A Comparative Study of the Application of Fluorescence Excitation-Emission Matrices Combined with Parallel Factor Analysis and Nonnegative Matrix Factorization in the Analysis of Zn Complexation by Humic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. HAs Chemical Characterization
2.2. Fluorescence Measurements in HA-Zn(II) Systems
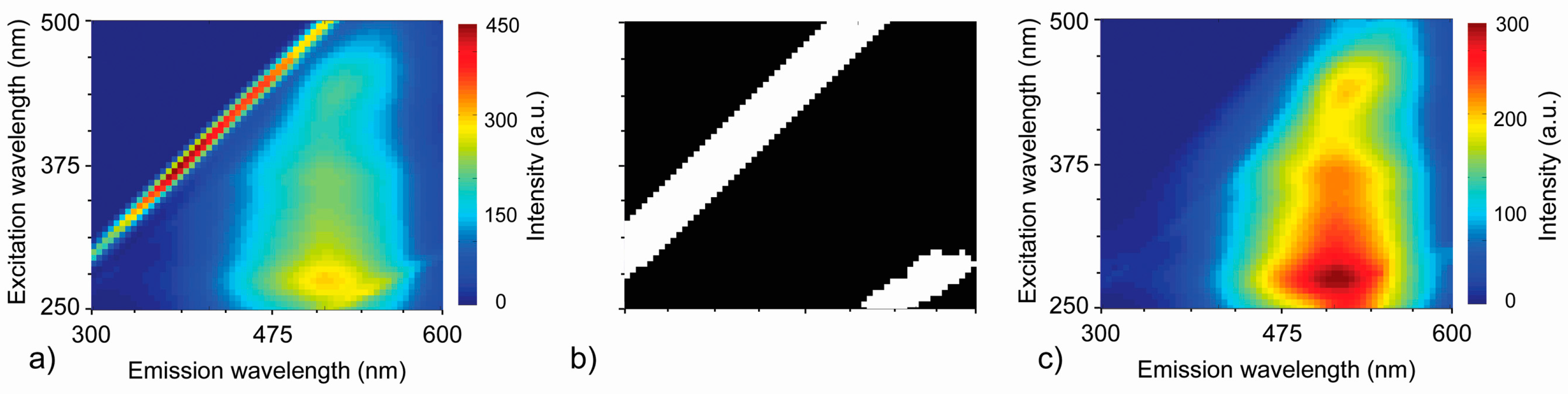
2.3. Fluorescence Data Pre-Processing
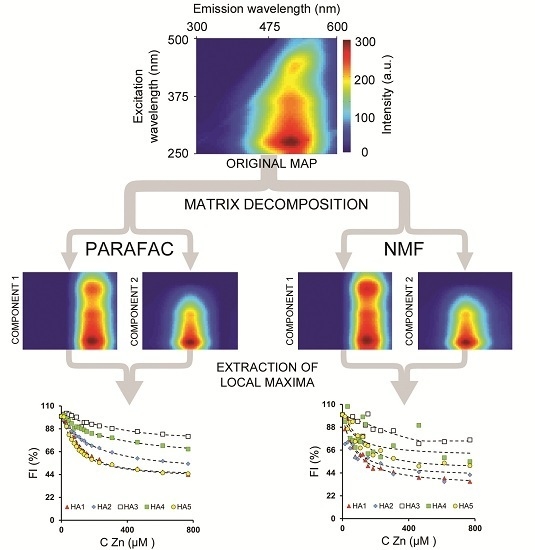
2.4. Fluorescence Data Unmixing
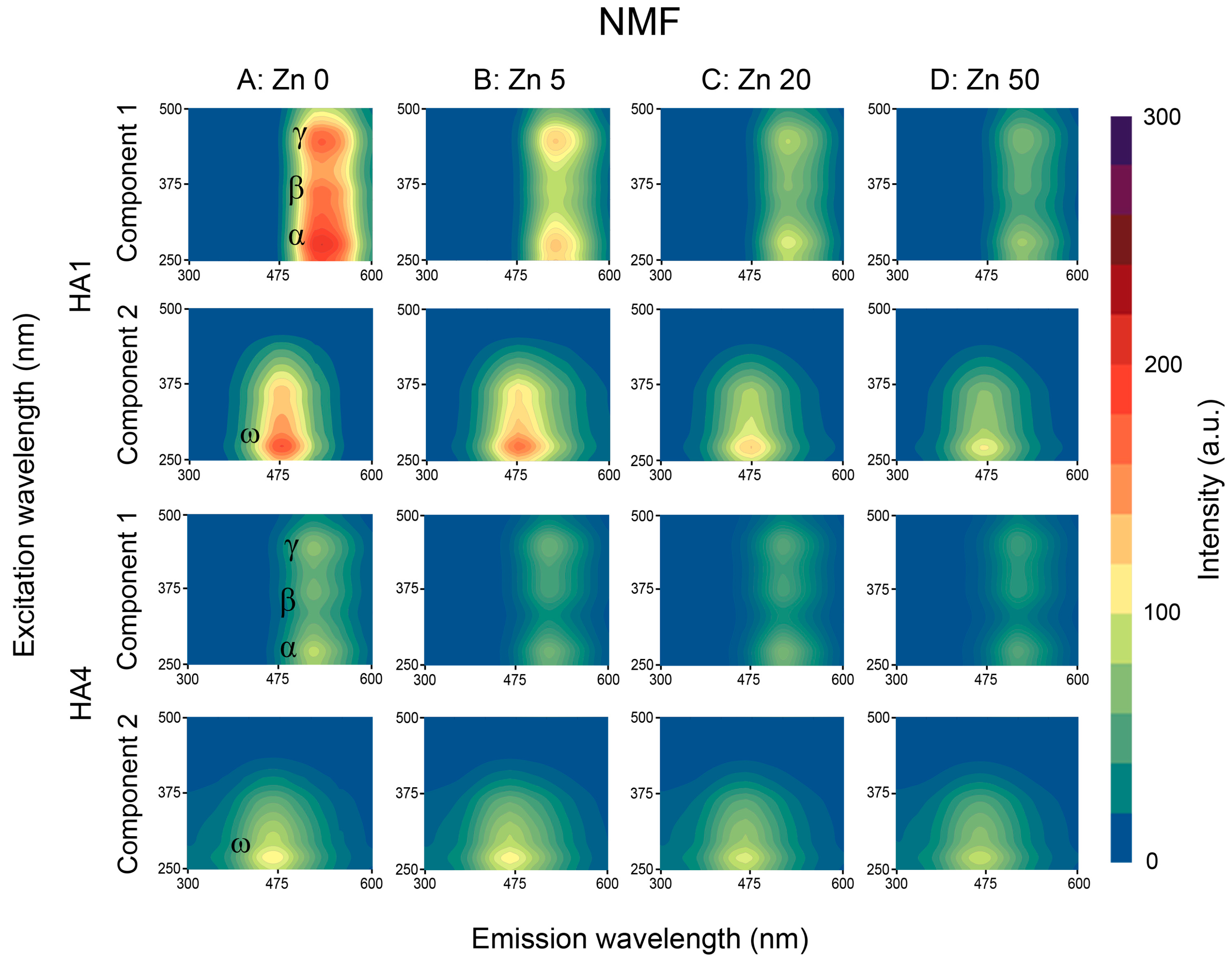
2.4.1. Nonnegative Matrix Factorization (NMF)
2.4.2. Parallel Factor Analysis Model (PARAFAC)
2.5. Calculation of Binding Parameters
3. Results and Discussion
3.1. Chemical Properties of Humic Acids
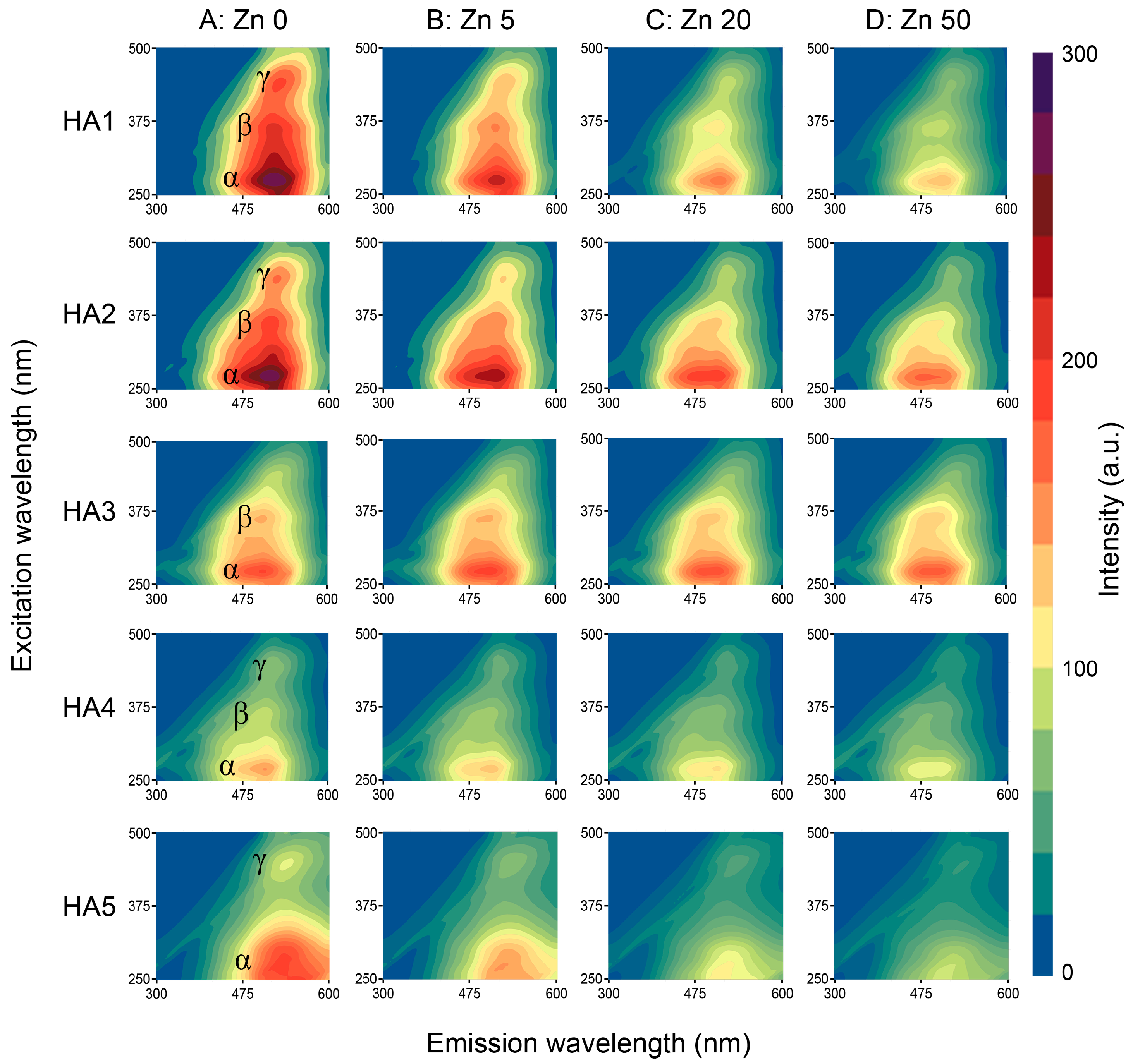
3.2. Characterization of Humic Acids Using Fluorescence Spectroscopy
3.2.1. RAW Data
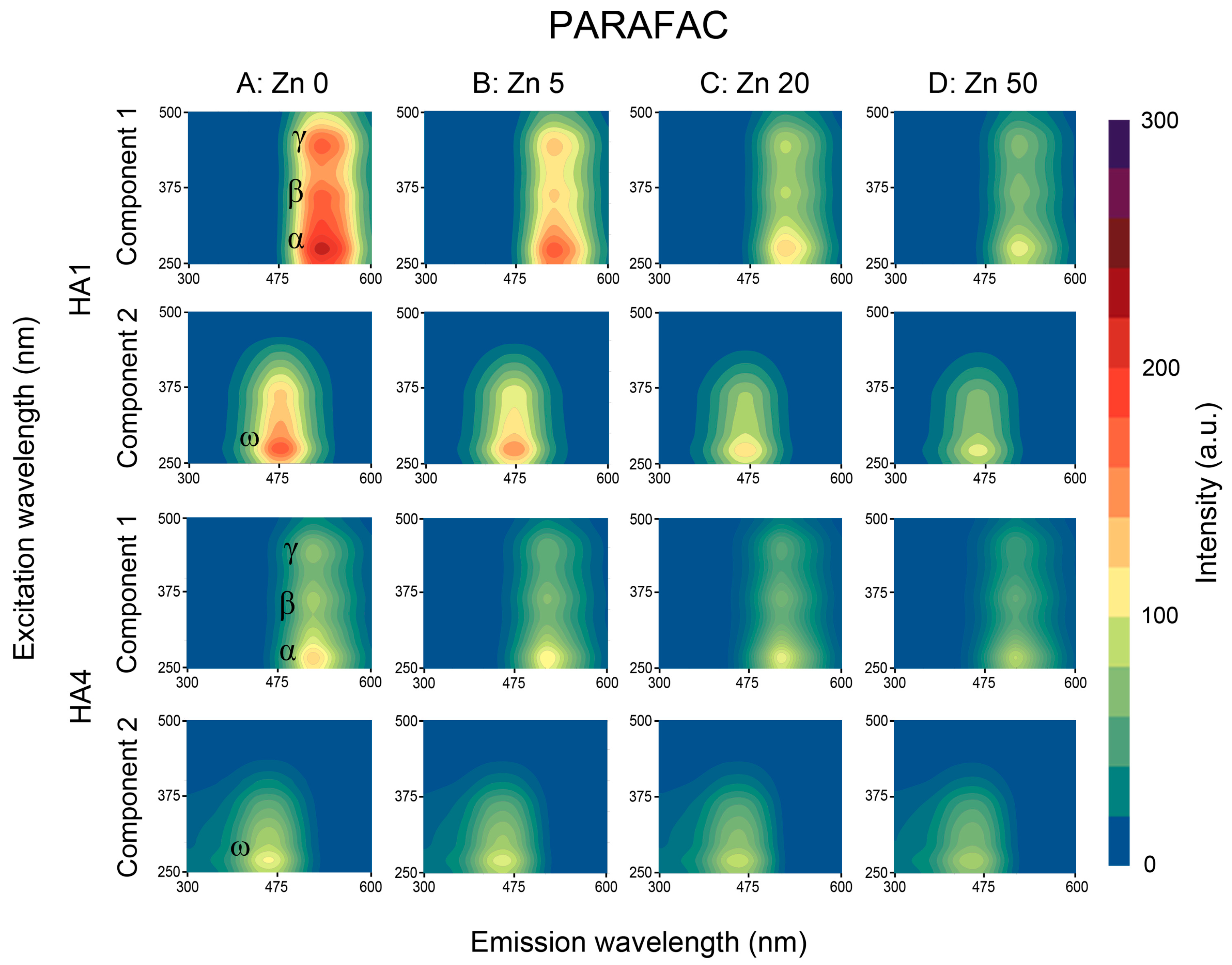
3.2.2. PARAFAC and NMF Processing
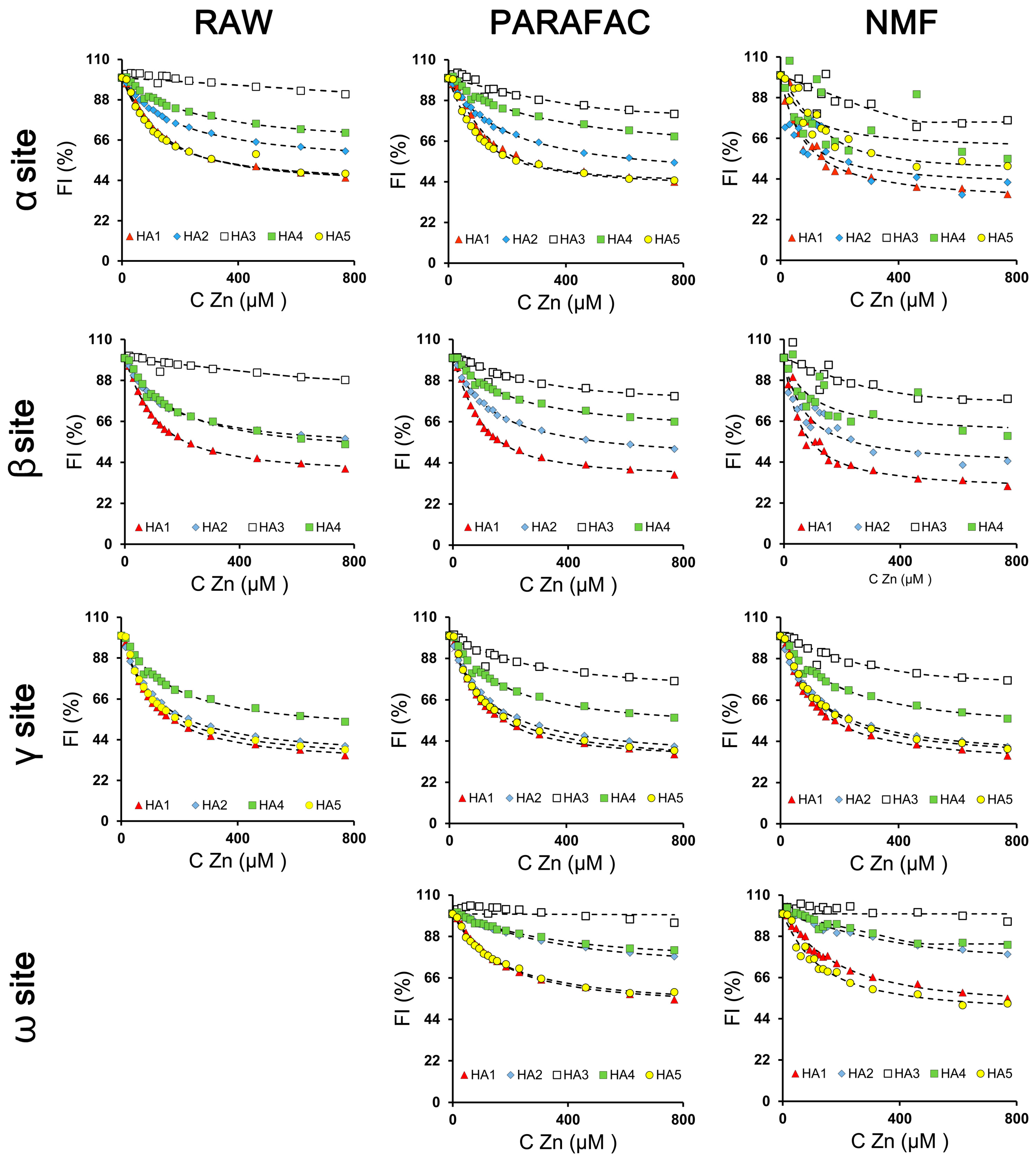
3.3. Quantification of Zn Complexation by Humic Acids
3.3.1. RAW Data
3.3.2. PARAFAC and NMF Processing
3.4. Summary of the PARAFAC and NMF Performance
3.5. Influence of Chemical Properties of HAs on the Complexation Process of Zinc(II) Ions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boguta, P.; Sokołowska, Z. Interactions of humic acids with metals. Acta Agroph. Monogr. 2013, 2, 1–113. [Google Scholar]
