High Frequency Sampling of TTL Pulses on a Raspberry Pi for Diffuse Correlation Spectroscopy Applications
Abstract
:1. Introduction
1.1. Diffuse Correlation Spectroscopy
1.2. Raspberry Pi
2. Experimental Section
2.1. DCS Instrumentation
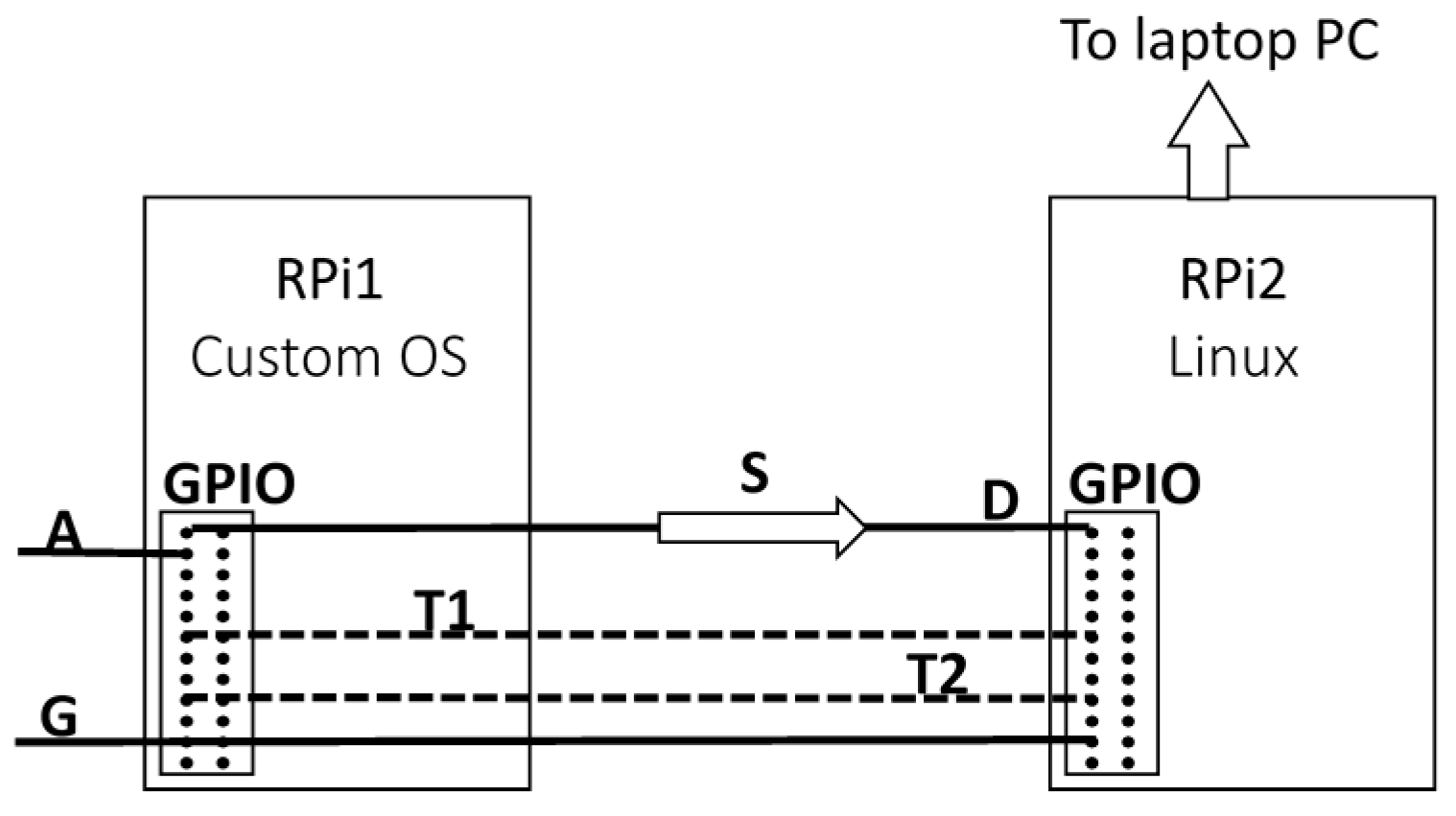
2.2. Acquisition of an Input Digital Signal Using the Raspberry Pi
2.3. Software Autocorrelation
2.4. Pump-Controlled Flow Experiments
2.5. In Vivo Cuff-Occlusion Experiments
2.6. Processing and Quantification of Acquired Autocorrelation Signals
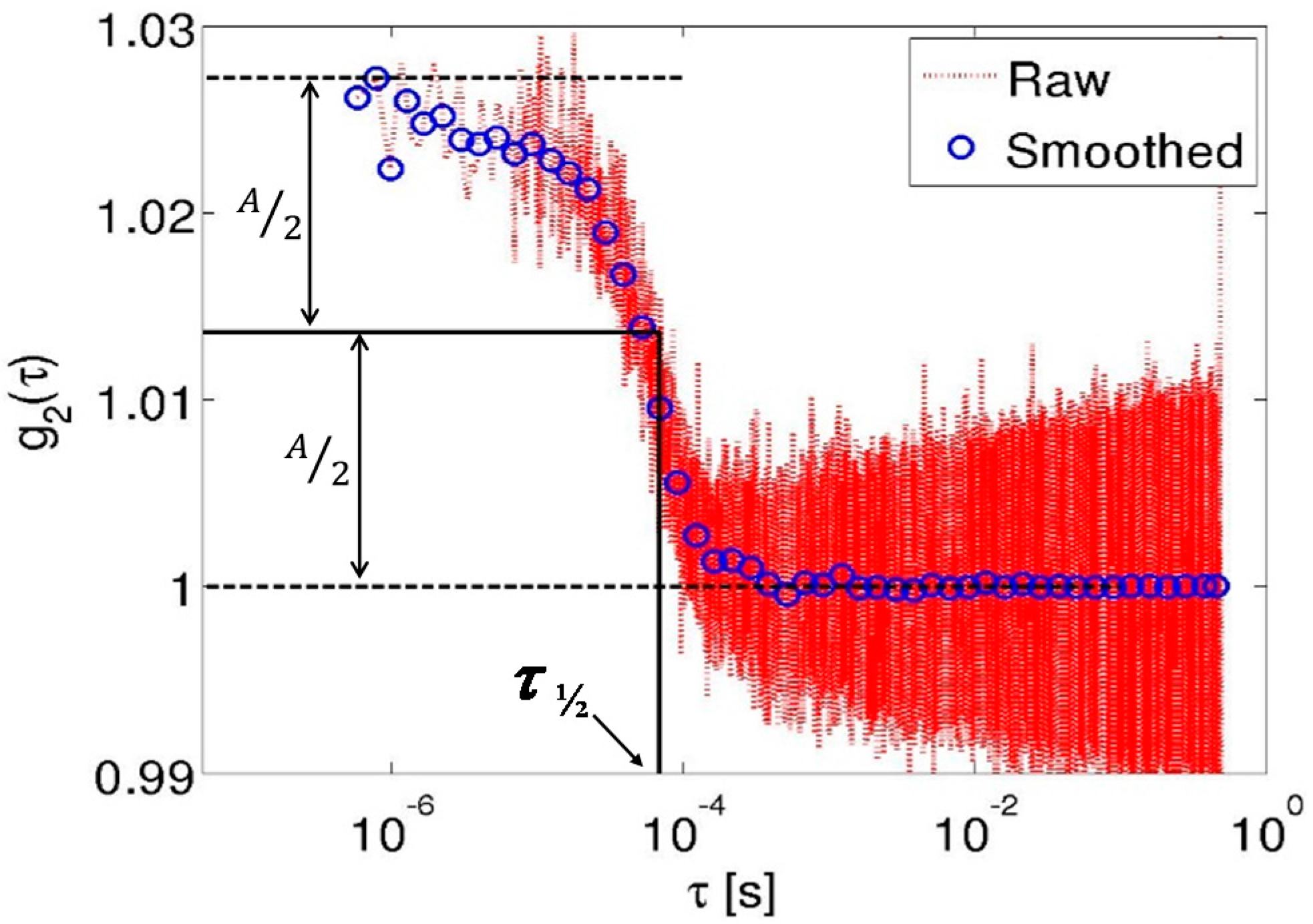
3. Results and Discussion
3.1. Pump-Controlled Flow Experiments
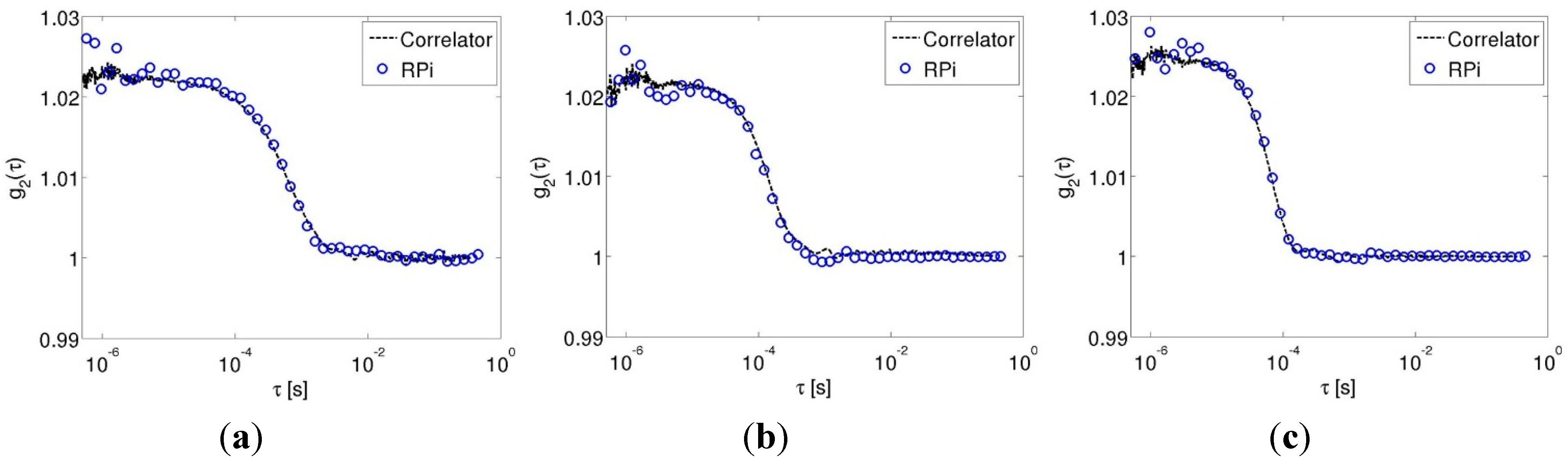
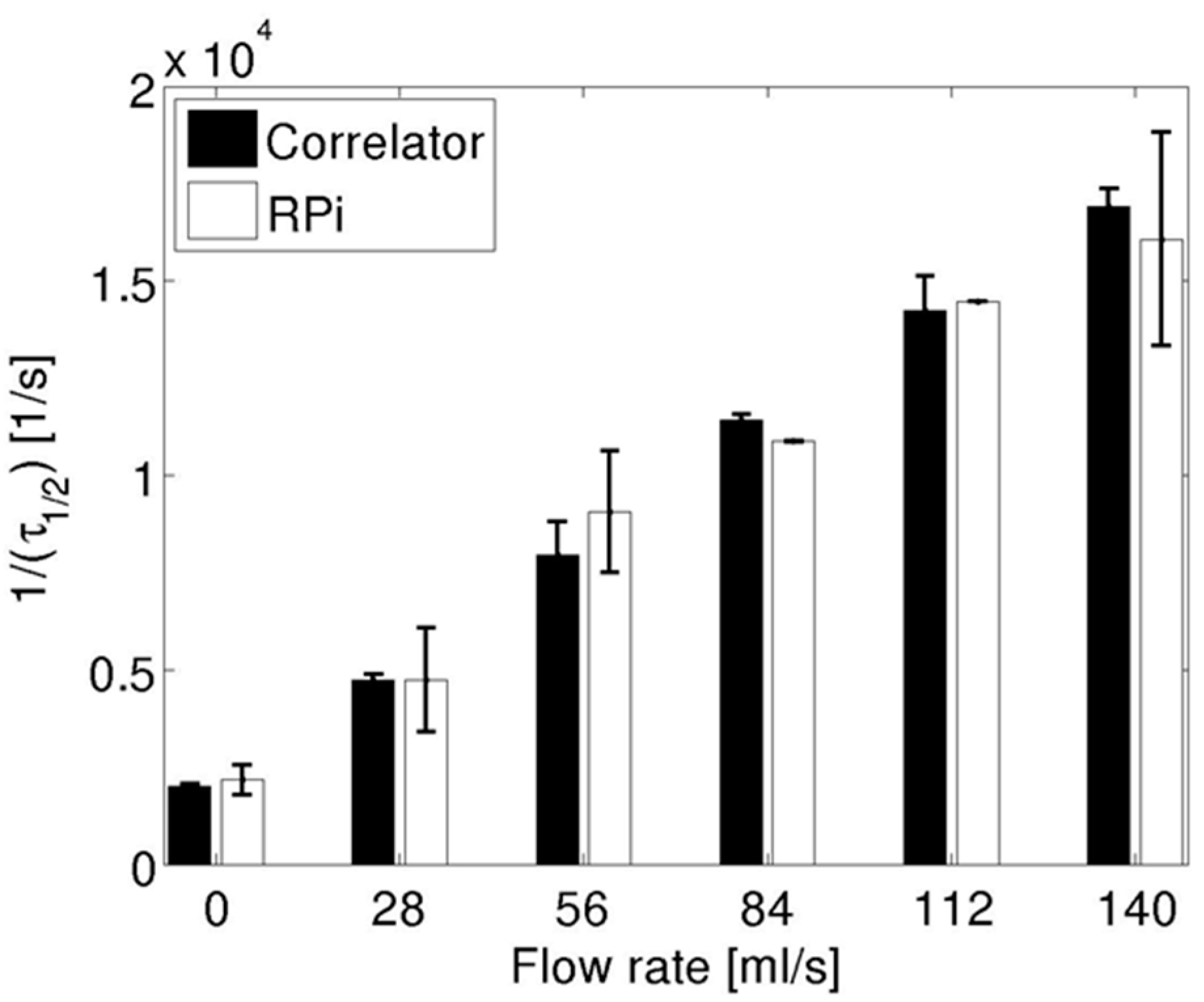
3.2. Cuff-Occlusion Experiments
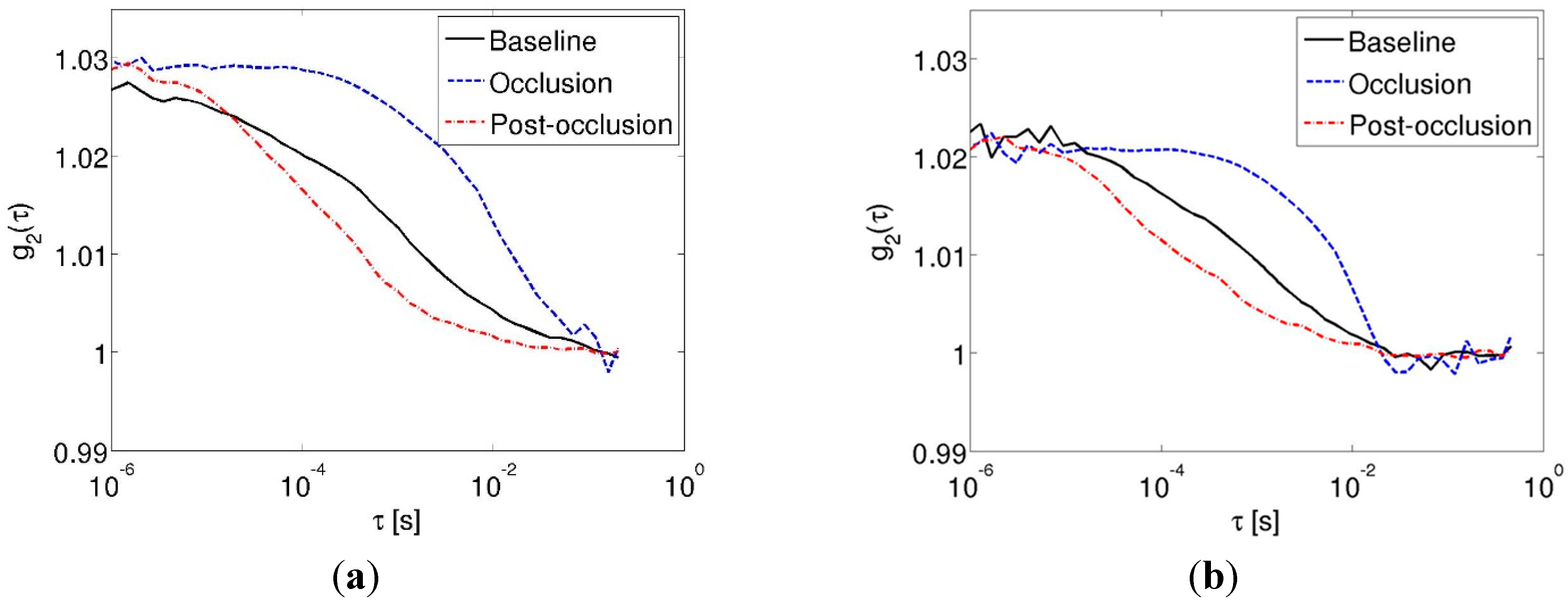
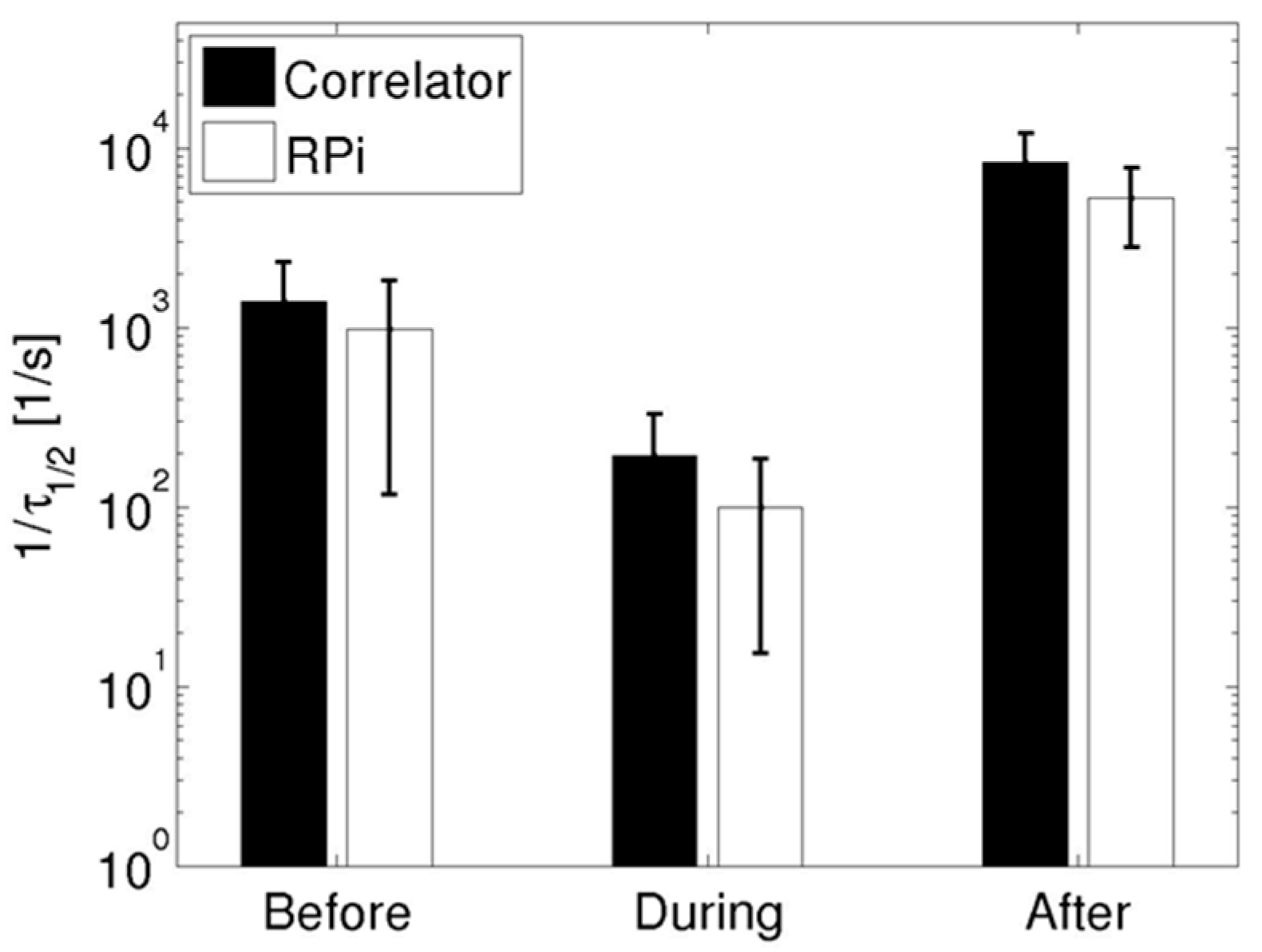
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
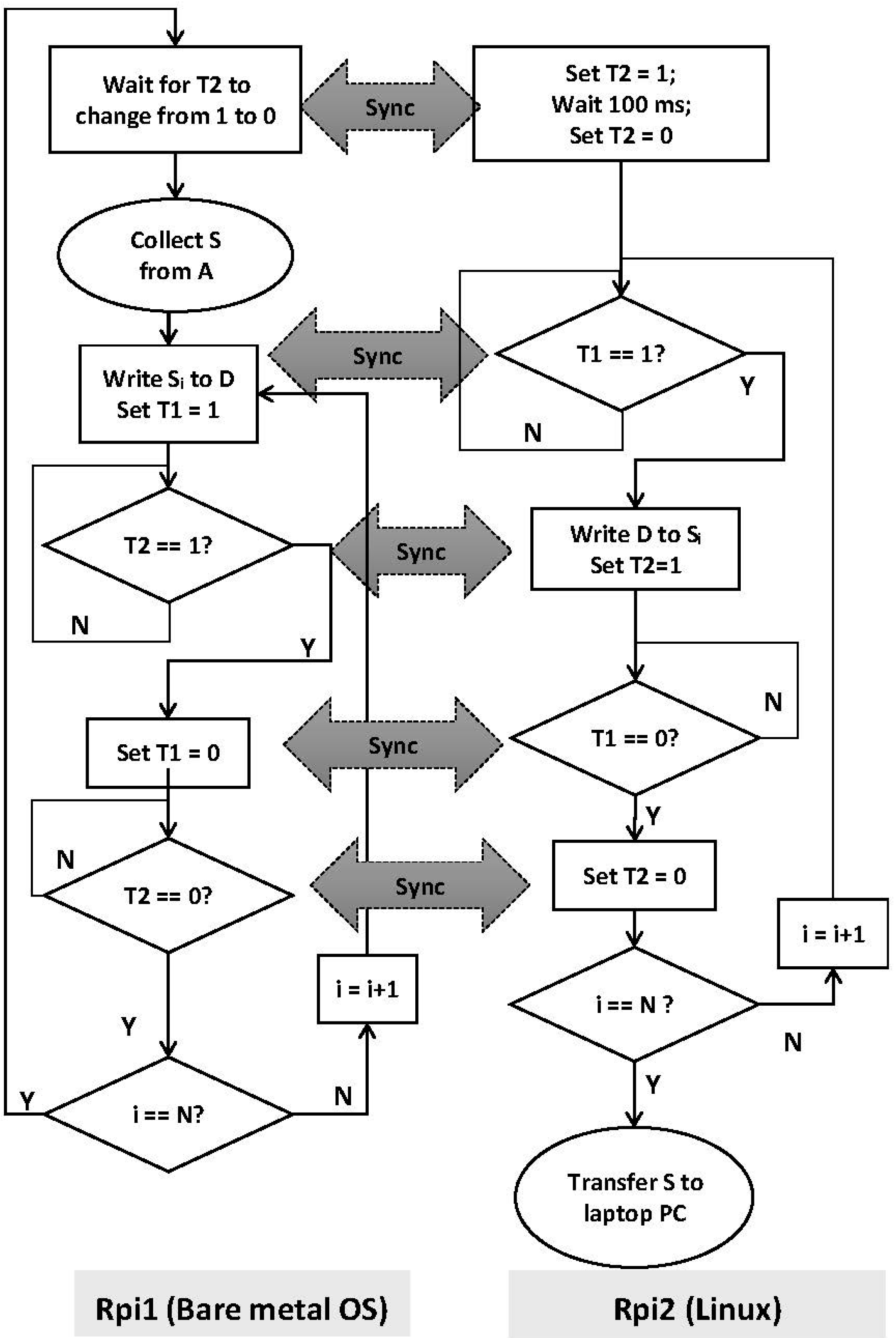
Initialize pins A (17) and T2 (23) as input
