An Overview on Marine Sponge-Symbiotic Bacteria as Unexhausted Sources for Natural Product Discovery
Abstract
1. Introduction
2. Symbiosis in Marine Environments
3. The Sponge Host and Its Symbiotic Microbial Community
4. Diversity of Sponge Symbiotic Microorganisms
5. Sponge-Derived Bioactive Compounds
6. Production of Natural Products by Sponge Symbionts
7. Detection and Isolation of Sponge-Symbiotic Bacteria
8. Genomic Advances Changing the Scene of Marine Biodiscovery
9. Metagenomics
10. Cryptic/Silent Biosynthetic Pathways
11. Altering Growth Conditions and Co-Cultivation of Microorganisms
12. Status of Marine Sponge-Microbial Natural Product Discovery
13. Ecological Impact of the Surrounding Environment on Sponges and Their Microbial Communities
14. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davies, J. Where have all the antibiotics gone? Can. J. Infect. Dis Med. Microbiol. 2006, 17, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Tulp, M.; Bohlin, L. Rediscovery of known natural compounds: Nuisance or goldmine? Bioorgan. Med. Chem. 2005, 13, 5274–5282. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. Eg49 and Nocardiopsis sp. Rv163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Fernandes, P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs 2010, 8, 705–727. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.S. New delhi metallo-beta-lactamases: A wake-up call for microbiologists. Indian J. Med. Microbiol. 2010, 28, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotics: A new hope. Chem. Biol. 2012, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. R. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.H.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Fenical, W. Chemical studies of marine bacteria: Developing a new resource. Chem. Rev. 1993, 93, 1673–1683. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kim, S.-K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid.-Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Wright, G. Introduction: Antibiotic resistance. Chem. Rev. 2005, 105, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Fenical, W. Strategies for the discovery of secondary metabolites from marine bacteria: Ecological perspectives. Annu. Rev. Microbiol. 1994, 48, 559–584. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.F.; Vagstad, A.L.; Piel, J. Polytheonamide biosynthesis showcasing the metabolic potential of sponge-associated uncultivated ‘entotheonella’bacteria. Curr. Opin. Chem. Biol. 2016, 31, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Fujimoto, J.; Nishisaka, F.; Matsumoto, S.; Harunari, E.; Imada, C.; Matsuzaki, T. Bacterial production of the tunicate-derived antitumor cyclic depsipeptide didemnin b. J. Nat. Prod. 2011, 74, 2329–2331. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elela, G.; Abd-Elnaby, H.; Ibrahim, H.; Okbah, M. Marine natural products and their potential applications as anti-infective agents. World Appl. Sci. J. 2009, 7, 872–880. [Google Scholar]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Dobler, I.; Beil, W.; Lang, S.; Meiners, M.; Laatsch, H. Integrated approach to explore the potential of marine microorganisms for the production of bioactive metabolites. Adv. Biochem. Eng. Biotechnol. 2002, 74, 207–238. [Google Scholar] [PubMed]
- Mukherjee, J.; Llewellyn, L.E.; Evans-Illidge, E.A. A tropical marine microbial natural products geobibliography as an example of desktop exploration of current research using web visualisation tools. Mar. Drugs 2008, 6, 550–577. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.B.; Mayer, A.M. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Talapatra, S.; Swarnakar, S. Bioactive compounds from marine invertebrates for potential medicines—An overview. Int. Lett. Nat. Sci. 2015, 34, 42–61. [Google Scholar] [CrossRef]
- Salomon, C.E.; Magarvey, N.A.; Sherman, D.H. Merging the potential of microbial genetics with biological and chemical diversity: An even brighter future for marine natural product drug discovery. Nat. Prod. Rep. 2004, 21, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, M.; Martens, D.; Wijffels, R.H. Towards commercial production of sponge medicines. Mar. Drugs 2009, 7, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E.; Sackett, D.L.; Vourloumis, D.; Nicolaou, K.C. The coral-derived natural products eleutherobin and sarcodictyins A and B: Effects on the assembly of purified tubulin with and without microtubule-associated proteins and binding at the polymer taxoid site. Biochemistry 1999, 38, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.K.; Haygood, M.G. Identification of sibling species of the bryozoan bugula neritina that produce different anticancer bryostatins and harbor distinct strains of the bacterial symbiont “candidatus endobugula sertula”. Biol. Bull. 1999, 196, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Muller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, D.J. Marine pharmacology. Antonie Van Leeuwenhoek 2000, 77, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A. Farming sponges to supply bioactive metabolites and bath sponges: A review. Mar. Biotechnol. 2009, 11, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Schippers, K.J.; Sipkema, D.; Osinga, R.; Smidt, H.; Pomponi, S.A.; Martens, D.E.; Wijffels, R.H. 6 cultivation of sponges, sponge cells and symbionts: Achievements and future prospects. Adv. Mar. Biol. 2012, 62, 273. [Google Scholar] [PubMed]
- Leelavathi, M.; Kumar, S.; Vani, L. Molecular phylogeny of marine symbiotic bacteria associated with sponges from the water off the coast south east of india. WJPPS 2014, 3, 894–902. [Google Scholar]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Deerinck, T.; Ellisman, M.; Faulkner, D. The cellular localization of dercitamide in the palauan sponge oceanapia sagittaria. Mar. Biol. 2001, 139, 313–319. [Google Scholar]
- Davidson, S.; Allen, S.; Lim, G.; Anderson, C.; Haygood, M. Evidence for the biosynthesis of bryostatins by the bacterial symbiont “candidatus endobugula sertula” of the bryozoanbugula neritina. Appl. Environ. Microbiol. 2001, 67, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Kjelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, C.C.P.; Costa, R. Microbial communities and bioactive compounds in marine sponges of the family irciniidae—A review. Mar. Drugs 2014, 12, 5089–5122. [Google Scholar] [CrossRef] [PubMed]
- De Bary, A. Die Erscheinung der Symbiose; Verlag von Karl J. Trübner: Strassburg, France, 1879. [Google Scholar]
- Webster, N.S.; Taylor, M.W. Marine sponges and their microbial symbionts: Love and other relationships. Environ. Microbiol. 2012, 14, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; Yan, L.; Boyd, K.G.; Wright, P.C.; Burgess, J.G. The symbiotic role of marine microbes on living surfaces. Hydrobiologia 2001, 461, 37–40. [Google Scholar] [CrossRef]
- Lopanik, N.B. Chemical defensive symbioses in the marine environment. Funct. Ecol. 2014, 28, 328–340. [Google Scholar] [CrossRef]
- Weis, V.M.; Reynolds, W.S.; Krupp, D.A. Host-symbiont specificity during onset of symbiosis between the dinoflagellates symbiodinium spp. And planula larvae of the scleractinian coral fungia scutaria. Coral Reefs 2001, 20, 301–308. [Google Scholar] [CrossRef]