- Tan, K.H. Principles of Soil Chemistry, 4th ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Chen, J.; LeBoeuf, E.J.; Dai, S.; Gu, B. Fluorescence spectroscopic studies of natural organic matter fractions. Chemosphere 2003, 50, 639–647. [Google Scholar] [CrossRef]
- Aryal, R.K.; Duong, T.T.T.; Lee, B.K.; Hossain, M.A.; Kandel, D.; Kamruzzaman, M.; Beecham, S.; Chong, M.N. Organic matter composition variability in road sediment and its role in binding heavy metals. Sustain. Environ. Res. 2014, 24, 81–91. [Google Scholar]
- Garcia-Gil, J.C.; Plaza, C.; Senesi, N.; Brunetti, G.; Polo, A. Effects of sewage amendment on humic acids and microbiological properties of a semiarid mediterranean soil. Biol. Fertil. Soils 2004, 39, 320–328. [Google Scholar]
- Hernández, D.; Plaza, C.; Senesi, N.; Polo, A. Detection of Copper(II) and Zinc(II) binding to humic acids from pig slurry and amended soils by fluorescence spectroscopy. Environ. Pollut. 2006, 143, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Jaffé, R. Characterizing the Interactions between Trace Metals and Dissolved Organic Matter Using Excitation-Emission Matrix and Parallel Factor Analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association and International Fertilizer Industry Association: Brussels, Belgium; Paris, France, 2008. [Google Scholar]
