Persistent Gaps of Knowledge for Naming and Distinguishing Multiple Species of Crown-of-Thorns-Seastar in the Acanthaster planci Species Complex
Abstract
:1. Introduction
2. Nomenclatorical Status and Problems
3. Why Taxonomy of COTS Does Matter—Differences between A. planci Species
3.1. Morphology
3.2. Toxicity
3.3. Outbreaks
3.4. Conclusions
4. The Usage of BINs for the Species of the Acanthaster planci Complex
5. Recommendations
- (1)
- Provide and deposit a whole- or partial specimen voucher or tissue sample to your local public museum or collection with the exact sampling locality and inventory data, and request a voucher number. Add voucher number and metadata to any publication that arises. These vouchers, or at least parts thereof, should be suitable for molecular analyses, for example, stored in high percentage (>95%) alcohol, but may be freeze-dried or frozen. The issue of voucher deposition is of particular importance for molecular studies [49,50] and genome sequencing project such that future studies do not mismatch different species and one can go back to the actual specimens for (morphological) verification. Regrettably, no voucher specimens were kept from the two A. solaris specimens from which draft genomes were recently sequenced [42], so their morphology unfortunately cannot be examined.
- (2)
- If possible, provide a COI barcode of your specimens, larvae or tissues, and use the BIN-code for unequivocal classification until the nomenclatorial prerequisites for valid Linnean binomens of all species are fulfilled (see [10] and above). Meanwhile, a good number of institutions (and the labs of Gerhard Haszprunar and Gert Wörheide) offer DNA barcoding services for a small fee, e.g., to cover consumables and sequencing.
- (3)
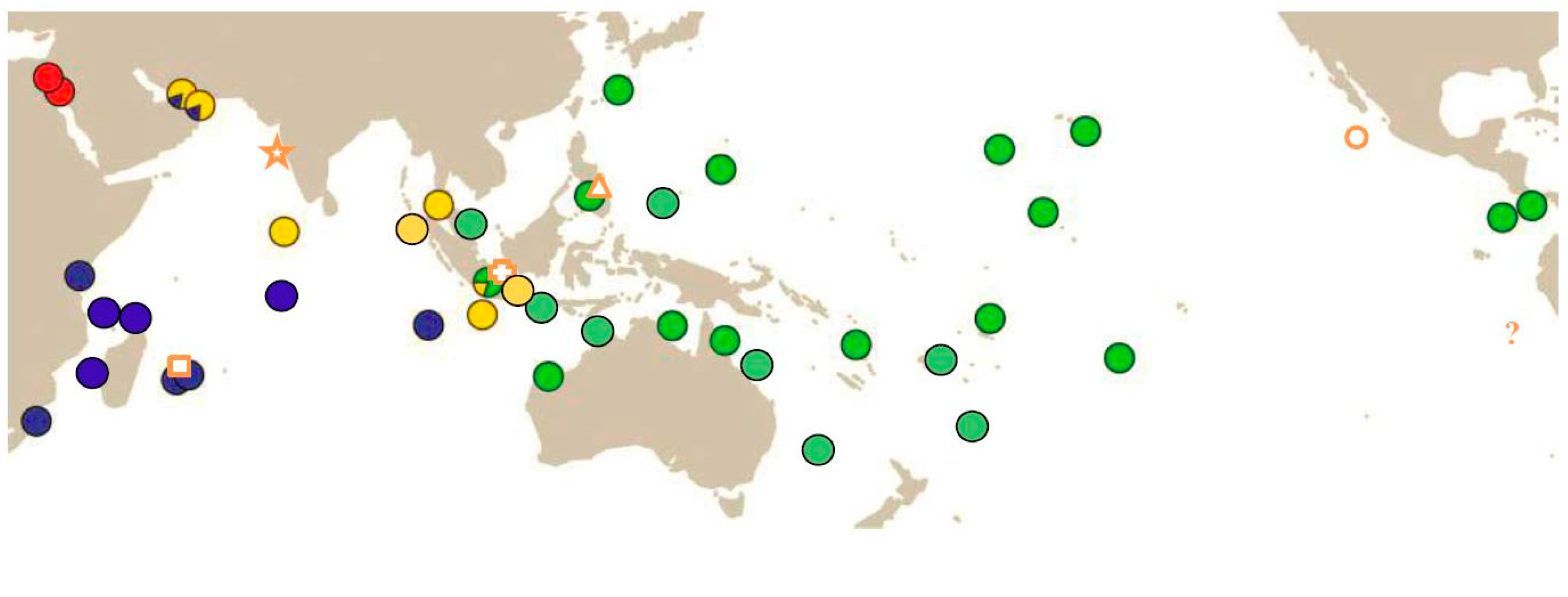
- Extend the knowledge about the biogeographic distribution of the at least four species of COTS. Of particular interest are potential areas of sympatry and areas that have not been covered so far (see [2,9,10,11]). Such areas include
- areas of putative species sympatry/clade overlap (e.g., the southern coast of Oman, Yemen, and Somalia; Gulf of Aden; Western Indonesia);
- sites close to the southern distribution limits of both Indian Ocean species;
- sites close to the northern and southern distribution limits of A. solaris;
- sites in the eastern Pacific to resolve the status of the putative species Acanthaster ellisii and Acanthaster ellisii pseudoplanci.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Ann. Rev. 2014, 52, 133–200. [Google Scholar]
- Haszprunar, G.; Spies, M. An integrative approach of the crown-of-thorns starfish species group (Asteroidea: Acanthaster): A review of names and comparison to recent molecular data. Zootaxa 2014, 3841, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Fisher, W.K. Starfishes of the Philippine seas and adjacent waters. Bull. US Natl. Mus. 1919, 3, 100. [Google Scholar]
- Nishida, M.; Lucas, J.S. Genetic differences between geographic populations of the crown-of-thorns starfish throughout the Pacific Ocean. Mar. Biol. 1990, 98, 359–368. [Google Scholar] [CrossRef]