- Lindquist, N.; Barber, P.H.; Weisz, J.B. Episymbiotic microbes as food and defence for marine isopods: Unique symbioses in a hostile environment. Proc. R Soc. Lond. B Biol. Sci. 2005, 272, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Rusch, D.; DeMaere, M.Z.; Yung, P.Y.; Lewis, M.; Halpern, A.; Heidelberg, K.B.; Egan, S.; Steinberg, P.D.; Kjelleberg, S. Functional genomic signatures of sponge bacteria reveal unique and shared features of symbiosis. ISME J. 2010, 4, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.J.; Thacker, R.W. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol. Oceanogr. 2011, 56, 1577–1586. [Google Scholar] [CrossRef]
- Chaston, J.; Goodrich-Blair, H. Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol. Rev. 2010, 34, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J. Ind. Microbiol. Biot. 2006, 33, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.J.; Arthur, K.E.; Ritson-Williams, R.; Ross, C.; Sharp, K. Chemical defenses: From compounds to communities. Biol. Bull. 2007, 213, 226–251. [Google Scholar] [CrossRef] [PubMed]
- Haygood, M.G.; Schmidt, E.W.; Davidson, S.K.; Faulkner, D.J. Microbial symbionts of marine invertebrates: Opportunities for microbial biotechnology. J. Mol. Microb. Biotechnol. 1999, 1, 33–43. [Google Scholar]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef] [PubMed]
- Gil-Turnes, M.S.; Hay, M.E.; Fenical, W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 1989, 246, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Müller, W.E.G. Molecular approaches to study stress adaptation, bioactivity and phylogenetic relationships within the porifera. Fundam. Gen. Proc. Mech. 2002, 20, 67–69. [Google Scholar]
- Hill, M.; Hill, A.; Lopez, N.; Harriott, O. Sponge-specific bacterial symbionts in the caribbean sponge, chondrilla nucula (demospongiae, chondrosida). Mar. Biol. 2006, 148, 1221–1230. [Google Scholar] [CrossRef]
- Cebrian, E.; Uriz, M.J.; Turon, X. Sponges as biomonitors of heavy metals in spatial and temporal surveys in northwestern mediterranean: Multispecies comparison. Environ. Toxicol. Chem. 2007, 26, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Monaco, R.; Quinlan, R. Novel natural product discovery from marine sponges and their obligate symbiotic organisms. Biorxiv 2014. [Google Scholar] [CrossRef]
- Rosenberg, E.; Sharon, G.; Atad, I.; Zilber-Rosenberg, I. The evolution of animals and plants via symbiosis with microorganisms. Environ. Microbiol. Rep. 2010, 2, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, U.; Hopke, J.; Horn, M.; Friedrich, A.B.; Wagner, M.; Hacker, J.; Moore, B.S. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 2002, 68, 4431–4440. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global diversity of sponges (porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.B.; Fischer, I.; Proksch, P.; Hacker, J.; Hentschel, U. Temporal variation of the microbial community associated with the mediterranean sponge aplysina aerophoba. FEMS Microbiol. Ecol. 2001, 38, 105–113. [Google Scholar] [CrossRef]
- Webster, N.S.; Wilson, K.J.; Blackall, L.L.; Hill, R.T. Phylogenetic diversity of bacteria associated with the marine sponge rhopaloeides odorabile. Appl. Environ. Microbiol. 2001, 67, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Negri, A.P.; Munro, M.M.H.G.; Battershill, C.N. Diverse microbial communities inhabit antarctic sponges. Environ. Microbiol. 2004, 6, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.B.; McCarthy, P.J. Associated bacterial communities of two deep-water sponges. Aquat. Microb. Ecol. 2005, 39, 47–55. [Google Scholar] [CrossRef]
- Thiel, V.; Neulinger, S.C.; Staufenberger, T.; Schmaljohann, R.; Imhoff, J.F. Spatial distribution of sponge-associated bacteria in the mediterranean sponge tethya aurantium. FEMS Microbiol. Ecol. 2007, 59, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Wichels, A.; Würtz, S.; Döpke, H.; Schütt, C.; Gerdts, G. Bacterial diversity in the breadcrumb sponge halichondria panicea (pallas). FEMS Microbial. Ecol. 2006, 56, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Wehrl, M.; Steinert, M.; Hentschel, U. Bacterial uptake by the marine sponge aplysina aerophoba. Microb. Ecol. 2007, 53, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, U.; Piel, J.; Degnan, S.M.; Taylor, M.W. Genomic insights into the marine sponge microbiome. Nat. Rev. Microbiol. 2012, 10, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Thacker, R.W.; Freeman, C.J. 2 Sponge-microbe symbioses: Recent advances and new directions. Adv. Mar. Boil. 2012, 62, 57. [Google Scholar]
- Selvin, J.; Ninawe, A.; Seghal Kiran, G.; Lipton, A. Sponge-microbial interactions: Ecological implications and bioprospecting avenues. Crit. Rev. Microbiol. 2010, 36, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Schupp, P.J.; Dahllöf, I.; Kjelleberg, S.; Steinberg, P.D. Host specificity in marine sponge-associated bacteria, and potential implications for marine microbial diversity. Environ. Microbiol. 2004, 6, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Althoff, K.; Schütt, C.; Steffen, R.; Batel, R.; Mueller, W.E.G. Evidence for a symbiosis between bacteria of the genus rhodobacter and the marine sponge halichondria panicea: Harbor also for putatively toxic bacteria? Mar. Biol. 1998, 130, 529–536. [Google Scholar] [CrossRef]
- Olson, J.B.; Gochfeld, D.J.; Slattery, M. Aplysina red band syndrome: A new threat to caribbean sponges. Dis. Aquat. Organ. 2006, 71, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl. Acad. Sci. USA 2007, 104, 8627–8633. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, J.-H.; Lee, H.K. Microbial symbiosis in marine sponges. J. Microbiol. 2001, 39, 254–264. [Google Scholar]
- Hill, R.T. Microbes from marine sponges: A treasure trove of biodiversity for natural products discovery. In Microbial Diversity and Bioprospecting; Bull, A.T., Ed.; ASM Press: Washington, DC, USA, 2004; pp. 177–190. [Google Scholar]
- Ereskovsky, A.V.; Gonobobleva, E.; Vishnyakov, A. Morphological evidence for vertical transmission of symbiotic bacteria in the viviparous sponge halisarca dujardini johnston (porifera, demospongiae, halisarcida). Mar. Biol. 2005, 146, 869–875. [Google Scholar] [CrossRef]
- Enticknap, J.J.; Kelly, M.; Peraud, O.; Hill, R.T. Characterization of a culturable alphaproteobacterial symbiont common to many marine sponges and evidence for vertical transmission via sponge larvae. Appl. Environ. Microb. 2006, 72, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Weisz, J.B.; Lindquist, N.; Hentschel, U. Vertical transmission of a phylogenetically complex microbial consortium in the viviparous sponge ircinia felix. Appl. Environ. Microb. 2007, 73, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Sharp, K.H.; Eam, B.; Faulkner, D.J.; Haygood, M.G. Vertical transmission of diverse microbes in the tropical sponge Corticium sp. Appl. Environ. Microb. 2007, 73, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Taylor, M.W.; Behnam, F.; Lucker, S.; Rattei, T.; Whalan, S.; Horn, M.; Wagner, M. Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ. Microbiol. 2010, 12, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.M.; Colman, A.S.; Tal, Y.; Hill, R.T. Diversity and expression of nitrogen fixation genes in bacterial symbionts of marine sponges. Environ. Microbial. 2008, 10, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Yang, C.; Horn, H.; Hajjar, D.; Ravasi, T.; Hentschel, U. Actinomycetes from red sea sponges: Sources for chemical and phylogenetic diversity. Mar. Drugs 2014, 12, 2771–2789. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, L.; Hentschel, U. An environmental genomics perspective on the diversity and function of marine sponge-associated microbiota. Curr. Opin. Microbiol. 2007, 10, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Tsai, P.; Bell, J.; Fromont, J.; Ilan, M.; Lindquist, N.; Perez, T.; Rodrigo, A.; Schupp, P.J.; Vacelet, J. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. ISME J. 2012, 6, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Fieseler, L.; Horn, M.; Wagner, M.; Hentschel, U. Discovery of the novel candidate phylum "poribacteria" in marine sponges. Appl. Environ. Microbiol. 2004, 70, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Mori, T.; Rückert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.E.; Heycke, N.; Schmitt, S. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.O.; Wong, Y.H.; Qian, P.-Y. Inter-and intraspecific variations of bacterial communities associated with marine sponges from San Juan Island, Washington. Appl. Environ. Microbiol. 2009, 75, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Magnino, G.; Sarà, A.; Lancioni, T.; Gaino, E. Endobionts of the coral reef sponge theonella swinhoei (porifera, demospongiae). Invertebr. Biol. 1999, 118, 213–220. [Google Scholar] [CrossRef]
