Arbitrarily Accessible 3D Microfluidic Device for Combinatorial High-Throughput Drug Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
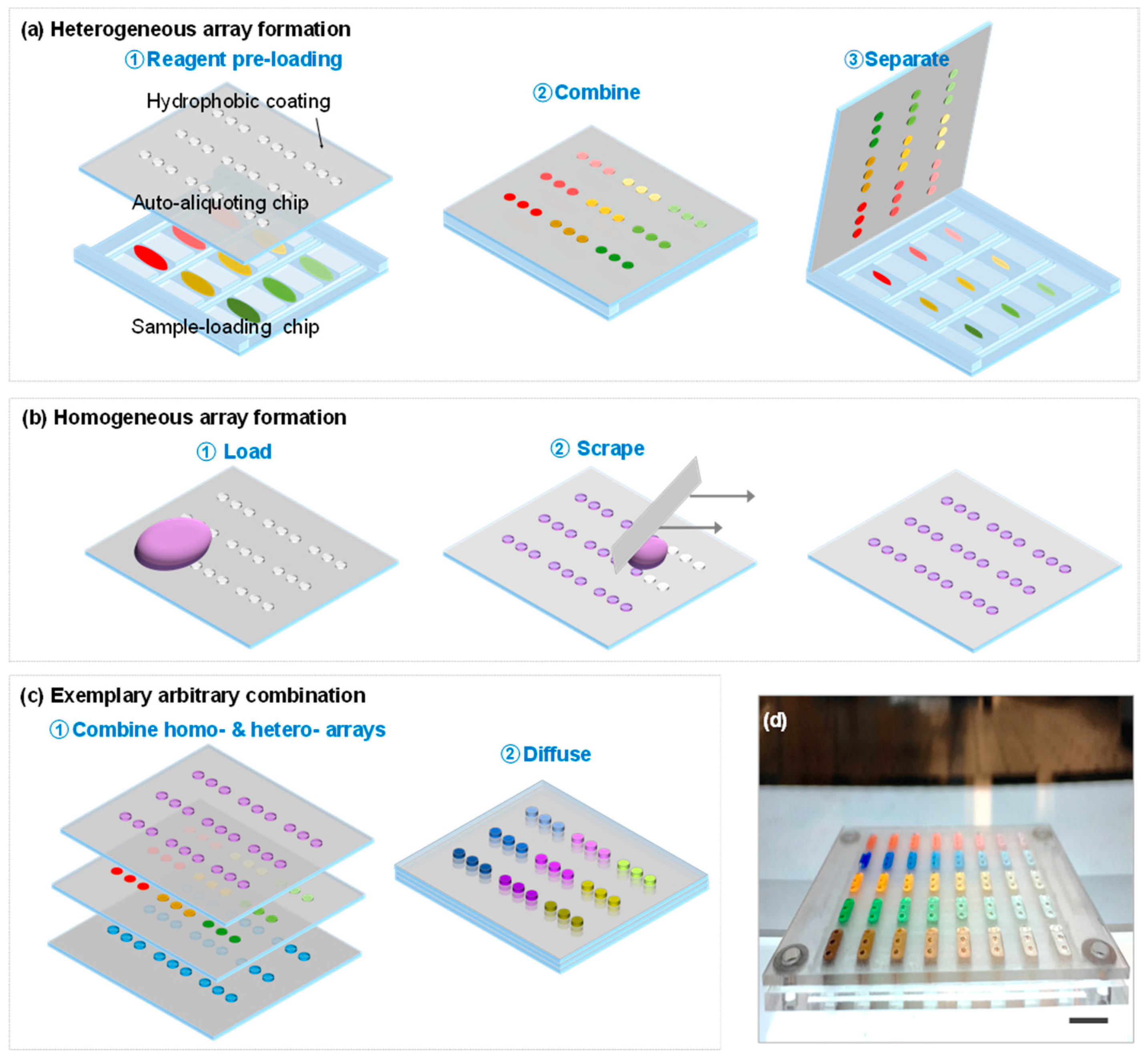
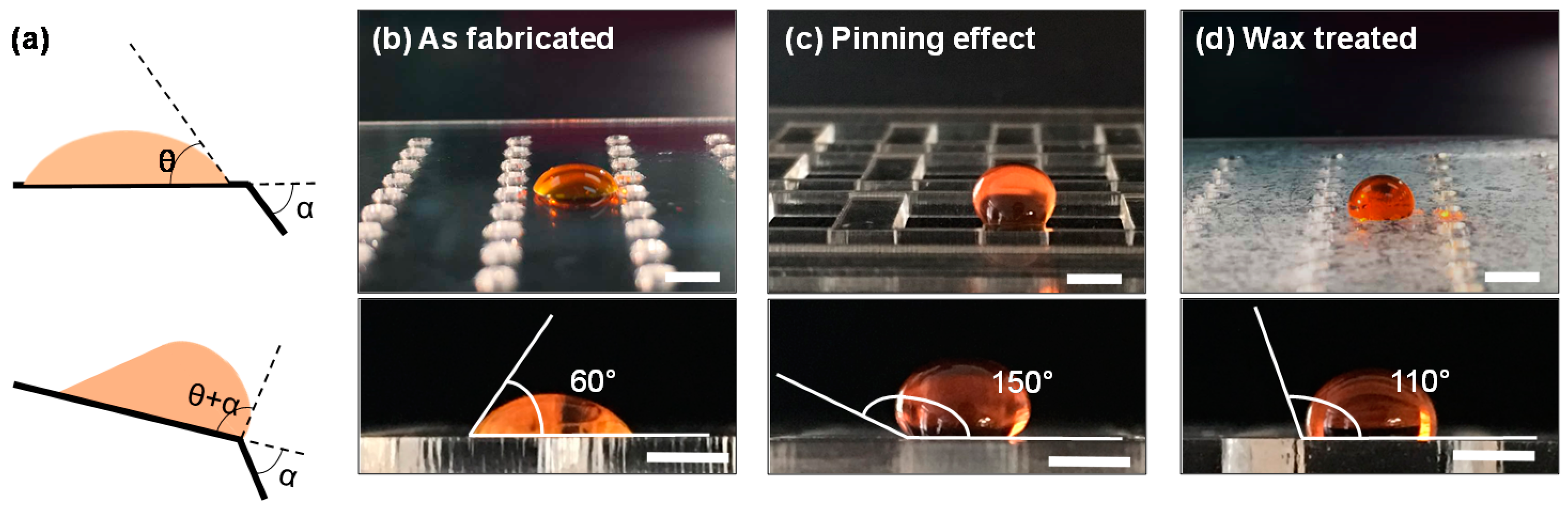
2.2. Device Design and Fabrication
2.3. Device Operation
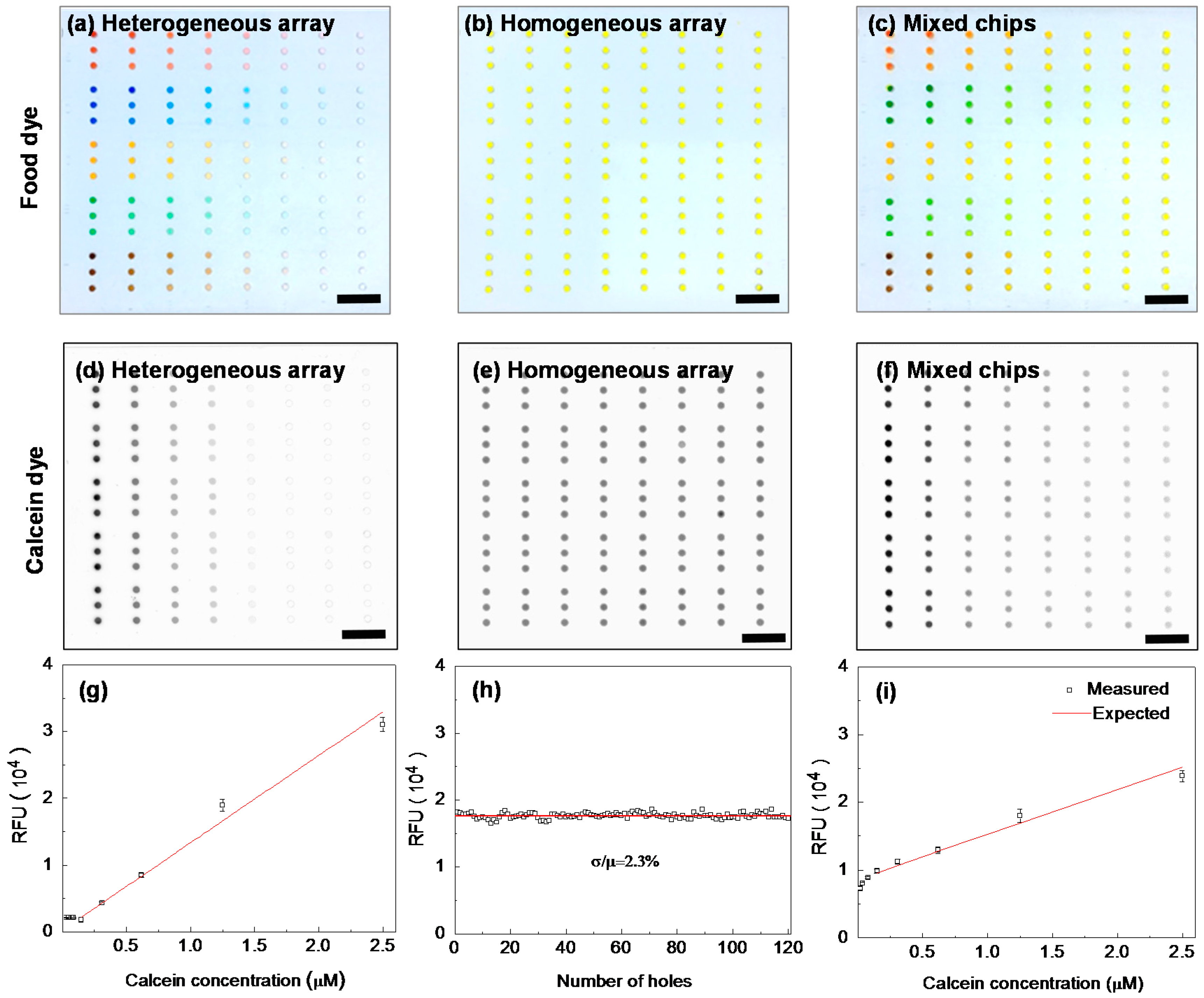
2.4. Device Validation
2.5. PfGCN5 Enzyme Purification
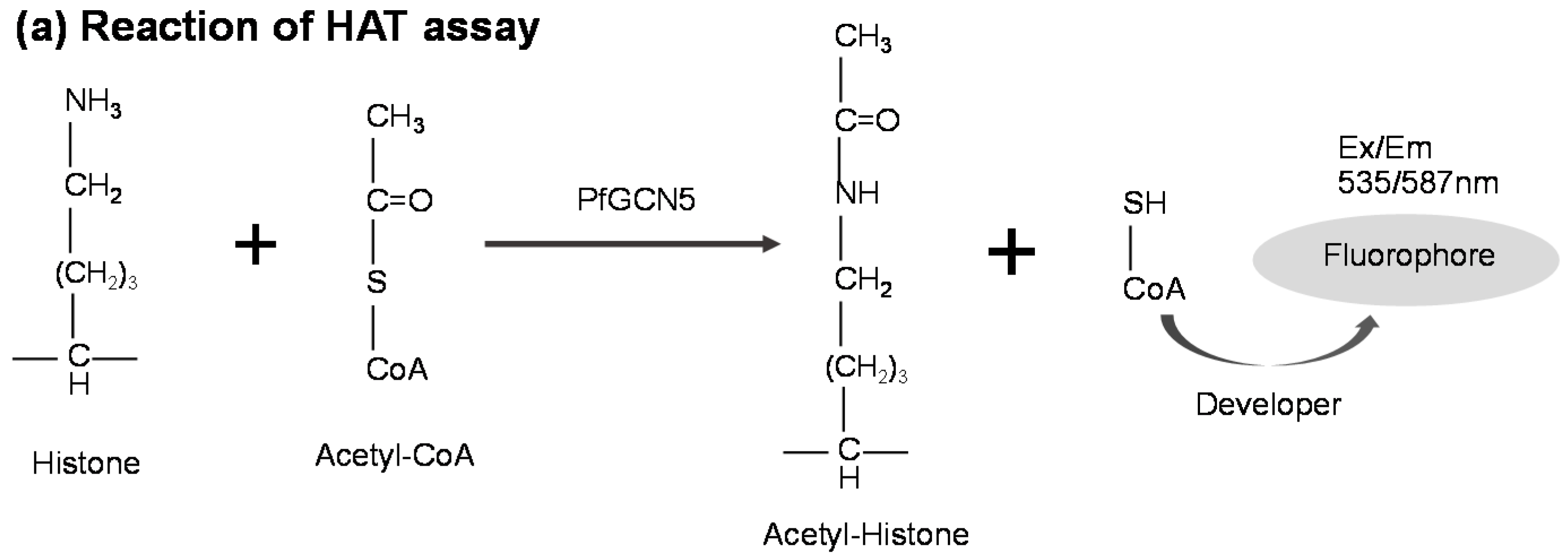
2.6. HAT Assay for PfGCN5-Based Drug Screening
3. Results and Discussion
3.1. Device Design Theoretical Considerations
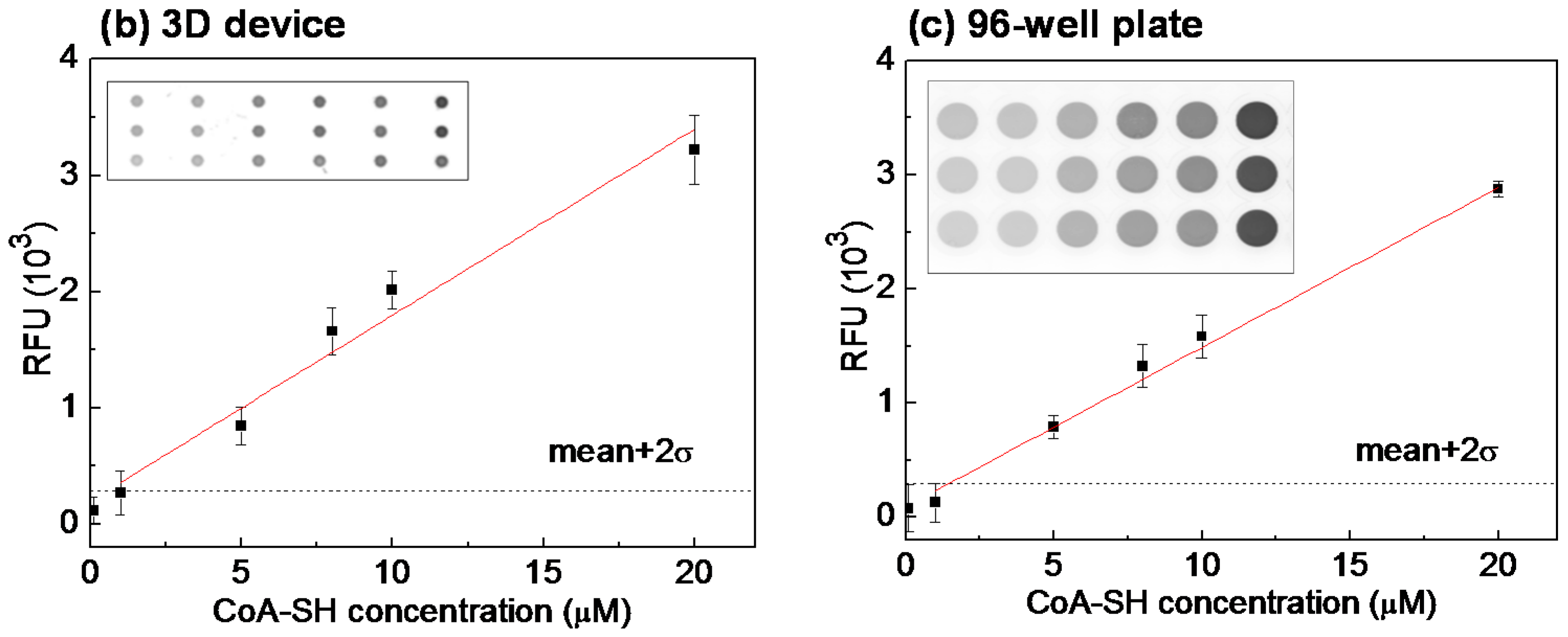
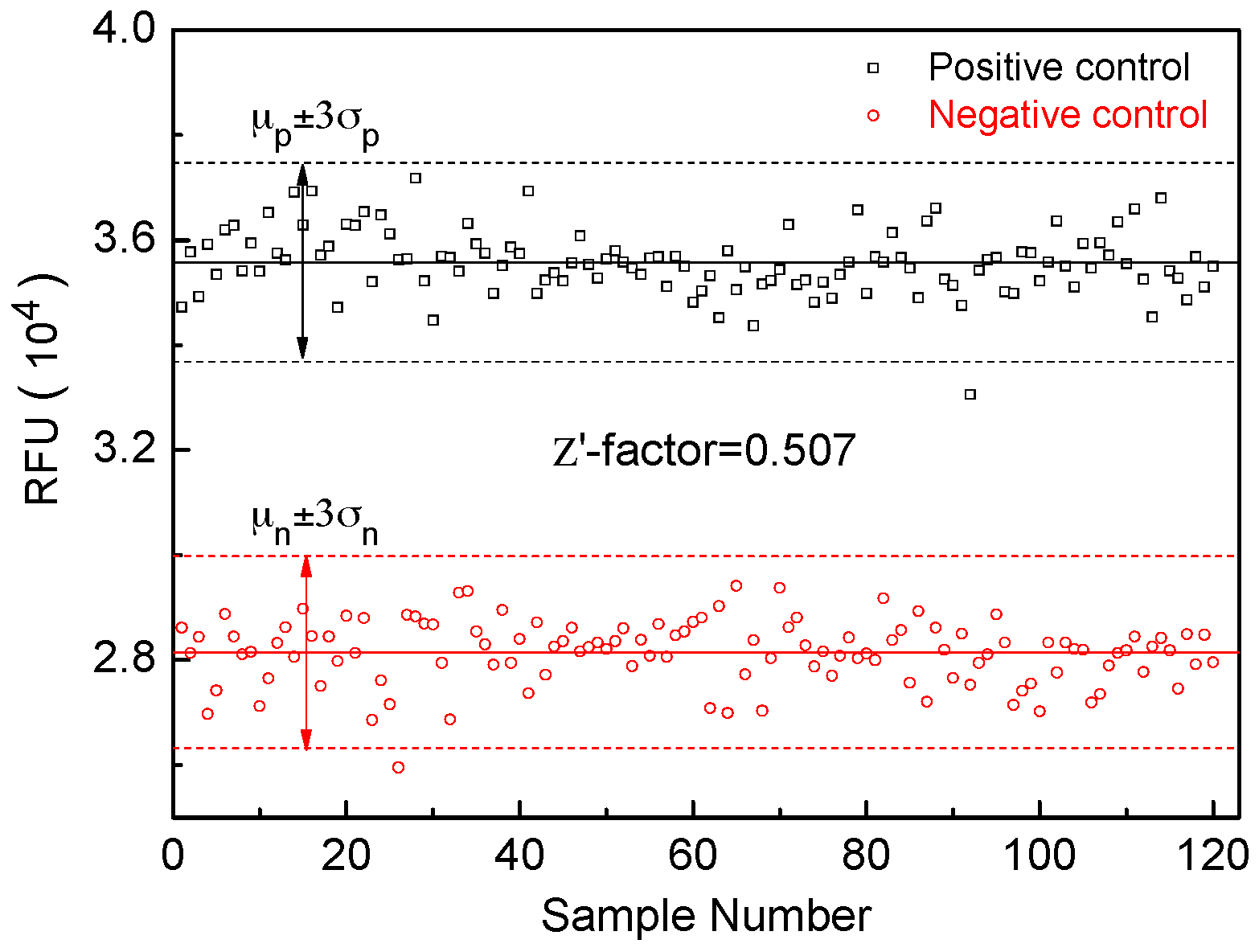
3.2. Quantitative Performances of the 3D Device
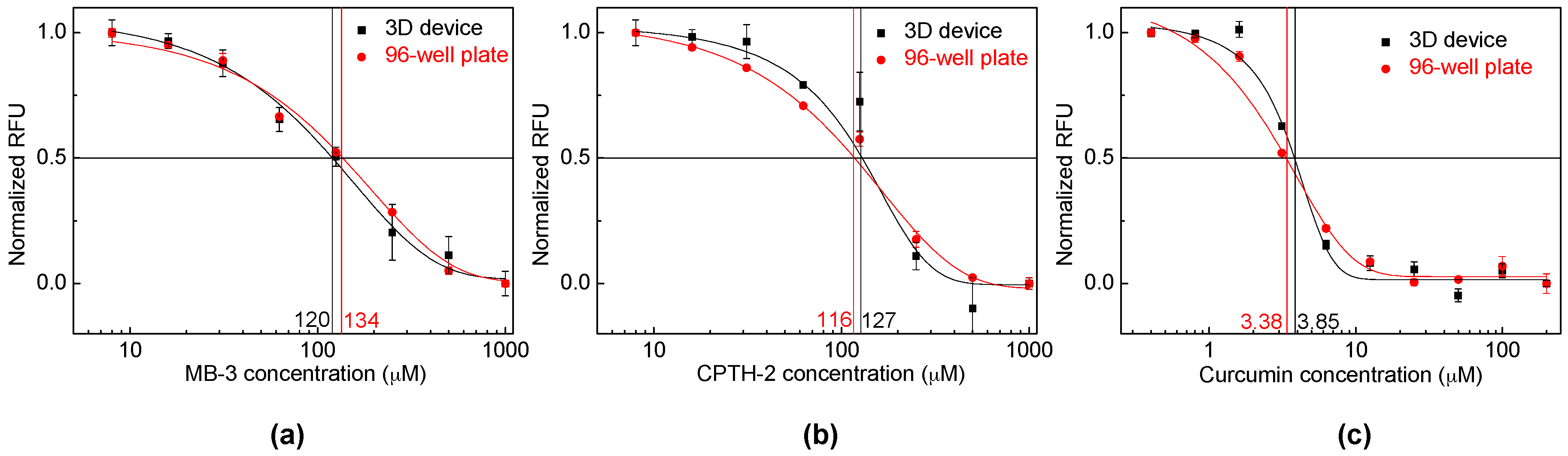
3.3. PfGCN5-Based Malaria Drug Screening
3.4. Versatility and Adaptability of the 3D Microfluidic Device
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, X.X.; Sha, S.S.; Liu, L.K.; Li, X.; Ma, Y.F. A 96-well microtiter plate assay for high-throughput screening of Mycobacterium tuberculosis dTDP-d-glucose 4,6-dehydratase inhibitors. Anal. Biochem. 2016, 498, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A novel colorimetric assay of beta-d-glucans in basidiomycete strains by alcian blue dye in a 96-well microtiter plate. Biotechnol. Prog. 2015, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Booij, P.; Vethaak, A.D.; Leonards, P.E.G.; Sjollema, S.B.; Kool, J.; de Voogt, P.; Lamoree, M.H. Identification of photosynthesis inhibitors of pelagic marine algae using 96-well plate microfractionation for enhanced throughput in effect-directed analysis. Environ. Sci. Technol. 