Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis
Abstract
:1. Introduction: Beta Cell as a Sensor
2. Part I: Assessing Beta Cell Function Using MP and [Ca2+]i Sensors
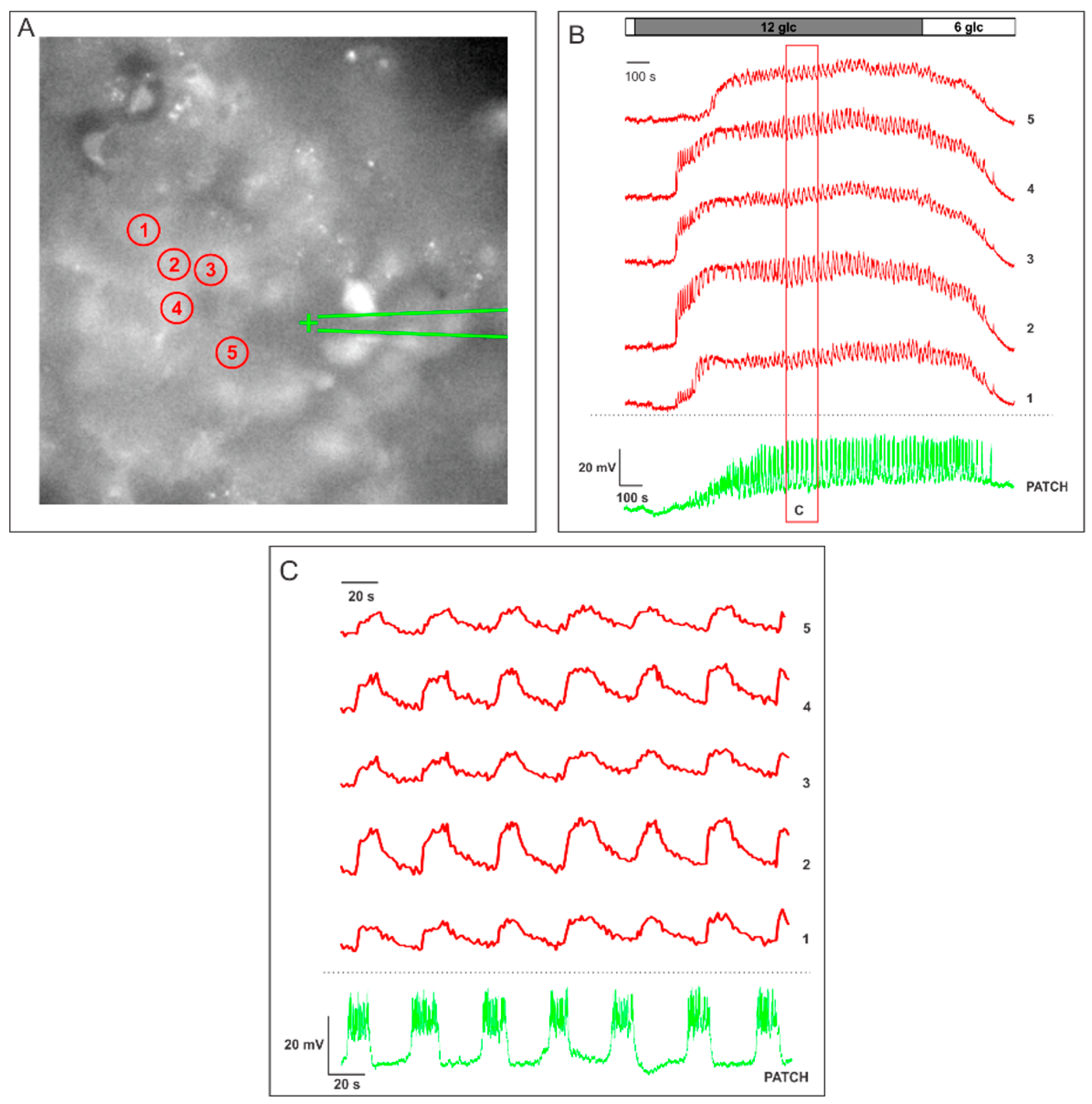
2.1. Measuring MP with a Patch-Clamp Pipette and [Ca2+]i with Fluorescent Dyes Using a CCD Camera
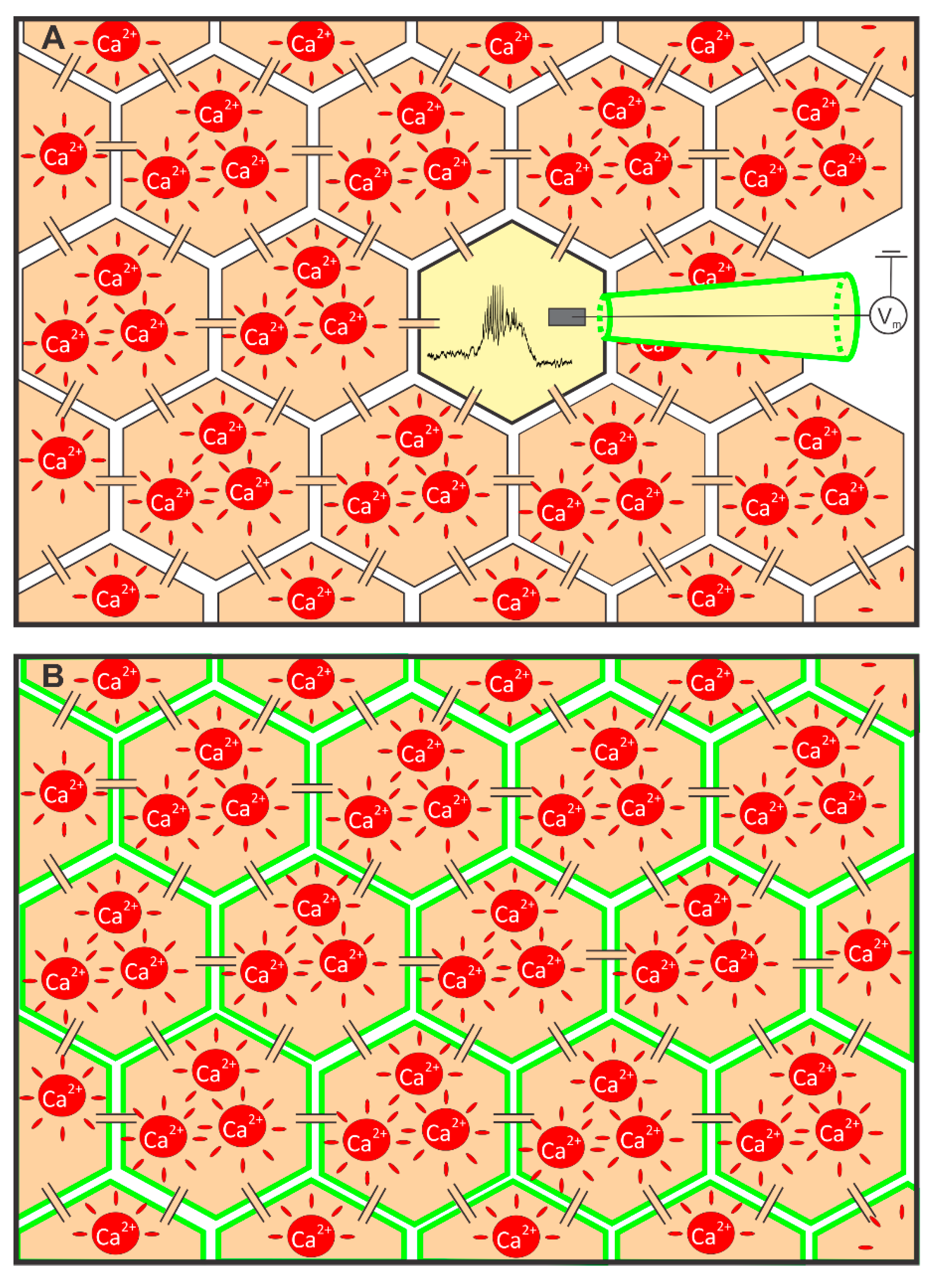
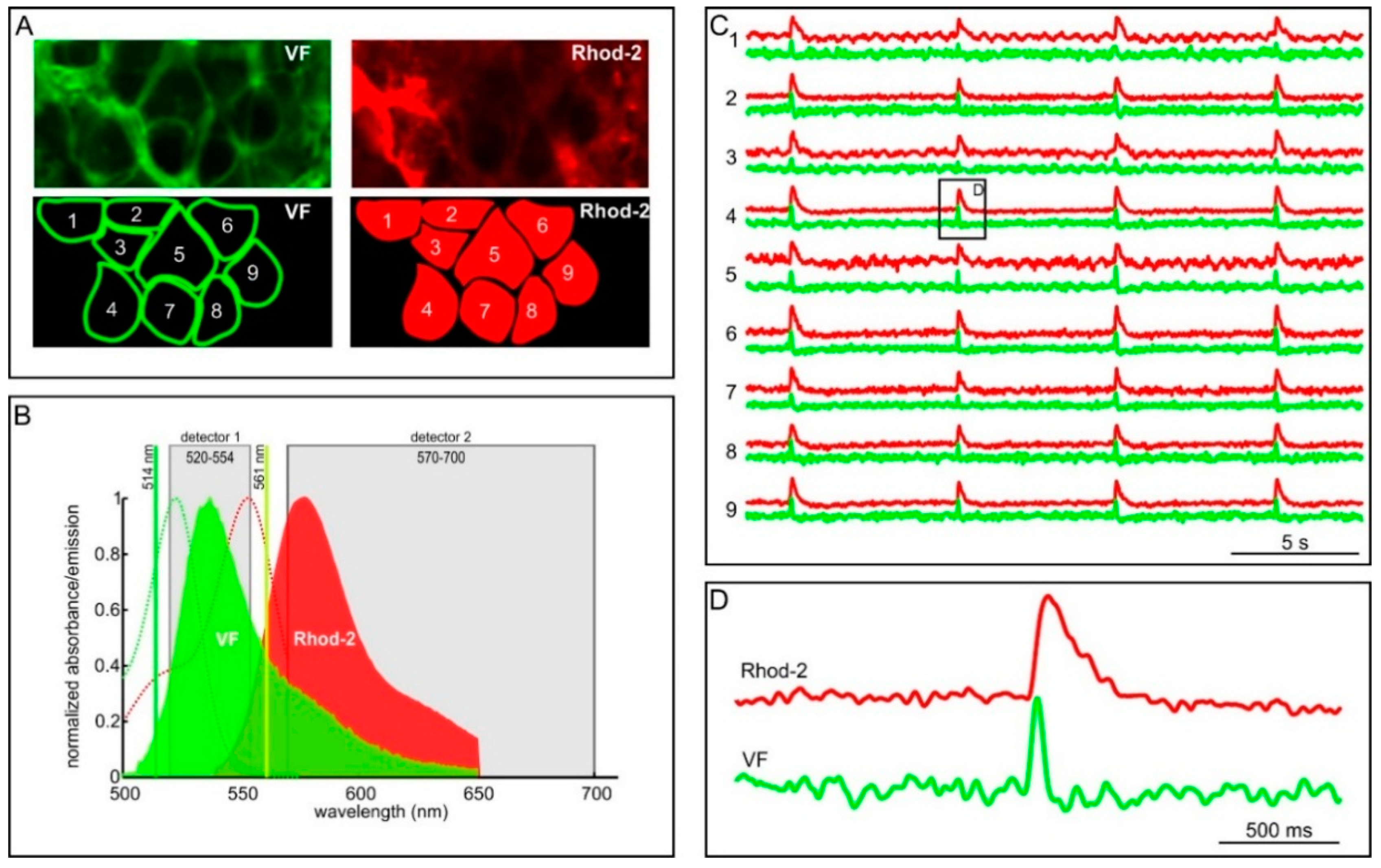
2.2. Measuring MP with Novel Voltage-Sensitive Dyes and [Ca2+]i with Fluorescent Dyes Using Confocal Microscope
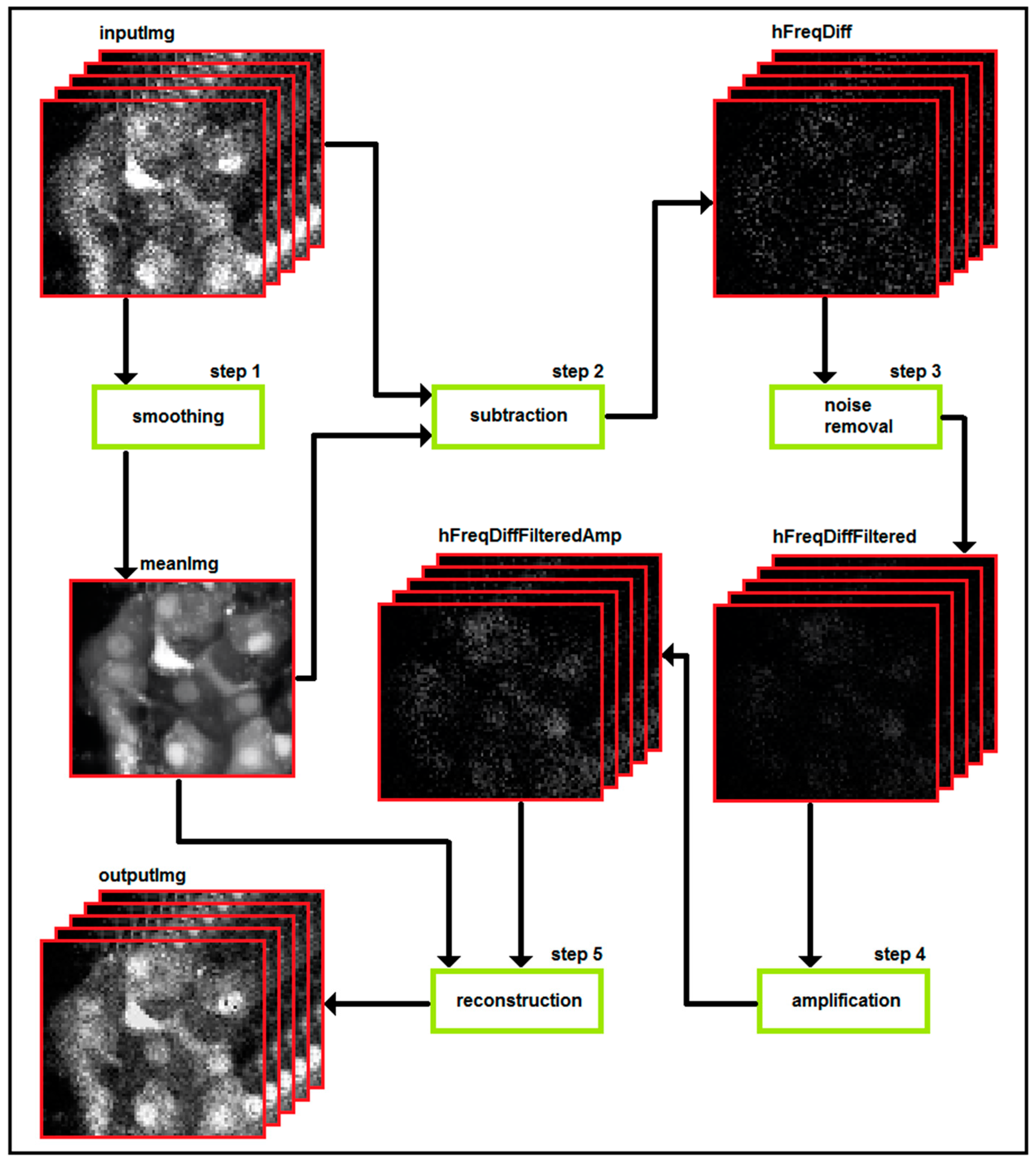
3. Part II: Analytical Methods Used to Analyze Noisy Signals of MP and [Ca2+]i
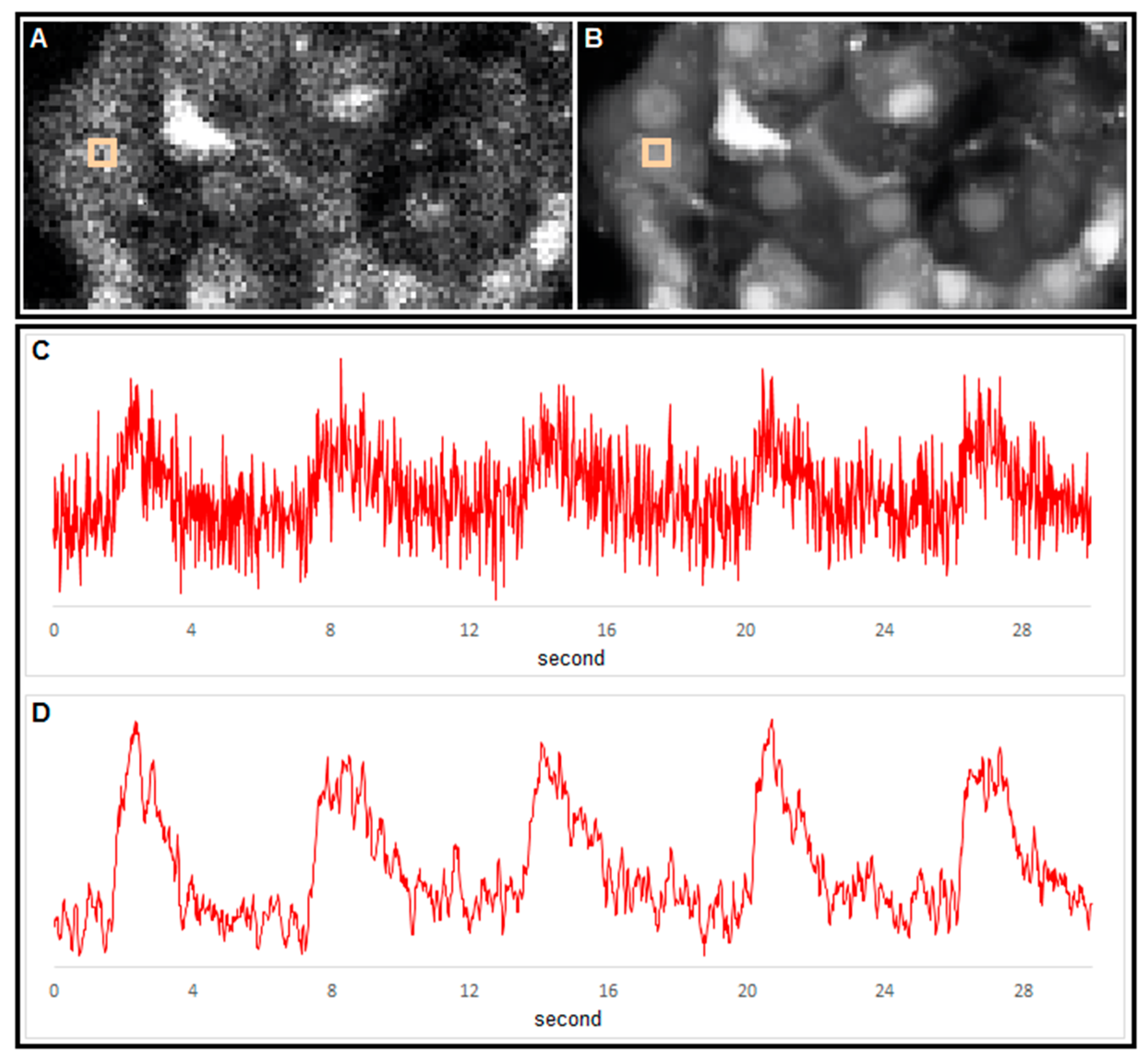
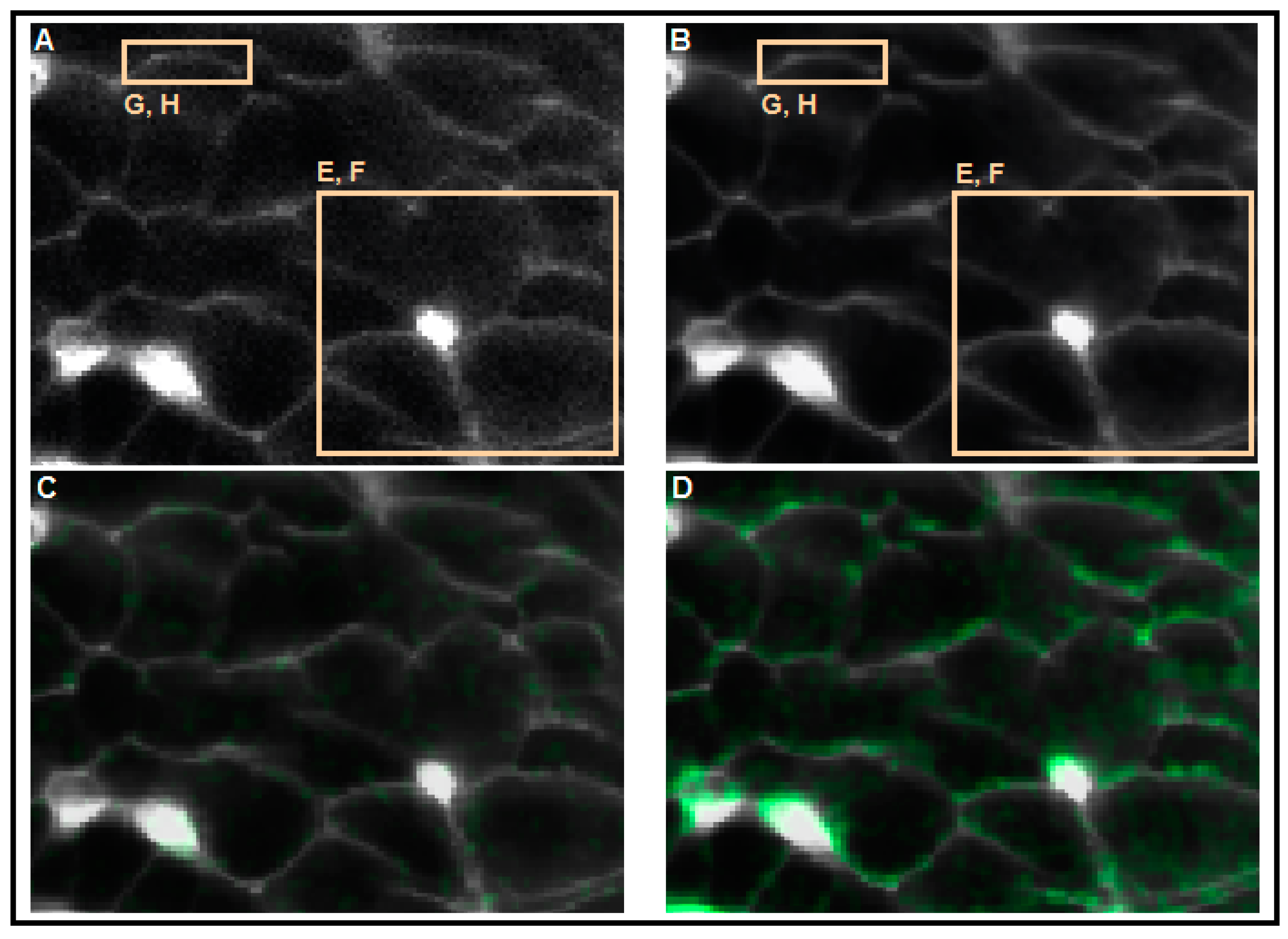
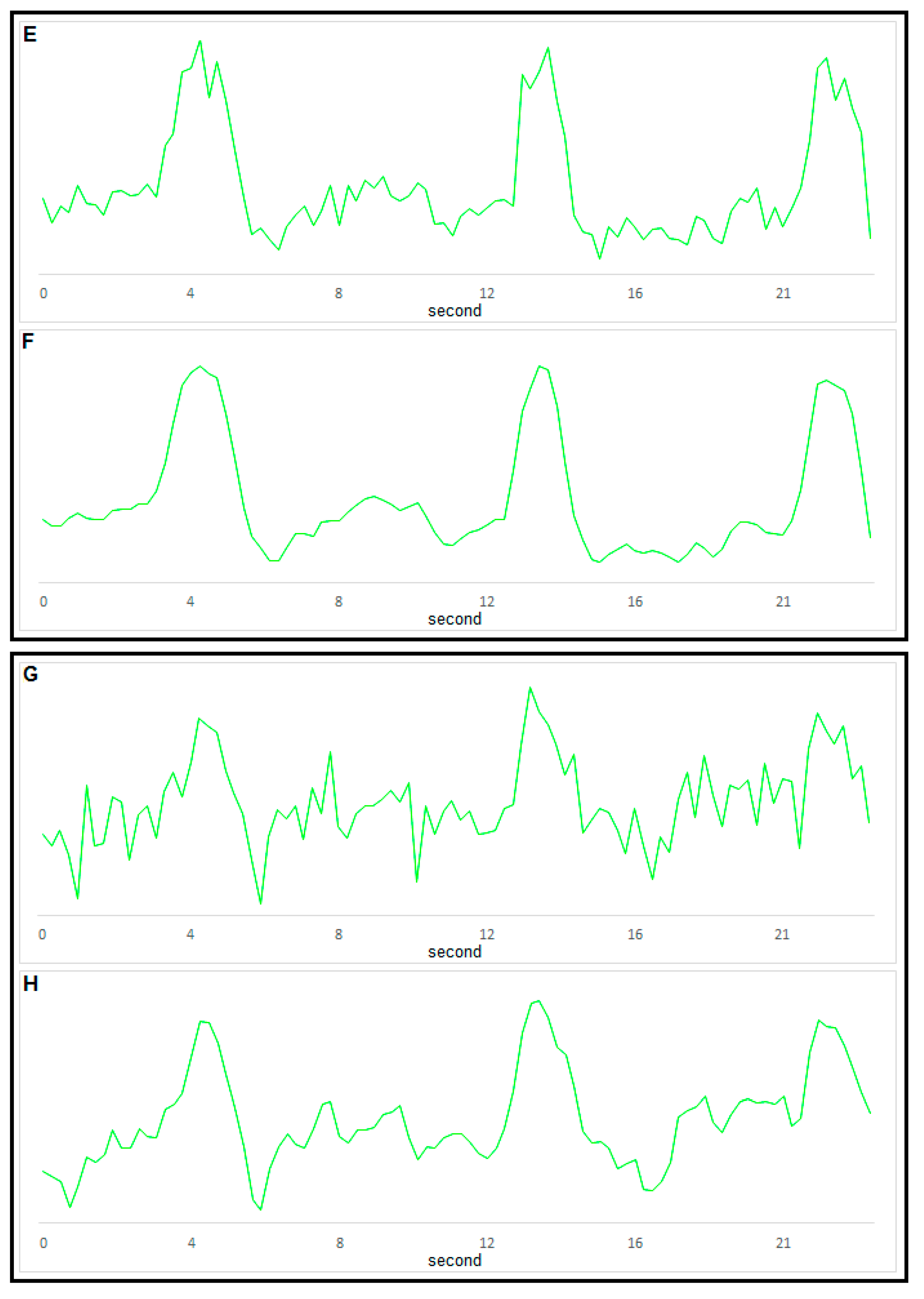
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Henquin, J. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Diabetologia 2009, 52, 739–751. [Google Scholar] [PubMed]
- Henquin, J.-C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S27–S31. [Google Scholar] [CrossRef]
- Huypens, P.R.; Huang, M.; Joseph, J.W. Overcoming the spatial barriers of the stimulus secretion cascade in pancreatic beta-cells. Islets 2012, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C.; Nenquin, M.; Ravier, M.A.; Szollosi, A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes. Metab. 2009, 11, 168–179. [Google Scholar] [PubMed]
- Rorsman, P.; Braun, M. Regulation of insulin secretion in human pancreatic islets. Ann. Rev. Physiol. 2013, 75, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Halban, P. 50 years forward: Beta cells. Diabetologia 2015, 58, 1688–1692. [Google Scholar] [PubMed]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. B-cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014, 37, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.; Newsholme, P. Metabolic regulation of insulin secretion. Vitam. Horm. 2014, 95, 1–33. [Google Scholar] [PubMed]
- Nolan, C.J.; Prentki, M. The islet β-cell: Fuel responsive and vulnerable. Trends Endocrinol. Metab. 2008, 19, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Matschinsky, F.M.; Madiraju, S.R.M. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013, 18, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Calcium signaling in the islets. In The Islets of Langerhans. Advances in Experimental Medicine and Biology; Islam, M.S., Ed.; Springer Netherlands: Heidelberg, Germany, 2010; Volume 654, pp. 235–259. [Google Scholar]
- Gilon, P.; Chae, H.-Y.; Rutter, G.A.; Ravier, M.A. Calcium signaling in pancreatic β-cells in health and in type 2 diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [PubMed]
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015, 466, 203–218. [Google Scholar] [PubMed]
- Rorsman, P.; Braun, M.; Zhang, Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium 2012, 51, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.-C.; Nenquin, M.; Stiernet, P.; Ahren, B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse. Diabetes 2006, 55, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.V.; Joseph, J.W.; Ronnebaum, S.M.; Burgess, S.C.; Sherry, A.D.; Newgard, C.B. Metabolic cycling in control of glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1287–E1297. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E. Signal integration at the level of ion channel and exocytotic function in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1065–E1069. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Joseph, J.W.; Rorsman, P. Glucose-sensing mechanisms in pancreatic β-cells. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Gaudel, C.; McClenaghan, N.H. Nutrient regulation of insulin secretion and beta-cell functional integrity. In Islets of Langerhans; Islam, M.S., Ed.; Springer-Verlag Berlin: Berlin, Germany, 2010; Volume 654, pp. 91–114. [Google Scholar]
- Newsholme, P.; Krause, M. Nutritional regulation of insulin secretion: Implications for diabetes. Clin. Biochem. Rev. Aust. Assoc. Clin. Biochem. 2012, 33, 35–47. [Google Scholar]
- Kojima, I.; Nakagawa, Y.; Ohtsu, Y.; Hamano, K.; Medina, J.; Nagasawa, M. Return of the glucoreceptor: Glucose activates the glucose-sensing receptor T1R3 and facilitates metabolism in pancreatic β-cells. J. Diabetes Investig. 2015, 6, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, G.A.; Soundarapandian, M.M.; Tyrberg, B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc. Natl. Acad. Sci. USA 2012, 109, E524–E532. [Google Scholar] [CrossRef] [PubMed]