- Simister, R.L.; Deines, P.; Botte, E.S.; Webster, N.S.; Taylor, M.W. Sponge-specific clusters revisited: A comprehensive phylogeny of sponge-associated microorganisms. Environ. Microbiol. 2012, 14, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Takagi, M.; Shin-Ya, K. Diversity, salt requirement, and antibiotic production of actinobacteria isolated from marine sponges. Actinomycetologica 2010, 24, 18–23. [Google Scholar] [CrossRef]
- Webster, N.S.; Hill, R.T. The culturable microbial community of the Great Barrier Reef sponge rhopaloeides odorabile is dominated by an α-proteobacterium. Mar. Biol. 2001, 138, 843–851. [Google Scholar] [CrossRef]
- Kennedy, J.; Baker, P.; Piper, C.; Cotter, P.D.; Walsh, M.; Mooij, M.J.; Bourke, M.B.; Rea, M.C.; O’Connor, P.M.; Ross, R.P. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge haliclona simulans collected from irish waters. Mar. Biotechnol. 2009, 11, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Kennedy, J.; Margassery, L.M.; Morrissey, J.P.; O’Gara, F.; Dobson, A.D.W. Diversity and antimicrobial activities of microbes from two irish marine sponges, Suberites carnosus and Leucosolenia sp. J. Appl. Microbiol. 2012, 112, 289–301. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, J.A.; Barbosa, T.M.; Morrissey, J.P.; Kennedy, J.; O’Gara, F.; Dobson, A.D.W. Diversity and antimicrobial activity of Pseudovibrio spp. From irish marine sponges. J. Appl. Microbiol. 2011, 110, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Margassery, L.M.; Kennedy, J.; O’Gara, F.; Dobson, A.D.; Morrissey, J.P. Diversity and antibacterial activity of bacteria isolated from the coastal marine sponges amphilectus fucorum and eurypon major. Lett. Appl. Microbiol. 2012, 55, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.I.S.; Hardoim, C.C.P.; Xavier, J.R.; Gonçalves, J.M.S.; Costa, R. Molecular richness and biotechnological potential of bacteria cultured from irciniidae sponges in the north-east atlantic. FEMS Microbiol. Ecol. 2013, 85, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.B.A.; Bonugli-Santos, R.C.; Miqueletto, P.B.; Passarini, M.R.Z.; Silva, C.H.D.; Justo, M.R.; Leal, R.R.; Fantinatti-Garboggini, F.; Oliveira, V.M.; Berlinck, R.G.S. Microbial diversity associated with algae, ascidians and sponges from the north coast of São Paulo State, Brazil. Microbiol. Res. 2010, 165, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.M.; Cicirelli, E.M.; Kan, J.; Chen, F.; Fuqua, C.; Hill, R.T. Diversity and quorum-sensing signal production of proteobacteria associated with marine sponges. Environ. Microbial. 2008, 10, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Simister, R.; Taylor, M.W.; Rogers, K.M.; Schupp, P.J.; Deines, P. Temporal molecular and isotopic analysis of active bacterial communities in two New Zealand sponges. FEMS Microbiol. Ecol. 2013, 85, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Erwin, P.M.; Pita, L.; López-Legentil, S.; Turon, X. Stability of sponge-associated bacteria over large seasonal shifts in temperature and irradiance. Appl. Environ. Microb. 2012, 78, 7358–7368. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, U.; Fieseler, L.; Wehrl, M.; Gernert, C.; Steinert, M.; Hacker, J.; Horn, M. Microbial diversity of marine sponges. Prog. Mol. Subcell. Biol. 2003, 37, 59–88. [Google Scholar] [PubMed]
- Gazave, E.; Lapébie, P.; Ereskovsky, A.V.; Vacelet, J.; Renard, E.; Cárdenas, P.; Borchiellini, C. No longer demospongiae: Homoscleromorpha formal nomination as a fourth class of porifera. Hydrobiologia 2012, 687, 3–10. [Google Scholar] [CrossRef]
- Uriz, M.J.; Martin, D.; Rosell, D. Relationships of biological and taxonomic characteristics to chemically mediated bioactivity in mediterranean littoral sponges. Mar. Biol. 1992, 113, 287–297. [Google Scholar]
- Hay, M.E.; Fenical, W. Chemical ecology and marine biodiversity: Insights and products from the sea. Oceanography 1996, 9, 10–20. [Google Scholar] [CrossRef]
- Hill, R.; Peraud, O.; Hamann, M.; Kasanah, N. Manzamine-Producing Actinomycetes. U.S. Patent Application No. 10/522454, 3 November 2005. [Google Scholar]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Reynolds, D.; Liu, M.; Stark, M.; Kjelleberg, S.; Webster, N.S.; Thomas, T. Functional equivalence and evolutionary convergence in complex communities of microbial sponge symbionts. Proc. Natl. Acad. Sci. USA 2012, 109, E1878–E1887. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Hu, Y.; Huang, Y.Q.; Huang, Y. Isolation and phylogenetic analysis of the biologically active bacteria associated with three south china sea sponges. Microbiology 2007, 76, 494–499. [Google Scholar] [CrossRef]
- Zhang, L.; An, R.; Wang, J.; Sun, N.; Zhang, S.; Hu, J.; Kuai, J. Exploring novel bioactive compounds from marine microbes. Curr. Opin. Chem. Biol. 2005, 8, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.C.; Cardellina Ii, J.H.; Singleton, F.L. A marinemicrococcus produces metabolites ascribed to the spongetedania ignis. Experientia 1988, 44, 1021. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, T.; Egami, Y.; Nakashima, Y.; Wakimoto, Y.; Mori, T.; Awakawa, T.; Ito, T.; Kenmoku, H.; Asakawa, Y.; Piel, J.; et al. Calyculin biogenesis from a pyrophosphate protoxin produced by a sponge symbiont. Nat. Chem. Biol. 2014, 10, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Oclarit, J.M.; Okada, H.; Ohta, S.; Kaminura, K.; Yamaoka, Y.; Iizuka, T.; Miyashiro, S.; Ikegami, S. Anti-bacillus substance in the marine sponge, hyatella species, produced by an associated vibrio species bacterium. Microbios 1994, 78, 7–16. [Google Scholar] [PubMed]
- Schmidt, E.W.; Bewley, C.A.; Faulkner, D.J. Theopalauamide, a bicyclic glycopeptide from filamentous bacterial symbionts of the lithistid sponge theonella swinhoei from palau and mozambique. J. Org. Chem. 1998, 63, 1254–1258. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Obraztsova, A.Y.; Davidson, S.K.; Faulkner, D.J.; Haygood, M.G. Identification of the antifungal peptide-containing symbiont of the marine sponge theonella swinhoei as a novel δ-proteobacterium,“candidatus entotheonella palauensis”. Mar. Biol. 2000, 136, 969–977. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Knight, J.C.; Collins, J.C.; Herald, D.L.; Pettit, R.K.; Boyd, M.R.; Young, V.G. Antineoplastic agents 430. Isolation and structure of cribrostatins 3, 4, and 5 from the republic of maldives cribrochalina species 1. J. Nat. Prod. 2000, 63, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three new manzamine alkaloids from a common indonesian sponge and their activity against infectious and tropical parasitic diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Okada, Y.; Fusetani, N.; van Soest, R.W.M. An antimicrobial c14 acetylenic acid from a marine sponge oceanapia species. J. Nat. Prod. 2000, 63, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Gulavita, N.K.; Gunasekera, S.P.; Pomponi, S.A.; Longley, R.E.; McCarthy, P.J. Antitumor and Antibacterial Peptide and Methods of Use. U.S. Patent 5,516,755, 14 May 1996. [Google Scholar]