- Benzie, J.A.H. Major genetic differences between crown-of-thorns starfish (Acanthaster planci) populations in the Indian and Pacific Oceans. Evolution 1999, 53, 1782–1795. [Google Scholar] [CrossRef]
- Benzie, J.A.H. The detection of spatial variation in widespread marine species: methods and bias in the analysis of population structure in the crown of thorns starfish (Echinodermata: Asteroidea). Hydrobiologia 2000, 420, 1–14. [Google Scholar] [CrossRef]
- Gérard, K.; Roby, C.; Chevalier, N.; Thomassin, B.; Chenuil, A.; Féral, J.-P. Assessment of three mitochondrial loci variability for the crown-of-thorns starfish: A first insight into Acanthaster phylogeography. C. R. Biol. 2008, 331, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, N.; Nagai, S.; Hamaguchi, M.; Okaji, K.; Gérard, K.; Nadaoka, K. Gene flow of Acanthaster planci (L.) in relation to ocean currents revealed by microsatellite analysis. Molec. Ecol. 2009, 18, 1574–1590. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.; Benzie, J.; Lessios, H.; Barber, P.; Wörheide, G. A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol. Lett. 2008, 4, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.; Benzie, J.; Barber, P.H.; Erdmann, M.V.; Ambariyanto, S.C.; Tenggardjaja, K.; Gérard, K.; Wörheide, G. Phylogeography of the crown-of-thorns starfish in the Indian Ocean. PLoS ONE 2012, 7, e43499. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.; Benzie, J.A.H.; Tenggardjaja, K.; Ambariyanto, S.C.; Barber, P.H.; Wörheide, G. Phylogeography of the crown-of-thorns starfish: Genetic structure within the Pacific species. Coral Reefs 2013, 32, 515–525. [Google Scholar] [CrossRef]
- Yasuda, N.; Hamaguchi, M.; Sasaki, M.; Nagai, S.; Saba, M.; Nadaoka, K. Complete mitochondrial genome sequences for crown-of-thorns starfish Acanthaster planci and Acanthaster brevispinus. BMC Genom. 2006, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Timmers, M.A.; Bird, C.E.; Skillings, D.J.; Smouse, P.E.; Toonen, R.J. There’s no place like home: Crown-of-thorns outbreaks in the central Pacific are regionally derived and independent events. PLoS ONE 2012, 7, e31159. [Google Scholar] [CrossRef] [PubMed]
- Tusso, S.; Morcinek, K.; Vogler, C.; Schupp, P.J.; Caballes, C.F.; Vargas, S.; Wörheide, G. Genetic structure of the crown-of-thorns sea star in the Pacific. Peer J. 2016, 5, e1970. [Google Scholar] [CrossRef] [PubMed]
- De Loriol, P. Catalogue raisonné des Echinodermes recuellis par M.V. de Robillard à l´Ile Maurice. II. Stellérides. Mem. Soc. Phys. Hist. Nat. Genéve 1885, 29, 1–84. (In French) [Google Scholar]
- Schreber, J.C.D. von Beschreibung der Seesonne, einer Art Seesterne, mit 21 Strahlen. Der Naturforscher Halle a/d Saale 1793, 27, 1–6. (In German) [Google Scholar]
- Ellis, J. The Natural History of Many Curious and Uncommon Zoophytes, Collected from Various Parts of The Globe; Benjamin White and Son: London, UK, 1786; pp. 1–12. [Google Scholar]
- Nakajima, R.; Nakatomi, N.; Kurihara, H.; Fox, M.D.; Smith, J.E.; Okaji, K. Crown-of-Thorns starfish larvae can feed on organic matter released from corals. Diversity 2016, 8, 18. [Google Scholar] [CrossRef]
- Nakamura, M.; Higa, Y.; Kumagai, N.H.; Okaji, K. Using long-term removal data to manage a Crown-of-Thorns Starfish population. Diversity 2016, 8, 24. [Google Scholar] [CrossRef]