2014, 48, 8003–8011. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.; Kavlock, R.; Martin, M.; Reif, D.; Houck, K.; Knudsen, T.; Richard, A.; Tice, R.R.; Whelan, M.; Xia, M.H.; et al. Perspectives on validation of high-throughput assays supporting 21st century toxicity testing. ALTEX 2013, 30, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Miller, N.; Huang, R.L.; Michael, S.; Itkin, M.; Kavlock, R.J.; Austin, C.P.; Shinn, P.; Simeonov, A.; Tice, R.R.; et al. The Tox21 robotic platform for the assessment of environmental chemicals—From vision to reality. Drug Discov. Today 2013, 18, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.K.; Gerger, M.J.; Balay, E.E.; O’Connell, R.; Hanson, S.; Daily, N.J.; Wakatsuki, T. Scalable 96-well plate based iPSC culture and production using a robotic liquid handling system. J. Vis. Exp. 2015, 99, e52755. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Fang, Q.; den Toonder, J.M. Microfluidics for cell-based high throughput screening platforms—A review. Anal. Chim. Acta 2016, 903, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Zhao, S.; Wang, J.; Liu, G.; Du, Y. Micro-scaffold array chip for upgrading cell-based high-throughput drug testing to 3D using benchtop equipment. Lab Chip 2014, 14, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Neuzi, P.; Giselbrecht, S.; Lange, K.; Huang, T.J.; Manz, A. Revisiting lab-on-a-chip technology for drug discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Li, L.; Nichols, K.P.; Ismagilov, R.F. SlipChip. Lab Chip 2009, 9, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Taylor, D.; Agrawal, N.; Wang, H.; Kim, H.; Han, A.; Rege, K.; Jayaraman, A. A programmable microfluidic cell array for combinatorial drug screening. Lab Chip 2012, 12, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Brown, A.C.; DiFeo, M.A.; Barker, T.H.; Lu, H. Continuously perfused, non-cross-contaminating microfluidic chamber array for studying cellular responses to orthogonal combinations of matrix and soluble signals. Lab Chip 2010, 10, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Kim, M.C.; Marquez, M.; Thorsen, T. High-density microfluidic arrays for cell cytotoxicity analysis. Lab Chip 2007, 7, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Kane, B.J.; Zinner, M.J.; Yarmush, M.L.; Toner, M. Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal. Chem. 2006, 78, 4291–4298. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.G.; Wu, Y.F.; Xu, Z.R.; Wang, J.H. A radial microfluidic concentration gradient generator with high-density channels for cell apoptosis assay. Lab Chip 2011, 11, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, S.; Sim, W.Y.; Kirn, S.B.; Jang, Y.H.; Lee, W.G.; Khabiry, M.; Bae, H.; Jambovane, S.; Hong, J.W.; Khademhosseini, A. Generating