- Tipping, E. Cation Binding by Humic Substances; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Adriano, D.; Vangronsveld, J.; Bolan, N.; Wenzel, W. Heavy metals. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 175–182. [Google Scholar]
- He, X.S.; Xi, B.D.; Jiang, Y.H.; He, L.S.; Li, D.; Pan, H.W.; Bai, S.G. Structural transformation study of water-extractable organic matter during the industrial composting of cattlemanure. Microchem. J. 2013, 106, 160–166. [Google Scholar] [CrossRef]
- Lai, T.M.; Shin, J.K.; Hur, J. Estimating the Biodegradability of Treated Sewage Samples Using Synchronous Fluorescence Spectra. Sensors 2011, 11, 7382–7394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, E.L.; Yin, Y.; VanDijk, M.A.; Feng, L.Q.; Shi, Z.Q.; Liu, M.L.; Qin, B.Q. Characteristics and sources of chromophoric dissolved organic matter in lakes of the Yungui Plateau, China, differing in trophic state and altitude. Limnol. Oceanogr. 2010, 55, 2645–2659. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, K.; Wen, Z.; Li, L.; Zang, S.; Shao, T.; Li, S.; Du, J. Seasonal characterization of CDOM for lakes in semiarid regions of Northeast China using excitation–emission matrix fluorescence and parallel factor analysis (EEM–PARAFAC). Biogeosci. Discuss. 2016, 13, 1635–1645. [Google Scholar] [CrossRef]