- Sigl, R.; Laforsch, C. The influence of water currents on movement patterns on sand in the Crown-of-Thorns Seastar (Acanthaster cf. solaris). Diversity 2016, 8, 25. [Google Scholar] [CrossRef]
- Cowan, Z.-L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M. Benthic predators influence microhabitat preferences and settlement success of Crown-of-Thorns Starfish (Acanthaster cf. solaris). Diversity 2016, 8, 27. [Google Scholar] [CrossRef]
- Buck, A.C.E.; Gardiner, N.M.; Bostrom-Einarsson, L. Citric acid injections: An accessible and efficient method for controlling outbreaks of the Crown-of-Thorns Starfish Acanthaster cf. solaris. Diversity 2016, 8, 28. [Google Scholar] [CrossRef]
- Hall, M.R.; Kocot, K.M.; Baughman, K.W.; Fernandez-Valverde, S.L.; Gauthier, M.E.A.; Hatleberg, W.L.; Krishnan, A.; McDougall, C.; Motti, C.A.; Shoguchi, E.; et al. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 2017, 544, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, A.W.; Dempsey, A.C. The status, threats, and resilience of reef-building corals of the Saudi Arabian Red Sea. In The Red Sea. The Formation, Morphology, Oceanography and Environment of a Young Ocean Basin; Rasul, N.M.A., Steward, C.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 470–486. [Google Scholar]
- Madsen, F.J. A note on the seastar genus Acanthaster. Vidensk. Medd. Dansk Naturh. Foren. 1955, 117, 179–192. [Google Scholar]
- Caso, M.E. Estudios sobre Astéridos de México. Observaciones sobre especies pacíficas del género Acanthaster y descripción de una subespecie nueva, Acanthaster ellisii pseudoplanci. Anales Inst. Biol. Univ. Nacl. Autón. México 1962, 32, 313–331. (In Spanish) [Google Scholar]
- Birkeland, C.; Lucas, J.S. Acanthaster Planci: Major Management Problem of Coral Reefs; CRC Press: Boca Raton, FL, USA, 1990; p. 267. [Google Scholar]
- Campbell, A.; Ormond, R.F.G. The threat of the “crown-of-thorns” starfish (Acanthaster planci) to coral reefs in the Indo-Pacific area: Observations on a normal population in the Red Sea. Biol. Conserv. 1970, 2, 246–251. [Google Scholar] [CrossRef]
- Sweatman, H. No-take reserves protect coral reefs from predatory starfish. Curr. Biol. 2008, 18, R598–R599. [Google Scholar] [CrossRef] [PubMed]
- Houk, P.; Raubani, J. Acanthaster planci outbreaks in Vanuatu coincide with ocean productivity, furthering trends throughout the Pacific Ocean. J. Oceanogr. 2011, 66, 435–438. [Google Scholar] [CrossRef]
- Houk, P.; Bograd, S.; van Woesik, R. The transition zone chlorophyll front can trigger Acanthaster planci outbreaks in the Pacific Ocean: Historical confirmation. J. Oceanogr. 2007, 63, 149–154. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Lucas, J.S.; Jones, M.M. Hybrid crown-of-thorns starfish (Acanthaster planci X A. brevispinus) reared to maturity in the laboratory. Nature 1976, 263, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. 2003, B270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.L.; van Oppen, M.J.H.; Romano, S.L.; Wörheide, G. Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Molec. Ecol. 2002, 11, 2475–2487. [Google Scholar] [CrossRef]
- Wörheide, G.; Erpenbeck, D. DNA taxonomy of sponges—Progress and perspectives. J. Mar. Biol. Ass. UK 2007, 87, 1629–1633. [Google Scholar] [CrossRef]
