PD generated SF6 decomposition gases adsorbed on carbon nanotubes. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 1200–1207. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Y.; Tang, J.; Sun, C.; Wan, L. Actuality and perspective of proximate analysis of SF6 decomposed products under partial discharge. High. Volt. Eng. 2008, 34, 664–669. [Google Scholar]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.; Li, Z.; Tsutsumi, T.; Shou, R.; Nakano, M.; Suehiro, J.; Ohtsuka, S. Detection of SF6 decomposition products generated by DC corona discharge using a carbon nanotube gas sensor. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 671–676. [Google Scholar] [CrossRef]
- Suehiro, J.; Zhou, G.; Hara, M. Detection of partial discharge in SF6 gas using a carbon nanotube-based gas sensor. Sens. Actuators B Chem. 2005, 105, 164–169. [Google Scholar] [CrossRef]
- Xiaoxing, Z.; Jing, T.; Jinbin, Z. A Pt-doped TiO2 nanotube arrays sensor for detecting SF6 decomposition products. Sensors 2013, 13, 14764–14776. [Google Scholar]
- Zhang, X.; Yu, L.; Tie, J.; Dong, X. Gas sensitivity and sensing mechanism studies on Au-doped TiO2 nanotube arrays for detecting SF6 decomposed components. Sensors 2014, 14, 19517–19532. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental sensing and density functional theory study of H2S and SOF2 adsorption on Au-modified graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Agbor, N.E.; Petty, M.C.; Monkman, A.P. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]