nonlinear concentration gradients in microfluidic devices for cell studies. Anal. Chem. 2011, 83, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Hattori, K.; Kanamori, T. Microfluidic serial dilution cell-based assay for analyzing drug dose response over a wide concentration range. Anal. Chem. 2010, 82, 8278–8282. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.N.; Qin, J.H.; Shi, W.W.; Liu, X.; Lin, B.C. Cell-based high content screening using an integrated microfluidic device. Lab Chip 2007, 7, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.H.; Ye, N.N.; Liu, X.; Lin, B.C. Microfluidic devices for the analysis of apoptosis. Electrophoresis 2005, 26, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.L.; Dertinger, S.K.W.; Chiu, D.T.; Choi, I.S.; Stroock, A.D.; Whitesides, G.M. Generation of solution and surface gradients using microfluidic systems. Langmuir 2000, 16, 8311–8316. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Baraban, L.; Bertholle, F.; Salverda, M.L.M.; Bremond, N.; Panizza, P.; Baudry, J.; de Visser, J.A.G.M.; Bibette, J. Millifluidic droplet analyser for microbiology. Lab Chip 2011, 11, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.; Doshi, A.; Kurup, G.K.; Ereifej, E.; Vandevord, P.J.; Basu, A.S. A modular approach for the generation, storage, mixing, and detection of droplet libraries for high throughput screening. Lab Chip 2010, 10, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Brouzes, E.; Medkova, M.; Savenelli, N.; Marran, D.; Twardowski, M.; Hutchison, J.B.; Rothberg, J.M.; Link, D.R.; Perrimon, N.; Samuels, M.L. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. USA 2009, 106, 14195–14200. [Google Scholar] [CrossRef] [PubMed]
- Clausell-Tormos, J.; Lieber, D.; Baret, J.C.; El-Harrak, A.; Miller, O.J.; Frenz, L.; Blouwolff, J.; Humphry, K.J.; Koster, S.; Duan, H.; et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 875. [Google Scholar] [CrossRef]
- Yan, X.; Wang, J.; Zhu, L.; Lowrey, J.J.; Zhang, Y.; Hou, W.; Dong, J.; Du, Y. A ready-to-use, versatile, multiplex-able three-dimensional scaffold-based immunoassay chip for high throughput hepatotoxicity evaluation. Lab Chip 2015, 15, 2634–2646. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Q.; Yi, X.; Xiao, K.; Li, S.; Kodzius, R.; Qin, J.H.; Wen, W.J. Wax-bonding 3D microfluidic chips. Lab Chip 2010, 10, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Chen, X.D.; Zhou, X.G.; Zhu, J.B.; Yu, Y.D. Droplet-in-oil array for picoliter-scale analysis based on sequential inkjet printing. Lab Chip 2015, 15, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, S.; Evans, S.R.; Aguilar, Z.P.; Mosbach, M.; Fritsch, I.; Schuhmann, W. Analysis in ultrasmall volumes: Microdispensing of picoliter droplets and analysis without protection from evaporation. Anal. Chem. 2004, 76, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.W.; Miao, J. Chromatin-mediated epigenetic regulation in the malaria parasite plasmodium falciparum. Eukaryot. Cell 2010, 9, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- LaCount, D.J.; Vignali, M.; Chettier, R.; Phansalkar, A.; Bell, R.; Hesselberth, J.R.; Schoenfeld, L.W.; Ota, I.; Sahasrabudhe, S.; Kurschner, C.; et al. A protein interaction network of the malaria parasite Plasmodium falciparum. Nature 2005, 438, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.J.; van den Bosch, T.; Martin, N.I. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov. Today 2014, 19, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bizzarri, B.; Maccioni, E.; Secci, D.; Bolasco, A.; Chimenti, P.; Fioravanti, R.; Granese, A.; Carradori, S.; Tosi, F.; et al. A novel histone acetyltransferase inhibitor modulating Gcn5 Network: Cyclopentylidene-[4-(4′-chlorophenyl)thiazol-2-yl)hydrazone. J. Med. Chem. 2009, 52, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Miao, J.; Furuya, T.; Fan, Q.; Li, X.; Rathod, P.K.; Su, X.Z.; Cui, L. Histone acetyltransferase inhibitor anacardic acid causes changes in global gene expression during in vitro Plasmodium falciparum development. Eukaryot. Cell 2008, 7, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Fan, Q.; Cui, L.; Li, X.L.; Wang, H.Y.; Ning, G.; Reese, J.C.; Cui, L.W. The MYST family histone acetyltransferase regulates gene expression and cell cycle in malaria parasite Plasmodium falciparum. Mol. Microbiol. 2010, 78, 883–902. [Google Scholar] [CrossRef] [PubMed]