Initialize pins D (22) and T1 (24) as output
Initialize CPU cycle counter
main:
wait1_for_rpi2:
if (pin T2 is LOW) {go to wait1_for_rpi2}
wait2_for_rpi2:
if (pin T2 is HIGH) {go to wait2_for_rpi2}
ti = read CPU cycle counter
i = 1
collect_signal:
read GPIO state and store to S[i]
i = i + 1
if (i < N + 1) {go to collect_signal}
tf = read CPU cycle counter
store (tf – ti) to S[0]
i = 0
j = 0
transfer:
if (i == 0) {
d = jth bit of S[0]
j = j + 1
if (j == 32) {i = i + 1}
}
if (i > 0) {
d = Value of Pin A from S[i]
i = i + 1
}
set pin D to 0
if (d = 1) {set pin D to 1}
set T1 to HIGH
handshake1:
if (T2 = LOW) {go to handshake1}
set T1 to LOW
handshake2:
if (T2 = HIGH) {go to handshake2}
if (i < N) {go to transfer}
if {i = N} {go to main}
References
- Boas, D.A.; Campbell, L.E.; Yodh, A.G. Scattering and imaging with diffusing temporal field correlations. Phys. Rev. Lett. 1995, 75, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Yodh, A.G. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 192–215. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, X.; Daugherty, A.; Shin, H.; Yu, G. Noninvasive quantification of postocclusive reactive hyperemia in mouse thigh muscle by near-infrared diffuse correlation spectroscopy. Appl. Opt. 2013, 52, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhao, Y.; Cheng, R.; Dong, L.; Irwin, D.; Yu, G. Portable optical tissue flow oximeter based on diffuse correlation spectroscopy. Opt. Lett. 2009, 34, 3556–3558. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Floyd, T.F.; Durduran, T.; Zhou, C.; Wang, J.; Detre, J.A.; Yodh, A.G. Validation of diffuse correlation spectroscopy for muscle blood flow with concurrent arterial spin labeled perfusion MRI. Opt. Express 2007, 15, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Culver, J.P.; Takahashi, K.; Greenberg, J.H.; Yodh, A.G. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys. Med. Biol. 2001, 46, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, L.; Desjardins, M.; Jehanne-Lacasse, J.; Bherer, L.; Lesage, F. Investigation of diffuse correlation spectroscopy in multi-layered media including the human head. Opt. Express 2008, 16, 15514–15530. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.C.; Schenkel, S.S.; Minkoff, D.L.; Lu, X.; Favilla, C.G.; Vora, P.M.; Busch, D.R.; Chandra, M.; Greenberg, J.H.; Detre, J.A.; et al. Influence of probe pressure on the diffuse correlation spectroscopy blood flow signal: Extra-cerebral contributions. Biomed. Opt. Express 2013, 4, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Verdecchia, K.; Lee, T.Y.; St Lawrence, K. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed. Opt. Express 2012, 3, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, K.; Diop, M.; Lee, T.Y.; St Lawrence, K. Quantifying the cerebral metabolic rate of oxygen by combining diffuse correlation spectroscopy and time-resolved near-infrared spectroscopy. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Durduran, T.; Zhou, C.; Cheng, R.; Yodh, A.G. Near-infrared diffuse correlation spectroscopy (dcs) for assessment of tissue blood flow. In Handbook of Biomedical Optics; Boas, D.A., Pitris, C., Ramanujam, N., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 195–216. [Google Scholar]
- Carp, S.A.; Dai, G.P.; Boas, D.A.; Franceschini, M.A.; Kim, Y.R. Validation of diffuse correlation spectroscopy measurements of rodent cerebral blood flow with simultaneous arterial spin labeling mri; towards mri-optical continuous cerebral metabolic monitoring. Biomed. Opt. Express 2010, 1, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Near-infrared diffuse correlation spectroscopy in cancer diagnosis and therapy monitoring. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Carp, S.A.; Roche-Labarbe, N.; Franceschini, M.A.; Srinivasan, V.J.; Sakadzic, S.; Boas, D.A. Due to intravascular multiple sequential scattering, diffuse correlation spectroscopy of tissue primarily measures relative red blood cell motion within vessels. Biomed. Opt. Express 2011, 2, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Raspberry Pi foundation. Available online: http://www.raspberrypi.org/ (accessed on 29 July 2015).
- Broadcom. BCM2835 arm peripherals. Available online: https://www.raspberrypi.org/wp-content/uploads/2012/02/BCM2835-ARM-Peripherals.pdf (accessed on 10 August 2015).
- Vishwanath, K.; Gurjar, R.; Kuo, S.; Fasi, A.; Roderick, K.; Feinberg, S.E.; Wolf, D.E. Sensing vascularization of ex vivo produced oral mucosal equivalent (evpome) skin grafts in nude mice using optical spectroscopy. In Proceedings of the SPIE Photonics West, San Francisco, CA, USA, 4 March 2014; pp. 8917–8926.
- Correlator.com. Available online: www.correlator.com (accessed on 10 August 2015).
- Tivnan, M. Rpi_autocorrelator. Available online: https://github.com/tivnanm/rpi_autocorrelator (accessed on 29 July 2015).
- Dong, J.; Bi, R.; Ho, J.H.; Thong, P.S.; Soo, K.C.; Lee, K. Diffuse correlation spectroscopy with a fast fourier transform-based software autocorrelator. J. Biomed. Opt. 2012, 17, 97001–97004. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Dong, J.; Lee, K. Multi-channel deep tissue flowmetry based on temporal diffuse speckle contrast analysis. Opt. Express 2013, 21, 22854–22861. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Dong, J.; Lee, K. Deep tissue flowmetry based on diffuse speckle contrast analysis. Opt. Lett. 2013, 38, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Frigo, M.; Johnson, S.G. The design and implementation of fftw3. IEEE Proc. 2005, 93, 216–231. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tivnan, M.; Gurjar, R.; Wolf, D.E.; Vishwanath, K. High Frequency Sampling of TTL Pulses on a Raspberry Pi for Diffuse Correlation Spectroscopy Applications. Sensors 2015, 15, 19709-19722. https://doi.org/10.3390/s150819709
Tivnan M, Gurjar R, Wolf DE, Vishwanath K. High Frequency Sampling of TTL Pulses on a Raspberry Pi for Diffuse Correlation Spectroscopy Applications. Sensors. 2015; 15(8):19709-19722. https://doi.org/10.3390/s150819709
Chicago/Turabian StyleTivnan, Matthew, Rajan Gurjar, David E. Wolf, and Karthik Vishwanath. 2015. "High Frequency Sampling of TTL Pulses on a Raspberry Pi for Diffuse Correlation Spectroscopy Applications" Sensors 15, no. 8: 19709-19722. https://doi.org/10.3390/s150819709
APA StyleTivnan, M., Gurjar, R., Wolf, D. E., & Vishwanath, K. (2015). High Frequency Sampling of TTL Pulses on a Raspberry Pi for Diffuse Correlation Spectroscopy Applications. Sensors, 15(8), 19709-19722. https://doi.org/10.3390/s150819709