- Plaza, C.; D’Orazio, V.; Senesi, N. Copper(II) complexation of humic acids from the first generation of EUROSOILS by total luminescence spectroscopy. Geoderma 2005, 125, 177–186. [Google Scholar] [CrossRef]
- Plaza, C.; Brunetti, G.; Senesi, N.; Polo, A. Molecular and quantitative analysis of metal ion binding to humic acids from sewage sludge and sludge-amended soils by fluorescence spectroscopy. Environ. Sci. Technol. 2006, 40, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Elkins, K.M.; Nelson, D.J. Spectroscopic approaches to the study of the interaction of aluminum with humic substances. Coord. Chem. Rev. 2002, 228, 205–225. [Google Scholar] [CrossRef]
- Yan, M.; Ma, X.; Cheng, J. Characterizing Interactions between Suwannee River Dissolved Organic Matter and Cu(II) Using Fluorescence Excitation−Emission Matrices and Parallel Factor Analysis. J. Water Sustain. 2013, 3, 165–177. [Google Scholar]
- Chen, M.; Price, R.M.; Yamashita, Y.; Jaffé, R. Comparative study of dissolved organic matter from groundwater and surface water in the Florida coastal Everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Appl. Geochem. 2010, 25, 872–880. [Google Scholar] [CrossRef]
- Wei, Y.; Chu, H.Q.; Dong, B.Z.; Li, X. Evaluation of humic acid removal by a flat submerged membrane photoreactor. Chin. Sci. Bull. 2011, 56, 3437–3444. [Google Scholar] [CrossRef]
- Yu, G.H.; He, P.J.; Shao, L.M. Novel insights into sludge dewaterability by fluorescence excitation–emission matrix combined with parallel factor analysis. Water Res. 2010, 44, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Herzi, F.; Jean, N.; Sakka, H.A.; Mounier, S. Three-dimensional (3-D) fluorescence spectroscopy analysis of the fluorescent dissolved organic matter released by the marine toxic dinoflagellate Alexandrium catenella exposed to metal stress by zinc or lead. J. Phycol. 2014, 50, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; He, P.J.; Shao, L.M. Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res. 2011, 45, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, G.; Yang, L.; Jiang, H. Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments. J. Hazard. Mater. 2013, 2, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Gaussier, E.; Goutte, C. Relation between PLSA and NMF and implications. In Proceedings of the 28th Annual International ACM SIGIR Conference on Research and Development in Information Retrieval, New York, NY, USA, 15–19 August 2005; pp. 601–602.
