PEG Functionalization of Whispering Gallery Mode Optical Microresonator Biosensors to Minimize Non-Specific Adsorption during Targeted, Label-Free Sensing
Abstract
:1. Introduction
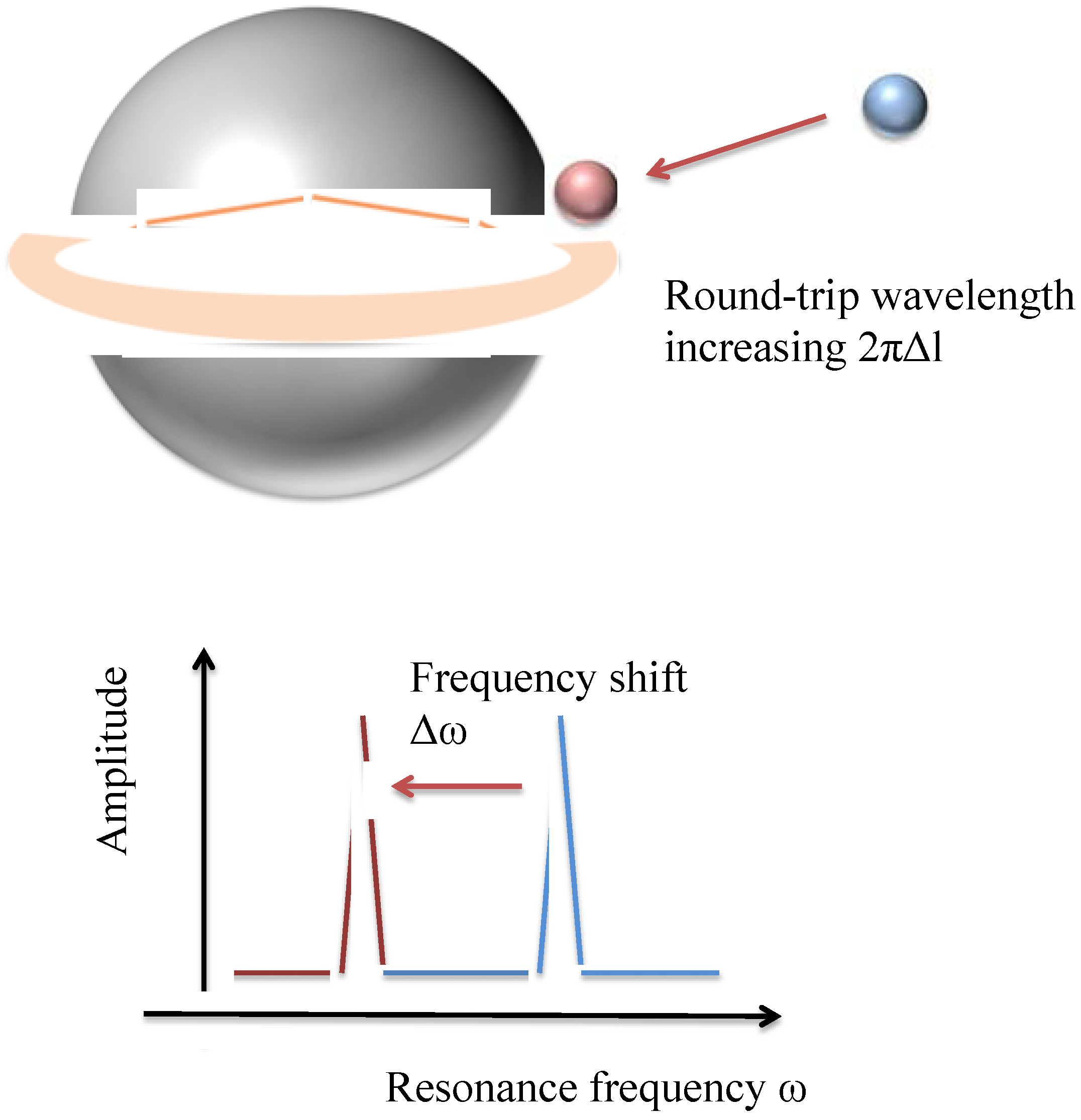
2. Experimental Section
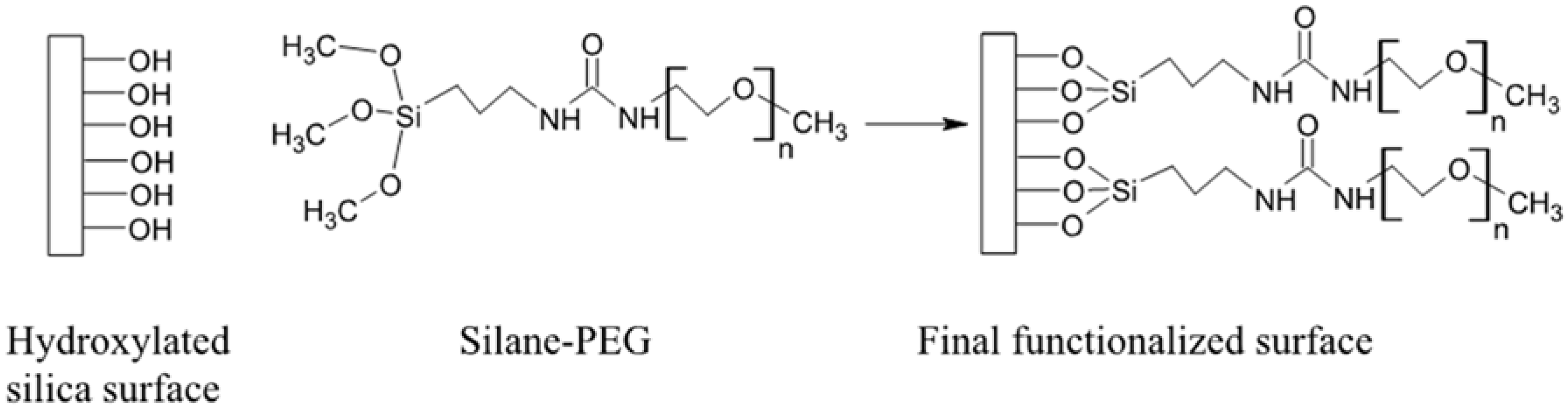
2.1. Synthesis and Characterization of Functionalized (100) Silica on Silicon Wafers
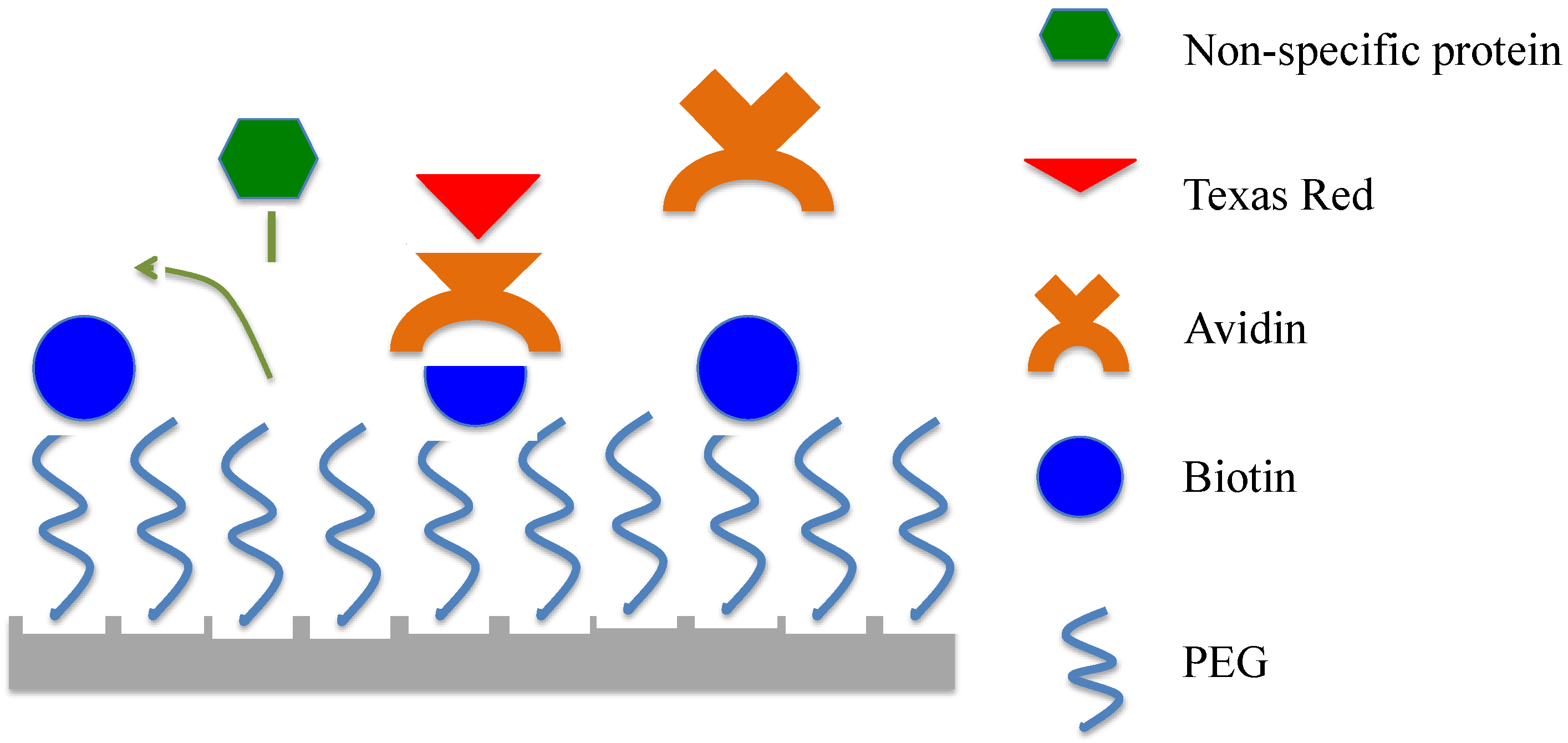
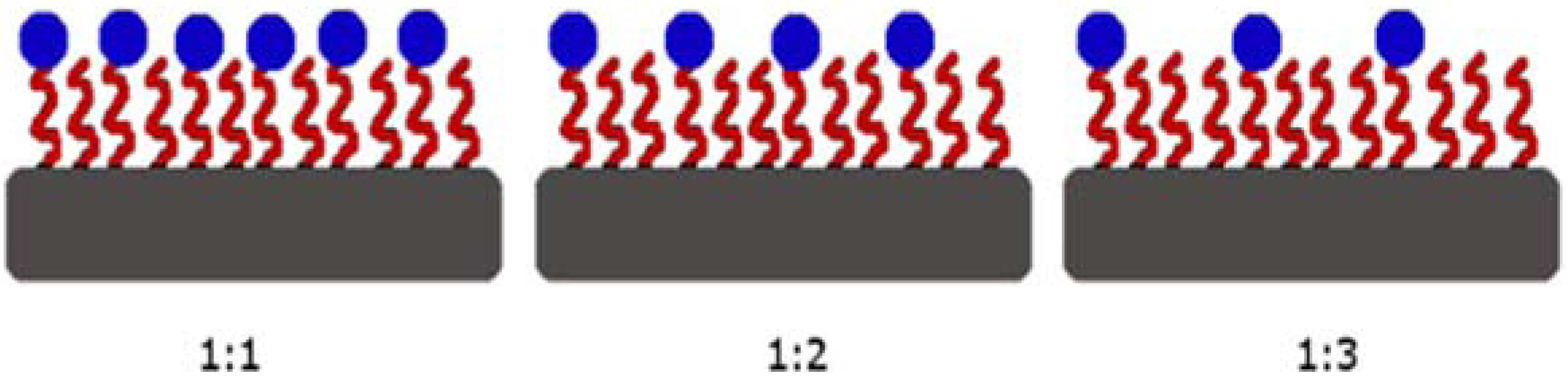
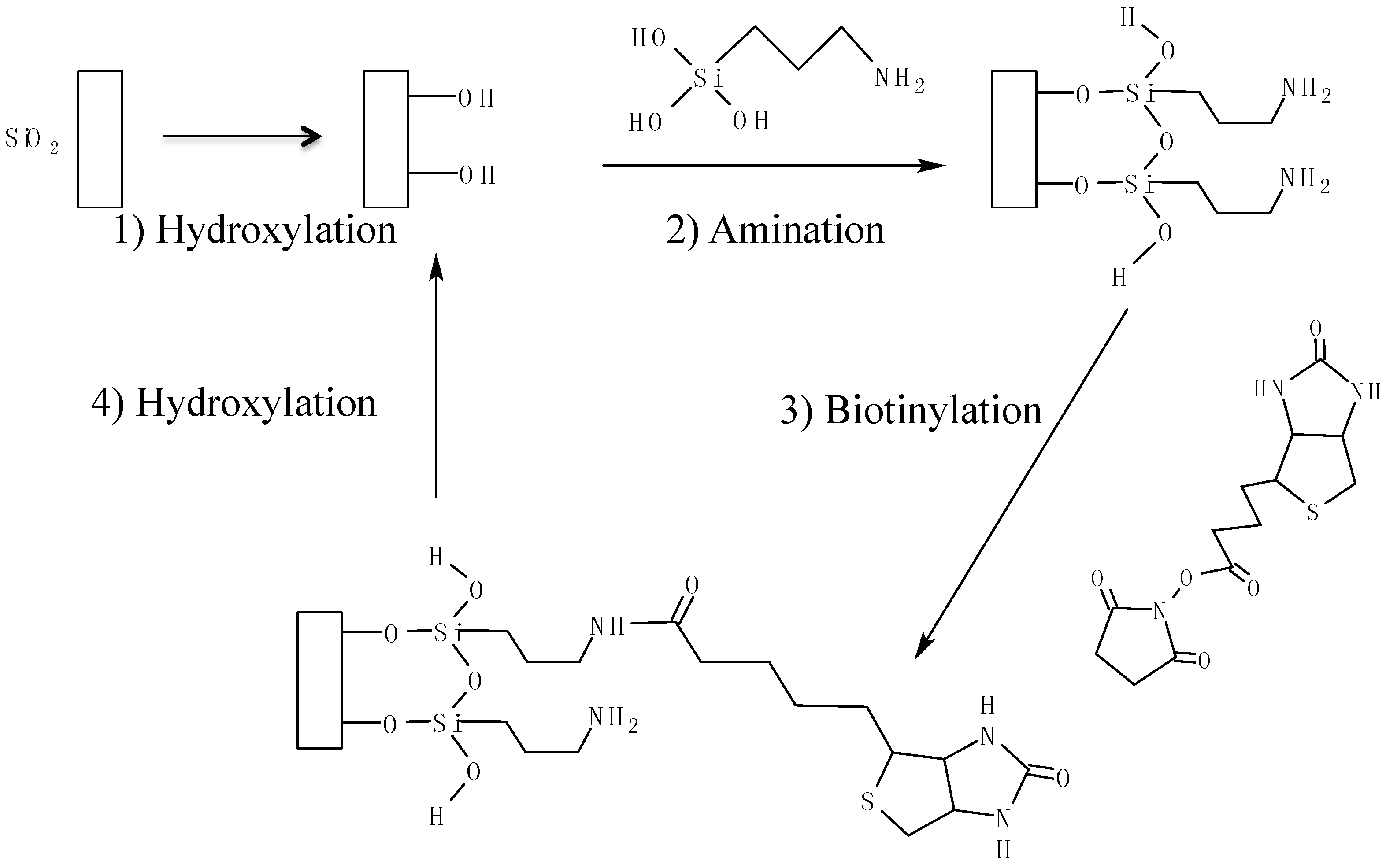