- Niki, A.; Niki, H.; Miwa, I.; Okuda, J. Insulin secretion by anomers of d-glucose. Science 1974, 186, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.-C. Do pancreatic beta cells “taste” nutrients to secrete insulin? Sci. Signal. 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferdaoussi, M.; Bergeron, V.; Zarrouki, B.; Kolic, J.; Cantley, J.; Fielitz, J.; Olson, E.N.; Prentki, M.; Biden, T.; MacDonald, P.E.; et al. G protein-coupled receptor (GPR) 40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 2012, 55, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.A.; Alquier, T.; Latour, M.G.; Poitout, V. Lipid receptors and islet function: Therapeutic implications? Diabetes Obes. Metab. 2009, 11, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.; Alquier, T.; Latour, M.G.; Semache, M.; Tremblay, C.; Poitout, V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 2008, 57, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Feng, Y.; Zhou, Y.P.; Eiermann, G.J.; Petrov, A.; Zhou, C.; Lin, S.; Salituro, G.; Meinke, P.; Mosley, R.; et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes 2008, 57, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Oya, M.; Suzuki, H.; Watanabe, Y.; Sato, M.; Tsuboi, T. Amino acid taste receptor regulates insulin secretion in pancreatic beta-cell line MIN6 cells. Genes Cells Devoted Mol. Cell. Mech. 2011, 16, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, A. Paracrine and autocrine interactions in the human islet: More than meets the eye. Semin. Cell Dev. Biol. 2013, 24, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.S.; Cho, J.H.; Chen, L.Y. Paracrine interactions within islets of langerhans. J. Mol. Neurosci. 2012, 48, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Menegaz, D.; Caicedo, A. Neurotransmitters act as paracrine signals to regulate insulin secretion from the human pancreatic islet. J. Physiol. 2014, 592, 3413–3417. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Orci, L. Paracrinology of islets and the paracrinopathy of diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 16009–16012. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B. Autonomic regulation of islet hormone secretion—Implications for health and disease. Diabetologia 2000, 43, 393–410. [Google Scholar] [PubMed]
- Dai, C.; Brissova, M.; Reinert, R.B.; Nyman, L.; Liu, E.H.; Thompson, C.; Shostak, A.; Shiota, M.; Takahashi, T.; Powers, A.C. Pancreatic islet vasculature adapts to insulin resistance through dilation and not angiogenesis. Diabetes 2013, 62, 4144–4153. [Google Scholar] [CrossRef] [PubMed]
- Dolensek, J.; Rupnik, M.S.; Stozer, A. Structural similarities and differences between the human and the mouse pancreas. Islets 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Gylfe, E.; Tengholm, A. Neurotransmitter control of islet hormone pulsatility. Diabetes Obes. Metab. 2014, 16 (Suppl. 1), 102–110. [Google Scholar] [CrossRef] [PubMed]
- Ilegems, E.; Dicker, A.; Speier, S.; Sharma, A.; Bahow, A.; Edlund, P.K.; Leibiger, I.B.; Berggren, P.O. Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proc. Natl. Acad. Sci. USA 2013, 110, 20581–20586. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Abdulreda, M.H.; Formoso, A.L.; Gans, I.; Ricordi, C.; Berggren, P.-O.; Caicedo, A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Caicedo, A. Novel approaches to studying the role of innervation in the biology of pancreatic islets. Endocrinol. Metab. Clin. North Am. 2013, 42, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Kailey, B.; van de Bunt, M.; Cheley, S.; Johnson, P.R.; MacDonald, P.E.; Gloyn, A.L.; Rorsman, P.; Braun, M. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. Am. J. Physiol.-Endoc. M 2012, 303, E1107–E1116. [Google Scholar]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2009, 8, 369–385. [Google Scholar] [CrossRef] [PubMed]
- De Azua, I.R.; Gautam, D.; Guettier, J.-M.; Wess, J. Novel insights into the function of β-cell M 3 muscarinic acetylcholine receptors: Therapeutic implications. Trends Endocrinol. Metab. TEM 2011, 22, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Rodriguez-Diaz, R.; Fachado, A.; Jacques-Silva, M.C.; Berggren, P.O.; Caicedo, A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014, 63, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Peterhoff, M.; Sieg, A.; Brede, M.; Chao, C.M.; Hein, L.; Ullrich, S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur. J. Endocrinol. 2003, 149, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Marquard, J.; Otter, S.; Welters, A.; Stirban, A.; Fischer, A.; Eglinger, J.; Herebian, D.; Kletke, O.; Klemen, M.S.; Stozer, A.; et al. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat. Med. 2015, 21, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wollheim, C.B.; Maechler, P. Beta cell glutamate receptor antagonists: Novel oral antidiabetic drugs? Nat. Med. 2015, 21, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Clark, A.; Walker, J.N.; Johnson, P.R.; Rorsman, P. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 2010, 59, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Ramracheya, R.; Rorsman, P. Autocrine regulation of insulin secretion. Diabetes Obes. Metab. 2012, 14, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Taneera, J.; Jin, Z.; Jin, Y.; Muhammed, S.J.; Zhang, E.; Lang, S.; Salehi, A.; Korsgren, O.; Renstrom, E.; Groop, L.; et al. Gamma-aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia 2012, 55, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Rorsman, P. KATP channels and islet hormone secretion: New insights and controversies. Nat. Rev. Endocrinol. 2013, 9, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Vetere, A.; Choudhary, A.; Burns, S.M.; Wagner, B.K. Targeting the pancreatic beta-cell to treat diabetes. Nat. Rev. Drug Discov. 2014, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L.; Ma, X.S.; Renstrom, E.; Barg, S.; Berggren, P.O.; Galvanovskis, J.; Gromada, J.; Jing, X.J.; Lundquist, I.; Salehi, A.; et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J. Gen. Physiol. 2003, 121, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L.; Renstrom, E.; Ammala, C.; Berggren, P.O.; Bertorello, A.M.; Bokvist, K.; Chibalin, A.; Deeney, J.T.; Flatt, P.R.; Gabel, J.; et al. PKC-dependent stimulation of exocytosis by sulfonylureas in pancreatic beta cells. Science 1996, 271, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Katoh, M.; Shibasaki, T.; Minami, K.; Sunaga, Y.; Takahashi, H.; Yokoi, N.; Iwasaki, M.; Miki, T.; Seino, S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009, 325, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.H.; Cradock, S.; Fisher, M.; Hall, G.; Hughes, E.; Middleton, A. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. Int. J. Clin. Pract. 2010, 64, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Benninger, R.K.; Piston, D.W. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol. Metab. TEM 2014, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Benninger, R.K.P.; Head, W.S.; Zhang, M.; Satin, L.S.; Piston, D.W. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J. Physiol. 2011, 589, 5453–5466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, D.; Haefliger, J.-A.; Meda, P. Connexins: Key mediators of endocrine function. Physiol. Rev. 2011, 91, 1393–1445. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, F.C.; Henquin, J.-C. Measurements of cytoplasmic Ca2+ in islet cell clusters show that glucose rapidly recruits β-cells and gradually increases the individual cell response. Diabetes 2001, 50, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Meda, P.; Schuit, F. Glucose-stimulated insulin secretion: The hierarchy of its multiple cellular and subcellular mechanisms. Diabetologia 2013, 56, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Pipeleers, D.; Kiekens, R.; Ling, Z.; Wilikens, A.; Schuit, F. Physiologic relevance of heterogeneity in the pancreatic beta-cell population. Diabetologia 1994, 37, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Ravier, M.A.; Güldenagel, M.; Charollais, A.; Gjinovci, A.; Caille, D.; Söhl, G.; Wollheim, C.B.; Willecke, K.; Henquin, J.-C.; Meda, P. Loss of Connexin36 channels alters β-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005, 54, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.; Meda, P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp. Cell Res. 1986, 162, 507–520. [Google Scholar] [CrossRef]
- Speier, S.; Gjinovci, A.; Charollais, A.; Meda, P.; Rupnik, M. Cx36-mediated coupling reduces β-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 2007, 56, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Head, W.S.; Orseth, M.L.; Nunemaker, C.S.; Satin, L.S.; Piston, D.W.; Benninger, R.K. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 2012, 61, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Bavamian, S.; Klee, P.; Britan, A.; Populaire, C.; Caille, D.; Cancela, J.; Charollais, A.; Meda, P. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes. Metab. 2007, 9, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, N.L.; Benninger, R.K.P. New insights into the role of connexins in pancreatic islet function and diabetes. FEBS Lett. 2014, 588, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Charollais, A.; Gjinovci, A.; Huarte, J.; Bauquis, J.; Nadal, A.; Martin, F.; Andreu, E.; Sanchez-Andres, J.V.; Calabrese, A.; Bosco, D.; et al. Junctional communication of pancreatic beta cells contributes to the control of insulin secretion and glucose tolerance. J. Clin. Investig. 2000, 106, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Benninger, R.K.; Zhang, M.; Head, W.S.; Satin, L.S.; Piston, D.W. Gap junction coupling and calcium waves in the pancreatic islet. Biophys. J. 2008, 95, 5048–5061. [Google Scholar] [CrossRef] [PubMed]