- Zhang, J.S.; Wang, C.P.; Yang, Y.Q. Learning latent features by nonnegative matrix factorization combining similarity judgments. Neurocomputing 2015, 155, 43–52. [Google Scholar] [CrossRef]
- Yin, H.; Liu, H. Nonnegative matrix factorization with bounded total variational regularization for face recognition. Pattern Recog. Lett. 2010, 31, 2468–2473. [Google Scholar] [CrossRef]
- Kopriva, I.; Jerić, I. Blind separation of analytes in nuclear magnetic resonance spectroscopy: Improved model for nonnegative matrix factorization. Chemometr. Intell. Lab. 2014, 137, 47–56. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, H.; Tang, J.; Luo, B. A sparse nonnegative matrix factorization technique for graph matching problems. Pattern Recogit. 2014, 47, 736–747. [Google Scholar] [CrossRef]
- Kırbız, S.; Günsel, B. Perceptually enhanced blind single-channel music source separation by Non-negative Matrix Factorization. Digit. Signal Process. 2013, 23, 646–658. [Google Scholar] [CrossRef]
- Skic, K.; Boguta, P.; Sokołowska, Z. Analysis of the sorption properties of different soils using water vapour adsorption and potentiometric titration methods. Int. Agrophys. 2016, 30, 369–374. [Google Scholar] [CrossRef]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1018–1020. [Google Scholar]
- Boguta, P.; Sokołowska, Z. Interactions of Zn(II) Ions with Humic Acids Isolated from Various Type of Soils. Effect of pH, Zn Concentrations and Humic Acids Chemical Properties. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Harshman, R.A. Foundations of the PARAFAC procedure: Model and conditions for an “explanatory” multi-mode factor analysis. UCLA Work. Pap. Phon. 1970, 16, 1–84. [Google Scholar]
- Carroll, J.D.; Chang, J. Analysis of individual differences in multidimensional scaling via an N-way generalization of and Eckart-Young decomposition. Psychometrika 1970, 35, 283–319. [Google Scholar] [CrossRef]
- Andersson, C.A.; Bro, R. The N-way Toolbox for MATLAB. Chemom. Intell. Lab. Syst. 2000, 52, 1–4. [Google Scholar] [CrossRef]
- Ryan, D.K.; Weber, J.K. Fluorescence quenching titration for determination of complexing capacities and stability constants of fulvic acid. Anal. Chem. 1982, 54, 986–990. [Google Scholar] [CrossRef]
- He, X.S.; He, X.S.; Xi, B.D.; Pan, H.W.; Li, X.; Li, D.; Cui, D.Y.; Tang, W.B.; Yuan, Y. Characterizing the heavy metal-complexing potential of fluorescent water-extractable organic matter from composted municipal solid wastes using fluorescence excitation–emission matrix spectra coupled with parallel factor analysis. Environ. Sci. Pollut. Res. 2014, 21, 7973–7984. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.S.; Lau, I.L.; Chong, W.N.; Asing, J.; Faizal, M.; Nor, M.; Satirawaty, A.; Pauzan, M. Characterization of the coal derived humic acids from Mukah, Sarawak as soil conditioner. J. Braz. Chem. Soc. 2006, 17, 582–587. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Implementation of spectroscopic parameters for practical monitoring of natural organic matter. Desalination 2005, 176, 47–55. [Google Scholar] [CrossRef]
- Purmalis, O.; Klavins, M. Formation and Changes of Humic Acid Properties during Peat Humification Process within Ombrotrophic Bogs. Open J. Soil Sci. 2012, 2, 100–110. [Google Scholar] [CrossRef]
- Hosen, J.D.; McDonough, O.T.; Febria, C.M.; Palmer, M.A. Dissolved Organic Matter Quality and Bioavailability Changes across an Urbanization Gradient in Headwater Streams. Environ. Sci. Technol. 2014, 48, 7817–7824. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.H.; Nicolodelli, G.; Romano, R.A.; Tadini, A.M.; Villas-Boas, P.R.; Montes, C.R.; Mouniere, S.; Miloria, D.M.B.P. Structure of Humic Substances from Some Regions of the Amazon Assessed Coupling 3D Fluorescence Spectroscopy and CP/PARAFAC. J. Braz. Chem. Soc. 2015, 26, 1136–1142. [Google Scholar] [CrossRef]
- Senesi, N.; Miano, T.M.; Provenzano, M.R.; Brunetti, G. Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil Sci. 1991, 152, 259–271. [Google Scholar] [CrossRef]
- Wu, F.; Midorikawa, T.; Tanoue, E. Fluorescence properties of organic ligands for Copper(II) in Lake Biwa and its rivers. Geochem. J. 2001, 35, 333–346. [Google Scholar] [CrossRef]
