Highly Selective and Ultrasensitive Turn-on Luminescence Chemosensor for Mercury (II) Determination Based on the Rhodamine 6G Derivative FC1 and Au Nanoparticles
Abstract
:1. Introduction
2. Material and Methods
2.1. Instruments
2.2. Reagents and Procedures
2.3. Sensor Development and Characterization
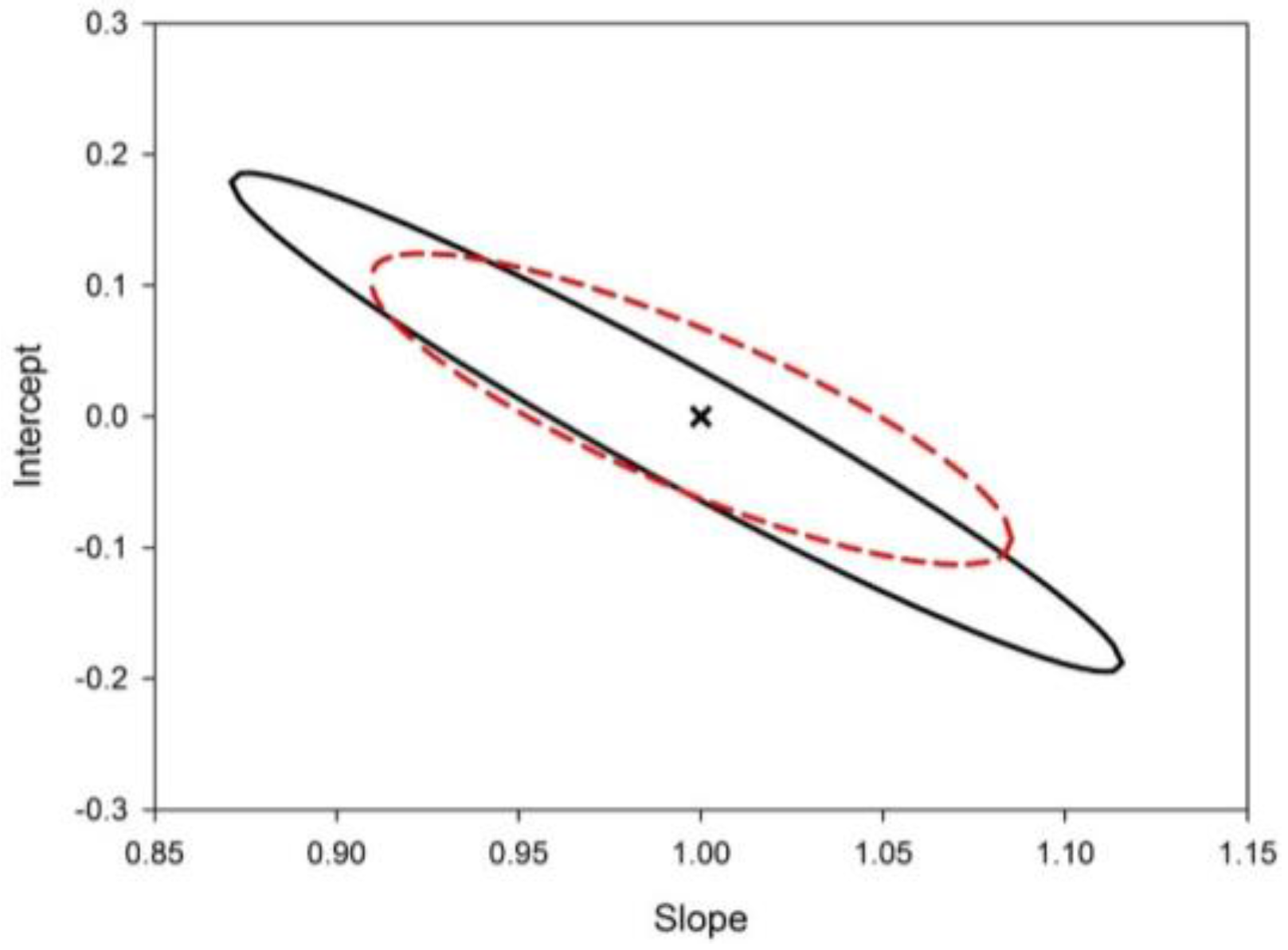
2.4. Method Validation: Limit of Detection (LOD), Limit of Quantitation (LOQ), Linearity, Precision and Accuracy
3. Results and Discussion
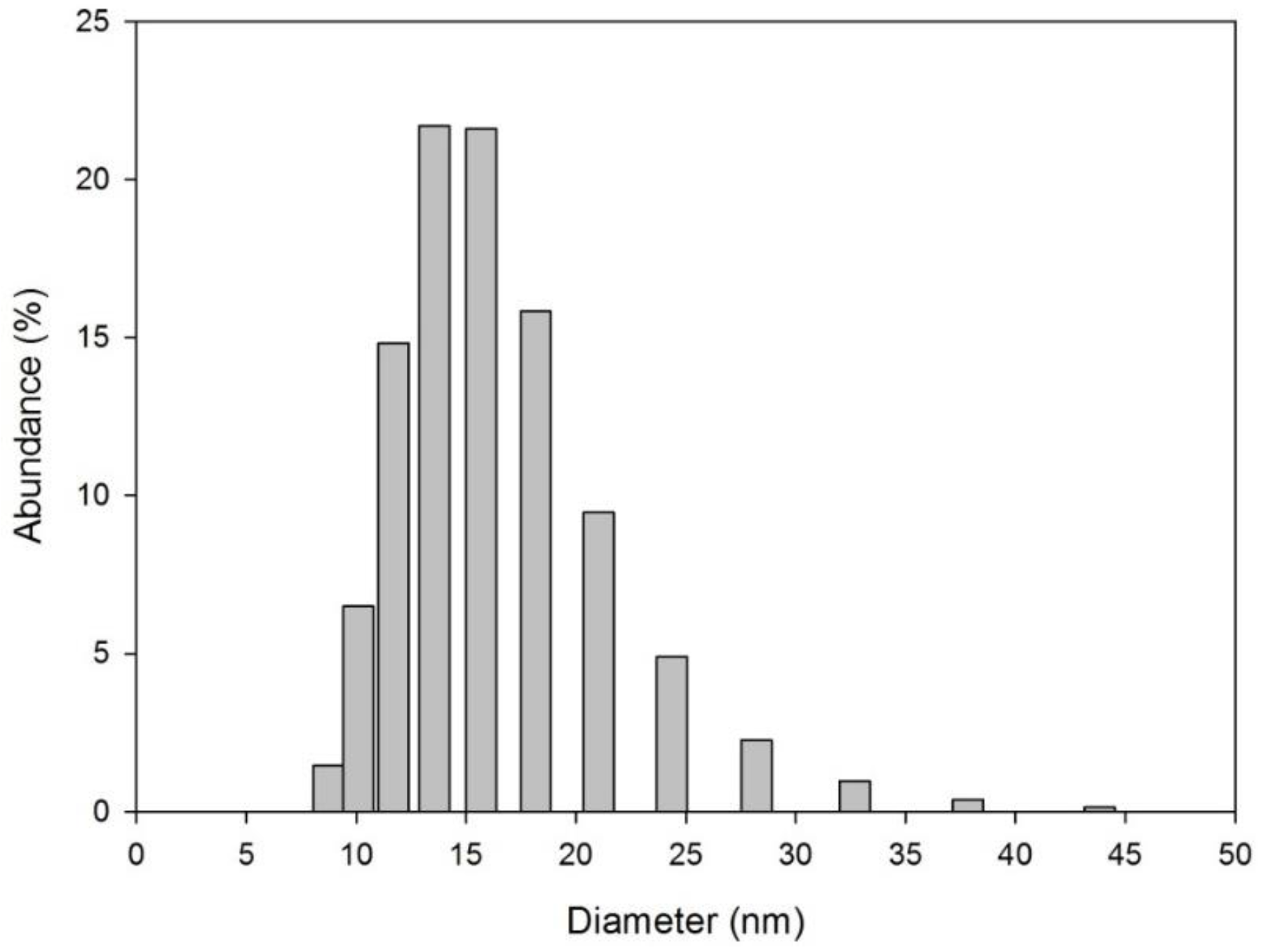