- Smolen, P.; Rinzel, J.; Sherman, A. Why pancreatic islets burst but single beta cells do not. The heterogeneity hypothesis. Biophys. J. 1993, 64, 1668–1680. [Google Scholar] [CrossRef]
- Félix-Martínez, G.J.; Godínez-Fernández, J.R. Mathematical models of electrical activity of the pancreatic β-cell: A physiological review. Islets 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.G.; Toffolo, G.M.; Cobelli, C. Cellular modeling: Insight into oral minimal models of insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E597–E601. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.G.; Corradin, A.; Toffolo, G.M.; Cobelli, C. A subcellular model of glucose-stimulated pancreatic insulin secretion. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2008, 366, 3525–3543. [Google Scholar] [CrossRef]
- Stožer, A.; Gosak, M.; Dolenšek, J.; Perc, M.; Marhl, M.; Rupnik, M.S.; Korošak, D. Functional connectivity in islets of langerhans from mouse pancreas tissue slices. PLoS Comput. Biol. 2013, 9, e1002923. [Google Scholar] [CrossRef] [PubMed]
- Gosak, M.; Stožer, A.; Markovič, R.; Dolenšek, J.; Marhl, M.; Slak Rupnik, M.; Perc, M. The relationship between node degree and dissipation rate in networks of diffusively coupled oscillators and its significance for pancreatic beta cells. Chaos (Woodbury, NY) 2015, 25. [Google Scholar] [CrossRef] [PubMed]
- Gosak, M.; Dolenšek, J.; Markovič, R.; Slak Rupnik, M.; Marhl, M.; Stožer, A. Multilayer network representation of membrane potential and cytosolic calcium concentration dynamics in beta cells. Chaos Solitons Fractals 2015, 80, 76–82. [Google Scholar] [CrossRef]
- Markovic, R.; Stozer, A.; Gosak, M.; Dolensek, J.; Marhl, M.; Rupnik, M.S. Progressive glucose stimulation of islet beta cells reveals a transition from segregated to integrated modular functional connectivity patterns. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Hodson, D.J.; Tarasov, A.I.; Gimeno Brias, S.; Mitchell, R.K.; Johnston, N.R.; Haghollahi, S.; Cane, M.C.; Bugliani, M.; Marchetti, P.; Bosco, D.; et al. Incretin-modulated beta cell energetics in intact islets of langerhans. Mol. Endocrinol. 2014, 28, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Hodson, D.J.; Mitchell, R.K.; Bellomo, E.A.; Sun, G.; Vinet, L.; Meda, P.; Li, D.; Li, W.H.; Bugliani, M.; Marchetti, P.; et al. Lipotoxicity disrupts incretin-regulated human beta cell connectivity. J. Clin. Investig. 2013, 123, 4182–4194. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Hodson, D.J. Beta cell connectivity in pancreatic islets: A type 2 diabetes target? Cell. Mol. Life Sci. CMLS 2015, 72, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Speier, S.; Rupnik, M. A novel approach to in situ characterization of pancreatic ß-cells. Pflügers Arch. Eur. J. Physiol. 2003, 446, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Dolenšek, J.; Stožer, A.; Skelin Klemen, M.; Miller, E.W.; Slak Rupnik, M. The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLoS ONE 2013, 8, e82374. [Google Scholar] [CrossRef] [PubMed]
- Stožer, A.; Dolenšek, J.; Rupnik, M.S. Glucose-stimulated calcium dynamics in islets of langerhans in acute mouse pancreas tissue slices. PLoS ONE 2013, 8, e54638. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Mitchell, J.; Do, O.; Bax, J.; Rawlings, A.; Zavortink, M.; Morgan, G.; Parton, R.; Gaisano, H.; Thorn, P. Glucose principally regulates insulin secretion in mouse islets by controlling the numbers of granule fusion events per cell. Diabetologia 2013, 56, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kishimoto, T.; Nemoto, T.; Kadowaki, T.; Kasai, H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science 2002, 297, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Do, O.H.; Low, J.T.; Gaisano, H.Y.; Thorn, P. The secretory deficit in islets from db/db mice is mainly due to a loss of responding beta cells. Diabetologia 2014, 57, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Low, J.T.; Zavortink, M.; Mitchell, J.M.; Gan, W.J.; Do, O.H.; Schwiening, C.J.; Gaisano, H.Y.; Thorn, P. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia 2014, 57, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Chen, S.W.; Bellomo, E.A.; Tarasov, A.I.; Kaut, C.; Rutter, G.A.; Li, W.H. Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR). Proc. Natl. Acad. Sci. USA 2011, 108, 21063–21068. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, J.; Hodson, D.J.; Jobe, K.; Rutter, G.A.; Goldup, S.M.; Watkinson, M. Biologically targeted probes for Zn2+: A diversity oriented modular “click-snar-click” approach. Chem. Sci. 2014, 5, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Huang, Z.J.; Chen, S.W.; Hu, Z.P.; Li, W.H. Glp-1 receptor mediated targeting of a fluorescent Zn2+ sensor to beta cell surface for imaging insulin/Zn2+ release. Bioconjug. Chem. 2015, 26, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Almaca, J.; Liang, T.; Gaisano, H.Y.; Nam, H.G.; Berggren, P.O.; Caicedo, A. Spatial and temporal coordination of insulin granule exocytosis in intact human pancreatic islets. Diabetologia 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Q.; Ahooghalandari, P.; Gribble, F.M.; Reimann, F.; Tengholm, A.; Gylfe, E. Submembrane atp and Ca2+ kinetics in alpha-cells: Unexpected signaling for glucagon secretion. FASEB J. 2015, 29, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Rocheleau, J.V.; Head, W.S.; Piston, D.W. Quantitative NAD(P)H/Flavoprotein Autofluorescence Imaging Reveals Metabolic Mechanisms of Pancreatic Islet Pyruvate Response. J. Biol. Chem. 2004, 279, 31780–31787. [Google Scholar] [CrossRef] [PubMed]
- Tengholm, A. Cyclic amp dynamics in the pancreatic beta-cell. Ups. J. Med. Sci. 2012, 117, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Arrojo e Drigo, R.; Ali, Y.; Diez, J.; Srinivasan, D.; Berggren, P.-O.; Boehm, B. New insights into the architecture of the islet of langerhans: A focused cross-species assessment. Diabetologia 2015, 58, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Weir, S.; Sullivan, B.A.; Weir, G.C. Human islet morphology revisited: Human and rodent islets are not so different after all. J. Histochem. Cytochem. 2015, 63, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Armanet, M.; Morel, P.; Niclauss, N.; Sgroi, A.; Muller, Y.D.; Giovannoni, L.; Parnaud, G.; Berney, T. Unique arrangement of α- and β-cells in human islets of langerhans. Diabetes 2010, 59, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Brissova, M.; Fowler, M.J.; Nicholson, W.E.; Chu, A.; Hirshberg, B.; Harlan, D.M.; Powers, A.C. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 2005, 53, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.-O.; Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2339. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekera, P.C.; Pippin, J.J. Of rodents and men: Species-specific glucose regulation and type 2 diabetes research. Altex 2014, 31, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Levetan, C.S.; Pierce, S.M. Distinctions between the islets of mice and men: Implications for new therapies for type 1 and 2 diabetes. Endocr. Pract. 2013, 19, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.J.; Kim, A.; Miller, K.; Hara, M. Pancreatic islet plasticity interspecies comparison of islet architecture and composition. Islets 2010, 2, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Cohrs, C.M.; Tsata, V.; Chouinard, J.A.; Selck, C.; Stertmann, J.; Reichelt, S.; Rose, T.; Ehehalt, F.; Weitz, J.; et al. Using pancreas tissue slices for in situ studies of islet of langerhans and acinar cell biology. Nat. Protoc. 2014, 9, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- Rupnik, M. The physiology of rodent beta-cells in pancreas slices. Acta Physiol. 2009, 195, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Ogden, D. Microelectrode Techniques: The Plymouth Workshop Handbook, 2nd ed.; Company of Biologists: Cambridge, UK, 1994; p. 448. [Google Scholar]
- Neher, E. Ion channels for communication between and within cells. Science 1992, 256, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Speier, S.; Yang, S.B.; Sroka, K.; Rose, T.; Rupnik, M. Katp-channels in beta-cells in tissue slices are directly modulated by millimolar atp. Mol. Cell. Endocrinol. 2005, 230, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Efendic, S.; Rupnik, M. Ca2+-secretion coupling is impaired in diabetic goto kakizaki rats. J. Gen. Physiol. 2007, 129, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Sedej, S.; Tsujimoto, T.; Zorec, R.; Rupnik, M. Voltage-activated Ca2+ channels and their role in the endocrine function of the pituitary gland in newborn and adult mice. J. Physiol. 2004, 555, 769–782. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Rorsman, P. Oscillations, intercellular coupling, and insulin secretion in pancreatic β cells. PLoS Biol. 2006, 4. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.; Unger, R.H. Functional subdivision of islets of langerhans and possible role of D cells. Lancet 1975, 2, 1243–1244. [Google Scholar] [CrossRef]
