A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips
Abstract
:1. Introduction
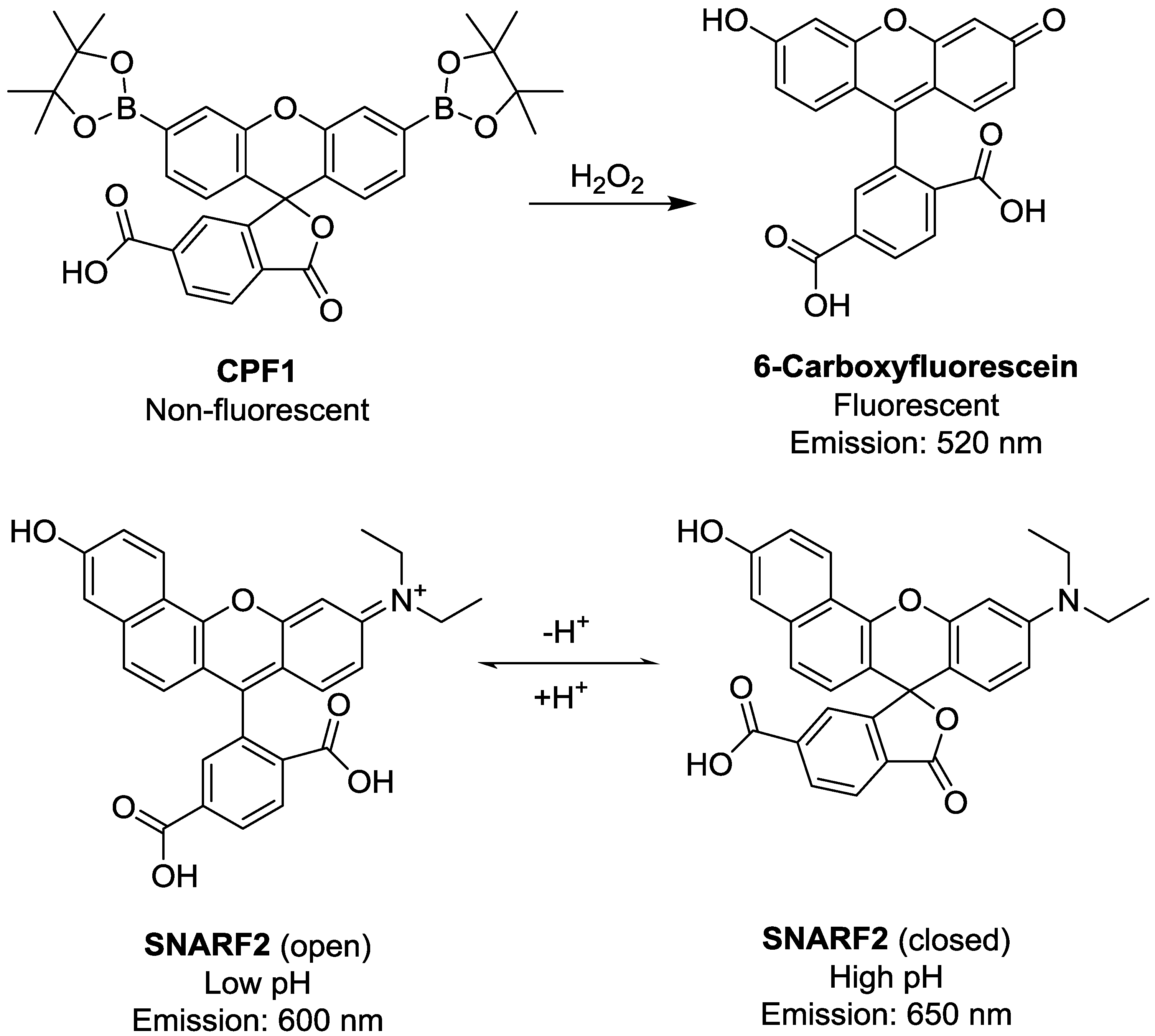
2. Experimental Section
2.1. Materials
2.2. Polyacrylamide Photo-Polymerisation on Optical Fibre Tips
2.3. Optical Measurements
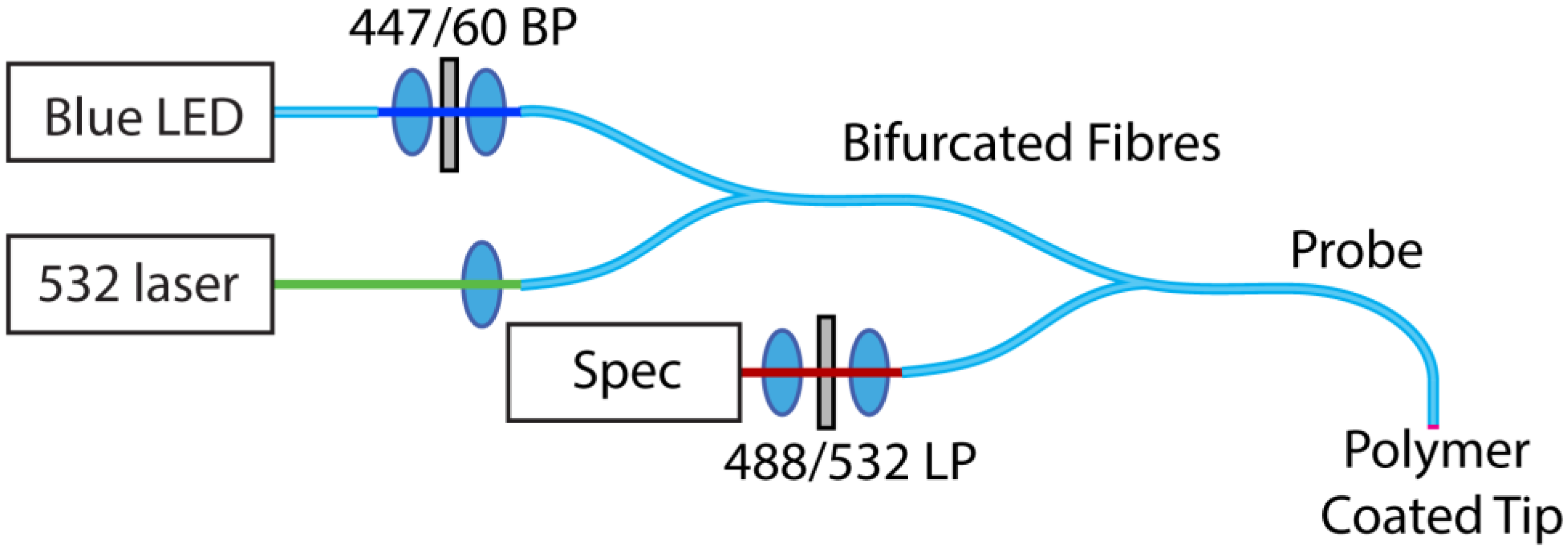
3. Results and Discussion
3.1. Hydrogen Peroxide Detection
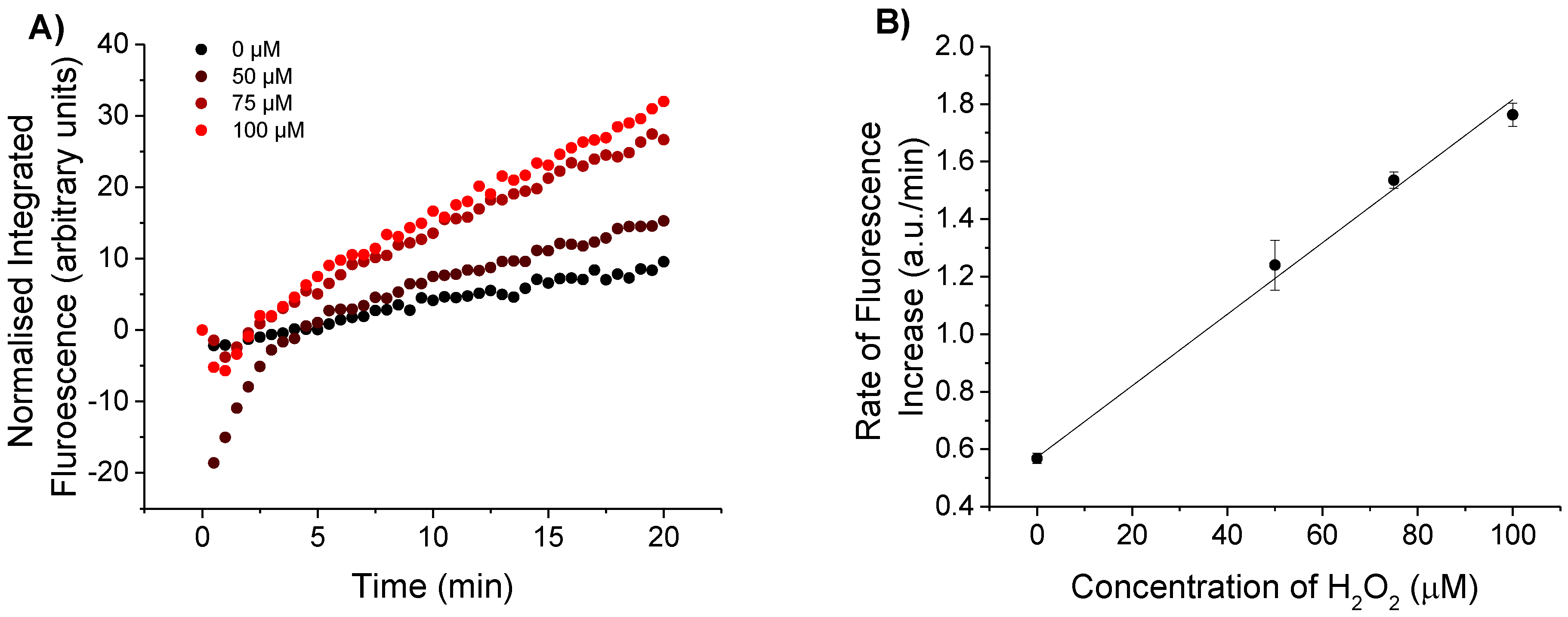
3.1.1. Detection of Biologically Relevant H2O2 Concentrations
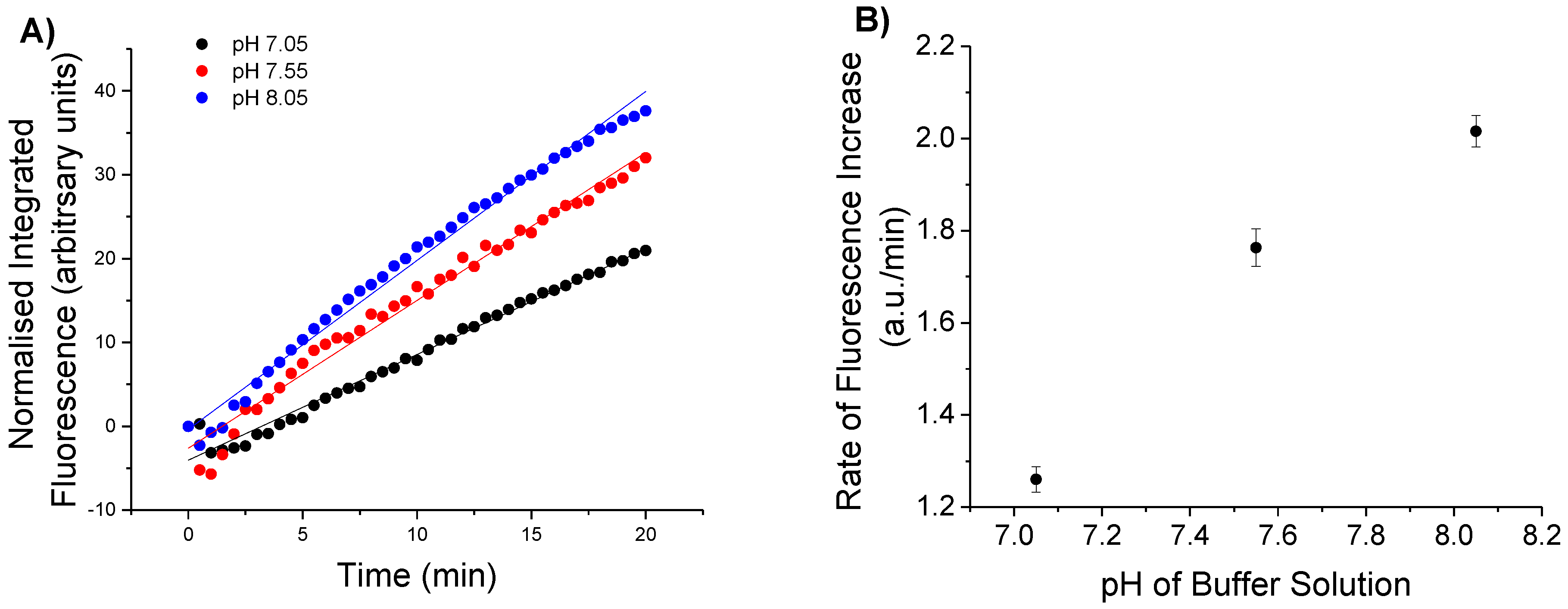
3.1.2. Effect of Change in pH on Detection of H2O2
3.2. pH Sensing
3.2.1. Initial pH Sensing
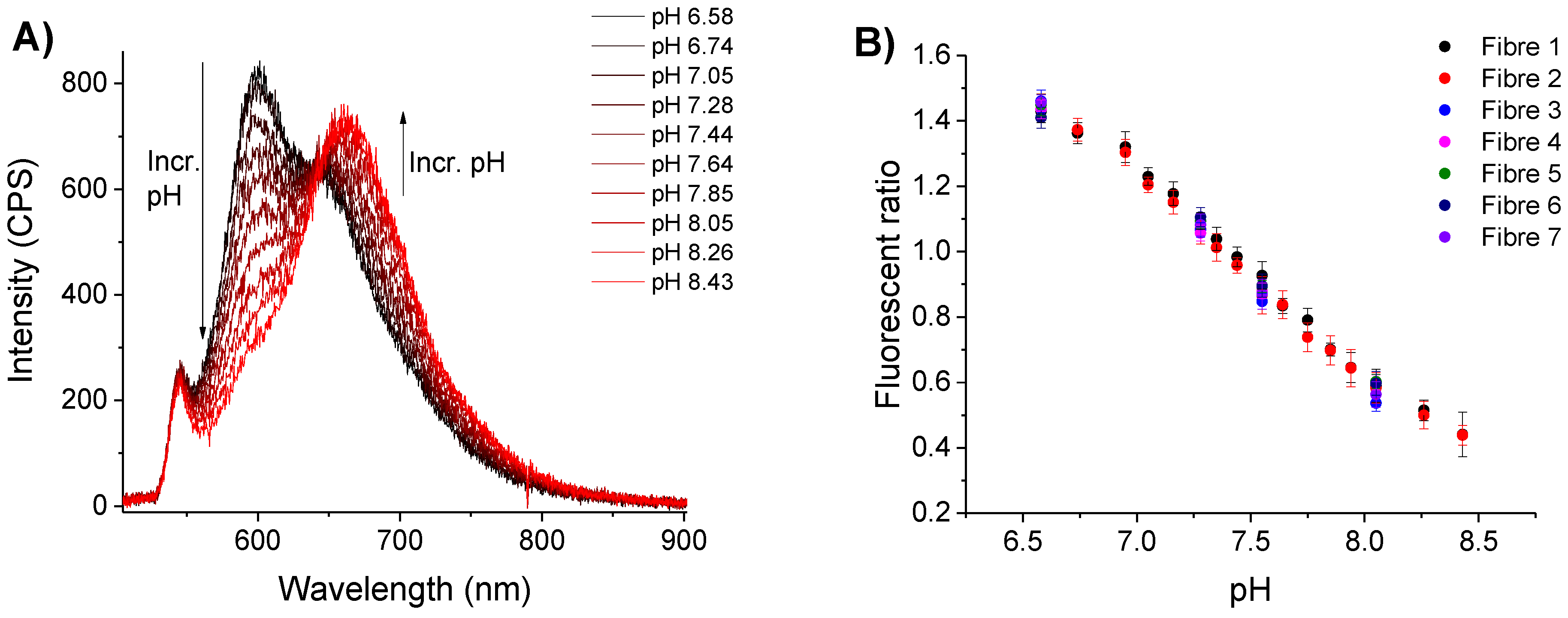
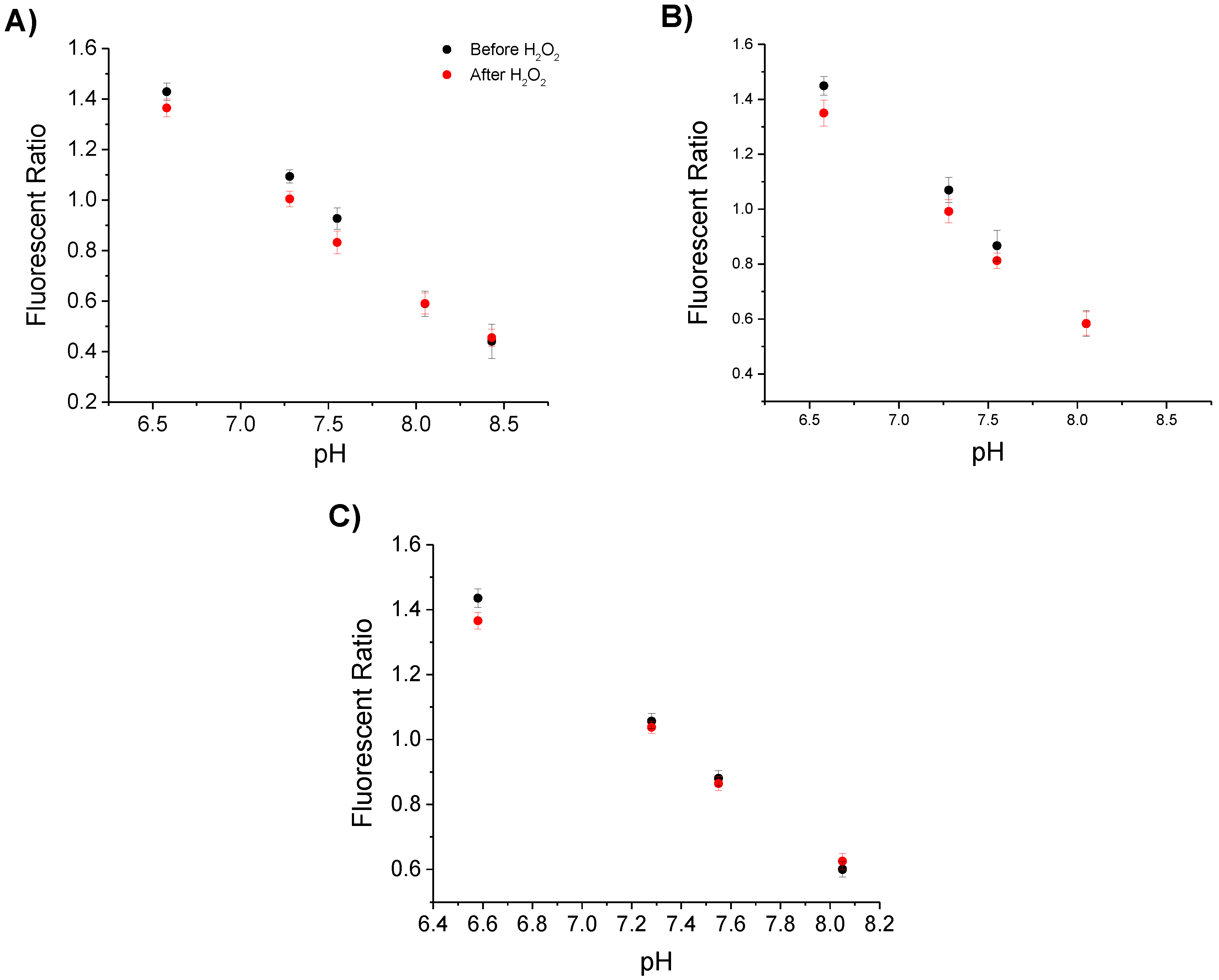
3.2.2. pH Sensing before and after Detection of Hydrogen Peroxide
4. Conclusions/Outlook
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gough, D.R.; Cotter, T.G. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Burdon, R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; McFarlane, J.D. Extracellular pH distribution in human tumours. Int. J. Hyperth. 