- Gul, W.; Hammond, N.L.; Yousaf, M.; Peng, J.; Holley, A.; Hamann, M.T. Chemical transformation and biological studies of marine sesquiterpene (s)-(+)-curcuphenol and its analogs. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.H.H.L.; Grube, A.; Köck, M.; Berlinck, R.G.S.; Macedo, M.L.; Ferreira, A.G.; Hajdu, E. Ingenamine G and cyclostellettamines G−I, K, and L from the New Brazilian species of marine sponge Pachychalina sp. J. Nat. Prod. 2004, 67, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.H.H.L.; Seleghim, M.H.R.; Timm, C.; Grube, A.; Köck, M.; Nascimento, G.G.F.; Martins, A.C.T.; Silva, E.G.O.; De Souza, A.O.; Minarini, P.R.R. Antimicrobial and antimycobacterial activity of cyclostellettamine alkaloids from sponge Pachychalina sp. Mar. Drugs 2006, 4, 1–8. [Google Scholar] [CrossRef]
- Grube, A.; Assmann, M.; Lichte, E.; Sasse, F.; Pawlik, J.R.; Köck, M. Bioactive metabolites from the caribbean sponge aka coralliphagum. J. Nat. Prod. 2007, 70, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Wang, B.-G.; Wiryowidagdo, S.; Wray, V.; van Soest, R.; Steube, K.G.; Guan, H.-S.; Proksch, P.; Ebel, R. Melophlins C−O, thirteen novel tetramic acids from the marine sponge melophlus sarassinorum. J. Nat. Prod. 2003, 66, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; Dilip de Silva, E.; Lassota, P.; Allen, T.M. Papuamides A−D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in papua new guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Sobel, C.; Diehl-Seifert, B.; Maidhof, A.; Schröder, H.C. Influence of the antileukemic and anti-human immunodeficiency virus agent avarol on selected immune responses in vitro and in vivo. Biochem. Pharmacol. 1987, 36, 1489–1494. [Google Scholar] [CrossRef]
- Wellington, K.D.; Cambie, R.C.; Rutledge, P.S.; Bergquist, P.R. Chemistry of sponges. 19. Novel bioactive metabolites from Hamigera tarangaensis. J. Nat. Prod. 2000, 63, 79–85. [Google Scholar]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G.; Thompson, A.M. Antiviral and antitumor agents from a New Zealand sponge, Mycale sp. 2. Structures and solution conformations of mycalamides a and b. J. Org. Chem. 1990, 55, 223–227. [Google Scholar] [CrossRef]
- Yousaf, M.; Hammond, N.L.; Peng, J.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.S.; Hamann, M.T. New manzamine alkaloids from an indo-pacific sponge. Pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, aids opportunistic infections, and inflammatory diseases. J. Med. Chem. 2004, 47, 3512. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Kelly-Borges, M. Hamacanthins A and B, new antifungal bis indole alkaloids from the deep-water marine sponge, Hamacantha sp. J. Nat. Prod. 1994, 57, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, D.C.; Rimoldi, J.M.; Clark, A.M.; Kelly, M.; Hamann, M.T. Anti-cryptococcal and nitric oxide synthase inhibitory imidazole alkaloids from the calcareous sponge Leucetta cf chagosensis. Tetrahedron 2000, 56, 8795–8798. [Google Scholar] [CrossRef]
- Zhou, G.-X.; Molinski, T.F. Manoalide derivatives from a sponge, Luffariella sp. J. Asian Nat. Prod. Res. 2006, 8, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, P.; Zidane, M.; Abdala, H.; Moré, M.-T. A glycoprotein isolated from the sponge, Pachymatisma johnstonii, has anti-leishmanial activity. Cell. Biol. Int. 2000, 24, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, H.; Shimomura, M.; Kimura, H.; Yamada, Y.; Kim, H.-S.; Yusuke, W. Antimalarial activity of kalihinol a and new relative diterpenoids from the okinawan sponge, Acanthella sp. Tetrahedron 1998, 54, 13467–13474. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Angerhofer, C.K. Novel potent antimalarial diterpene isocyanates, isothiocyanates, and isonitriles from the tropical marine sponge Cymbastela hooperi. J. Org. Chem. 1996, 61, 3259–3267. [Google Scholar] [CrossRef]
- Hua, H.M.; Peng, J.; Fronczek, F.R.; Kelly, M.; Hamann, M.T. Crystallographic and NMR studies of antiinfective tricyclic guanidine alkaloids from the sponge Monanchora unguifera. Bioorgan. Med. Chem. 2004, 12, 6461–6464. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.P.; Bondoso, J.; Gaspar, H.; Xavier, J.R.; Monteiro, M.C.; de la Cruz, M.; Oves-Costales, D.; Vicente, F.; Lage, O.M. Antimicrobial activity of heterotrophic bacterial communities from the marine sponge Erylus discophorus (astrophorida, geodiidae). PLoS ONE 2013, 8, e78992. [Google Scholar] [CrossRef] [PubMed]
- Omura, S. Philosophy of new drug discovery. Microbiol. Rev. 1986, 50, 259. [Google Scholar] [PubMed]
- Lancini, G.; Lorenzetti, R. Antibiotics and bioactive microbial metabolites. In Biotechnology of Antibiotics and Other Bioactive Microbial Metabolites; Springer: New York, NY, USA, 1993; pp. 1–18. [Google Scholar]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Hochmuth, T.; Piel, J. Polyketide synthases of bacterial symbionts in sponges–evolution-based applications in natural products research. Phytochemistry 2009, 70, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Sperstad, S.V.; Haug, T.; Blencke, H.-M.; Styrvold, O.B.; Li, C.; Stensvåg, K. Antimicrobial peptides from marine invertebrates: Challenges and perspectives in marine antimicrobial peptide discovery. Biotechnol. Adv. 2011, 29, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Morton, D.; Kettle, C. Structural characteristics of bioactive marine natural products. In Marine Biomaterials: Characterization, Isolation and Applications; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 173. [Google Scholar]
- Uzair, B.; Ahmed, N.; Ahmad, V.U.; Kousar, F. A new antibacterial compound produced by an indigenous marine bacteria—Fermentation, isolation, and biological activity. Nat. Prod. Res. 2006, 20, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.T.; Šturdíková, M.; Šturdík, E. Natural products of marine origin and their perspectives in the discovery of new anticancer drugs. Acta Chim. Slov. 2009, 2, 63–74. [Google Scholar]
- Kumar, P.S.; Krishna, E.R.; Sujatha, P.; Kumar, B.V. Screening and isolation of associated bioactive microorganisms from fasciospongia cavernosa from of visakhapatnam coast, bay of bengal. Electron. J. Chem. 2012, 9, 2166–2176. [Google Scholar]
- Nicacio, K.J.; Ióca, L.P.; Fróes, A.M.; Leomil, L.; Appolinario, L.R.; Thompson, C.C.; Thompson, F.L.; Ferreira, A.G.; Williams, D.E.; Andersen, R.J.; et al. Cultures of the marine bacterium Pseudovibrio denitrificans ab134 produce bromotyrosine-derived alkaloids previously only isolated from marine sponges. J. Nat. Prod. 2017, 80, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Reen, F.J.; Romano, S.; Dobson, A.D.W.; O’Gara, F. The sound of silence: Activating silent biosynthetic gene clusters in marine microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Blanton, J.M.; Podell, S.; Taton, A.; Schorn, M.A.; Busch, J.; Lin, Z.; Schmidt, E.W.; Jensen, P.R.; Paul, V.J.; et al. Metagenomic discovery of polybrominated diphenyl ether biosynthesis by marine sponges. Nat. Chem. Biol. 2017, 13, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, S.; Ichiba, T.; Kohmoto, S.; Saucy, G.; Higa, T. Isolation and structure elucidation of onnamide A, a new bioactive metabolite of a marine sponge, Theonella sp. J. Am. Chem. Soc. 1988, 110, 4851–4853. [Google Scholar] [CrossRef]