- Ku, S.K.; Lee, H.S.; Lee, J.H. An immunohistochemical study on the pancreatic endocrine cells of the C57BL/6 mouse. J. Vet. Sci. 2002, 3, 327–333. [Google Scholar] [PubMed]
- Pfeifer, C.R.; Shomorony, A.; Aronova, M.A.; Zhang, G.; Cai, T.; Xu, H.; Notkins, A.L.; Leapman, R.D. Quantitative analysis of mouse pancreatic islet architecture by serial block-face SEM. J. Struct. Biol. 2015, 189, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Speier, S.; Nyqvist, D.; Kohler, M.; Caicedo, A.; Leibiger, I.B.; Berggren, P.O. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat. Protoc. 2008, 3, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Speier, S.; Nyqvist, D.; Cabrera, O.; Yu, J.; Molano, R.D.; Pileggi, A.; Moede, T.; Kohler, M.; Wilbertz, J.; Leibiger, B.; et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat. Med. 2008, 14, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Ilegems, E.; van Krieken, P.P.; Edlund, P.K.; Dicker, A.; Alanentalo, T.; Eriksson, M.; Mandic, S.; Ahlgren, U.; Berggren, P.O. Light scattering as an intrinsic indicator for pancreatic islet cell mass and secretion. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Camacho, P.; Lechleiter, J.D.; Herman, B. Measurement of intracellular calcium. Physiol. Rev. 1999, 79, 1089–1125. [Google Scholar] [PubMed]
- Miyawaki, A.; Griesbeck, O.; Heim, R.; Tsien, R.Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl. Acad. Sci. USA 1999, 96, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Hatsugai, N.; Horikawa, K.; Kobayashi, K.; Matsu-ura, T.; Mikoshiba, K.; Nagai, T. Auto-luminescent genetically-encoded ratiometric indicator for real-time Ca2+ imaging at the single cell level. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Pothier, P.; D’Orleans-Juste, P.; Simaan, M.; Jacques, D.; Jaalouk, D.; Belzile, F.; Hassan, G.; Boutin, C.; Haddad, G.; et al. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol. Cell. Biochem. 1997, 172, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Valdeolmillos, M.; Santos, R.M.; Contreras, D.; Soria, B.; Rosario, L.M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of langerhans. FEBS Lett. 1989, 259, 19–23. [Google Scholar] [CrossRef]
- Valdeolmillos, M.; Nadal, A.; Soria, B.; Garciasancho, J. Fluorescence digital image-analysis of glucose-induced Ca2+ oscillations in mouse pancreatic islets of langerhans. Diabetes 1993, 42, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Rosario, L.M.; Nadal, A.; Garcia-Sancho, J.; Soria, B.; Valdeolmillos, M. Widespread synchronous Ca oscillations due to bursting electrical activity in single pancreatic islets. Pflügers Arch. Eur. J. Physiol. 1991, 418, 417–422. [Google Scholar] [CrossRef]
- Gilon, P.; Henquin, J.C. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic b-cell. J. Biol. Chem. 1992, 267, 20713–20720. [Google Scholar] [PubMed]
- Zarkovic, M.; Henquin, J.-C. Synchronization and entrainment of cytoplasmic Ca2+ oscillations in cell clusters prepared from single or multiple mouse pancreatic islets. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E340–E347. [Google Scholar] [CrossRef] [PubMed]
- Ravier, M.R.; Sehlin, J.S.; Henquin, J.H. Disorganization of cytoplasmic Ca2+ oscillations and pulsatile insulin secretion in islets from ob/ob mice. Diabetologia 2002, 45, 1154–1163. [Google Scholar] [PubMed]
- Nadal, A.; Quesada, I.; Soria, B. Homologous and heterologous asynchronicity between identified α-, β- and δ-cells within intact islets of langerhans in the mouse. J. Physiol. 1999, 517, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Quesada, I.; Nadal, A.; Soria, B. Different effects of tolbutamide and diazoxide in alpha, beta-, and delta-cells within intact islets of langerhans. Diabetes 1999, 48, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Shibuya, I.; Iwanaga, T.; Niwa, K.; Kanno, T. Identification of alpha- and beta-cells in intact isolated islets of langerhans by their characteristic cytoplasmic Ca2+ concentration dynamics and immunocytochemical staining. Diabetes 1998, 47, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Lin, J.Y.; Frady, E.P.; Steinbach, P.A.; Kristan, W.B.; Tsien, R.Y. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc. Natl. Acad. Sci. USA 2012, 109, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P.; Shepherd, R.M.; Henquin, J.C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidenced in single pancreatic islets. J. Biol. Chem. 1993, 268, 22265–22268. [Google Scholar] [PubMed]