1995, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Bize, I.; Santander, G.; Cabello, P.; Driscoll, D.; Sharpe, C. Hydrogen peroxide is involved in hamster sperm capacitation in vitro. Biol. Reprod. 1991, 44, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Nasr-Esfahani, M.H.; Aitken, J.R.; Johnson, M.H. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 1990, 109, 501–507. [Google Scholar] [PubMed]
- Armstrong, J.S.; Rajasekaran, M.; Chamulitrat, W.; Gatti, P.; Hellstrom, W.J.; Sikka, S.C. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic. Biol. Med. 1999, 26, 869–880. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C.G. The Effect of Reactive Oxygen Species on Equine Sperm Motility, Viability, Acrosomal Integrity, Mitochondrial Membrane Potential, and Membrane Lipid Peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [PubMed]
- Morado, S.; Cetica, P.; Beconi, M.; Thompson, J.G.; Dalvit, G. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction 2013, 145, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ocon, O.M.; Hansen, P.J. Disruption of Bovine Oocytes and Preimplantation Embryos by Urea and Acidic pH. J. Dairy Sci. 2003, 86, 1194–1200. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C.J. Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Recent development and applications of optical and fiber-optic pH sensors. TrAC Trends Anal. Chem. 2000, 19, 541–552. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2009, 110, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Nguyen, M.-C.; Kostecki, R.; Monro, T.M.; Abell, A.D. Nanoliter-scale, regenerable ion sensor: Sensing with a surface functionalized microstructured optical fibre. RSC Adv. 2013, 3, 8308–8317. [Google Scholar] [CrossRef]
- Foo, H.T.C.; Ebendorff-Heidepriem, H.; Sumby, C.J.; Monro, T.M. Towards microstructured optical fibre sensors: surface analysis of silanised lead silicate glass. J. Mater. Chem. C 2013, 1, 6782–6789. [Google Scholar] [CrossRef]
- Heng, S.; Mak, A.M.; Stubing, D.B.; Monro, T.M.; Abell, A.D. Dual Sensor for Cd(II) and Ca(II): Selective Nanoliter-Scale Sensing of Metal Ions. Anal. Chem. 2014, 86, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Shi, Z.Y.; Smith, S.; Birnbaum, D.; Kopelman, R. Submicrometer intracellular chemical optical fiber