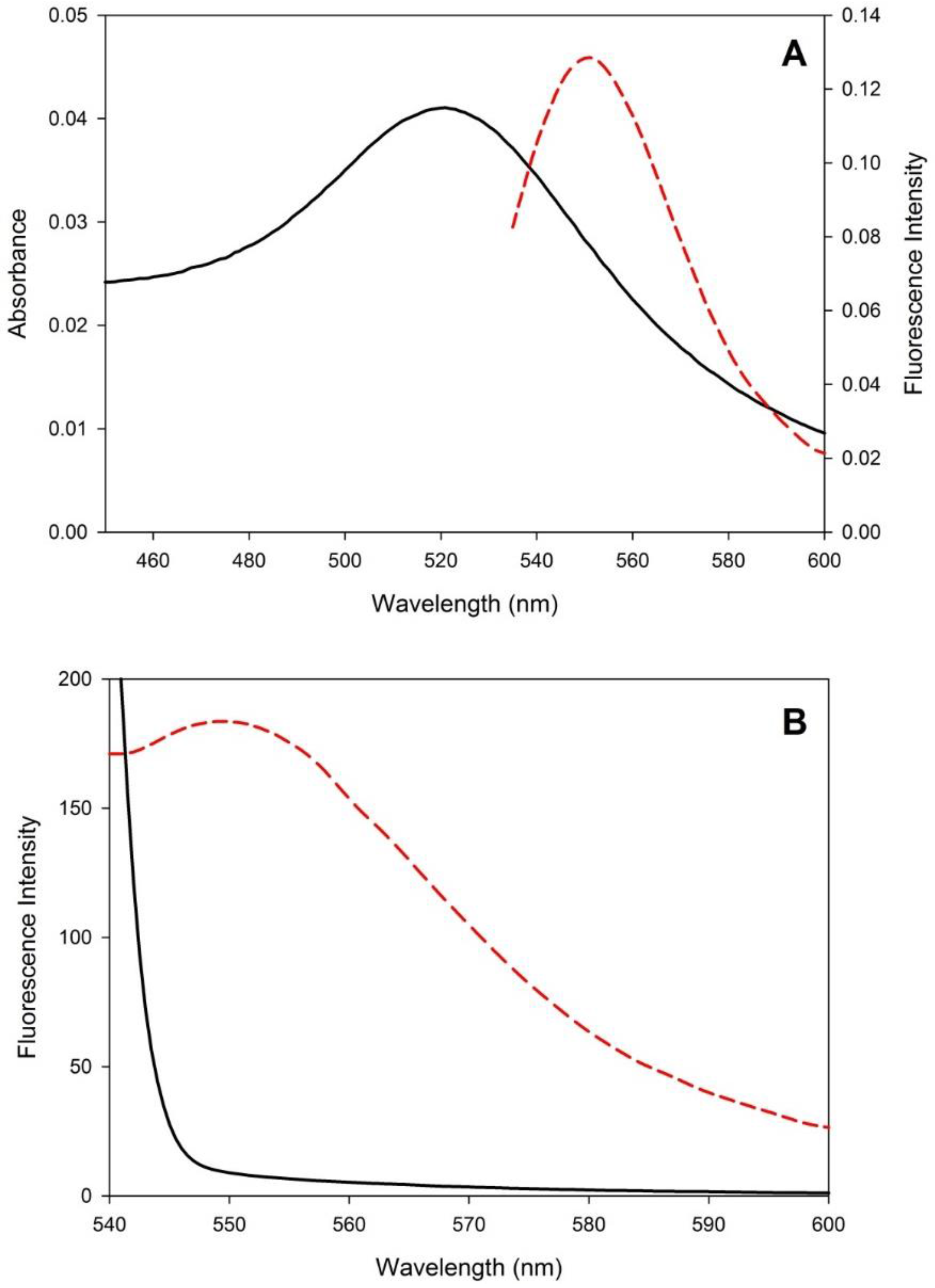
3.1. Sensor Development and Characterization
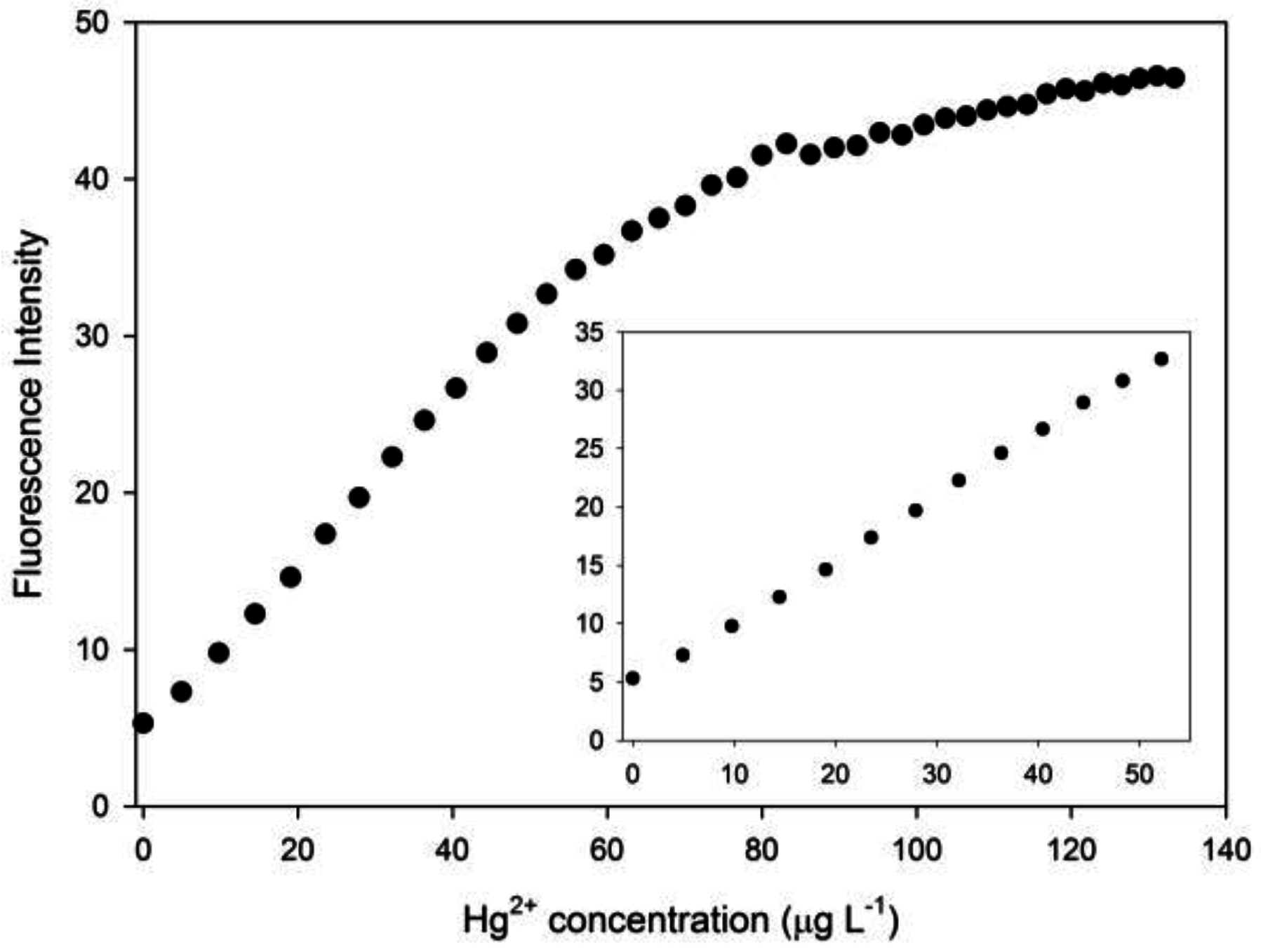
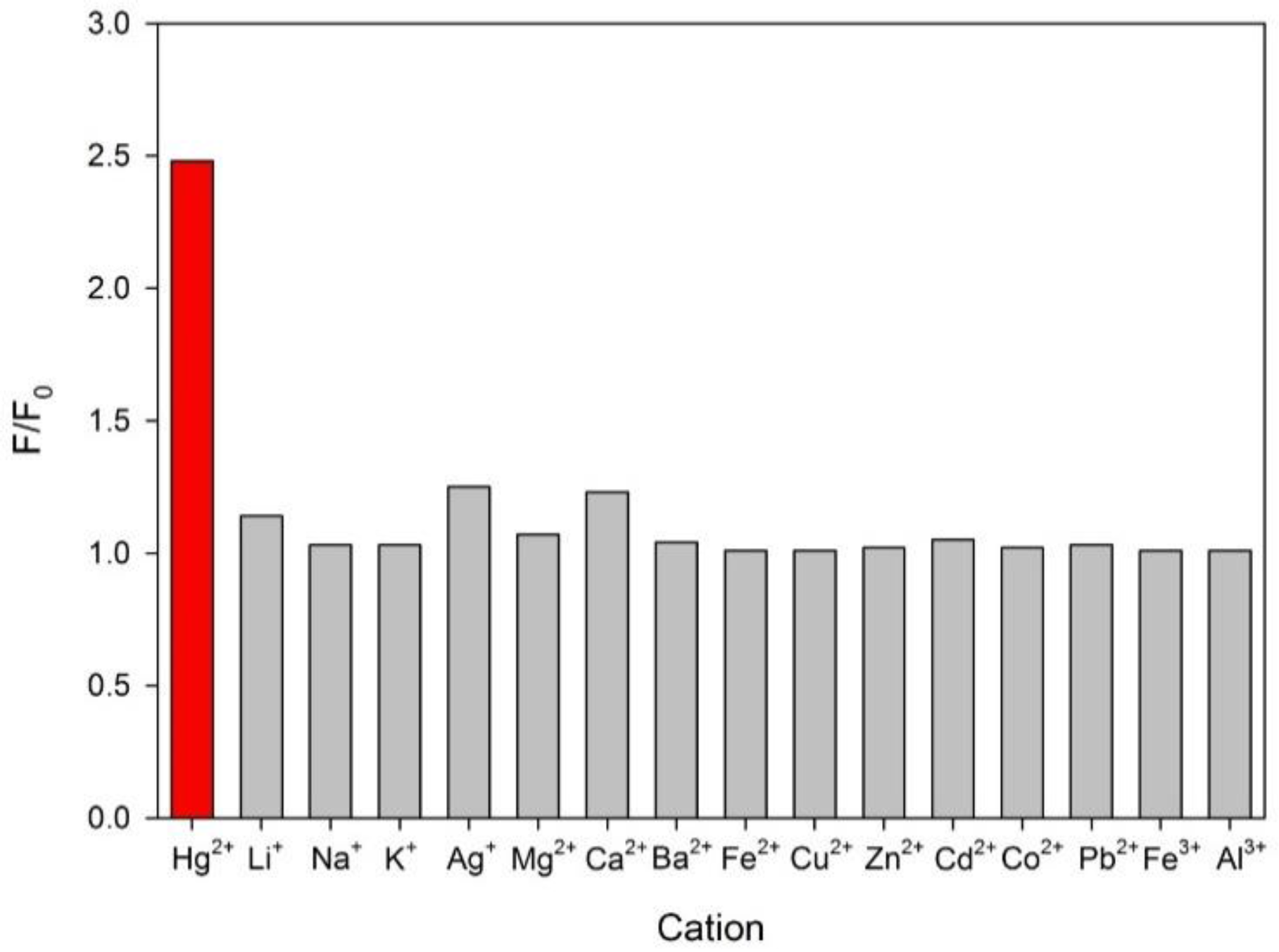
3.2. Optimization of the Chemodosimeter Response
3.3. Analytical Parameters and Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- National Primary Drinking Water Regulations. Available online: https://www.epa.gov/dwreg-dev/drinking-water-regulations-and-contaminants#Primary (accessed on 1 October 2016).
- Guidelines for Drinking-Water Quality. Available online: http://www.who.int/water_sani-tation_health/dwq/fulltext.pdf (accessed on 1 October 2016).
- Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption as Amended by Regulations 1882/2003/EC and 596/2009/EC. Available online: http://rod.eionet.europa.eu/instruments/545 (accessed on 1 October 2016).