- Fidock, D.A.; Wellems, T.E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 1997, 94, 10931–10936. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Li, J.F.; Fan, Q.; Li, X.L.; Li, X.Y.; Cui, L.W. The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J. Cell. Sci. 2010, 123, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Secci, D.; Carradori, S.; Bizzarri, B.; Bolasco, A.; Ballario, P.; Patramani, Z.; Fragapane, P.; Vernarecci, S.; Canzonetta, C.; Filetici, P. Synthesis of a novel series of thiazole-based histone acetyltransferase inhibitors. Bioorgan. Med. Chem. 2014, 22, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Singh, A.; Singh, A.; Pathak, L.P.; Shrivastava, N.; Tripathi, P.K.; Singh, M.P.; Singh, K. Inhibition of P. falciparum PFATP6 by curcumin and its derivatives: A bioinformatic study. Cell Mol. Biol. 2012, 58, 182–186. [Google Scholar] [PubMed]
- Kurogi, K.; Yan, H.; Tsujii, K. Importance of pinning effect of wetting in super water-repellent surfaces. Colloid Surface A 2008, 317, 592–597. [Google Scholar] [CrossRef]
- Gao, L.C.; McCarthy, T.J. Contact angle hysteresis explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.F.; Huh, C.; Mason, S.G. Resistance to spreading of liquids by sharp edges. J. Colloid Interface Sci. 1977, 59, 568–581. [Google Scholar] [CrossRef]