sensors. Science 1992, 258, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Schartner, E.; Monro, T. Fibre Tip Sensors for Localised Temperature Sensing Based on Rare Earth-Doped Glass Coatings. Sensors 2014, 14, 21693–21701. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, T.; Prudenzano, F.; Warren-Smith, S.C.; Monro, T.M. Design of exposed-core fiber for methadone monitoring in biological fluids. J. Non Cryst. Solids 2011, 357, 2000–2004. [Google Scholar] [CrossRef]
- Smolka, S.; Barth, M.; Benson, O. Highly efficient fluorescence sensing with hollow core photonic crystal fibers. Opt. Express 2007, 15, 12783–12791. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2008, 80, 4269–4283. [Google Scholar] [CrossRef] [PubMed]
- Schartner, E.P.; Tsiminis, G.T.; Henderson, M.R.; Monro, T.M. A Comparison Between Multimode Tip and Suspended Core Fluorescence Optical Fibre Sensors; Optical Society of America: Munich, Germany, 2015. [Google Scholar]
- Purdey, M.S.; Schartner, E.P.; Sutton-McDowall, M.L.; Ritter, L.J.; Thompson, J.G.; Monro, T.M.; Abell, A.D. Localised hydrogen peroxide sensing for reproductive health. Proc. SPIE 2015, 9506. [Google Scholar] [CrossRef]