- Liu, H.; Kameoka, J.; Czaplewski, D.A.; Craighead, H.G. Polymeric nanowire chemical sensor. Nano Lett. 2004, 4, 671–675. [Google Scholar] [CrossRef]
- Virji, S.; Kaner, R.B.; Weiller, B.H. Hydrogen sensors based on conductivity changes in polyaniline nanofibers. J. Phys. Chem. B 2006, 110, 22266–22270. [Google Scholar]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. Preparation and characterization of chemically synthesized polyaniline–polystyrene blends as a carbon dioxide gas sensor. Synth. Met. 2013, 181, 27–36. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Yu, L.; Yang, B.; Zhou, J. Highly sensitive and selective polyaniline thin-film sensors for detecting SF6, decomposition products at room temperature. Synth. Met. 2015, 200, 74–79. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
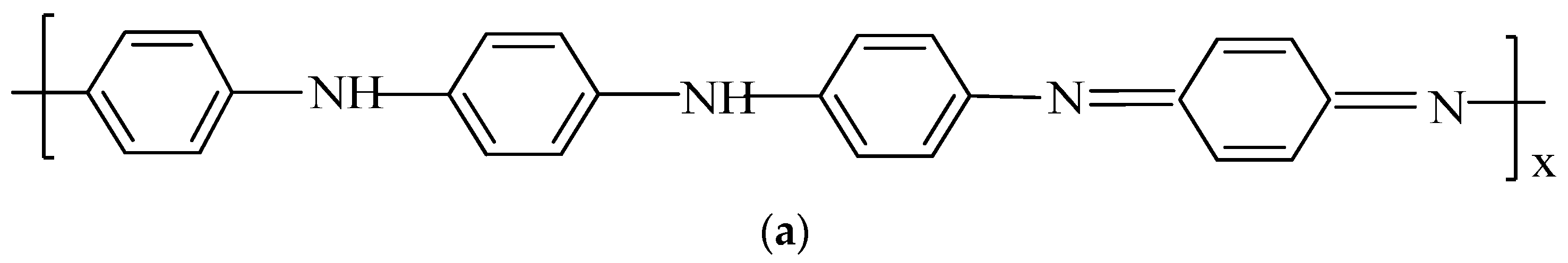
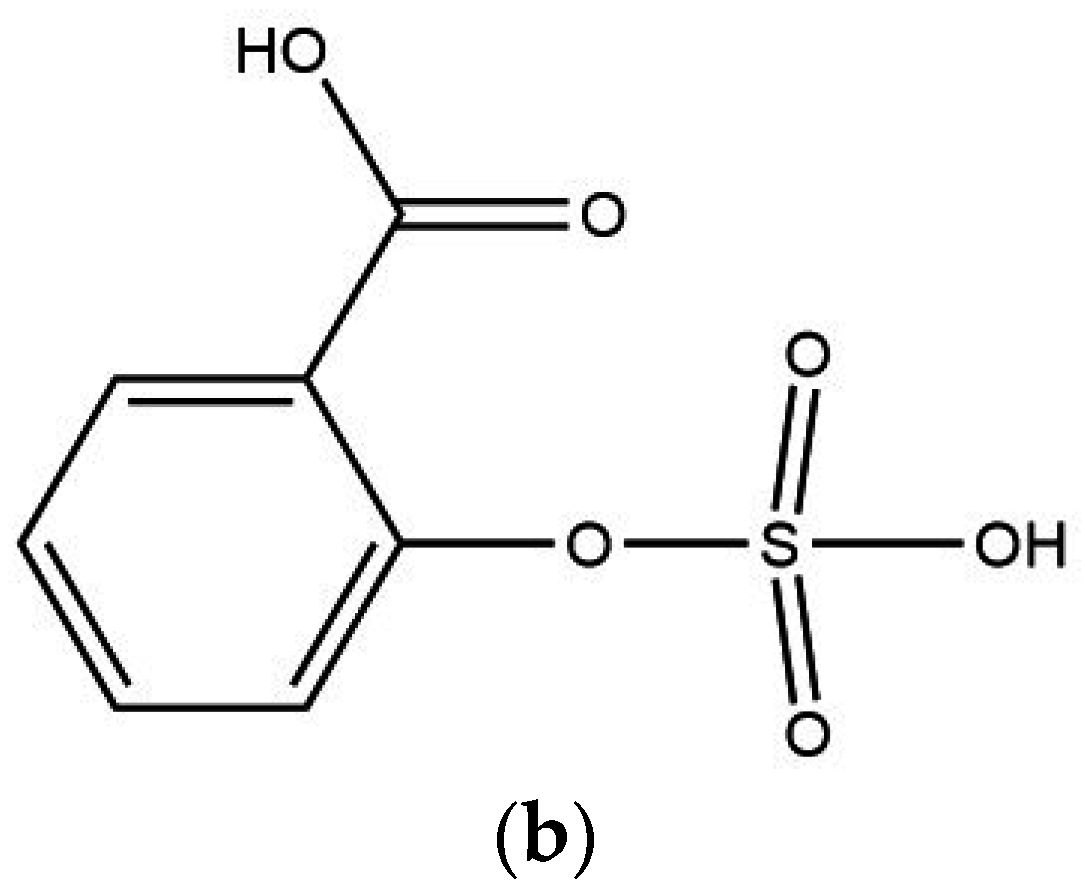
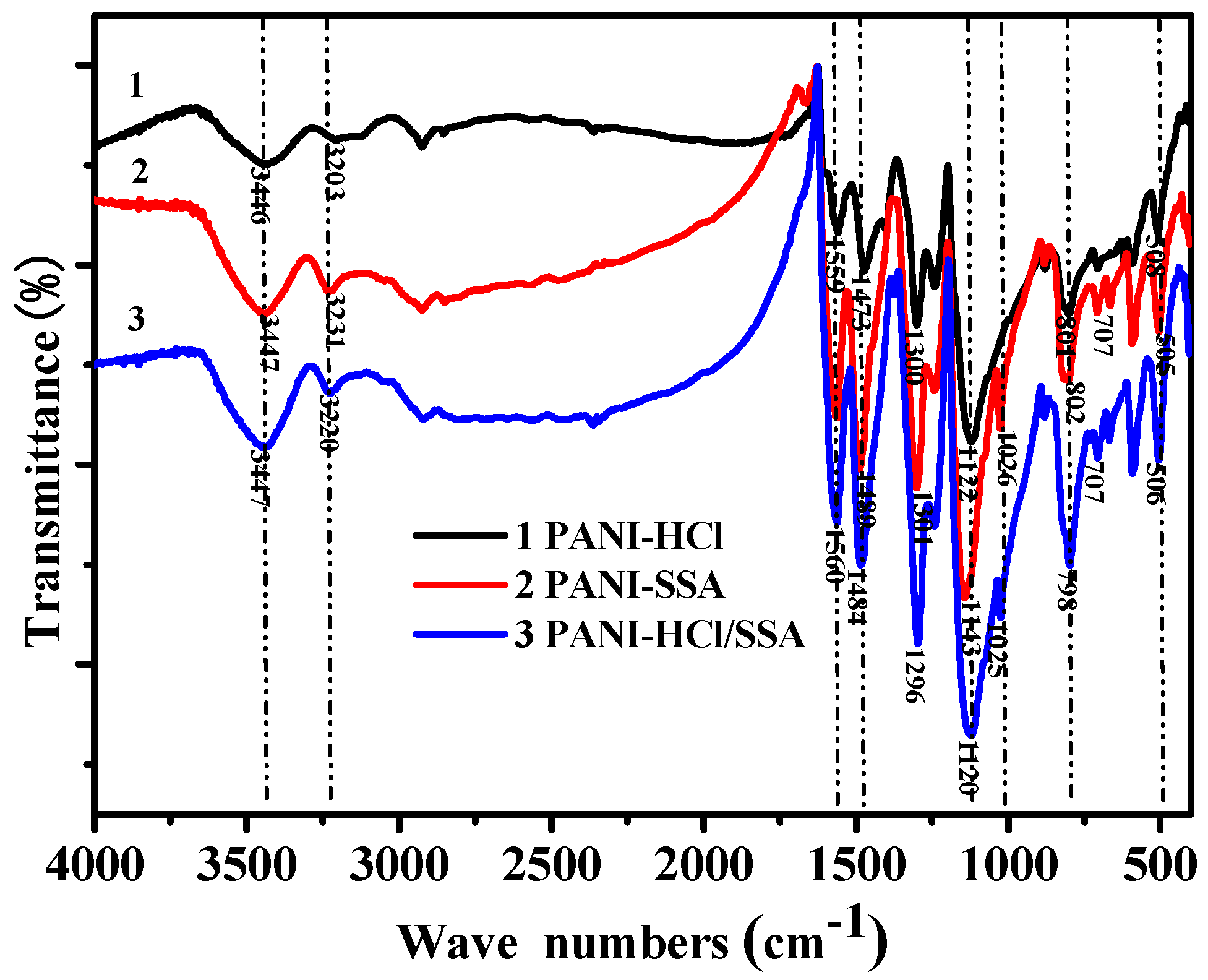
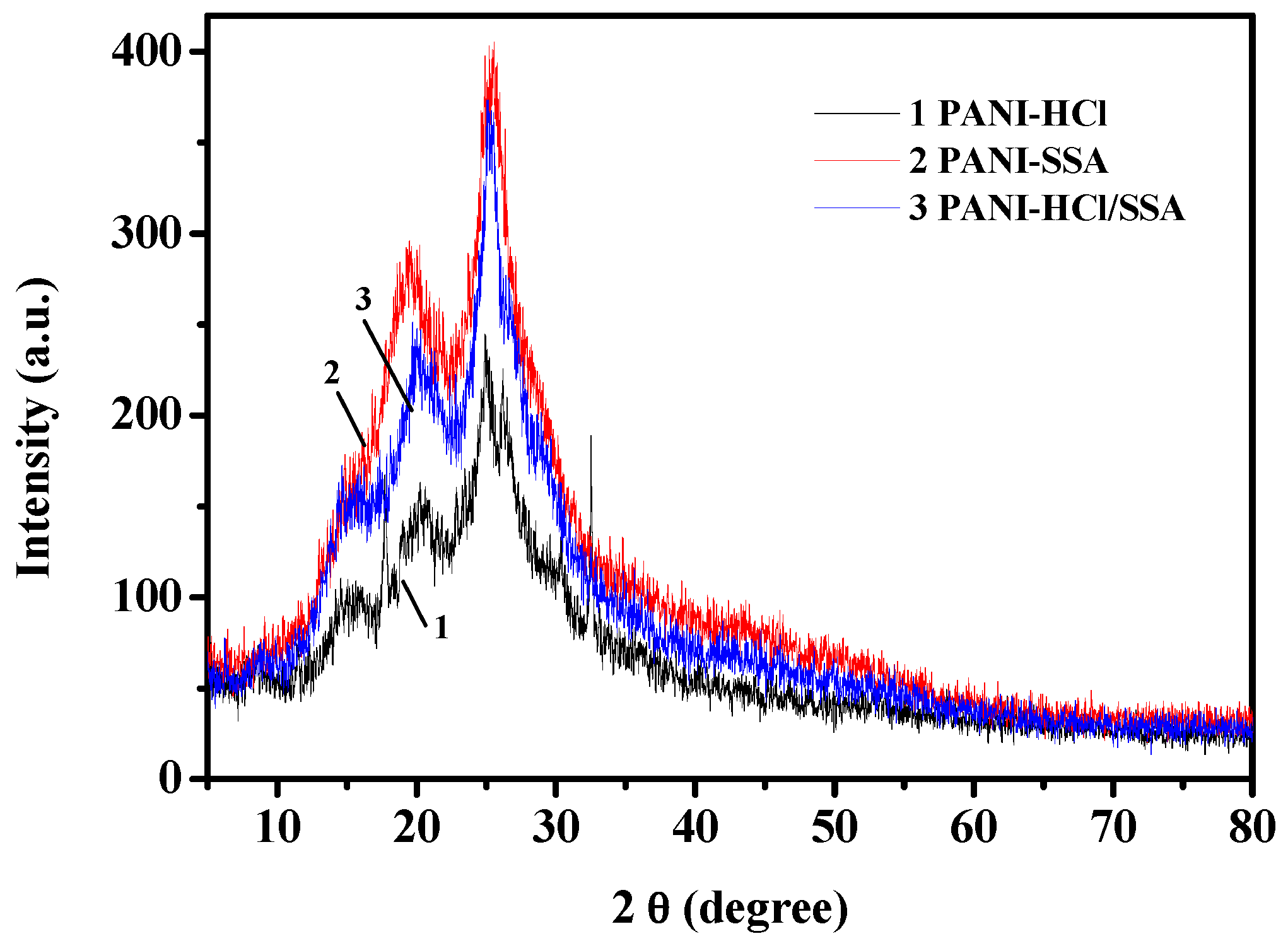

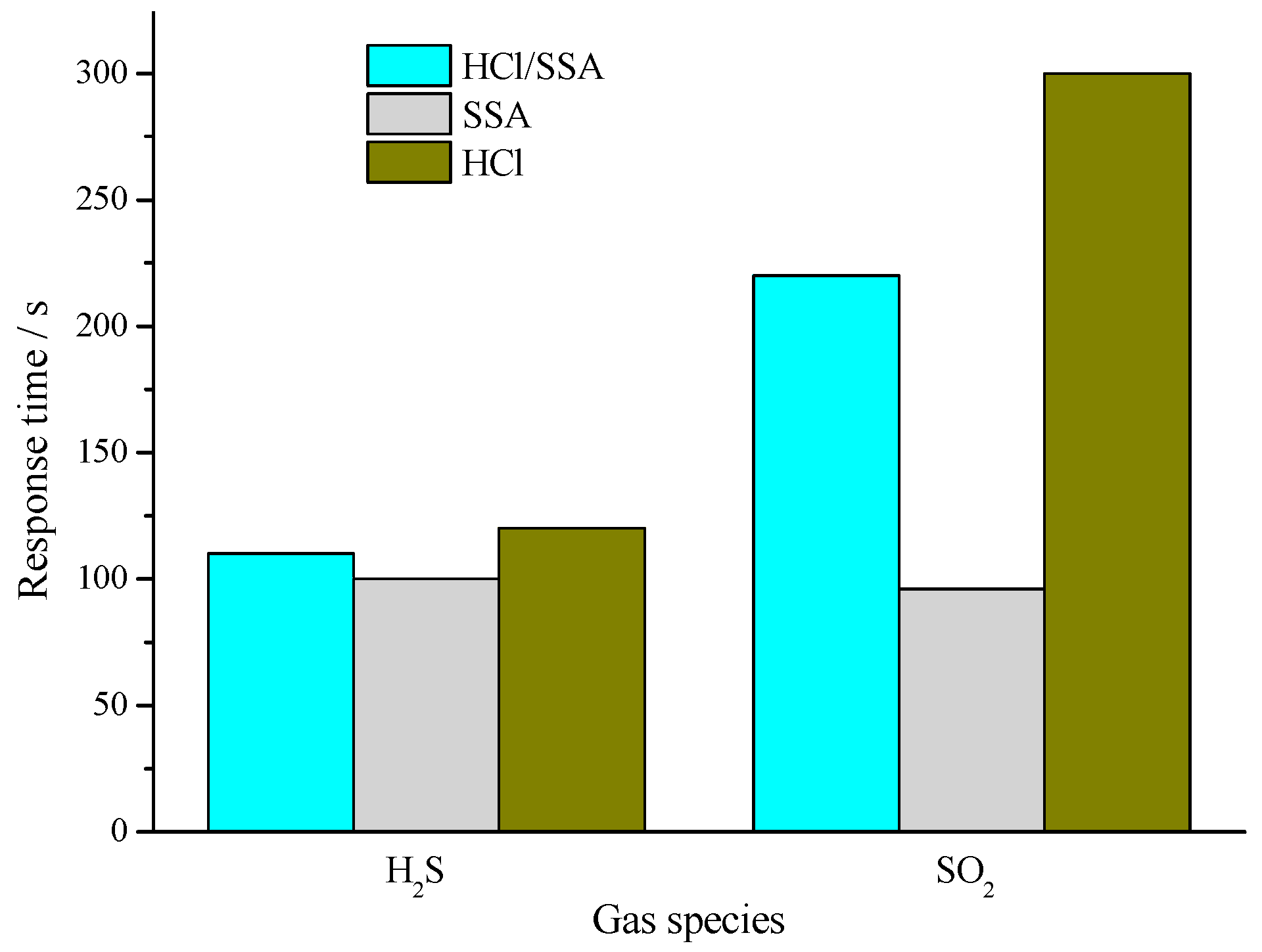
| Vibration Group | PANI-HCl (cm−1) | PANI-SSA (cm−1) | PANI-HCl/SSA (cm−1) |
|---|---|---|---|
| N-H stretching vibration | 3203 | 3231 | 3220 |
| Quinone ring frame vibration | 1559 | 1561 | 1560 |
| Benzene skeleton vibration | 1473 | 1489 | 1484 |
| N-B-N stretching vibration | 1300 | 1301 | 1296 |
| B-NH-B | 1122 | 1143 | 1120 |
| O=S=O | - | 1026 | 1025 |
| Out-of-ring bending vibration of benzene ring | 801 | 802 | 798 |
| Aromatic ring bending vibration | 508 | 505 | 506 |
| Gas Sensors | 1.0 μL/L SO2 | 0.5 μL/L H2S |
|---|---|---|
| PANI-SSA | 1%~2% | about 2% |
| PANI-HCl/SSA | about 10% | about 20% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhang, X.; Wu, X.; Cui, H.; Chen, D. Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2. Sensors 2016, 16, 1889. https://doi.org/10.3390/s16111889
Dong X, Zhang X, Wu X, Cui H, Chen D. Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2. Sensors. 2016; 16(11):1889. https://doi.org/10.3390/s16111889
Chicago/Turabian StyleDong, Xingchen, Xiaoxing Zhang, Xiaoqing Wu, Hao Cui, and Dachang Chen. 2016. "Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2" Sensors 16, no. 11: 1889. https://doi.org/10.3390/s16111889
APA StyleDong, X., Zhang, X., Wu, X., Cui, H., & Chen, D. (2016). Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2. Sensors, 16(11), 1889. https://doi.org/10.3390/s16111889