- Fernandez, J.; Valdeolmillos, M. Synchronous glucose-dependent [Ca2+]i oscillations in mouse pancreatic islets of langerhans recorded in vivo. FEBS Lett. 2000, 477, 33–36. [Google Scholar] [CrossRef]
- Bertuzzi, F.; Davalli, A.M.; Nano, R.; Socci, C.; Codazzi, F.; Fesce, R.; Di Carlo, V.; Pozza, G.; Grohovaz, F. Mechanisms of coordination of Ca2+ signals in pancreatic islet cells. Diabetes 1999, 48, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.; Carroll, P.B.; Ricordi, C.; Boschero, A.C.; Stojilkovic, S.S.; Atwater, I. Control of cytosolic free calcium in cultured human pancreatic beta-cells occurs by external calcium-dependent and independent mechanisms. Endocrinology 1994, 134, 1771–1781. [Google Scholar] [PubMed]
- Hellman, B.; Gylfe, E.; Bergsten, P.; Grapengiesser, E.; Lund, P.; Berts, A.; Tengholm, A.; Pipeleers, D.; Ling, Z. Glucose induces oscillatory Ca2+ signalling and insulin release in human pancreatic beta cells. Diabetologia 1994, 37, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Soria, B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium 1996, 20, 409–414. [Google Scholar] [CrossRef]
- Quesada, I.; Todorova, M.G.; Alonso-Magdalena, P.; Beltrá, M.; Carneiro, E.M.; Martin, F.; Nadal, A.; Soria, B. Glucose induces opposite intracellular Ca2+ concentration oscillatory patterns in identified α- and β-cells within intact human islets of langerhans. Diabetes 2006, 55, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Hodson, D.J. Minireview: Intraislet regulation of insulin secretion in humans. Mol. Endocrinol. 2013, 27, 1984–1995. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.; Fromherz, P. Anellated hemicyanine dyes in a neuron membrane: Molecular stark effect and optical voltage recording. J. Phys. Chem. B 2003, 107, 7903–7913. [Google Scholar] [CrossRef]
- Grinvald, A.; Fine, A.; Farber, I.C.; Hildesheim, R. Fluorescence monitoring of electrical responses from small neurons and their processes. Biophys. J. 1983, 42, 195–198. [Google Scholar] [CrossRef]
- Merglen, A.; Theander, S.; Rubi, B.; Chaffard, G.; Wollheim, C.B.; Maechler, P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 2004, 145, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Hjortoe, G.M.; Hagel, G.M.; Terry, B.R.; Thastrup, O.; Arkhammar, P.O.G. Functional identification and monitoring of individual α and β cells in cultured mouse islets of langerhans. Acta Diabetol. 2004, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Heart, E.; Yaney, G.C.; Corkey, R.F.; Schultz, V.; Luc, E.; Liu, L.; Deeney, J.T.; Shirihai, O.; Tornheim, K.; Smith, P.J.S.; et al. Ca2+, NAD(P)H and membrane potential changes in pancreatic β-cells by methyl succinate: Comparison with glucose. Biochem. J. 2007, 403, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Goehring, I.; Gerencser, A.A.; Schmidt, S.; Brand, M.D.; Mulder, H.; Nicholls, D.G. Plasma membrane potential oscillations in insulin secreting INS-1 832/13 cells do not require glycolysis and are not initiated by fluctuations in mitochondrial bioenergetics. J. Biol. Chem. 2012, 287, 15706–15717. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.E.; Tsien, R.Y. Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem. Biol. 1997, 4, 269–277. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Bindokas, V.P.; Marks, J.D.; Philipson, L.H. Fret-based voltage probes for confocal imaging: Membrane potential oscillations throughout pancreatic islets. Am. J. Physiol. Cell Physiol. 2005, 289, C224–C229. [Google Scholar] [CrossRef] [PubMed]
- Woodford, C.R.; Frady, E.P.; Smith, R.S.; Morey, B.; Canzi, G.; Palida, S.F.; Araneda, R.C.; Kristan, W.B., Jr.; Kubiak, C.P.; Miller, E.W.; et al. Improved pet molecules for optically sensing voltage in neurons. J. Am. Chem. Soc. 2015, 137, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, F.; Marshall, J.D.; Yang, Y.; Gong, Y.Y.; Schnitzer, M.J.; Lin, M.Z. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 2014, 17, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Akemann, W.; Sasaki, M.; Mutoh, H.; Imamura, T.; Honkura, N.; Knopfel, T. Two-photon voltage imaging using a genetically encoded voltage indicator. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Akemann, W.; Mutoh, H.; Perron, A.; Park, Y.K.; Iwamoto, Y.; Knopfel, T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 2012, 108, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S. Fluorescence probes for membrane potentials based on mesoscopic electron transfer. Nano Lett. 2007, 7, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Walker, A.S.; Miller, E.W. A photostable silicon rhodamine platform for optical voltage sensing. J. Am. Chem. Soc. 2015, 137, 10767–10776. [Google Scholar] [CrossRef] [PubMed]
