Organic Vapour Sensing Properties of Area-Ordered and Size-Controlled Silicon Nanopillar
Abstract
:1. Introduction
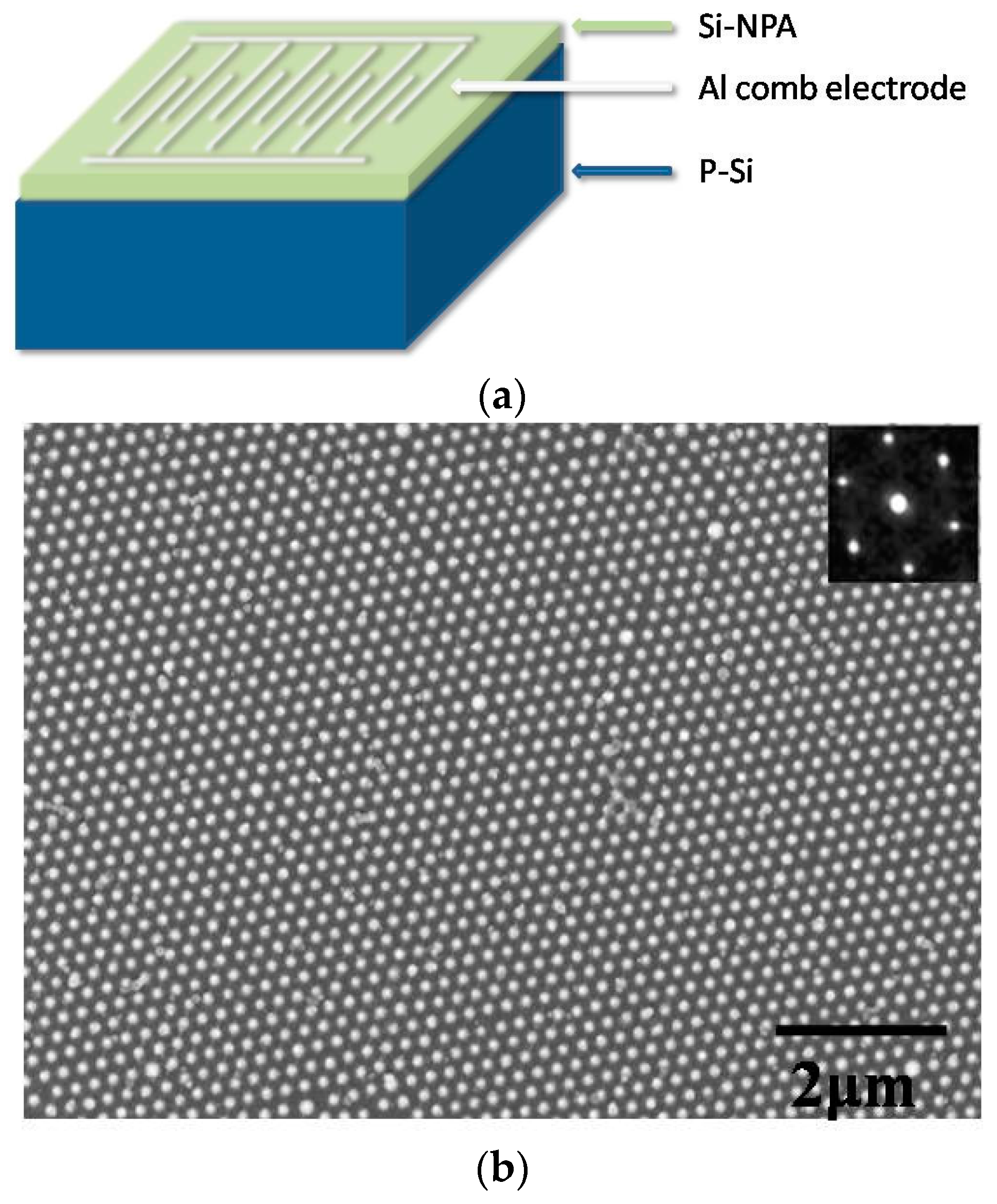
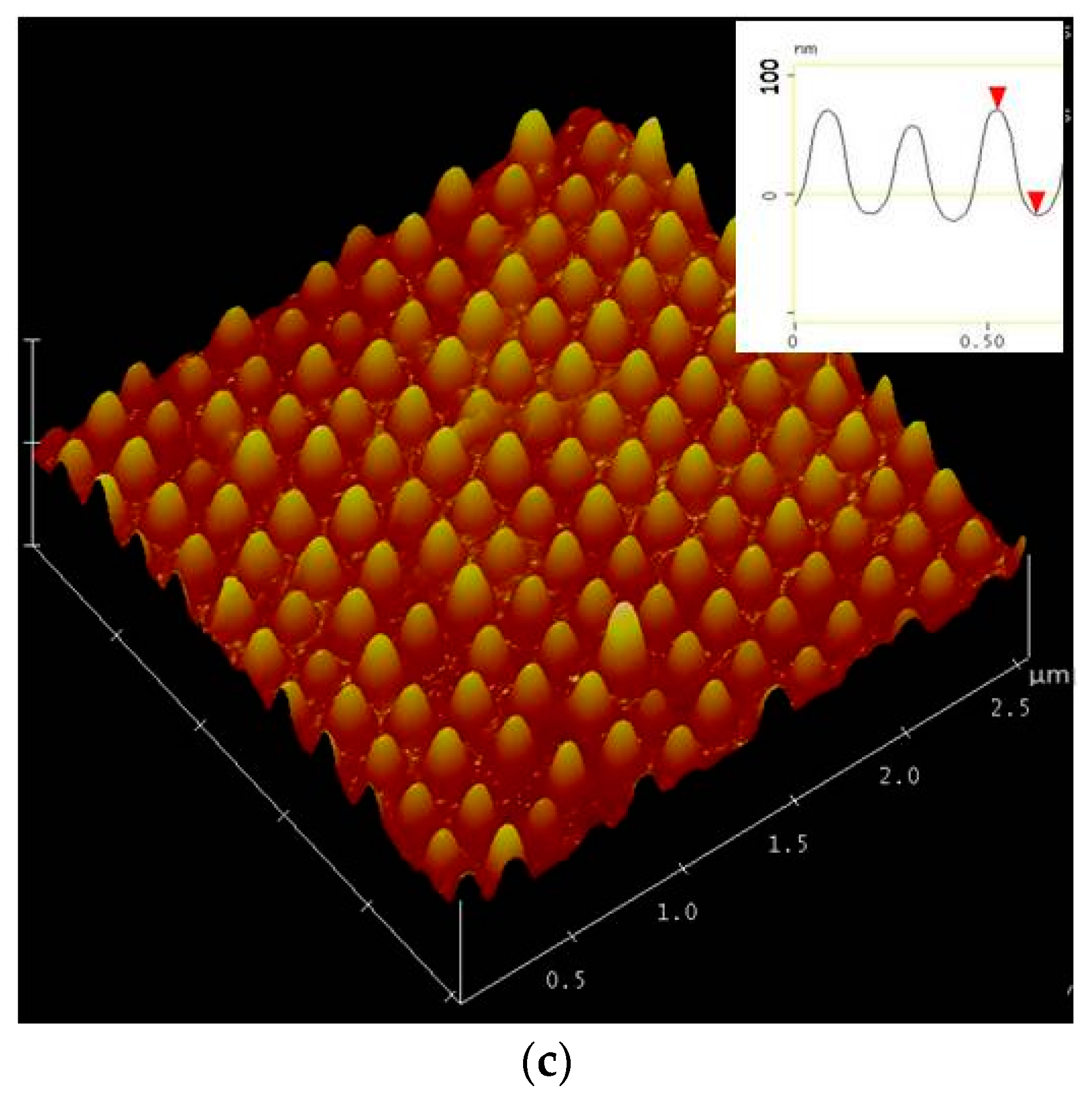
2. Experiment
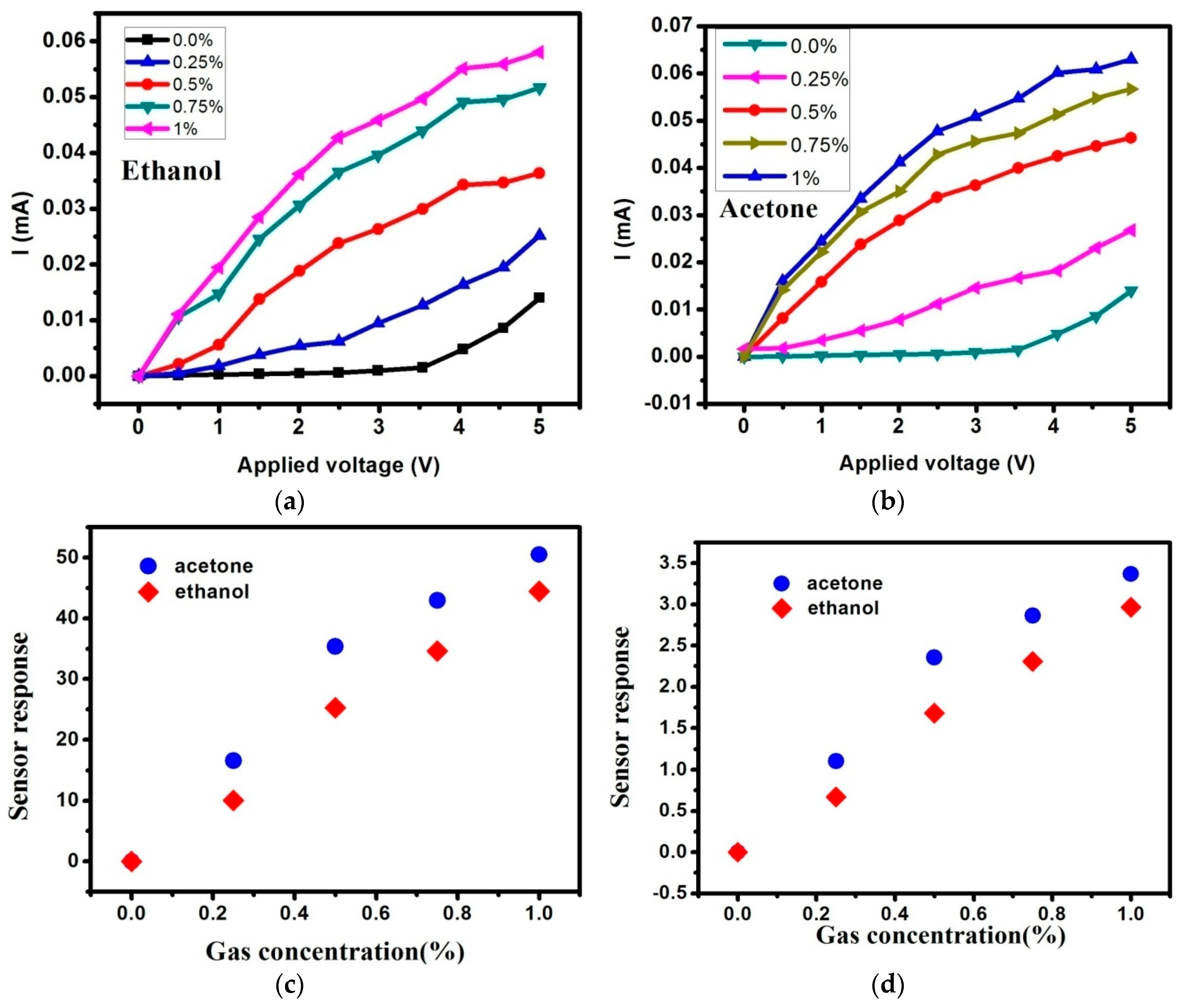
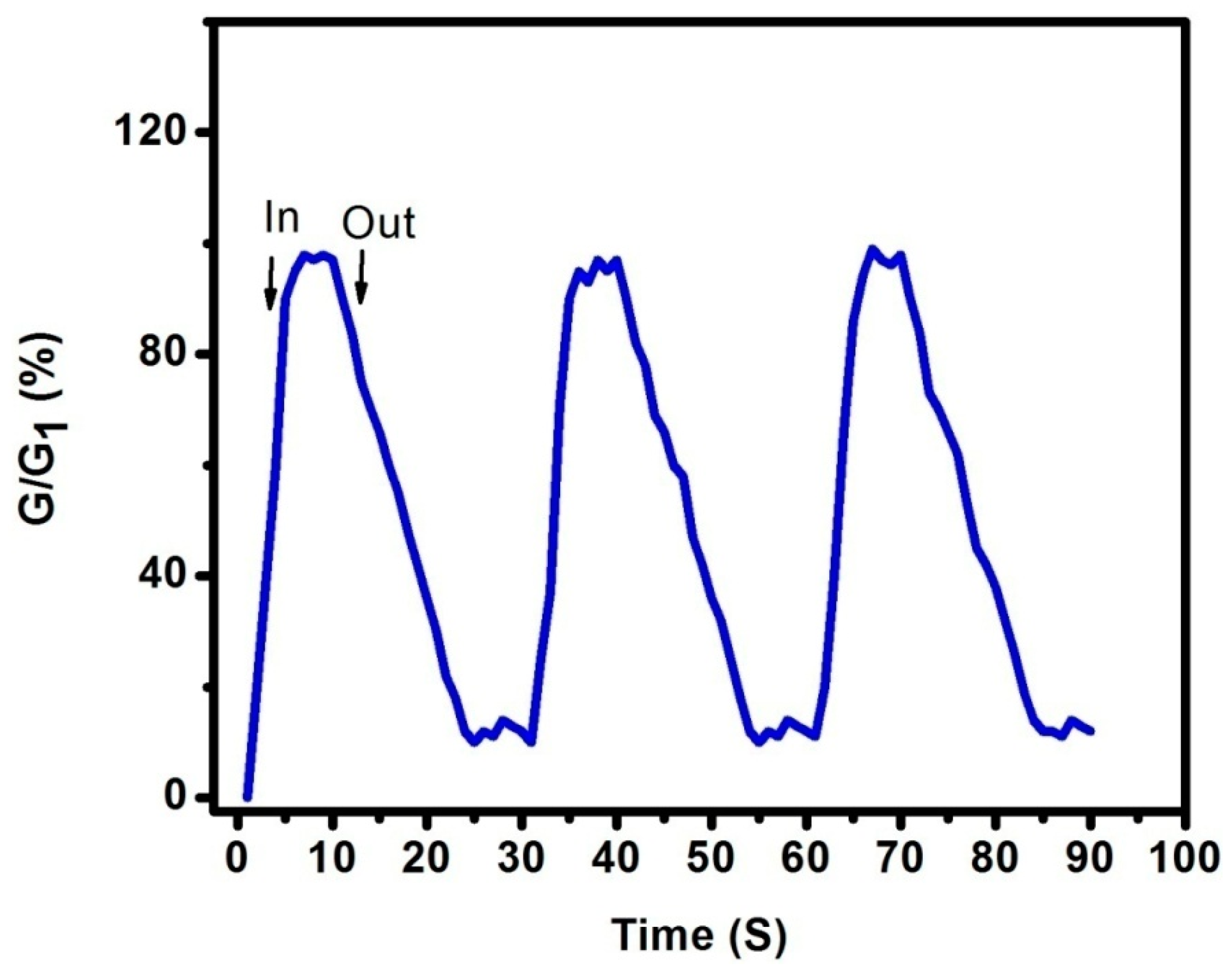
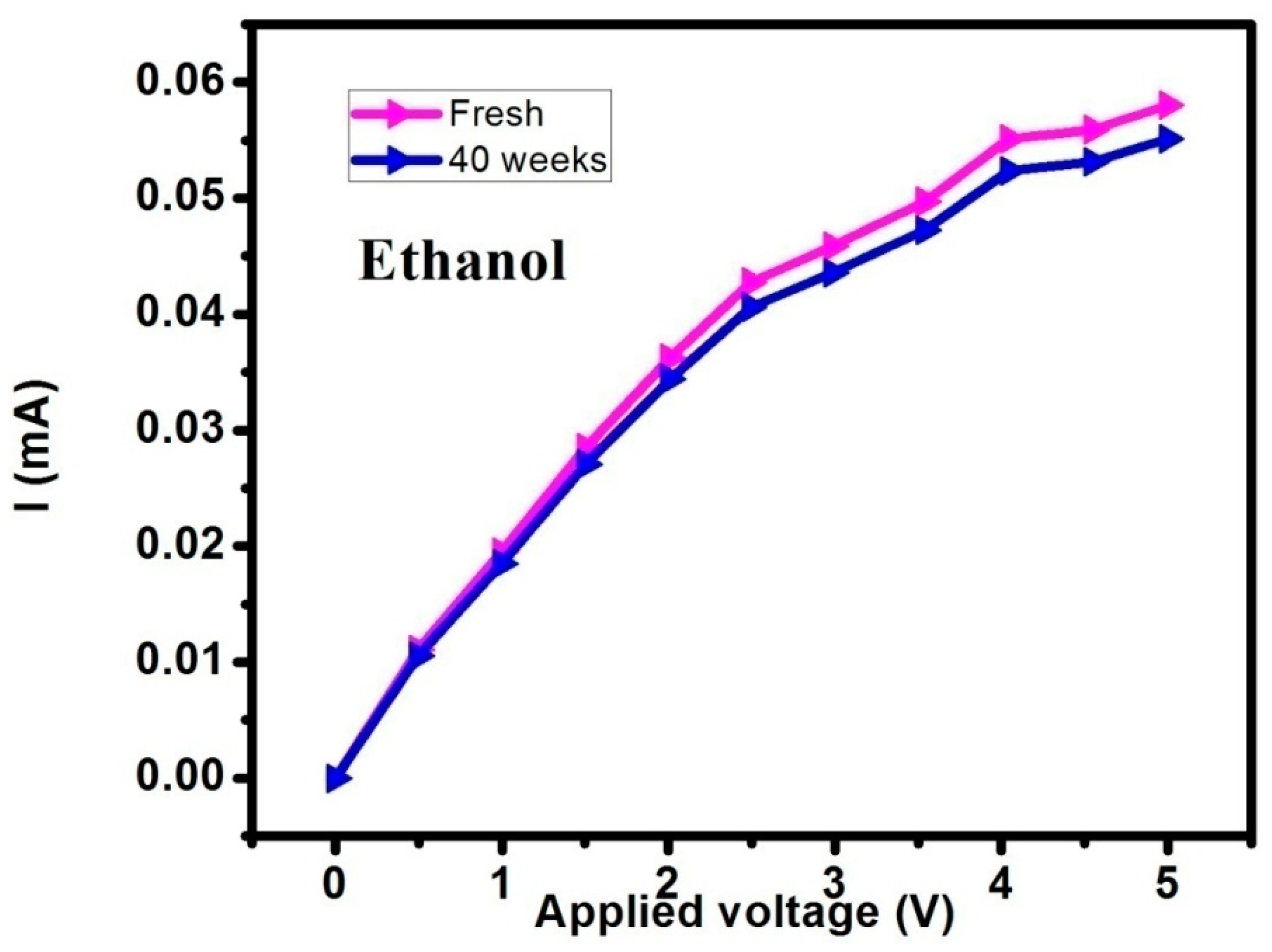
3. Results and Discussions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, S.M.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.J.; Cho, E.; Shin, H.J.; Park, J.H.; Hwang, K.S. Enhancing the sensitivity of a micro-diaphragm resonating sensor by effectively positioning the mass on the membrane. Sci. Rep. 2015, 5, 17096. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Topalian, Z.; Sarkar, A.D.; Österlund, L.; Ahuja, R. TiO2-Based Gas Sensor: A Possible Application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Anantachaisilp, S.; Smith, S.M.; Ton-That, C.; Osotchan, T.; Moon, A.R.; Phillips, M.R. Tailoring Deep Level Surface Defects in ZnO Nanorods for High Sensitivity Ammonia Gas Sensing. J. Phys. Chem. C 2014, 118, 27150–27156. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Voznyy, O.; Hu, L.; Fu, Q.; Zhou, D.; Xia, Z.; Sargent, E.H.; Tang, J. Physically Flexible, Rapid-Response Gas Sensor Based on Colloidal Quantum Dot Solids. Adv. Mater. 2014, 26, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.W.; Mayrhofer, L.; Casals, O.; Caccamo, L.; Hernandez-Ramirez, F.; Lilienkamp, G.; Daum, W.; Moseler, M.; Waag, A.; Shen, H. A Highly Selective and Self-Powered Gas Sensor via Organic Surface Functionalization of p-Si/n-ZnO Diodes. Adv. Mater. 2014, 26, 8017–8022. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hwang, I.S.; Park, J.G.; Choi, K.J.; Park, J.H.; Lee, J.H. Novel fabrication of an SnO2 nanowire gas sensor with high sensitivity. Nanotechnology 2008, 19, 095508. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Zeng, J.; Hu, M.; Wang, W.D.; Chen, H.; Qin, Y. NO2-sensing properties of porous WO3 gas sensor based on anodized sputtered tungsten thin film. Sens. Actuators B Chem. 2012, 161, 447–452. [Google Scholar] [CrossRef]
- Nishiyama, A.; Hua, Z.; Suematsu, K.; Yuasa, M.; Shimanoe, K. WO3 Nanolamella Gas Sensor: Porosity Control Using SnO2 Nanoparticles for Enhanced NO2 Sensing. Langmuir 2014, 30, 2571–2579. [Google Scholar]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.J.; Shi, G.Q. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Late, D.J.; Huang, Y.K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.J.; Park, S.G.; Kwon, J.D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based Gas Sensing Using Atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Moh, L.C.H.; Swager, T.M. Single-Walled Carbon Nanotube—Metalloporphyrin Chemiresistive Gas Sensor Arrays for Volatile Organic Compounds. Chem. Mater. 2015, 27, 3560–3563. [Google Scholar] [CrossRef]
- Peng, N.; Zhang, Q.; Chow, C.L.; Tan, O.K.; Marzari, N. Sensing Mechanisms for Carbon Nanotube Based NH3 Gas Detection. Nano Lett. 2009, 9, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Donato, N.; Saitta, G.; Bonavita, A.; Rizzo, G.; Neri, G. Flexible ethanol sensors on glossy paper substrates operating at room temperature. Sens. Actuators B Chem. 2010, 145, 488–494. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Tan, W.; Yu, Q.; Ruan, X.; Huang, X. Design of SnO2-based highly sensitive ethanol gas sensor based on quasi molecular-cluster imprinting mechanism. Sens. Actuators B Chem. 2015, 212, 47–54. [Google Scholar] [CrossRef]
- Peng, K.Q.; Wang, X.; Lee, S.T. Gas sensing properties of single crystalline porous silicon nanowires. Appl. Phys. Lett. 2009, 95, 243112. [Google Scholar] [CrossRef]
- Jalkanen, T.; Mäkilä, E.; Määttänen, A.; Tuura, J.; Kaasalainen, M.; Lehto, V.; Ihalainen, P.; Peltonen, J.; Salonen, J. Porous silicon micro- and nanoparticles for printed humidity sensors. Appl. Phys. Lett. 2012, 101, 263110. [Google Scholar] [CrossRef]
- Li, W.; Hu, M.Y.; Ge, P.P.; Wang, J.; Guo, Y.Y. Humidity sensing properties of morphology-controlled ordered silicon nanopillar. Appl. Surf. Sci. 2014, 317, 970–973. [Google Scholar] [CrossRef]
- Li, W.; Dai, E.; Bang, G.; Xu, J. Depth-dependent humidity sensing properties of silicon nanopillararray. Sens. Actuators B Chem. 2016, 237, 526–533. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; He, S.; Wang, J.; Guo, Y.Y.; Guo, Y.F. Enhanced photoluminescence from CdS with SiO2 nanopillar arrays. Sci. Rep. 2015, 5, 11375. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, S.; Hu, M.; He, S.; Ge, P.; Wang, J.; Guo, Y.Y.; Zhaowei, L. Enhancement of electroluminescence from embedded Si quantum dots/SiO2 multilayers film by localized-surface-plasmon and surface roughening. Sci. Rep. 2015, 5, 11881. [Google Scholar] [CrossRef] [PubMed]
- Galeazzo, E.; Peres, H.E.M.; Santos, G.; Peixoto, N.; Ramirez-Fernandez, F.J. Gas sensitive porous silicon devices: Responses to organic vapors. Sens. Actuators B Chem. 2003, 93, 384–390. [Google Scholar] [CrossRef]
- Baratto, C.; Comini, E.; Faglia, G.; Sberveglieri, G.; di Francia, G.; de Filippo, F.; la Ferrara, V.; Quercia, L.; Lancellotti, L. Gas detection with a porous silicon based sensor. Sens. Actuators B Chem. 2000, 65, 257–259. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, S.J.; Feng, C.Y. Characterization of silicon nanoporous pillar array as room temperature capacitive ethanol gas sensor. Sens. Actuators B Chem. 2007, 123, 461–465. [Google Scholar] [CrossRef]
- Schechter, I.; Ben-Chorin, M.; Kux, A. Gas sensing properties of porous silicon. Anal. Chem. 1995, 67, 3727–3732. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Feng, Z.; Dai, E.; Xu, J.; Bai, G. Organic Vapour Sensing Properties of Area-Ordered and Size-Controlled Silicon Nanopillar. Sensors 2016, 16, 1880. https://doi.org/10.3390/s16111880
Li W, Feng Z, Dai E, Xu J, Bai G. Organic Vapour Sensing Properties of Area-Ordered and Size-Controlled Silicon Nanopillar. Sensors. 2016; 16(11):1880. https://doi.org/10.3390/s16111880
Chicago/Turabian StyleLi, Wei, Zhilin Feng, Enwen Dai, Jie Xu, and Gang Bai. 2016. "Organic Vapour Sensing Properties of Area-Ordered and Size-Controlled Silicon Nanopillar" Sensors 16, no. 11: 1880. https://doi.org/10.3390/s16111880
APA StyleLi, W., Feng, Z., Dai, E., Xu, J., & Bai, G. (2016). Organic Vapour Sensing Properties of Area-Ordered and Size-Controlled Silicon Nanopillar. Sensors, 16(11), 1880. https://doi.org/10.3390/s16111880