2.2. Device Fabrication, Functionalization, and Surface Characterization
2.3. Device Characterization
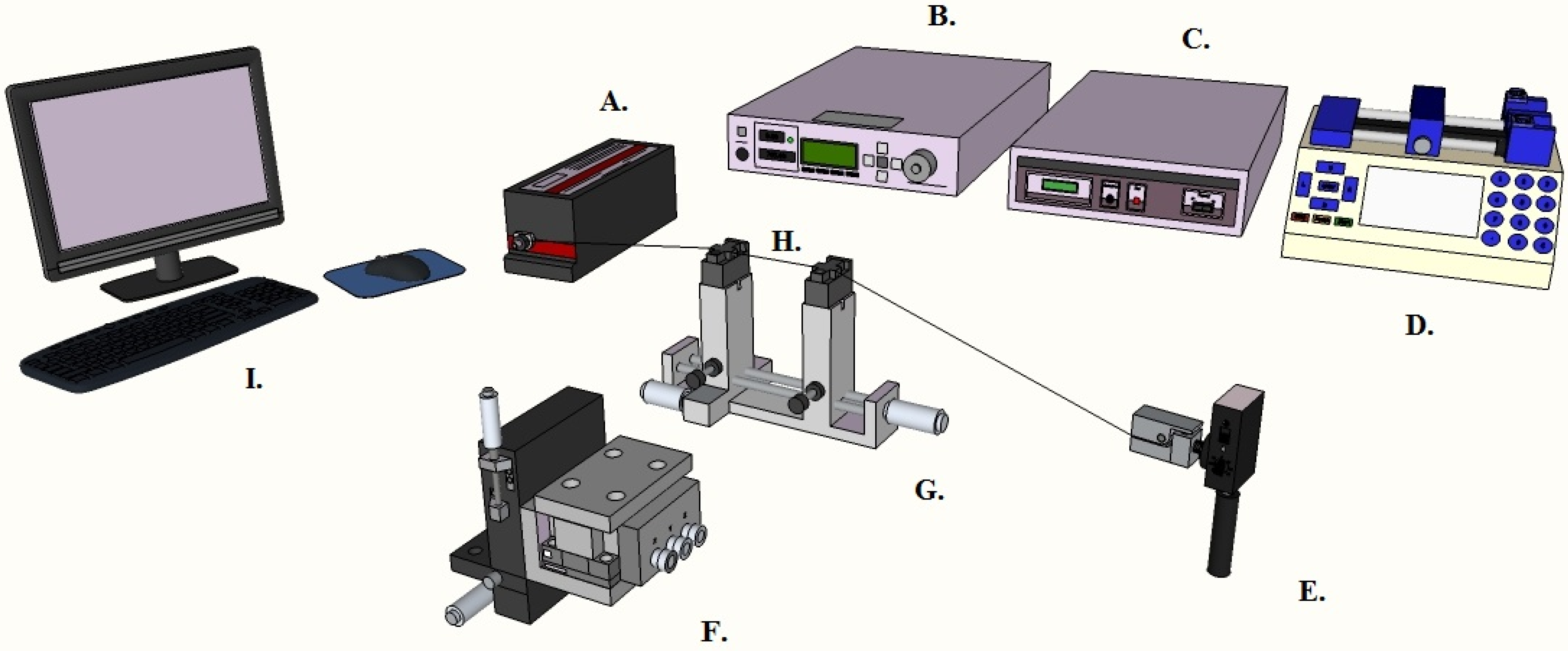
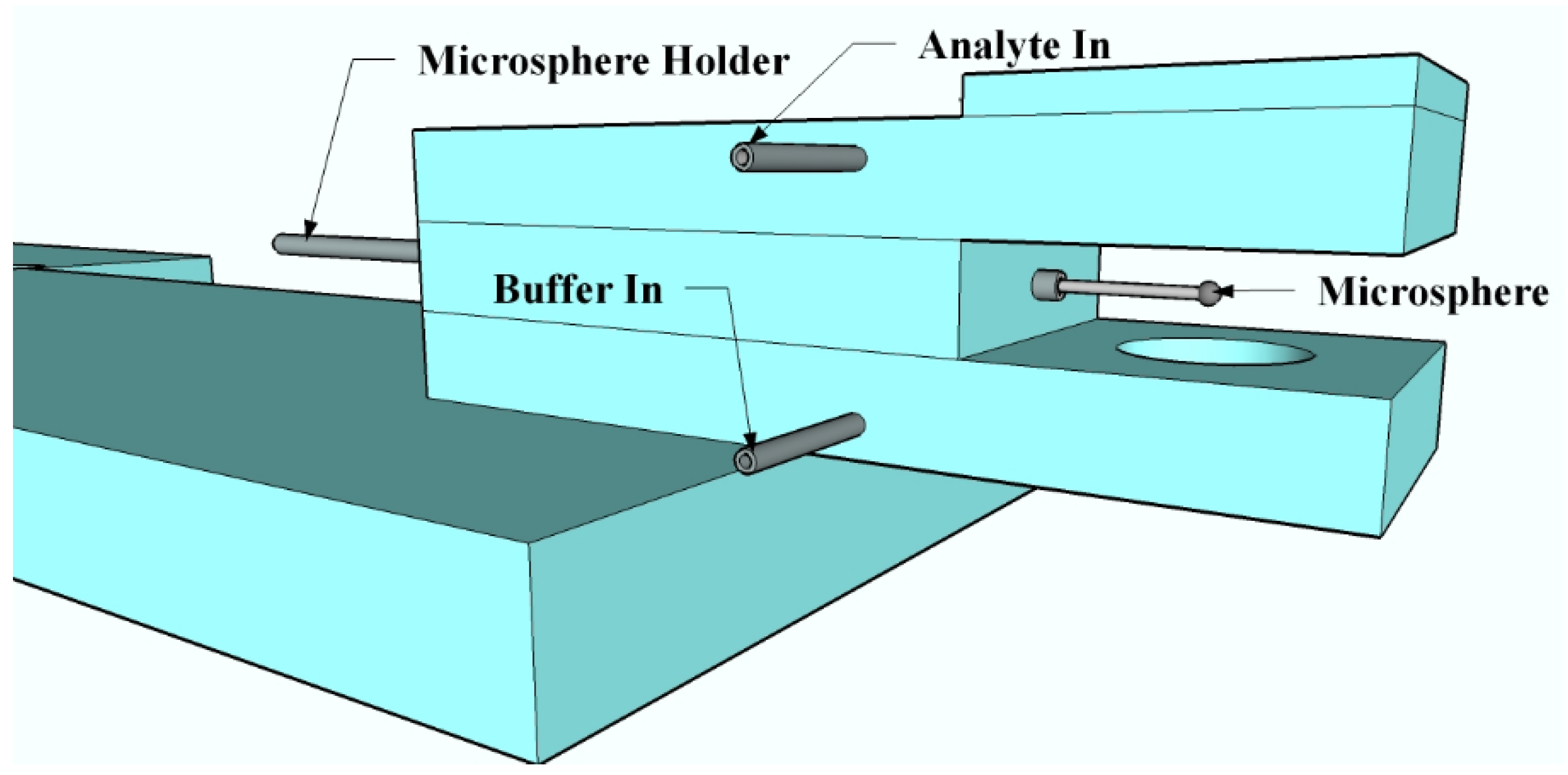
2.4. Sensing
3. Results and Discussion
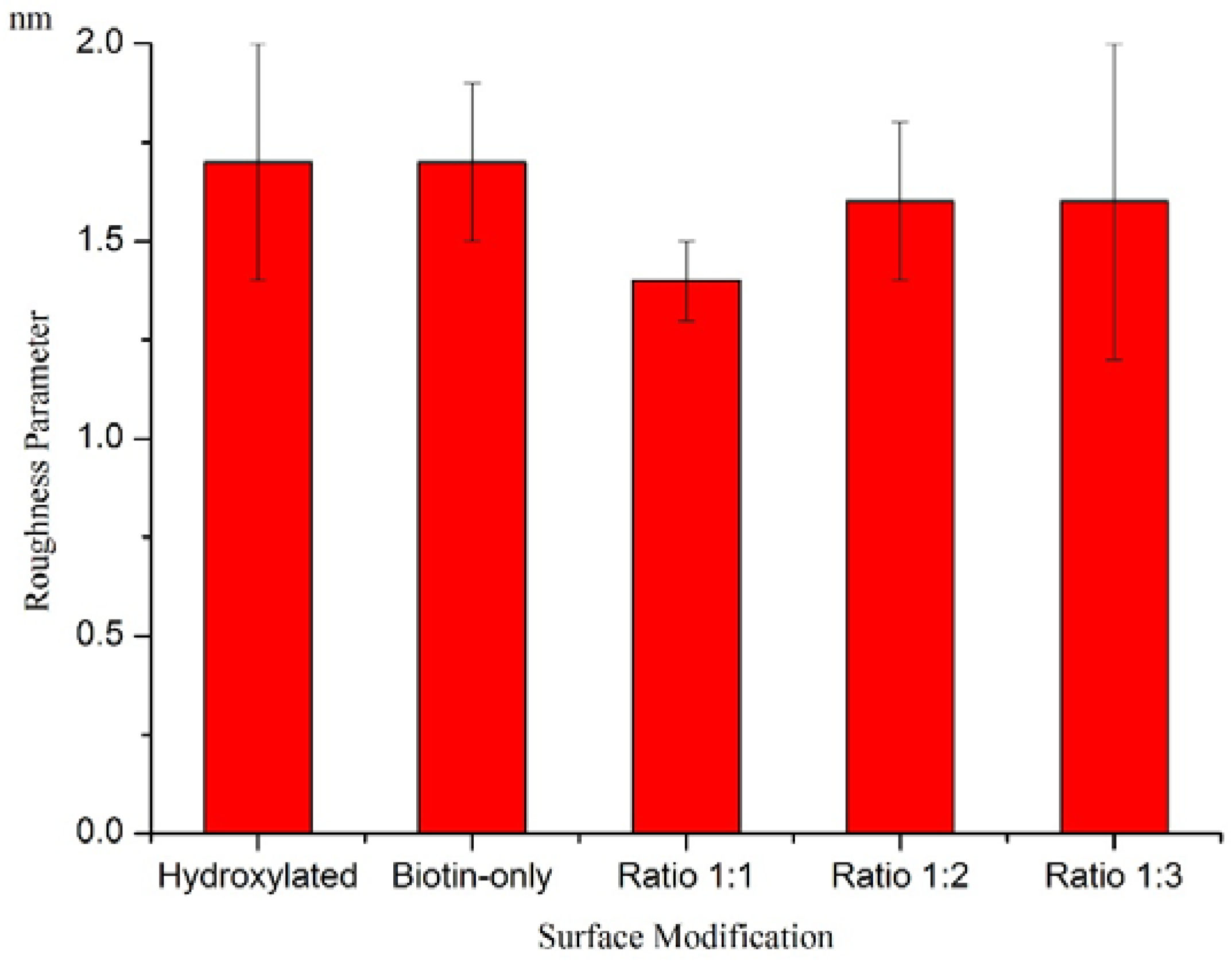
Characterization of Functionalized Wafers
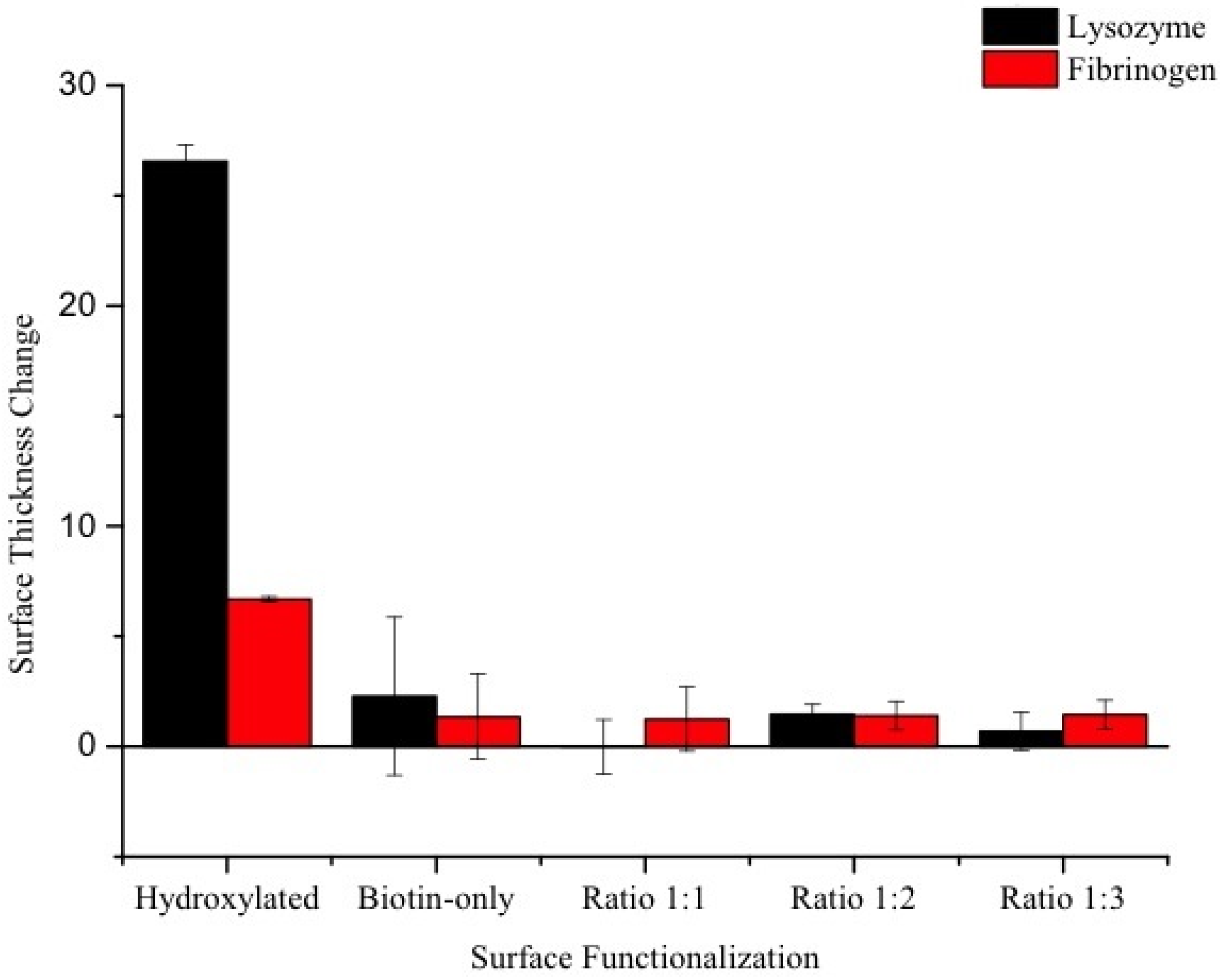
| Contact Angle Measurement | |||
|---|---|---|---|
| Pre-Adsorption | Post-Adsorption (L) | Post-Adsorption (F) | |
| Hydroxylated | 65.8 ± 5.0 | 129.2 ± 5.7 | 90.3 ± 3.5 |