- Provenzano, M.R.; D’razio, V.; Jerzykiewicz, M.; Senesi, N. Fluorescence behaviour of Zn and Ni complexes of humic acids from different sources. Chemosphere 2004, 55, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Mounier, S.; Zhao, H.; Garnier, C.; Redon, R. Copper complexing properties of dissolved organic matter: PARAFAC treatment of fuorescence quenching. Biogeochemistry 2011, 106, 107–116. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Boguta, P.; D’Orazio, V.; Sokołowska, Z.; Senesi, N. Effects of selected chemical and physicochemical properties of humic acids from peat soils on their interaction mechanisms with copper ions at various pHs. J. Geochem. Explor. 2016, 168, 119–126. [Google Scholar] [CrossRef]
- Dudali, Y.; Holgado, R.; Maestri, G.; Guillon, E.; Dupont, L. Rapid screening of DOM’s metal-binding ability using a fluorescence-based microplate assay. Sci. Total Environ. 2006, 354, 286–291. [Google Scholar]
- Pehlivan, E.; Arslan, G. Uptake of metal ions on humic acids. Energy Sour. Part A Recovery Util. Environ. Eff. 2006, 28, 1099–1112. [Google Scholar] [CrossRef]
- Abate, G.; Masini, J.C. Acid-basic and complexation properties of a sedimentary humic acid. A study on the Barra Bonita reservoir of Tietê River, São Paulo State, Brazil. J. Braz. Chem. Soc. 2001, 12, 109–116. [Google Scholar] [CrossRef]
- Ciardelli, F.; Tsuchida, E.; Wöhrle, D. Macromolecule-Metal Complexes; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]

| HA No. | Raw Soil Material | Soil location | N (%) | C (%) | N/C | COOH (cmol·kg−1) | COOH + OH (cmol·kg−1) | Q6 (cmol·kg−1) | E4/E6 | E254/E436 | ∆logK |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HA1 | Haplic Fluvisol (Alluvial soil) | 51°09′ N/22°59 ′E | 5.11 | 54.81 | 0.080 | 321 | 650 | 284 | 5.35 | 5.00 | 0.70 |
| HA2 | Haplic Cambisol (Brown soil) | 50°32′ N/24°01′ E | 3.70 | 51.24 | 0.062 | 411 | 741 | 266 | 5.69 | 5.59 | 0.67 |
| HA3 | Mollic Gleysol (Black Earth) | 50°22′ N/23°39′ E | 4.30 | 53.28 | 0.069 | 424 | 670 | 254 | 7.08 | 8.32 | 0.89 |
| HA4 | Stagnic Luvisol (Grey-brown soil) | 50°38′ N/22°41′ E | 3.35 | 58.97 | 0.049 | 260 | 499 | 261 | 6.17 | 6.48 | 0.75 |
| HA5 | Haplic Chernozem (Chernozem) | 51°23′ N/22°35′ E | 4.69 | 56.1 | 0.072 | 196 | 454 | 278 | 4.8 | 5.28 | 0.65 |
| α-Site RAW | α-Site PARAFAC | α-Site NMF | ||||||||||||||||
| HAs No. | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) |
| HA1 | 275/500 | 112 | 4.11 | 1.00 | 60 | 2.44 | 275/520 | 91 | 4.41 | 0.99 | 58 | 3.85 | 280/515 | 62 | 4.14 | 0.95 | 69 | 0.25 |
| HA2 | 270/495 | 139 | 3.76 | 1.00 | 49 | 0.38 | 275/510 | 92 | 3.75 | 1.00 | 56 | 0.44 | 505/275 | 73 | 4.22 | 0.63 | 61 | 0.25 |
| HA3 | 275/490 | n/a | 270/515 | 103 | 4.32 | 0.87 | 22 | 12.5 | 270/515 | 71 | 5.91 | 0.67 | 25 | 10.5 | ||||
| HA4 | 270/490 | 86 | 3.71 | 0.99 | 38 | 2.17 | 270/505 | 65 | 3.52 | 0.98 | 43 | 0.60 | 275/505 | 48 | 4.16 | 0.34 | 40 | 0.41 |
| HA5 | 270/505 | 182 | 4.07 | 0.99 | 59 | 1.44 | 265/525 | 184 | 4.19 | 1.00 | 60 | 1.63 | 280/525 | 127 | 4.37 | 0.91 | 52 | 3.89 |
| β-Site RAW | β-Site PARAFAC | β-Site NMF | ||||||||||||||||
| HAs No. | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML | logK | R | f % | CL (mmol/g) |
| HA1 | 360/500 | 75 | 4.15 | 1.00 | 64 | 1.57 | 360/520 | 60 | 4.31 | 1.00 | 65 | 2.05 | 360/515 | 45 | 4.29 | 0.96 | 72 | 0.25 |
| HA2 | 365/495 | 94 | 3.88 | 1.00 | 50 | 0.25 | 365/510 | 70 | 3.88 | 1.00 | 56 | 0.25 | 365/505 | 61 | 4.17 | 0.85 | 58 | 0.25 |
| HA3 | 365/490 | 114 | 5.08 | 0.92 | 13 | 17.8 | 365/515 | 77 | 4.02 | 0.96 | 24 | 8.00 | 370/515 | 59 | 4.36 | 0.76 | 25 | 8.48 |
| HA4 | 360/490 | 32 | 3.87 | 0.99 | 53 | 1.40 | 365/505 | 43 | 3.73 | 0.99 | 42 | 0.86 | 370/505 | 34 | 4.06 | 0.61 | 42 | 0.25 |
| HA5 | n/d | n/d | n/d | |||||||||||||||
| γ-Site RAW | γ-Site PARAFAC | γ-Site NMF | ||||||||||||||||
| HAs No. | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) |
| HA1 | 435/510 | 56 | 4.13 | 1.00 | 70 | 1.50 | 445/520 | 56 | 4.22 | 1.00 | 67 | 2.33 | 445/515 | 51 | 4.10 | 1.00 | 69 | 1.38 |
| HA2 | 435/505 | 53 | 3.97 | 1.00 | 67 | 0.62 | 440/510 | 51 | 3.96 | 1.00 | 67 | 0.68 | 440/505 | 50 | 3.93 | 1.00 | 67 | 0.65 |