- Fowkes, F.M.; Harkins, W.D. The state of monolayers adsorbed at the interface solid-aqueous solution. J. Am. Chem. Soc. 1940, 62, 3377–3386. [Google Scholar] [CrossRef]
- Rodriguez-Valverde, M.A.; Tirado Miranda, M. Derivation of Jurin’s law revisited. Eur. J. Phys. 2011, 32, 49–54. [Google Scholar] [CrossRef]
- You, L.Y.; Nie, J.Y.; Sun, W.J.; Zheng, Z.Q.; Yang, X.J. Lysine acetylation: Enzymes, bromodomains and links to different diseases. Essays Biochem. 2012, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Li, W.; Choi, G.; Yang, X.; Miao, J.; Cui, L.; Guan, W. Arbitrarily Accessible 3D Microfluidic Device for Combinatorial High-Throughput Drug Screening. Sensors 2016, 16, 1616. https://doi.org/10.3390/s16101616
Chen Z, Li W, Choi G, Yang X, Miao J, Cui L, Guan W. Arbitrarily Accessible 3D Microfluidic Device for Combinatorial High-Throughput Drug Screening. Sensors. 2016; 16(10):1616. https://doi.org/10.3390/s16101616
Chicago/Turabian StyleChen, Zhuofa, Weizhi Li, Gihoon Choi, Xiaonan Yang, Jun Miao, Liwang Cui, and Weihua Guan. 2016. "Arbitrarily Accessible 3D Microfluidic Device for Combinatorial High-Throughput Drug Screening" Sensors 16, no. 10: 1616. https://doi.org/10.3390/s16101616
APA StyleChen, Z., Li, W., Choi, G., Yang, X., Miao, J., Cui, L., & Guan, W. (2016). Arbitrarily Accessible 3D Microfluidic Device for Combinatorial High-Throughput Drug Screening. Sensors, 16(10), 1616. https://doi.org/10.3390/s16101616