- Chang, M.C.Y.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am.Chem. Soc. 2004, 126, 15392–15393. [Google Scholar] [CrossRef] [PubMed]
- Purdey, M.S.; Connaughton, H.S.; Whiting, S.; Schartner, E.P.; Monro, T.M.; Thompson, J.G.; Aitken, R.J.; Abell, A.D. Boronate probes for the detection of hydrogen peroxide release from human spermatozoa. Free Radic. Biol. Med. 2015, 81, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sutton-McDowall, M.L.; Purdey, M.; Brown, H.M.; Abell, A.D.; Mottershead, D.G.; Cetica, P.D.; Dalvit, G.C.; Goldys, E.M.; Gilchrist, R.B.; Gardner, D.K.; et al. Redox and anti-oxidant state within cattle oocytes following in vitro maturation with bone morphogenetic protein 15 and follicle stimulating hormone. Mol. Reprod. Dev. 2015, 82, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.E.; Haugland, R.P.; Prendergast, F.G. Spectral and photophysical studies of benzo[c]xanthene dyes: Dual emission pH sensors. Anal. Biochem. 1991, 194, 330–344. [Google Scholar] [CrossRef]
- Srikun, D.; Albers, A.E.; Chang, C.J. A dendrimer-based platform for simultaneous dual fluorescence imaging of hydrogen peroxide and pH gradients produced in living cells. Chem. Sci. 2011, 2, 1156–1165. [Google Scholar] [CrossRef]
- Song, A.; Parus, S.; Kopelman, R. High-Performance Fiber-Optic pH Microsensors for Practical Physiological Measurements Using a Dual-Emission Sensitive Dye. Anal. Chem. 1997, 69, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Zielonka, J.; Lopez, M.; Joseph, J.; Kalyanaraman, B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic. Biol. Med. 2009, 47, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hennink, E.J.; Young, I.T.; Tanke, H.J. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. Biophys. J. 1995, 68, 2588–2600. [Google Scholar] [CrossRef]
- Platkov, M.; Tirosh, R.; Kaufman, M.; Zurgil, N.; Deutsch, M. Photobleaching of fluorescein as a probe for oxidative stress in single cells. J. Photochem. Photobiol. B Biol. 2014, 140, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Yue, Y.; Guo, Y.; Shao, S. A water-soluble BODIPY derivative as a highly selective “Turn-On” fluorescent sensor for H2O2 sensing in vivo. Biosens. Bioelectron. 2014, 56, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Leung, A.; Shankar, P.M.; Mutharasan, R. A review of fiber-optic biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purdey, M.S.; Thompson, J.G.; Monro, T.M.; Abell, A.D.; Schartner, E.P. A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips. Sensors 2015, 15, 31904-31913. https://doi.org/10.3390/s151229893
Purdey MS, Thompson JG, Monro TM, Abell AD, Schartner EP. A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips. Sensors. 2015; 15(12):31904-31913. https://doi.org/10.3390/s151229893
Chicago/Turabian StylePurdey, Malcolm S., Jeremy G. Thompson, Tanya M. Monro, Andrew D. Abell, and Erik P. Schartner. 2015. "A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips" Sensors 15, no. 12: 31904-31913. https://doi.org/10.3390/s151229893
APA StylePurdey, M. S., Thompson, J. G., Monro, T. M., Abell, A. D., & Schartner, E. P. (2015). A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips. Sensors, 15(12), 31904-31913. https://doi.org/10.3390/s151229893