- Leermakers, M.; Baeyens, W.; Quevauviller, P.; Horvat, M. Mercury in environmental samples: Speciation, artifacts and validation. TrAC Trends Anal. Chem. 2005, 24, 383–393. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3433–3480. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Selid, P.D.; Xu, H.; Collins, E.M.; Face-Collins, S.M.; Zhao, J.X. Sensing mercury for biomedical and environmental monitoring. Sensors 2009, 9, 5446–5459. [Google Scholar] [CrossRef] [PubMed]
- Culzoni, M.J.; Muñoz de la Peña, A.; Machuca, A.; Goicoechea, H.C.; Babiano, R. Rhodamine and BODIPY chemodosimeters and chemosensors for the detection of Hg2+, based on fluorescence enhancement effects. Anal. Methods 2013, 5, 30–49. [Google Scholar] [CrossRef]
- Yang, Y.K.; Yook, K.J.; Tae, J. A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J. Am. Chem. Soc. 2015, 127, 16760–16761. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.K.; Yang, Y.K.; Tae, J.; Shin, I. In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J. Am. Chem. Soc. 2006, 128, 14150–14155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Ko, S.K.; Shin, I.; Tae, J. Synthesis of a highly metal-selective rhodamine-based probe and its use for the in vivo monitoring of mercury. Nat. Protoc. 2007, 2, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Francis, M.B. Practical synthetic route to functionalized rhodamine dyes. Org. Lett. 2003, 5, 3245–3248. [Google Scholar] [CrossRef] [PubMed]
- Dujols, V.; Ford, F.; Czarnik, A.W. A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J. Am. Chem. Soc. 1997, 119, 7386–7387. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Jang, Y.; Lee, J.Y.; Kim, K.M.; Seo, M.S.; Nam, W.; Yoon, J. A highly selective fluorescent chemosensor for Pb2+. J. Am. Chem. Soc. 2005, 127, 10107–10111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Wei, B.; Yang, J. Synthesis of 2-(4-methoxylphenyloxy-acetylamido)-5-aryloxymethyl-1,3,4-oxadiazoles under microwave irradiation. Synth. Commun. 2002, 32, 1097–1103. [Google Scholar] [CrossRef]
- Zou, X.; Jin, G. Synthesis of pyridazinone-substituted 1,3,4-thiadiazoles, -1,3,4-oxadiazoles and -1,2,4-triazoles. J. Heterocycl. Chem. 2001, 38, 993–996. [Google Scholar] [CrossRef]
- Bohoyo, D.; Rodriguez, M.I.; Hurtado, M.C.; Muñoz de la Peña, A. Fluorescent determination of Hg2+ in water and fish samples using a chemodosimeter based in a rhodamine 6G derivative and a portable fiber-optic spectrofluorimeter. Appl. Spec. 2010, 64, 520–527. [Google Scholar]
- Yang, Y.K.; Ko, S.K.; Shin, I.; Tae, J. Fluorescent detection of methylmercury by desulfurization reaction of rhodamine hydrazide derivatives. Org. Biomol. Chem. 2009, 7, 4590–4593. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lee, S.J.; Jung, J.H.; Lim, H.; Kim, J.S. Luminophore-immobilized mesoporous silica for selective Hg2+ sensing. Tetrahedron 2007, 63, 12087–12092. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Liu, S. Hg2+-reactive double hydrophilic block copolymer assemblies as novel multifunctional fluorescent probes with improved performance. Langmuir 2010, 26, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, P.; Du, D.; He, C.; Qiu, B.; Chen, X.; Chen, G. Rhodamine-based ratiometric fluorescence sensing for the detection of mercury(II) in aqueous solution. Talanta 2010, 81, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Muñoz de la Peña, A.; Rodriguez Cáceres, M.I.; Hurtado, M.C.; Bohoyo, D. A novel application of Hg2+ based in a spirocyclic rhodamine 6G phenyl-thiosemicarbazide derivative. Luminiscence 2010, 25, 229–230. [Google Scholar]
- Lozano, V.A.; Escandar, G.M.; Mahedero, M.C.; Muñoz de la Peña, A. A novel nylon membrane–rhodamine 6G spirocyclicphenylthiosemicarbazide derivative system as a fluorimetric probe for mercury(II) ion. Anal. Methods 2012, 4, 2002–2008. [Google Scholar] [CrossRef]