- Fusetani, N.; Matsunaga, S.; Matsumoto, H.; Takebayashi, Y. Bioactive marine metabolites. 33. Cyclotheonamides, potent thrombin inhibitors, from a marine sponge Theonella sp. J. Am. Chem. Soc. 1990, 112, 7053–7054. [Google Scholar] [CrossRef]
- Kobayashi, J.; Itagaki, F.; Shigemori, H.; Ishibashi, M.; Takahashi, K.; Ogura, M.; Nagasawa, S.; Nakamura, T.; Hirota, H. Keramamides B. Apprx. D, novel peptides from the okinawan marine sponge Theonella sp. J. Am. Chem. Soc. 1991, 113, 7812–7813. [Google Scholar] [CrossRef]
- Kobayashi, J.I.; Sato, M.; Murayama, T.; Ishibashi, M.; Wälchi, M.R.; Kanai, M.; Shoji, J.; Ohizumi, Y. Konbamide, a novel peptide with calmoduiin antagonistic activity from the okinawan marine sponge Theonella sp. J. Chem. Soc. Chem. Commun. 1991, 1050–1052. [Google Scholar] [CrossRef]
- Lackner, G.; Peters, E.E.; Helfrich, E.J.N.; Piel, J. Insights into the lifestyle of uncultured bacterial natural product factories associated with marine sponges. Proc. Natl. Acad. Sci. USA 2017, 114, E347–E356. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Egami, Y.; Kimura, M.; Wakimoto, T.; Abe, I. Metagenomic analysis of the sponge discodermia reveals the production of the cyanobacterial natural product kasumigamide by ‘entotheonella’. PLoS ONE 2016, 11, e0164468. [Google Scholar] [CrossRef] [PubMed]
- Indraningrat, A.A.G.; Smidt, H.; Sipkema, D. Bioprospecting sponge-associated microbes for antimicrobial compounds. Mar. Drugs 2016, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kamigiri, K.; Arao, N.; Suzumura, K.; Kawano, Y.; Yamaoka, M.; Zhang, H.; Watanabe, M.; Suzuki, K. Ym-266183 and ym-266184, novel thiopeptide antibiotics produced by bacillus cereus isolated from a marine sponge. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological properties. J. Antibiot. (Tokyo) 2003, 56, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, K.; Yokoi, T.; Funatsu, M.; Nagai, K.; Tanaka, K.; Zhang, H.; Suzuki, K. Ym-266183 and ym-266184, novel thiopeptide antibiotics produced by bacillus cereus isolated from a marine sponge II. Structure elucidation. J. Antibiot. (Tokyo) 2003, 56, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Abdelmohsen, U.R.; Ibrahim, A.K.; Hassanean, H.A.; Hentschel, U.; Ahmed, S.A. New antibacterial xanthone from the marine sponge-derived Micrococcus sp. Eg45. Bioorg. Med. Chem. Lett. 2014, 24, 4939–4942. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Garson, M.J.; Fuerst, J.A. Marine actinomycetes related to the ‘salinospora’ group from the Great Barrier Reef sponge pseudoceratina clavata. Environ. Microbiol. 2005, 7, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.C.; Soares, A.R.; Machado, F.L.; Romanos, M.T.; Muricy, G.; Giambiagi-deMarval, M.; Laport, M.S. Investigation of biotechnological potential of sponge-associated bacteria collected in brazilian coast. Lett. Appl. Microbiol. 2015, 60, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Pabel, C.T.; Vater, J.; Wilde, C.; Franke, P.; Hofemeister, J.; Adler, B.; Bringmann, G.; Hacker, J.; Hentschel, U. Antimicrobial activities and matrix-assisted laser desorption/ionization mass spectrometry of bacillus isolates from the marine sponge aplysina aerophoba. Mar. Biotechnol. 2003, 5, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Skariyachan, S.; G Rao, A.; Patil, M.R.; Saikia, B.; Bharadwaj, K.N.; Rao, G.S. Antimicrobial potential of metabolites extracted from bacterial symbionts associated with marine sponges in coastal area of gulf of mannar biosphere, India. Lett. Appl. Microbiol. 2014, 58, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bultel-Ponce, V.V.; Berge, J.P.; Debitus, C.; Nicolas, J.L.; Guyot, M. Metabolites from the sponge-associated bacterium pseudomonas species. Mar. Biotechnol. 1999, 1, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Muhlbacher, J.; Schaumann, K.; Steffens, S.; Rytik, P.G.; Hentschel, U.; Morschhauser, J.; Muller, W.E. Sorbicillactone A: A structurally unprecedented bioactive novel-type alkaloid from a sponge-derived fungus. Prog. Mol. Subcell. Biol. 2003, 37, 231–253. [Google Scholar] [PubMed]
- Peng, J.; Jiao, J.; Li, J.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Pyronepolyene C-glucosides with NF-kappaB inhibitory and anti-influenza a viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg. Med. Chem. Lett. 2012, 22, 3188–3190. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with anti-HIV activity from the sponge-derived fungus stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Lin, X.P.; Qin, C.; Liao, S.R.; Wan, J.T.; Zhang, T.Y.; Liu, J.; Fredimoses, M.; Chen, H.; Yang, B.; et al. Antimicrobial and antiviral sesquiterpenoids from sponge-associated fungus, aspergillus sydowii zsds1-f6. J. Antibiot. (Tokyo) 2014, 67, 581–583. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, M.M.; El-Bondkly, A.M. Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya48. J. Ind. Microbiol. Biotechnol. 2010, 37, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kamigiri, K.; Matsumoto, H.; Kawano, Y.; Yamaoka, M.; Shimoi, H.; Watanabe, M.; Suzuki, K. Ym-202204, a new antifungal antibiotic produced by marine fungus Phoma sp. J. Antibiot. (Tokyo) 2002, 55, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Wahidullah, S.; Rodrigues, C.; Souza, L.D. The sponge-associated bacterium bacillus licheniformis SAB1: A source of antimicrobial compounds. Mar. Drugs 2010, 8, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Holler, U.; Konig, G.M.; Wright, A.D. Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999, 62, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.H.; Holmes, M.J.; Higa, T.; Hamann, M.T.; Kara, U.A.K. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob. Agents Chemother. 2000, 44, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Peraud, O. Isolation and Characterization of a Sponge-Associated Actinomycete that Produces Manzamines. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2006. [Google Scholar]
- Waters, A.L.; Peraud, O.; Kasanah, N.; Sims, J.W.; Kothalawala, N.; Anderson, M.A.; Abbas, S.H.; Rao, K.V.; Jupally, V.R.; Kelly, M. An analysis of the sponge acanthostrongylophora igens’ microbiome yields an actinomycete that produces the natural product manzamine A. Front. Mar. Sci. 2014, 1, 54. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Szesny, M.; Othman, E.M.; Schirmeister, T.; Grond, S.; Stopper, H.; Hentschel, U. Antioxidant and anti-protease activities of diazepinomicin from the sponge-associated Micromonospora strain RV115. Mar. Drugs 2012, 10, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins a and b from a marine sponge associated-Actinokineospora sp. Eg49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Elardo, S.M.; Kozytska, S.; Bugni, T.S.; Ireland, C.M.; Moll, H.; Hentschel, U. Anti-parasitic compounds from Streptomyces sp. Strains isolated from Mediterranean sponges. Mar. Drugs 2010, 8, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I.; Ludwig, W.; Schleifer, K.-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [PubMed]
- Eilers, H.; Pernthaler, J.; Glöckner, F.O.; Amann, R. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microb. 2000, 66, 3044–3051. [Google Scholar] [CrossRef]