| Biotin-only | 77.0 ± 5.7 | 73.1 ± 4.6 | 89.2 ± 4.6 |
| Ratio 1:1 | 62.2 ± 2.2 | 74.0 ± 2.7 | 81.3 ± 4.1 |
| Ratio 1:2 | 63.2 ± 0.8 | 78.5 ± 5.5 | 80.5 ± 3.3 |
| Ratio 1:3 | 65.8 ± 2.9 | 90.3 ± 9.3 | 86.5 ± 0.5 |
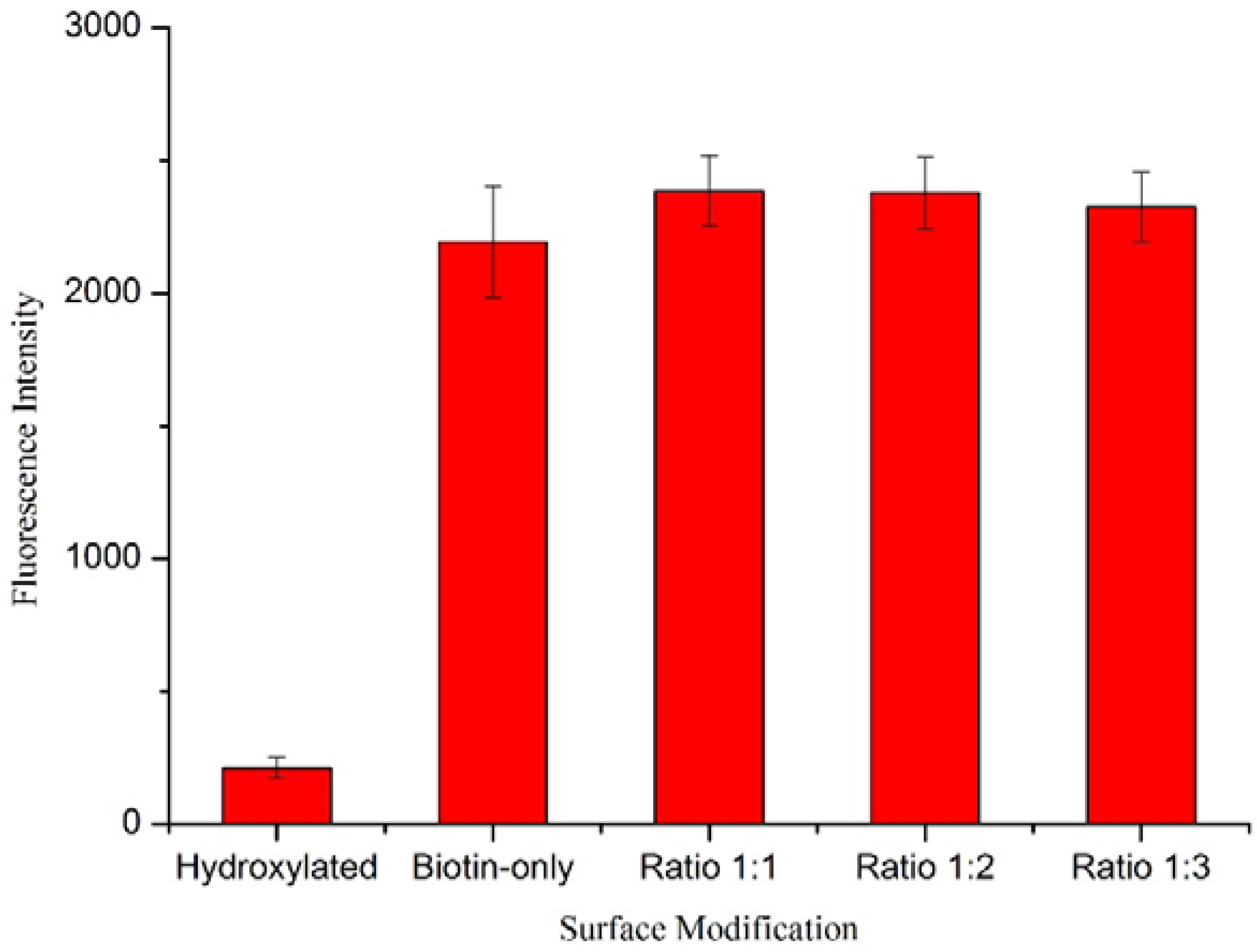
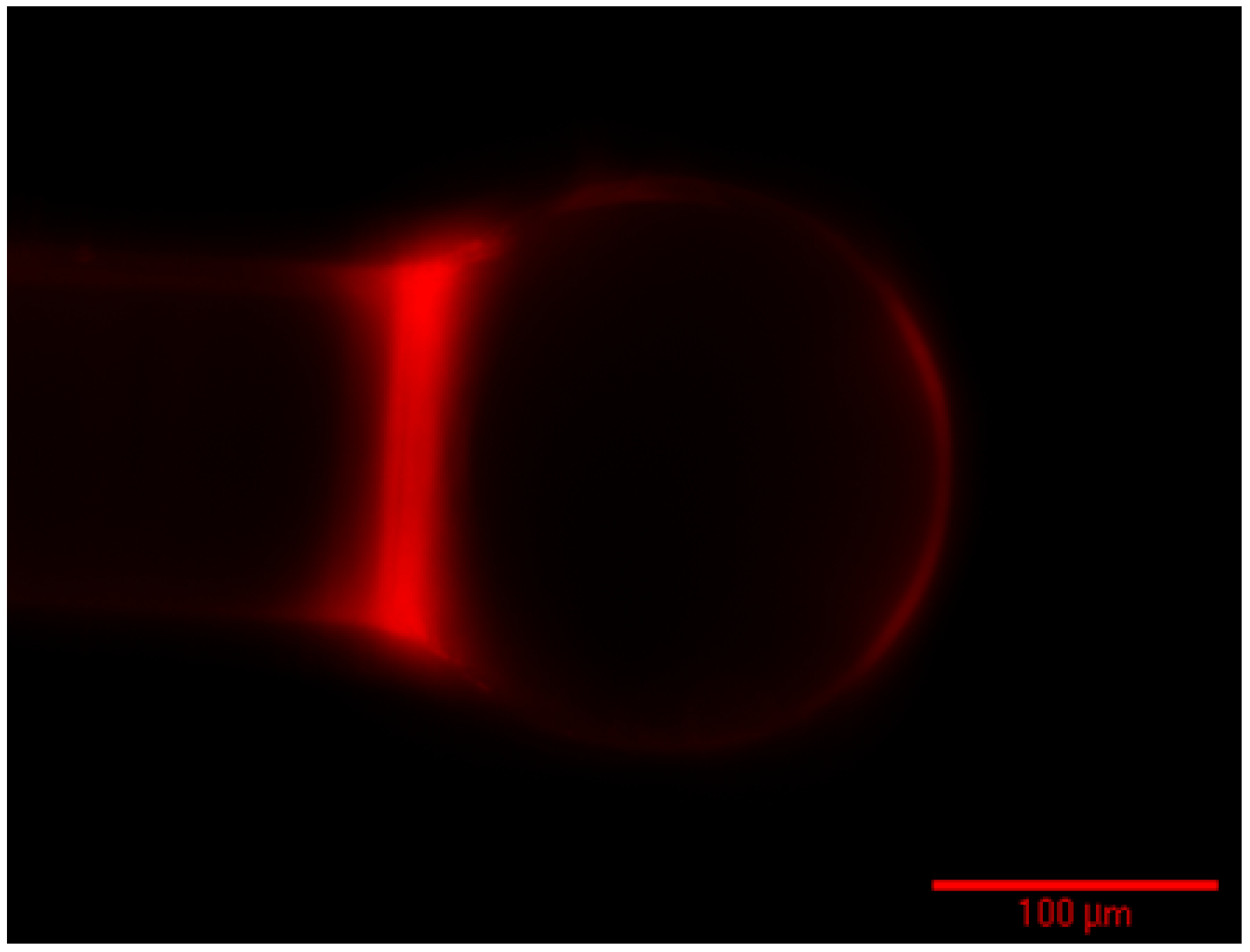
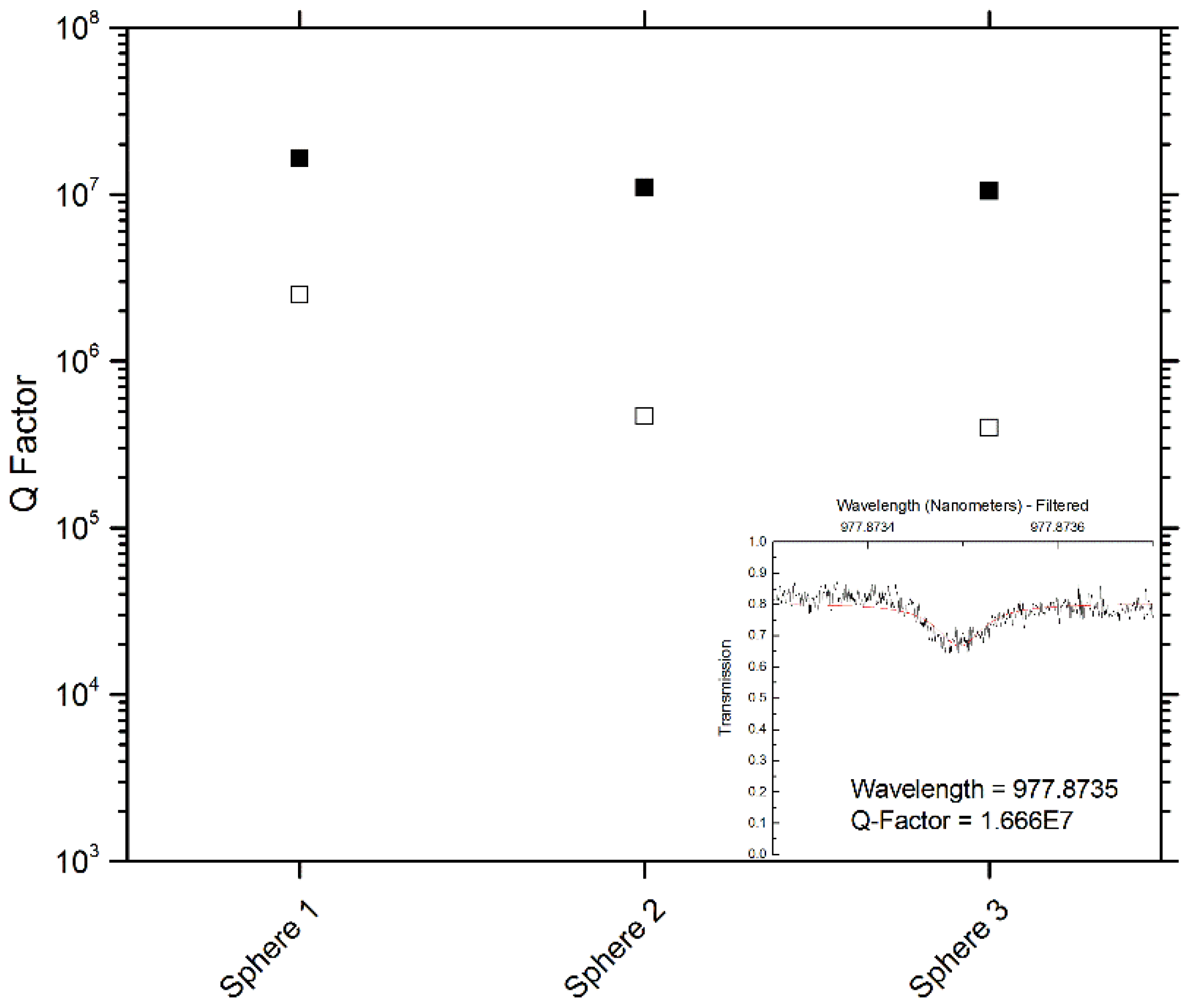

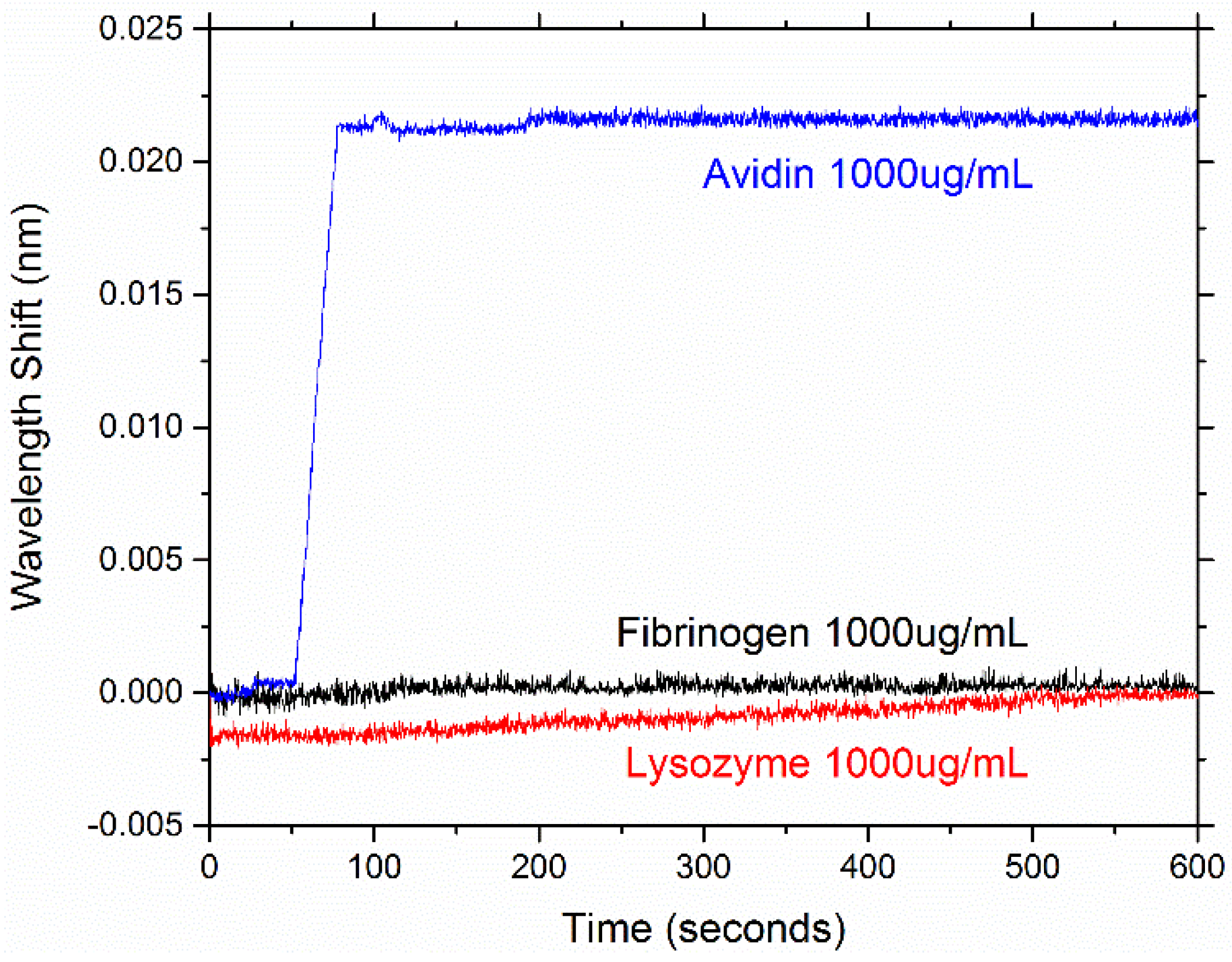
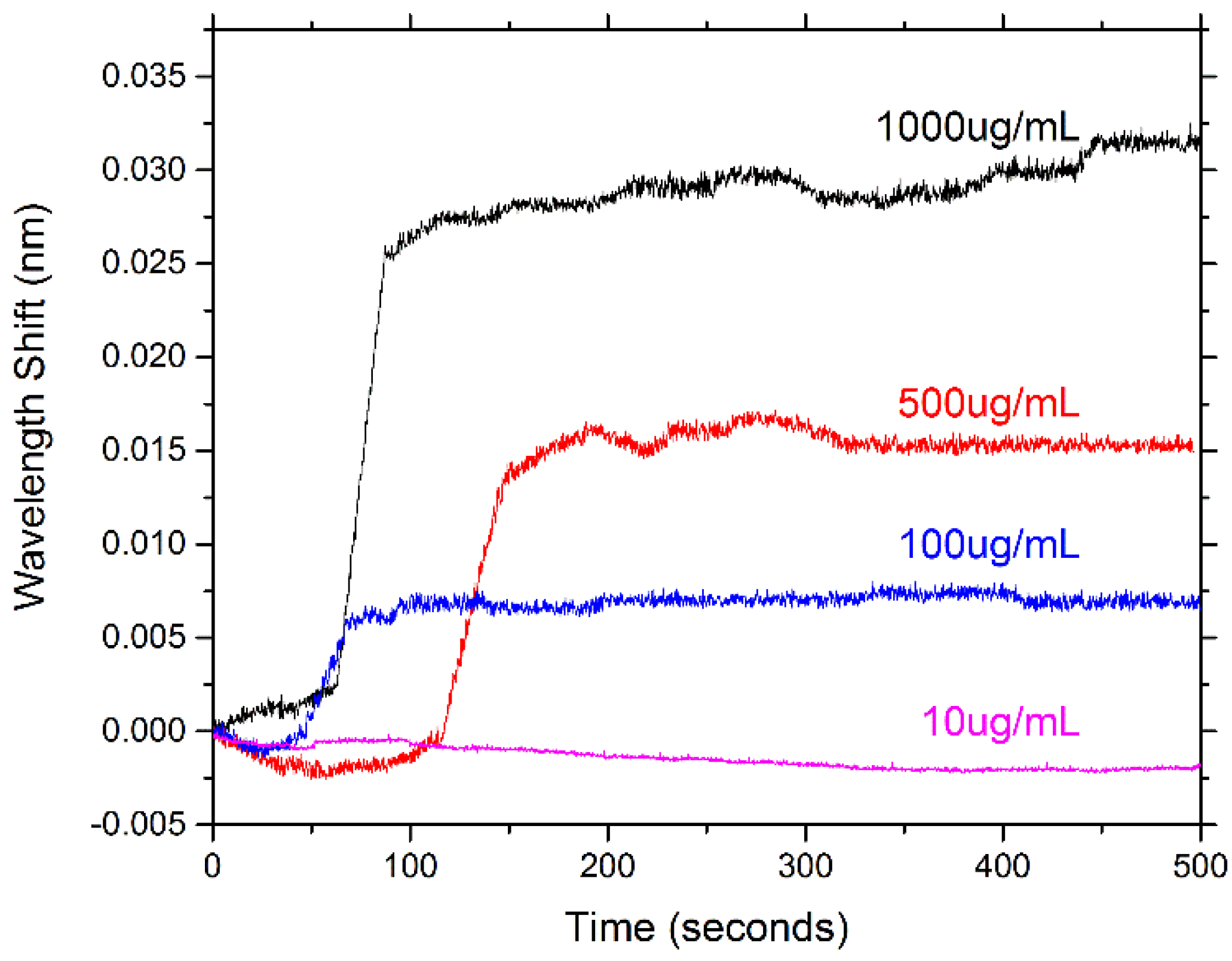
4. Conclusions/Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, E.L.; Tomlinson, I.D.; Mason, J.; Gresch, P.; Warnement, M.R.; Wright, D.; Sanders-Bush, E.; Blakely, R.; Rosenthal, S.J. Surface modification to reduce nonspecific binding of quantum dots in live cell assays. Bioconjugate Chem. 2005, 16, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Methods 2008, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y. The development of label-free cellular assays for drug discovery. Expert Opin. Drug Discov. 2011, 6, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, S. Recent advances in label-free biosensors applications in protein biosynthesis and hts screening. In Protein Biosynthesis; Esterhouse, T.E., Petrinos, L.B., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Situ, C.; Mooney, M.H.; Elliott, C.T.; Buijs, J. Advances in surface plasmon resonance biosensor technology towards high-throughput, food-safety analysis. TrAC Trends Anal. Chem. 2010, 29, 1305–1315. [Google Scholar] [CrossRef]
- Méjard, R.; Griesser, H.J.; Thierry, B. Optical biosensing for label-free cellular studies. TrAC Trends Anal. Chem. 2014, 53, 178–186. [Google Scholar] [CrossRef]
- Wilson, K.A.; Finch, C.A.; Anderson, P.; Vollmer, F.; Hickman, J.J. Whispering gallery mode biosensor quantification of fibronectin adsorption kinetics onto alkylsilane monolayers and interpretation of resultant cellular response. Biomaterials 2012, 33, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Maleki, L.; Ilchenko, V. Optical Sensing Based on Whispering-Gallery-Mode Microcavity. U.S. Patents 6490039 B2, 3 December 2002. [Google Scholar]
- Foreman, M.R.; Swaim, J.D.; Vollmer, F. Whispering gallery mode sensors. Adv. Opt. Photonics 2015, 7, 168–240. [Google Scholar] [CrossRef]
- Luchansky, M.S.; Bailey, R.C. High-Q Optical Sensors for Chemical and Biological Analysis. Anal. Chem. 2012, 84, 793–821. [Google Scholar] [CrossRef] [PubMed]
- Farca, G.; Shopova, S.I.; Elizondo, L.A.; Naweed, A.; Rosenberger, A.T. Evanescent-wave chemical sensing using WGM microresonators. In Proceedings of the 2004 Digest of the LEOS Summer Topical Meetings, San Diego, CA, USA, 28–30 June 2004.