| HA3 | n/d | 430/515 | 56 | 3.77 | 0.96 | 30 | 4.87 | 435/515 | 56 | 4.10 | 0.96 | 27 | 8.10 | |||||
| HA4 | 435/505 | 32 | 3.87 | 0.99 | 53 | 1.40 | 440/505 | 33 | 3.83 | 0.99 | 52 | 1.29 | 440/505 | 31 | 3.76 | 0.99 | 53 | 0.71 |
| HA5 | 445/525 | 29 | 4.03 | 1.00 | 69 | 0.85 | 450/525 | 27 | 4.00 | 1.00 | 69 | 0.85 | 455/525 | 28 | 4.07 | 1.00 | 66 | 1.70 |
| ω-Site RAW | ω-Site PARAFAC | ω-Site NMF | ||||||||||||||||
| HAs No. | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) | EEWP (nm/nm) | IML a.u | logK | R | f % | CL (mmol/g) |
| HA1 | n.d. | 275/450 | 85 | 3.97 | 1.00 | 51 | 2.41 | 275/450 | 85 | 3.92 | 0.99 | 52 | 2.87 | |||||
| HA2 | n.d. | 275/435 | 134 | 3.79 | 0.99 | 29 | 7.97 | 270/440 | 145 | 4.09 | 0.93 | 26 | 11.02 | |||||
| HA3 | n.d. | 275/440 | n/a | 275/445 | n/a | |||||||||||||
| HA4 | n.d. | 270/430 | 71 | 3.77 | 0.99 | 25 | 6.77 | 270/440 | 84 | 6.88 | 0.92 | 16 | 11.71 | |||||
| HA5 | n.d. | 260/455 | 44 | 3.84 | 0.99 | 51 | 0.59 | 260/470 | 48 | 4.01 | 0.96 | 55 | 1.29 | |||||
| EEM-RAW | |||||||
| N | N/C | COOH | COOH + OH | E4/E6 | E254/E436 | ∆logK | |
| α-area | |||||||
| f | 0.91 | 0.97 | −0.12 | 0.08 | −0.91 | −0.98 * | −0.74 |
| K | 0.97 * | 0.93 * | −0.39 | −0.19 | −0.85 | −0.86 | −0.50 |
| β-area | |||||||
| f | 0.09 | 0.00 | −0.65 | −0.21 | −0.94 | −0.95 * | −0.90 |
| K | 0.38 | 0.41 | 0.57 | 0.23 | 0.78 | 0.80 | 0.90 |
| γ-area | |||||||
| f | 0.22 | 0.24 | −0.48 | −0.15 | −0.94 * | −1.00 * | −0.97 * |
| K | 0.56 | 0.55 | −0.38 | −0.03 | −0.88 * | −0.95 * | −0.83 |
| α-area | |||||||
| f | 0.22 | 0.24 | −0.51 | −0.19 | −0.96 * | −0.99 * | −0.99 * |
| K | 0.93 * | 0.94 * | 0.11 | 0.15 | −0.09 | 0.02 | 0.20 |
| β-area | |||||||
| f | 0.31 | 0.33 | −0.28 | 0.22 | −0.99 * | −0.99 * | −0.93 |
| K | 0.99 * | 0.98 * | 0.17 | 0.39 | −0.32 | −0.29 | −0.03 |
| γ-area | |||||||
| f | 0.21 | 0.24 | −0.47 | −0.15 | −0.95 * | −0.99 * | −0.99 * |
| K | 0.69 | 0.67 | −0.25 | 0.08 | −0.74 | −0.84 | −0.65 |
| ω-area | |||||||
| f | 0.95 * | 0.92 * | −0.41 | −0.21 | −0.90 | −0.87 | −0.56 |
| K | 0.94 | 0.87 | 0.00 | 0.19 | −0.46 | −0.78 | −0.13 |
| EEM-NMF | |||||||
| N | N/C | COOH | COOH + OH | E4/E6 | E254/E436 | ∆logK | |
| α-area | |||||||
| f | 0.41 | 0.45 | −0.33 | 0.02 | −0.89 * | −0.97 * | −0.90 * |
| K | 0.06 | 0.12 | 0.59 | 0.32 | 0.81 | 0.91 * | 0.91 * |
| β-area | |||||||
| f | 0.41 | 0.42 | −0.26 | 0.24 | −0.98 * | −0.98 * | −0.88 |
| K | 0.75 | 0.83 | 0.65 | 0.56 | 0.33 | 0.36 | 0.53 |
| γ-area | |||||||
| f | 0.19 | 0.20 | −0.45 | −0.12 | −0.91 * | −0.99 * | −0.97 * |
| K | 0.87 | 0.95 * | 0.18 | 0.24 | −0.14 | −0.01 | 0.12 |
| ω-area | |||||||
| f | 0.93 | 0.92 | −0.38 | −0.18 | −0.93 | −0.89 | −0.63 |
| K | −0.67 | −0.86 | −0.25 | −0.42 | 0.78 | 0.94 | 0.87 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguta, P.; Pieczywek, P.M.; Sokołowska, Z. A Comparative Study of the Application of Fluorescence Excitation-Emission Matrices Combined with Parallel Factor Analysis and Nonnegative Matrix Factorization in the Analysis of Zn Complexation by Humic Acids. Sensors 2016, 16, 1760. https://doi.org/10.3390/s16101760
Boguta P, Pieczywek PM, Sokołowska Z. A Comparative Study of the Application of Fluorescence Excitation-Emission Matrices Combined with Parallel Factor Analysis and Nonnegative Matrix Factorization in the Analysis of Zn Complexation by Humic Acids. Sensors. 2016; 16(10):1760. https://doi.org/10.3390/s16101760
Chicago/Turabian StyleBoguta, Patrycja, Piotr M. Pieczywek, and Zofia Sokołowska. 2016. "A Comparative Study of the Application of Fluorescence Excitation-Emission Matrices Combined with Parallel Factor Analysis and Nonnegative Matrix Factorization in the Analysis of Zn Complexation by Humic Acids" Sensors 16, no. 10: 1760. https://doi.org/10.3390/s16101760
APA StyleBoguta, P., Pieczywek, P. M., & Sokołowska, Z. (2016). A Comparative Study of the Application of Fluorescence Excitation-Emission Matrices Combined with Parallel Factor Analysis and Nonnegative Matrix Factorization in the Analysis of Zn Complexation by Humic Acids. Sensors, 16(10), 1760. https://doi.org/10.3390/s16101760