- Kurtböke, D.I. Actinophages as indicators of actinomycete taxa in marine environments. Antonie Leeuwenhoek 2005, 87, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K. Springer Handbook of Marine Biotechnology; Springer: New York, USA, 2015; ISBN 9783642539718. [Google Scholar]
- Wilson, G.S.; Raftos, D.A.; Corrigan, S.L.; Nair, S.V. Diversity and antimicrobial activities of surface-attached marine bacteria from Sydney Harbour, Australia. Microbiol. Res. 2010, 165, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R.H. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis? Biotechnol. Bioeng. 2005, 90, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martin, J.; Harrington, C.; Dobson, A.D.; O’Gara, F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar. Drugs 2014, 12, 3516–3559. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, V.; Richter, M.; Romano, S.; Piel, J.; Schwedt, A.; Schulz-Vogt, H.N. The genus pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environ. Microbial. 2013, 15, 2095–2113. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.P.; O’Gara, F.; O’Sullivan, O.; Cotter, P.D.; Dobson, A.D.W. Marine Pseudovibrio sp. As a novel source of antimicrobials. Mar. Drugs 2014, 12, 5916–5929. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fan, L.; Zhong, L.; Kjelleberg, S.; Thomas, T. Metaproteogenomic analysis of a community of sponge symbionts. ISME J. 2012, 6, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Riesenfeld, C.S.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic analysis of microbial communities. Annu. Rev. Genet. 2004, 38, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Yung, P.Y.; Burke, C.; Lewis, M.; Kjelleberg, S.; Thomas, T. Novel antibacterial proteins from the microbial communities associated with the sponge cymbastela concentrica and the green alga Ulva Australis. Appl. Environ. Microbiol. 2011, 77, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; De Santi, C.; Palma Esposito, F.; Tedesco, P.; Galati, F.; Visone, M.; Di Scala, A.; De Pascale, D. Marine metagenomics, a valuable tool for enzymes and bioactive compounds discovery. Front. Mar. Sci. 2014, 1, 38. [Google Scholar] [CrossRef]
- Trindade, M.; van Zyl, L.J.; Navarro-Fernández, J.; Abd Elrazak, A. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front. Microbiol. 2015, 6, 890. [Google Scholar] [CrossRef] [PubMed]
- Atikana, A.; Naim, M.A.; Sipkema, D. Detection of keto synthase (ks) gene domain in sponges and bacterial sponges. Ann. Bogor. 2013, 17, 27–33. [Google Scholar]
- Schirmer, A.; Gadkari, R.; Reeves, C.D.; Ibrahim, F.; DeLong, E.F.; Hutchinson, C.R. Metagenomic analysis reveals diverse polyketide synthase gene clusters in microorganisms associated with the marine sponge discodermia dissoluta. Appl. Environ. Microbiol. 2005, 71, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Fuerst, J.A. Diversity of polyketide synthase genes from bacteria associated with the marine sponge pseudoceratina clavata: Culture-dependent and culture-independent approaches. Environ. Microbiol. 2006, 8, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Elardo, S.M.; Grozdanov, L.; Proksch, S.; Hentschel, U. Diversity of nonribosomal peptide synthetase genes in the microbial metagenomes of marine sponges. Mar. Drugs 2012, 10, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.T.; Sturdikova, M.; Sturdik, E. Bioactive marine and terrestrial polyketide and peptide secondary metabolites and perspectives of their biotechnological production. Acta Chimica Slovaca 2010, 3, 103–119. [Google Scholar]
- Steindler, L.; Schuster, S.; Ilan, M.; Avni, A.; Cerrano, C.; Beer, S. Differential gene expression in a marine sponge in relation to its symbiotic state. Mar. Biotechnol. 2007, 9, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Radax, R.; Rattei, T.; Lanzen, A.; Bayer, C.; Rapp, H.T.; Urich, T.; Schleper, C. Metatranscriptomics of the marine sponge geodia barretti: Tackling phylogeny and function of its microbial community. Environ. Microbial. 2012, 14, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Beloqui, A.; Timmis, K.N.; Golyshin, P.N. Metagenomics for mining new genetic resources of microbial communities. J. Mol. Microb. Biotechnol. 2009, 16, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Miyazaki, K. Functional metagenomics for enzyme discovery: Challenges to efficient screening. Curr. Opin. Biotechnol. 2009, 20, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Dobson, A.D.W.; Adams, C.; O’Gara, F. Emerging concepts promising new horizons for marine biodiscovery and synthetic biology. Mar. Drugs 2015, 13, 2924–2954. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, P.; Tang, Y. Engineered polyketide biosynthesis and biocatalysis in Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 88, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A. Secondary metabolic gene clusters: Evolutionary toolkits for chemical innovation. Trends Genet. 2010, 26, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, T.; Egami, Y.; Abe, I. Calyculin: Nature’s way of making the sponge-derived cytotoxin. Nat. Prod. Rep. 2016, 33, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zazopoulos, E.; Huang, K.; Staffa, A.; Liu, W.; Bachmann, B.O.; Nonaka, K.; Ahlert, J.; Thorson, J.S.; Shen, B.; Farnet, C.M. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat. Biotechnol. 2003, 21, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Bergman, O.; Haber, M.; Mayzel, B.; Anderson, M.A.; Shpigel, M.; Hill, R.T.; Ilan, M. Marine-based cultivation of diacarnus sponges and the bacterial community composition of wild and maricultured sponges and their larvae. Mar. Biotechnol. 2011, 13, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Laureti, L.; Song, L.; Huang, S.; Corre, C.; Leblond, P.; Challis, G.L.; Aigle, B. Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in streptomyces ambofaciens. Proc. Natl. Acad. Sci. USA 2011, 108, 6258–6263. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Gessner, A.; Thaker, M.N.; Waglechner, N.; Zhu, X.; Szawiola, A.; Bechthold, A.; Wright, G.D.; Zechel, D.L. A cryptic polyene biosynthetic gene cluster in streptomyces calvus is expressed upon complementation with a functional blda gene. Chem. Biol. 2013, 20, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Q. Genome-based studies of marine microorganisms to maximize the diversity of natural products discovery for medical treatments. Evid.-Based Complement. Altern. Med. 2011, 2011, 384572. [Google Scholar] [CrossRef] [PubMed]
- Metzker, M.L. Sequencing technologies—The next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Schorn, M.; Zettler, J.; Noel, J.P.; Dorrestein, P.C.; Moore, B.S.; Kaysser, L. Genetic basis for the biosynthesis of the pharmaceutically important class of epoxyketone proteasome inhibitors. ACS Chem. Biol. 2013, 9, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escribano, J.P.; Song, L.; Bibb, M.J.; Challis, G.L. Posttranslational β-methylation and macrolactamidination in the biosynthesis of the bottromycin complex of ribosomal peptide antibiotics. Chem. Sci. 2012, 3, 3522–3525. [Google Scholar] [CrossRef]
- Izawa, M.; Kawasaki, T.; Hayakawa, Y. Cloning and heterologous expression of the thioviridamide biosynthesis gene cluster from streptomyces olivoviridis. Appl. Environ. Microb. 2013, 79, 7110–7113. [Google Scholar] [CrossRef] [PubMed]