- Orriach-Fernández, F.J.; Medina-Castillo, A.; Fernández-Sánchez, J.F.; Muñoz de la Peña, A.; Fernández-Gutiérrez, A. Hg2+-selective sensing film based on the incorporation of a rhodamine 6G derivative into a novel hydrophilic water-insoluble copolymer. Anal. Methods 2013, 5, 6642–6648. [Google Scholar] [CrossRef]
- Orriach-Fernández, F.J.; Medina-Castillo, A.L.; Díaz-Gómez, J.E.; Muñoz de la Peña, A.; Fernández-Sánchez, J.F.; Fernández-Gutiérrez, A. A sensing microfibre mat produced by electrospinning for the turn-on luminescence determination of Hg2+ in water samples. Sens. Actuators B Chem. 2014, 195, 8–14. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Kim, I.K.; Park, T.G. Fluorescent gold nanoprobe sensitive intracellular reactive oxygen species. Adv. Funct. Mat. 2009, 19, 1884–1890. [Google Scholar] [CrossRef]
- Yuan, M.J.; Li, Y.J.; Liu, H.B.; Li, Y.L. Chemical sensors based on π-conjugated organic molecules and gold nanoparticles. Sci. China Ser. B Chem. 2009, 52, 715–730. [Google Scholar] [CrossRef]
- Demchenko, A.P. Nanoparticles and nanocomposites for fluorescence sensing and imaging. Methods Appl. Fluoresc. 2013, 1, 022001. [Google Scholar] [CrossRef]
- Rex, M.; Hernández, F.E.; Campiglia, A.D. Pushing the limits of mercury sensors with gold nanorods. Anal. Chem. 2006, 78, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chang, H.T. Selective gold-nanoparticle-based “turn-on” fluorescent sensors for detection of mercury(II) in aqueous solution. Anal. Chem. 2006, 78, 8332–8338. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, A.F.; Chen, A.H.; Gao, Y.; He, C.; Kai, X.; Wu, G.; Chen, Y. A functionalized gold nanoparticles and Rhodamine 6G based fluorescent sensor for high sensitive and selective detection of mercury(II) in environmental water samples. Anal. Chim. Acta 2007, 599, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.X.; Jin, J.Y.; Yang, R.H. Gold nanoparticle-based colorimetric and “turn-on” fluorescent probe for mercury(II) ions in aqueous solution. Anal. Chem. 2008, 80, 9021–9028. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Huang, C.C.; Chang, H.T. Gold nanoparticle probes for the detection of mercury, lead and copper ions. Analyst 2011, 136, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Jiang, X. Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules. Nanoscale 2011, 3, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yuan, A.; Cai, N.; He, Y. Highly sensitive and selective detection of mercury (II) ions with fluorescent Au nanodots, GSTF intern. J. Chem. Sci. 2013, 1, 1–16. [Google Scholar]
- Förster, T. 10th Spiers Memorial Lecture Transfer Mechanisms of Electronic Excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Chance, R.R.; Prock, A.; Silbey, R. Molecular fluorescence and energy transfer near interfaces. Adv. Chem. Phys. 1978, 37, 1–65. [Google Scholar]
- Zheng, A.; Chen, J.; Wu, G.; Wei, H.; He, C.; Kai, X.; Wu, G.; Chen, Y. Optimization of a sensitive method for the “switch-on” determination of mercury(II) in waters using Rhodamine B capped gold nanoparticles as a fluorescence sensor. Microchim. Acta 2009, 164, 17–27. [Google Scholar] [CrossRef]
- Stobiecka, M.; Hepel, M. Multimodal coupling of optical transitions and plasmonic oscillations in rhodamine B modified gold nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Sadhu, S.; Patra, A. Surface energy transfer from rhodamine 6G to gold nanoparticles: A spectroscopic ruler. Appl. Phys. Letters 2007, 91, 043104. [Google Scholar] [CrossRef]
- Karthikeyan, B. Fluorescence quenching of rhodamine-6G in Au nanocomposite polymers. J. Appl. Phys. 2010, 108, 084311. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold, Discuss. Faraday Soc. 1951, 11, 55–59. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Sigma Aldrich. Gold Nanoparticles: Properties and Applications. Available online: http://www.sigmaaldrich.com/materials-science/nanomaterials/gold-nanoparticles.html (accessed on 1 October 2016).