- Coupe, P.; Hellier, P.; Kervrann, C.; Barillot, C. Nonlocal means-based speckle filtering for ultrasound images. IEEE Trans. Image Proc. Publ. IEEE Signal Proc. Soc. 2009, 18, 2221–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanfar, P. A tour of modern image filtering: New insights and methods, both practical and theoretical. Signal Proc. Mag. IEEE 2013, 30, 106–128. [Google Scholar] [CrossRef]
- Akgun, T.; Altunbasak, Y.; Mersereau, R.M. Super-resolution reconstruction of hyperspectral images. IEEE Trans. Image Proc. 2005, 14, 1860–1875. [Google Scholar] [CrossRef]
- Špelič, D.; Dolenšek, J.; Stožer, A.; Slak Rupnik, M.; Žalik, B.; Mongus, D. Improved analysis of membrane potential oscillations in the network of cells from islet of langerhans. In Proceedings of the GraphiCon’2013—23rd International Conference on Computer Graphics and Vision, Vladivostok, Russia, 16–20 September 2013.
- Portilla, J.; Strela, V.; Wainwright, M.J.; Simoncelli, E.P. Image denoising using scale mixtures of gaussians in the wavelet domain. IEEE Trans. Image Proc. 2003, 12, 1338–1351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Hirakawa, K. Minimum risk wavelet shrinkage operator for poisson image denoising. IEEE Trans. Image Proc. 2015, 24, 1660–1671. [Google Scholar] [CrossRef]
- Boutet de Monvel, J.; Le Calvez, S.; Ulfendahl, M. Image restoration for confocal microscopy: Improving the limits of deconvolution, with application to the visualization of the mammalian hearing organ. Biophys. J. 2001, 80, 2455–2470. [Google Scholar] [CrossRef]
- Kohler, M.; Zaitsev, S.V.; Zaitseva, I.I.; Leibiger, B.; Leibiger, I.B.; Turunen, M.; Kapelioukh, I.L.; Bakkman, L.; Appelskog, I.B.; de Monvel, J.B.; et al. On-line monitoring of apoptosis in insulin-secreting cells. Diabetes 2003, 52, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, J.; Kervrann, C.; Bouthemy, P.; Elbau, P.; Sibarita, J.B.; Salamero, J. Patch-based nonlocal functional for denoising fluorescence microscopy image sequences. IEEE Trans. Med. Imaging 2010, 29, 442–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggioni, M.; Sanchez-Monge, E.; Foi, A. Joint removal of random and fixed-pattern noise through spatiotemporal video filtering. IEEE Trans. Image Proc. 2014, 23, 4282–4296. [Google Scholar] [CrossRef] [PubMed]
- Janicek, R.; Hotka, M.; Zahradnikova, A., Jr.; Zahradnikova, A.; Zahradnik, I. Quantitative analysis of calcium spikes in noisy fluorescent background. PLoS ONE 2013, 8, e64394. [Google Scholar] [CrossRef] [PubMed]
- Janiek, R.; Zahradnikova, A., Jr.; Polakova, E.; Pavelkova, J.; Zahradnik, I.; Zahradnikova, A. Calcium spike variability in cardiac myocytes results from activation of small cohorts of ryanodine receptor 2 channels. J. Physiol. 2012, 590, 5091–5106. [Google Scholar] [CrossRef] [PubMed]
- Klee, P.; Allagnat, F.; Pontes, H.; Cederroth, M.; Charollais, A.; Caille, D.; Britan, A.; Haefliger, J.A.; Meda, P. Connexins protect mouse pancreatic beta cells against apoptosis. J. Clin. Investig. 2011, 121, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.P.F.; Oliveira, R.B.; Britan, A.; Santos-Silva, J.C.; Boschero, A.C.; Meda, P.; Collares-Buzato, C.B. Impaired β-cell-β-cell coupling mediated by Cx36 gap junctions in prediabetic mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E144–E151. [Google Scholar] [CrossRef] [PubMed]
- Cigliola, V.; Chellakudam, V.; Arabieter, W.; Meda, P. Connexins and β-cell functions. Diabetes Res. Clin. Pract. 2013, 99, 250–259. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolenšek, J.; Špelič, D.; Klemen, M.S.; Žalik, B.; Gosak, M.; Rupnik, M.S.; Stožer, A. Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis. Sensors 2015, 15, 27393-27419. https://doi.org/10.3390/s151127393
Dolenšek J, Špelič D, Klemen MS, Žalik B, Gosak M, Rupnik MS, Stožer A. Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis. Sensors. 2015; 15(11):27393-27419. https://doi.org/10.3390/s151127393
Chicago/Turabian StyleDolenšek, Jurij, Denis Špelič, Maša Skelin Klemen, Borut Žalik, Marko Gosak, Marjan Slak Rupnik, and Andraž Stožer. 2015. "Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis" Sensors 15, no. 11: 27393-27419. https://doi.org/10.3390/s151127393
APA StyleDolenšek, J., Špelič, D., Klemen, M. S., Žalik, B., Gosak, M., Rupnik, M. S., & Stožer, A. (2015). Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis. Sensors, 15(11), 27393-27419. https://doi.org/10.3390/s151127393