- Menzella, H.G.; Reeves, C.D. Combinatorial biosynthesis for drug development. Curr. Opin. Microbiol. 2007, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Tang, Y. Synthetic biological approaches to natural product biosynthesis. Curr. Opin. Biotechnol. 2012, 23, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Uria, A.; Piel, J. Cultivation-independent approaches to investigate the chemistry of marine symbiotic bacteria. Phytochem. Rev. 2009, 8, 401–414. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. Antismash: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Medema, M.H.; Kazempour, D.; Fischbach, M.A.; Breitling, R.; Takano, E.; Weber, T. Antismash 2.0—A versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013, 41, W204–W212. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.J.; Schwartz, S.L.; Lee, J.; Korobeynikov, A.; Dorrestein, P.C.; Gerwick, L.; Gerwick, W.H. Spongosine production by a vibrio harveyi strain associated with the sponge tectitethya crypta. J. Nat. Prod. 2015, 78, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.; Hentschel, U.; Abdelmohsen, U.R. Mining genomes of three marine sponge-associated actinobacterial isolates for secondary metabolism. Genome Announc. 2015, 3, e01106–e01115. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Marchesi, J.R.; Dobson, A.D.W. Metagenomic approaches to exploit the biotechnological potential of the microbial consortia of marine sponges. Appl. Microbiol. Biotechnol. 2007, 75, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, C.M.; McKiness, Z.P.; Newton, I.L.G.; Stewart, F.J. Marine chemosynthetic symbioses. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 475–507. [Google Scholar]
- Dubilier, N.; Bergin, C.; Lott, C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat. Rev. Microbiol. 2008, 6, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhu, T.; Wei, H.; Zhang, G.; Wang, H.; Gu, Q. Spicochalasin a and new aspochalasins from the marine-derived fungus spicaria elegans. Eur. J. Org. Chem. 2009, 2009, 3045–3051. [Google Scholar] [CrossRef]
- Martín, J.F. Phosphate control of the biosynthesis of antibiotics and other secondary metabolites is mediated by the phor-phop system: An unfinished story. J. Bacteriol. 2004, 186, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- König, C.C.; Scherlach, K.; Schroeckh, V.; Horn, F.; Nietzsche, S.; Brakhage, A.A.; Hertweck, C. Bacterium induces cryptic meroterpenoid pathway in the pathogenic fungus Aspergillus fumigatus. Chembiochem 2013, 14, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Marketon, M.M.; Glenn, S.A.; Eberhard, A.; González, J.E. Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 2003, 185, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, B.; Dulla, G.; Lindow, S.E. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. MPMI 2005, 18, 682–693. [Google Scholar] [CrossRef] [PubMed]
- González, J.E.; Keshavan, N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 2006, 70, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, V. Striking a balance: Inter-kingdom cell-to-cell signaling, friendship or war? Trends Immunol. 2004, 25, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Borneman, J. What Is the Evidence for the Loss of Microbial Diversity. In Microbial Diversity and Bioprospecting; Bull, AT., Ed.; ASM Press: Washington, DC, USA, 2004; pp. 421–428. [Google Scholar]
- Perry, R.I.; Cury, P.; Brander, K.; Jennings, S.; Möllmann, C.; Planque, B. Sensitivity of marine systems to climate and fishing: Concepts, issues and management responses. J. Mar. Syst. 2010, 79, 427–435. [Google Scholar] [CrossRef]
- Mendola, D. Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: Process developments and economics. Biomol. Eng. 2003, 20, 441–458. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Rao, V.; Hamann, M.T.; Kelly, M.; Hill, R.T. Monitoring bacterial diversity of the marine sponge ircinia strobilina upon transfer into aquaculture. Appl. Environ. Microbiol. 2008, 74, 4133–4143. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.A.; Northcote, P.T.; Page, M.J. Spatial and temporal variability of the bacterial community in different chemotypes of the New Zealand marine sponge mycale hentscheli. FEMS Microbiol. Ecol. 2010, 72, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Schupp, P.J.; Baillie, H.J.; Charlton, T.S.; De Nys, R.; Kjelleberg, S.; Steinberg, P.D. Evidence for acyl homoserine lactone signal production in bacteria associated with marine sponges. Appl. Environ. Microbiol. 2004, 70, 4387–4389. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W.; Nelson, J.T.; Rasko, D.A.; Sudek, S.; Eisen, J.A.; Haygood, M.G.; Ravel, J. Patellamide A and C biosynthesis by a microcin-like pathway in prochloron didemni, the cyanobacterial symbiont of lissoclinum patella. Proc. Natl. Acad. Sci. USA 2005, 102, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Clardy, J. Using genomics to deliver natural products from symbiotic bacteria. Genome Biol. 2005, 6, 232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luter, H.M.; Widder, S.; Botté, E.S.; Abdul Wahab, M.; Whalan, S.; Moitinho-Silva, L.; Thomas, T.; Webster, N.S. Biogeographic variation in the microbiome of the ecologically important sponge, Carteriospongia foliascens. PeerJ 2015, 3, e1435. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.J.; Davy, S.K.; Jones, T.; Taylor, M.W.; Webster, N.S. Could some coral reefs become sponge reefs as our climate changes? Global Chang. Biol. 2013, 19, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Webb, R.I.; Ridd, M.J.; Hill, R.T.; Negri, A.P. The effects of copper on the microbial community of a coral reef sponge. Environ. Microbiol. 2001, 3, 19–31. [Google Scholar] [CrossRef]
- Williams, P.G. Panning for chemical gold: Marine bacteria as a source of new therapeutics. Trends Biotechnol. 2009, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.T.; Fenical, W. Pharmaceuticals from marine natural products: Surge or ebb? Curr. Opin. Biotechnol. 2010, 21, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Evans-Illidge, E.A.; Logan, M.; Doyle, J.; Fromont, J.; Battershill, C.N.; Ericson, G.; Wolff, C.W.; Muirhead, A.; Kearns, P.; Abdo, D.; et al. Phylogeny drives large scale patterns in australian marine bioactivity and provides a new chemical ecology rationale for future biodiscovery. PLoS ONE 2013, 8, e73800. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Kjelleberg, S.; Thomas, T. Functional genomic analysis of an uncultured δ-proteobacterium in the sponge Cymbastela concentrica. ISME J. 2011, 5, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chem. Biol. Chem. 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Hentschel, U.; Usher, K.M.; Taylor, M.W. Marine sponges as microbial fermenters. FEMS Microbiol. Ecol. 2006, 55, 167–177. [Google Scholar] [CrossRef] [PubMed]
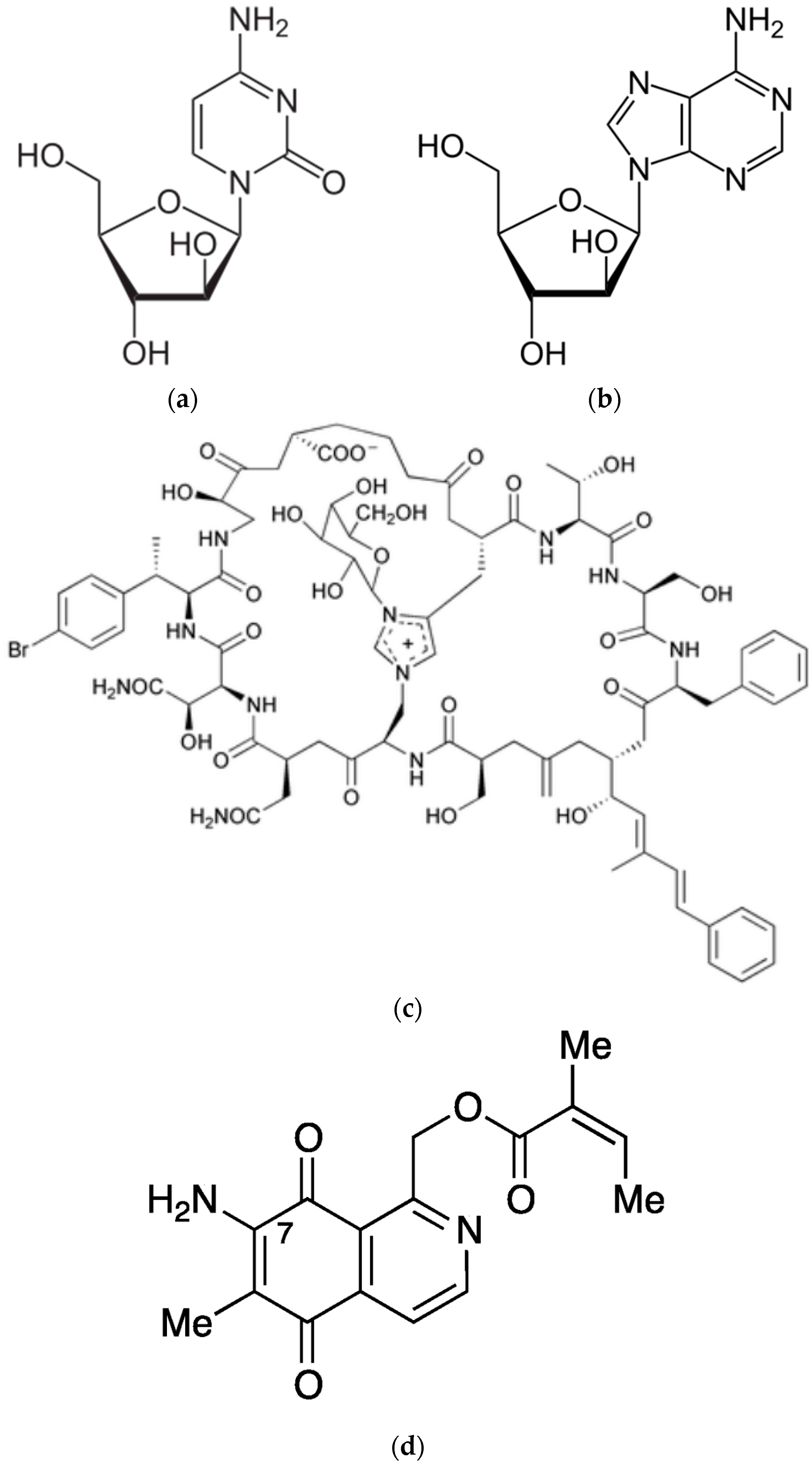
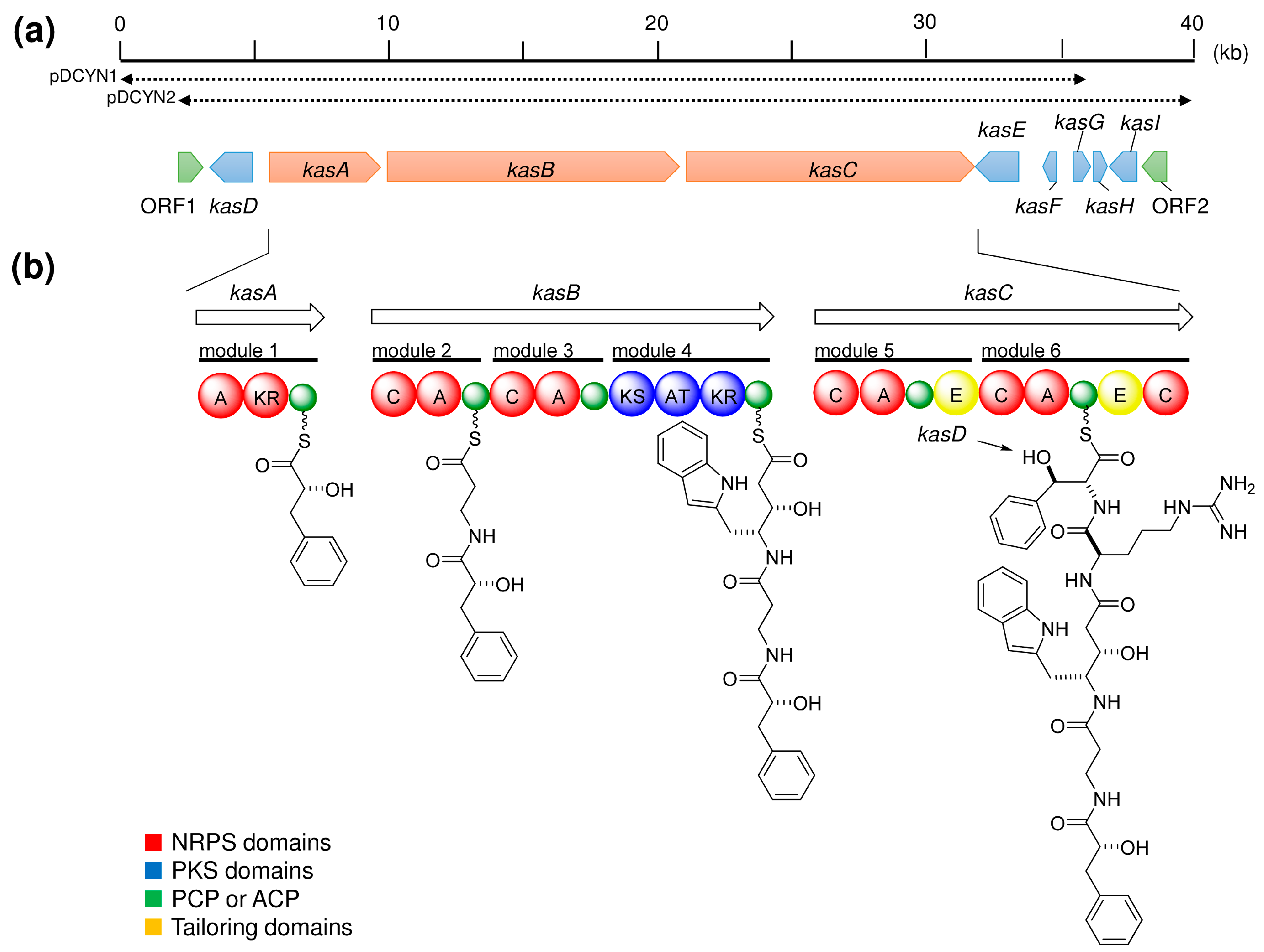
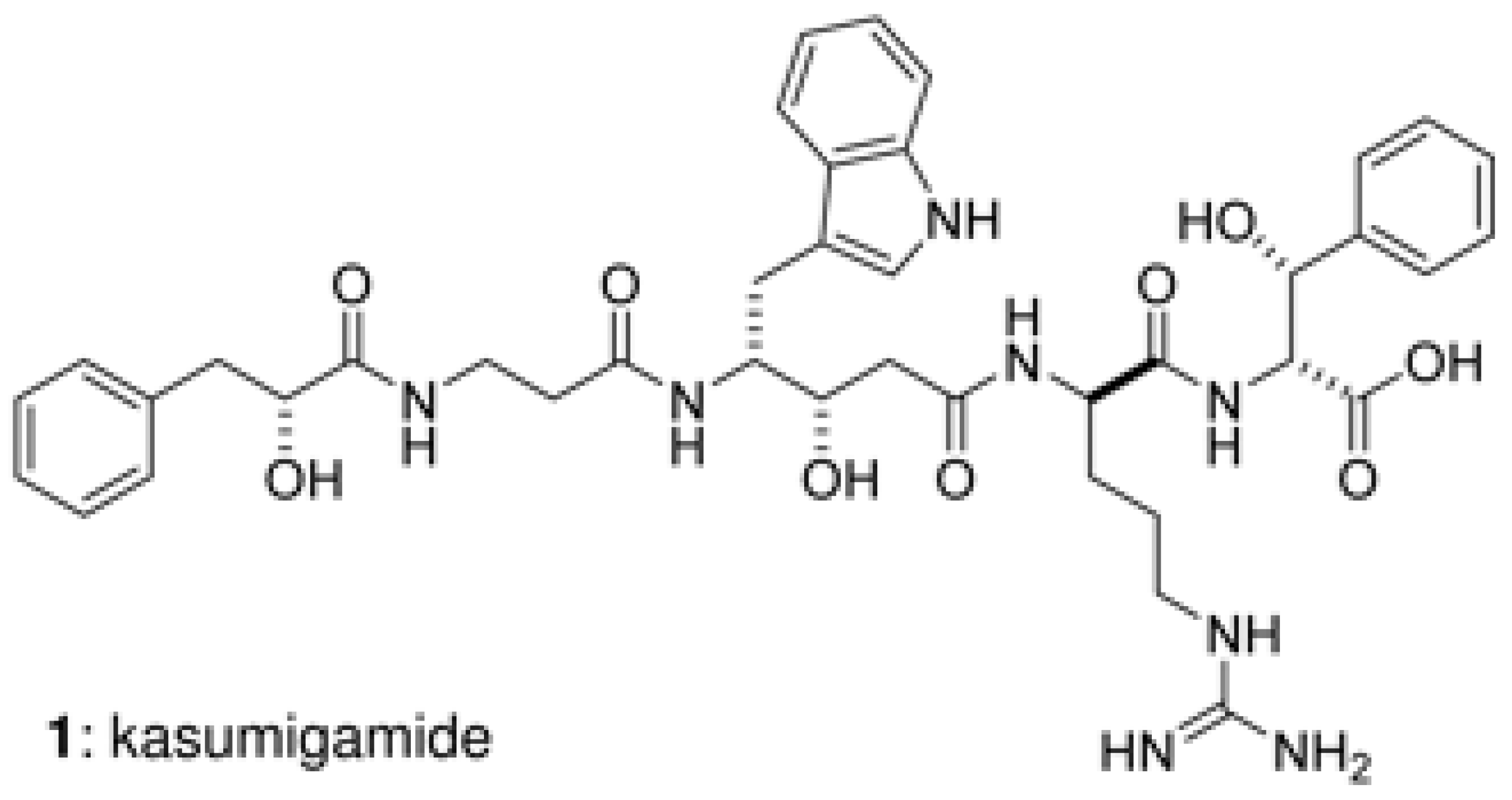
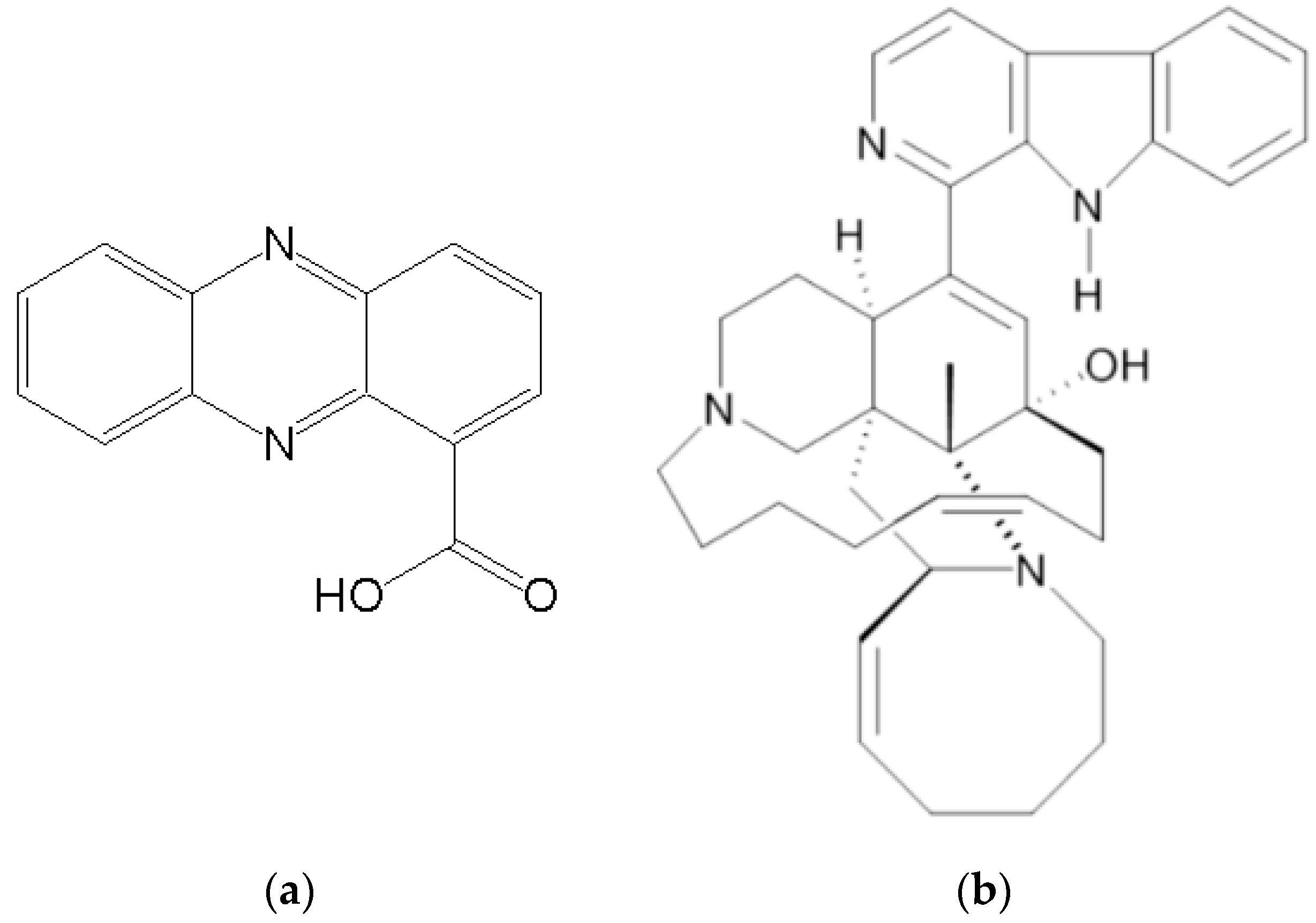
| Sponge | Compound | Class | Target | References |
|---|---|---|---|---|
| Antibacterial | ||||
| Acanthostrongylophora sp. | 6-hydroxymanzamine E | Alkaloid | Mycobacterium tuberculosis | [121] |
| Oceanapia sp. | C14 acetylenic acid | Fatty acid | E. coli, P. aeruginosa, B. subtillis and S. aureus | [122] |
| Discodermia sp. | Polydiscamide A | Peptide | B. subtilis | [123] |
| Cribrochalina sp. | Cribrostatin 3 | Alkaloid | N. gonorrheae (antibiotic-resistant strain) | [120] |
| Myrmekioderma styx | (S)-(+)-curcuphenol | Sesquiterpene | M. tuberculosis | [124] |