- Kavitha, S.R.; Umadevi, M.; Janani, S.R.; Balakrishnan, T.; Ramanibai, R. Fluorescence quenching and photocatalytic degradation of textile dyeing waste water by silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 127, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Li, Y.; Bai, Q. A highly selective and sensitive fluorescein-based chemodosimeter for Hg2+ in aqueous media. Anal. Chim. Acta 2007, 584, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.C. Analytical figures of merit: From univariate to multiway calibration. Chem. Rev. 2014, 114, 5358–5378. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Herrador, M.A.; Asuero, A.G. Intra-laboratory testing of method accuracy from recovery assays. Talanta 1999, 48, 729–736. [Google Scholar] [CrossRef]
| Figure of Merit | Value |
|---|---|
| Intercept (SD) a | 7.0 (0.2) |
| Slope (L·μg−1) (SD) a | 7.2 (0.1) |
| R | 0.9983 |
| Analytical sensitivity (γ) (L·μg−1) | 16.4 |
| γ−1 (μg·L−1) | 0.06 |
| LOD (μg·L−1) b | 0.15 |
| LOQ (μg·L−1) b | 0.43 |
| Linearity range (μg·L−1) | 0.43–3.00 |
| Fexp c | 0.77 |
| Parameter | Result |
|---|---|
| Week 1 (SD) a | 1.50 (0.03) |
| Week 2 (SD) a | 1.53 (0.04) |
| Week 3 (SD) a | 1.51 (0.05) |
| Intermediate precision (CV%) b | 2.94 |
| ANOVA c | F = 2.64 (p = 0.08) |
| Sample | Hg2+ Concentration (μg·L−1) | |
|---|---|---|
| Nominal | Found a | |
| Milli-Q | 1.00 | 0.99 (0.01)[99.0] |
| 1.50 | 1.49 (0.03)[99.1] | |
| 2.00 | 1.99 (0.07)[99.3] | |
| Tap water b | 0.00 | ND c |
| 1.00 | 0.99 (0.04)[99.3] | |
| 1.50 | 1.48 (0.08)[98.7] | |
| 2.00 | 2.0 (0.1)[101.2] | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brasca, R.; Onaindia, M.C.; Goicoechea, H.C.; Peña, A.M.d.l.; Culzoni, M.J. Highly Selective and Ultrasensitive Turn-on Luminescence Chemosensor for Mercury (II) Determination Based on the Rhodamine 6G Derivative FC1 and Au Nanoparticles. Sensors 2016, 16, 1652. https://doi.org/10.3390/s16101652
Brasca R, Onaindia MC, Goicoechea HC, Peña AMdl, Culzoni MJ. Highly Selective and Ultrasensitive Turn-on Luminescence Chemosensor for Mercury (II) Determination Based on the Rhodamine 6G Derivative FC1 and Au Nanoparticles. Sensors. 2016; 16(10):1652. https://doi.org/10.3390/s16101652
Chicago/Turabian StyleBrasca, Romina, María C. Onaindia, Héctor C. Goicoechea, Arsenio Muñoz de la Peña, and María J. Culzoni. 2016. "Highly Selective and Ultrasensitive Turn-on Luminescence Chemosensor for Mercury (II) Determination Based on the Rhodamine 6G Derivative FC1 and Au Nanoparticles" Sensors 16, no. 10: 1652. https://doi.org/10.3390/s16101652
APA StyleBrasca, R., Onaindia, M. C., Goicoechea, H. C., Peña, A. M. d. l., & Culzoni, M. J. (2016). Highly Selective and Ultrasensitive Turn-on Luminescence Chemosensor for Mercury (II) Determination Based on the Rhodamine 6G Derivative FC1 and Au Nanoparticles. Sensors, 16(10), 1652. https://doi.org/10.3390/s16101652