- Schweiger, G.; Horn, M. Effect of changes in size and index of refraction on the resonance wavelength of microspheres. JOSA B 2006, 23, 212–217. [Google Scholar] [CrossRef]
- Hu, J.; Sun, X.; Agarwal, A.; Kimerling, L.C. Design guidelines for optical resonator biochemical sensors. JOSA B 2009, 26, 1032–1041. [Google Scholar] [CrossRef]
- Vollmer, F.; Yang, L. Review Label-free detection with high-Q microcavities: A review of biosensing mechanisms for integrated devices. Nanophotonics 2012, 1, 267–291. [Google Scholar] [CrossRef]
- Armani, D.K.; Kippenberg, T.J.; Spillane, S.M.; Vahala, K.J. Ultra-high-Q toroid microcavity on a chip. Nature 2003, 421, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Kuwata-Gonokami, M. Laser emission from dye-doped polystyrene microsphere. In Proceedings of the 1993 LEOS’93 Conference, San Jose, CA, USA, 15–18 November 1993; pp. 300–301.
- Nöckel, J.U.; Stone, A.D. Ray and Wave Chaos in Asymmetric Resonant Optical Cavities. 1998, arXiv:chao-dyn/9806017. arXiv preprint. Available online: http://arxiv.org/pdf/chao-dyn/9806017 (accessed on 2 January 2015).
- Moon, H.-J.; Park, G.-W.; Lee, S.-B.; An, K.; Lee, J.-H. Laser oscillations of resonance modes in a thin gain-doped ring-type cylindrical microcavity. Opt. Commun. 2004, 235, 401–407. [Google Scholar] [CrossRef]
- Francois, A.; Himmelhaus, M. Optical biosensor based on whispering gallery mode excitations in clusters of microparticles. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Merhari, L. Hybrid Nanocomposites for Nanotechnology; Springer: Berlin, Germany, 2009. [Google Scholar]
- Yang, J.J.; Huang, M.; Yu, J.; Lan, Y.Z. Surface whispering-gallery mode. EPL 2011, 96. [Google Scholar] [CrossRef]
- Koyun, A.; Ahlatcolu, E.; Koca, Y.; Kara, S. Biosensors and their principles. In A Roadmap of Biomedical Engineers and Milestones; InTech: Rijeka, Croatia, Chapter 4. [CrossRef]
- Washburn, A.L.; Luchansky, M.S.; Bowman, A.L.; Bailey, R.C. Quantitative, Label-Free Detection of Five Protein Biomarkers Using Multiplexed Arrays of Silicon Photonic Microring Resonators. Anal. Chem. 2010, 82, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Himmelhaus, M.; Krishnamoorthy, S.; Francois, A. Optical Sensors Based on Whispering Gallery Modes in Fluorescent Microbeads: Response to Specific Interactions. Sensors 2010, 10, 6257–6274. [Google Scholar] [CrossRef] [PubMed]
- Bog, U.; Brinkmann, F.; Wondimu, S.F.; Wienhold, T.; Kraemmer, S.; Koos, C.; Kalt, H.; Hirtz, M.; Fuchs, H.; Koeber, S.; et al. Densely Packed Microgoblet Laser Pairs for Cross-Referenced Biomolecular Detection. Adv. Sci. 2015. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Dale, P.S.; Caldwell, C.W.; Fan, X.D. Rapid and Label-Free Detection of Breast Cancer Biomarker CA15–3 in Clinical Human Serum Samples with Optofluidic Ring Resonator Sensors. Anal. Chem. 2009, 81, 9858–9865. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Wurm, F.; Mangold, C.; Frey, H. Multifunctional poly(ethylene glycol)s. Angew. Chem. Int. Ed. 2011, 50, 7988–7997. [Google Scholar] [CrossRef] [PubMed]
- Golander, C.G.; Herron, J.N.; Lim, K.; Claesson, P.; Stenius, P.; Andrade, J.D.; Harris, J.M. Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Zalipsky, S.; Harris, J.M. Introduction to chemistry and biological applications of poly(ethylene glycol). Poly (ethyl. glycol) 1997, 680, 1–13. [Google Scholar]
- Chapman, R.G.; Ostuni, E.; Takayama, S.; Holmlin, R.E.; Yan, L.; Whitesides, G.M. Surveying for surfaces that resist the adsorption of proteins. J. Am. Chem. Soc. 2000, 122, 8303–8304. [Google Scholar] [CrossRef]
- Uchida, K.; Otsuka, H.; Kaneko, M.; Kataoka, K.; Nagasaki, Y. A reactive poly (ethylene glycol) layer to achieve specific surface plasmon resonance sensing with a high S/N ratio: The substantial role of a short underbrushed PEG layer in minimizing nonspecific adsorption. Anal. Chem. 2005, 77, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Soteropulos, C.E.; Zurick, K.M.; Bernards, M.T.; Hunt, H.K. Tailoring the Protein Adsorption Properties of Whispering Gallery Mode Optical Biosensors. Langmuir 2012, 28, 15743–15750. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.K.; Soteropulos, C.; Armani, A.M. Bioconjugation strategies for microtoroidal optical resonators. Sensors 2010, 10, 9317–9336. [Google Scholar] [CrossRef] [PubMed]
- Smolders, C.A.; Duyvis, E.M. Contact angles; wetting and de-wetting of mercury: Part I. A critical examination of surface tension measurement by the sessile drop method. Recl. Trav. Chim. Pays-Bas 1961, 80, 635–649. [Google Scholar] [CrossRef]
- Soteropulos, C.E.; Hunt, H.K. Attaching biological probes to silica optical biosensors using silane coupling agents. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Vahala, K.J. Optical microcavities. Nature 2003, 424, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [PubMed]
- Kippenberg, T.J.; Spillane, S.M.; Armani, D.K.; Vahala, K.J. Fabrication and coupling to planar high-Q silica disk microcavities. Appl. Phys. Lett. 2003, 83, 797–799. [Google Scholar] [CrossRef]
- Gorodetsky, M.L.; Savchenkov, A.A.; Ilchenko, V.S. Ultimate Q of optical microsphere resonators. Opt. Lett. 1996, 21, 453–455. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Anderson, M.; Bernards, M.T.; Hunt, H.K. PEG Functionalization of Whispering Gallery Mode Optical Microresonator Biosensors to Minimize Non-Specific Adsorption during Targeted, Label-Free Sensing. Sensors 2015, 15, 18040-18060. https://doi.org/10.3390/s150818040
Wang F, Anderson M, Bernards MT, Hunt HK. PEG Functionalization of Whispering Gallery Mode Optical Microresonator Biosensors to Minimize Non-Specific Adsorption during Targeted, Label-Free Sensing. Sensors. 2015; 15(8):18040-18060. https://doi.org/10.3390/s150818040
Chicago/Turabian StyleWang, Fanyongjing, Mark Anderson, Matthew T. Bernards, and Heather K. Hunt. 2015. "PEG Functionalization of Whispering Gallery Mode Optical Microresonator Biosensors to Minimize Non-Specific Adsorption during Targeted, Label-Free Sensing" Sensors 15, no. 8: 18040-18060. https://doi.org/10.3390/s150818040
APA StyleWang, F., Anderson, M., Bernards, M. T., & Hunt, H. K. (2015). PEG Functionalization of Whispering Gallery Mode Optical Microresonator Biosensors to Minimize Non-Specific Adsorption during Targeted, Label-Free Sensing. Sensors, 15(8), 18040-18060. https://doi.org/10.3390/s150818040