| Pachychalina sp. | Cyclostellettamines A-I, K-L | Nitrogenous | S. aureus (MRSA strain), P. aeruginosa (antibiotic-resistant strain), M. tuberculosis | [125,126] |
| Aka coralliphaga | Corallidictyals A-D | Hydroquinones | S. aureus | [127] |
| Melophlus sarassinorum | Melophlin C | Nitrogen heterocycles | B. subtilis and S. aureus | [128] |
| Antiviral | ||||
| Cryptotethya crypta | Ara-A | Nucleoside | HSV-1, HSV-2, VZV | [108] |
| Theonella sp. | Papuamides A–D | Cyclic depsipeptides | HIV-1 | [129] |
| Dysidea avara | Avarol | Sesquiterpene hydroquinone | HIV-1 | [130] |
| Hamigera tarangaensis | Hamigeran B | Phenolic macrolide | herpes and polio viruses | [131] |
| Mycale sp. | Mycalamide A-B | Nucleosides | A59 coronavirus, HSV-1 | [132] |
| Antifungal | ||||
| Acanthostrongylophora sp. | Manzamine A | Alkaloid | C. neoformans | [133] |
| Discodermia sp. | Discobahamin A-B | Peptides | C. albicans | [134] |
| Leucetta cf. chagosensis | Naamine D | Alkaloid | C. neoformans | [135] |
| Discodermia sp. | Discobahamin A-B | Peptides | C. albicans | [134] |
| Luffariella variabilis | Secomanoalide | Sesterterpenoid | C. glabrata, C. krusei and C. albicans | [136] |
| Antiprotozoal | ||||
| Pachymatisma johnstonii | Pachymatismin | Glycoprotein | Leishmania sp. | [137] |
| Acanthella sp. | Kalihinol A | Kalihinane diterpenoids | P. falciparum | [138] |
| Cymbastela hooperi | Diisocyanoadociane | Tetracyclic diterpene | P. falciparum | [139] |
| Monanchora unguifera | Mirabilin B | Alkaloid | L. donovani | [140] |
| Sponge | Location | Microorganism | Phylum | Compound | Target | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibacterial | Antibacterial | ||||||||||||
| Halichondria japonica | Iriomote island, Japan | Bacillus cereus QNO3323 | Firmicutes | Thiopeptide YM-266183 | Staphylococcus aureus | [164,165] | |||||||
| Spheciospongia vagabunda | Red Sea | Micrococcus sp. EG45 | Actinobacteria | Microluside A | S. aureus NCTC 8325 | [166] | |||||||
| Isodictya setifera | Ross island, Antartica (30–40 m) | Pseudomonas aeruginosa | Proteobacteria | Phenazine-1-carboxylic acid | S. aureus | [167] | |||||||
| Pseudoceratina clavata | Heron Island, Great Barrier Reef (14 m) | Salinispora sp. M102, M403, M412, M413, M414, SW10, SW15 and SW17 | Actinobacteria | Unidentified | S. aureus | [168] | |||||||
| Haliclona sp. | Cagarras Archipelago, Brazil (4–20 m) | Pseudomonas fluorescens H40, H41 and Pseudomonas aeruginosa H51 | Proteobacteria | Diketopipe-razine | S. aureus | [169] | |||||||
| Aplysina aerophoba | Banyuls-sur-Mer, France (5–15 m) | Bacillus subtilis A190 | Firmicutes | Surfactin | S. aureus, Staphylococcus epidermidis Bacillus megaterium, Clavibacter michiganensis, Proteus vulgaris and Escherichia coli | [170] | |||||||
| Haliclona sp. | Cagarras Archipelago, Brazil (4–20 m) | Pseudomonas fluorescens H40, H41 and Pseudomonas aeruginosa H51 | Proteobacteria | Diketopiperazine cyclo-(l-Leu-l-Pro) | P. aeruginosa | [169] | |||||||
| Isodictya setifera | Ross island, Antarctica (30–40 m) | Pseudomonas aeruginosa | Proteobacteria | Phenazine-1-carboxylic acid and phenazine-1-carboxamide | Micrococcus luteus, S. aureus, Bacillus cereus | [167] | |||||||
| Hyatella sp. | Unknown | Vibrio sp. | Proteobacteria | Polyketide-peptide compound | Bacillus sp. | [116] | |||||||
| Haliclonaocculata | Gulf of Mannar, India | Bacillus licheniformis T6-1 | Firmicutes | Fluorophore compound | Salmonella typhi | [171] | |||||||
| Antiviral | |||||||||||||
| Homophymia sp. | Touho, New Caledonia | Pseudomonas sp. 1531-E7 | Proteobacteria | 2-undecyl-4-quinolone | HIV-1 | [172] | |||||||
| Ircinia fasciculata | Bight of Fetovaia, Italy (17.5 m) | Penicillium chrysogenum | Ascomycota | Sorbicillactone A | HIV-1 | [173] | |||||||
| Callyspongia sp. | Sanya, China | Epicoccum sp. JJY40 | Ascomycota | Pyronepolyene C-glucoside iso-D8646-2-6 | H1N1 | [174] | |||||||
| Xestospongia testudinaria | Paracel Islands | Stachybotrys chartarum MXH-X73 | Ascomycota | Stachybotrin D | NNRTI resistant HIV-1RT-L100I, K103N | [175] | |||||||
| Unidentified | Paracel Islands | Aspergillus sydowii ZSDS1-F6 | Ascomycota | (Z)-5-(Hydroxymethyl)-2-(60)-methylhept-20-en-20-yl)-phenol | H3N2 | [176] | |||||||
| Antifungal | |||||||||||||
| Aplysina fistularis | Sharm El-Sheikh, Egypt | Streptomyces sp. Hedaya48 | Actinobacteria | Saadamycin | Candida albicans, Trichophyton rubrum, Microsporum gypseum, Epidermophyton floccosum, Fusarium oxysporum, Cryptococcus humicolus, Aspergillus fumigatus, Trichophyton mentagrophyte, Epidermophyton floccosum | [177] | |||||||
| Aplysina fistularis | Sharm El-Sheikh, Egypt | Streptomyces sp. Hedaya48 | Actinobacteria | 5,7-Dimethoxy-4-pmethoxylphenylcoumarin | T. rubrum, T. mentagrophyte, C. albicans, M. gypseum, E. floccosum, F. oxysporum, C. humicolus | [177] | |||||||
| Halichondria japonica | Iriomote Island, Japan | Phoma sp. Q60596 | Ascomycota | YM-202204 | C. albicans, Cryptococcus neoformans, Saccharomyces cerevisiae | [178] | |||||||
| Halichondria sp. | West Coast of India (10 m) | Bacillus sp. SAB1 | Firmicutes | 4,41-Oxybis (3-phenylpropionic acid) | C. albicans, Aspergillus niger, Rhodotorula sp., Vibrio cholerae | [179] | |||||||
| Halichondria sp. | West Coast of India (10 m) | Bacillus sp. SAB1 | Firmicutes | 3-Phenylpropionic acid | A. niger, Rhodotorula sp., C. albicans | [179] | |||||||
| Myxilla incrustans | The Caribbean Island of Dominica | Microsphaeropsis sp. | Ascomycota | Microsphaeropsisin | Eurotium repens, Ustilago violacea | [180] | |||||||
| Ectyoplasia ferox | The Caribbean Island of Dominica | Coniothyrium sp. | Ascomycota | (3R)-6-Methoxymellein | E. repens, U. violacea | [180] | |||||||
| Ectyoplasia ferox | The Caribbean Island of Dominica | Coniothyrium sp. | Ascomycota | Phenylethanol | U. violacea, E. repens | [180] | |||||||
| Antiprotozoal | |||||||||||||
| Acanthostrongylophora ingens | Manado, Indonesia | Micromonospora sp. M42 | Actinobacteria | Manzamine A | Plasmodium falciparum, Plasmodium berghei | [122,181,182,183,184] | |||||||
| Homophymia sp. | Touho, New Caledonia | Pseudomonas sp. 1531-E7 | Proteobacteria | 2-Undecyl-4-quinolone | P. falciparum | [172] | |||||||
| Aplysina aerophoba | Rovinj, Croatia (3–20 m) | Micromonospora sp. RV115 | Actinobacteria | Diazepinomicin | Trypanosoma brucei | [185] | |||||||
| Spheciospongia vagabunda | Red Sea | Actinokinespora sp. EG49 | Actinobacteria | Actinosporin A | T. brucei | [186] | |||||||
| Aplysina polypoides | Rovinj, Croatia (3–20 m) | Streptomyces sp. 34 | Actinobacteria | Valinomycin | T. brucei, Leishmania major | [187] | |||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinkmann, C.M.; Marker, A.; Kurtböke, D.İ. An Overview on Marine Sponge-Symbiotic Bacteria as Unexhausted Sources for Natural Product Discovery. Diversity 2017, 9, 40. https://doi.org/10.3390/d9040040
Brinkmann CM, Marker A, Kurtböke Dİ. An Overview on Marine Sponge-Symbiotic Bacteria as Unexhausted Sources for Natural Product Discovery. Diversity. 2017; 9(4):40. https://doi.org/10.3390/d9040040
Chicago/Turabian StyleBrinkmann, Candice M., Amberlee Marker, and D. İpek Kurtböke. 2017. "An Overview on Marine Sponge-Symbiotic Bacteria as Unexhausted Sources for Natural Product Discovery" Diversity 9, no. 4: 40. https://doi.org/10.3390/d9040040
APA StyleBrinkmann, C. M., Marker, A., & Kurtböke, D. İ. (2017). An Overview on Marine Sponge-Symbiotic Bacteria as Unexhausted Sources for Natural Product Discovery. Diversity, 9(4), 40. https://doi.org/10.3390/d9040040