- Ward, R.; Holmes, B.H.; O´Hara, T.D. DNA barcoding discriminates echinoderm species. Molec. Ecol. Res. 2008, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Janosik, A.M.; Mahon, A.R.; Halanych, K.M. Evolutionary history of Southern Ocean Odontaster sea star species (Odontasteridae; Asteroidea). Polar Biol. 2011, 34, 575–586. [Google Scholar] [CrossRef]
- Bucklin, A.; Steinke, D.; Blanco-Bercial, L. DNA barcoding of marine Metazoa. Ann. Rev. Mar. Sci. 2011, 3, 471–508. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The barcode of life data system (www.barcodinglife.org). Molec. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ratsasingham, S.; Hebert, P.D.N. BOLD’s role in barcode data management and analysis: A response. Molec. Ecol. Res. 2011, 11, 941–942. [Google Scholar] [CrossRef]
- BOLD Systems. Available online: http://www.boldsystems.org/ (accessed on 10 May 2017).
- Jones, M.; Ghoorah, A.; Blaxter, M. jMOTU and Taxonerator: Turning DNA barcode sequences into annotated operational taxonomic units. PLoS ONE 2011, 6, e19259. [Google Scholar] [CrossRef] [PubMed]
- Ratsasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar]
- Jörger, K.M.; Norenburg, J.L.; Wilson, N.G.; Schrödl, M. Barcoding against a paradox? Combined molecular species delineations reveal multiple cryptic lineages in elusive meiofaunal sea slugs. BMC Evol. Biol. 2012, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. Publ. Lib. Sci. Biol. 2005, 3, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, A.; Godfray, H.C.J.; Huemer, P.; Mutanen, M.; Rougerie, R.; van Nieukerken, E.J.; Ratnasingham, S.; Hebert, P.D.N. Genetic patterns in European geometrid moths revealed by the Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e84518. [Google Scholar] [CrossRef]
- BOLD-Handbook—Barcode Index Numbers. Available online: www.boldsystems.org/index.php/resources/handbook?chapter=2_databases.html§ion=bins (accessed on 10 May 2017).
- Pleijel, F.; Jondelius, U.; Norlinder, E.; Nygren, A.; Oxelman, B.; Schander, C.; Sundberg, P.; Thollesson, M. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol. Phylogenet. Evol. 2008, 48, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Astrin, J.J.; Zhou, X.; Misof, B. The importance of biobanking in molecular taxonomy, with proposed definitions for vouchers in a molecular context. ZooKeys 2013, 365, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Diversity of the Indopacific Network. Available online: http://diversityindopacific.net (accessed on 10 May 2017).

| OTU (Vogler et al., 2008 [9]) | NIO Species | SIO Species | Pacific Species | Red Sea Species |
| Name [proposed] | A. planci (Linnaeus, 1758) | A. mauritiensis de Loriol, 1885 | [A. solaris (Schreber, 1795)] | NN (not yet named) |
| Type locality | off Goa, West Indian coast | off Mauritius | Magellan Street, Philippines | - |
| BIN (BOLD) | AAA1633 | AAA1631 | AAA1630 | AAA1632 |
| Synonyms [proposed] | [A. echinites (Ellis & Solander in Watt, 1786)] | - | A. ellisii (Gray, 1840) A. e. pseudoplanci Caso, 1962 | - |
| Distribution | North Indian Ocean | South Indian Ocean (except West Australian coast ) | Pacific Ocean and West Australian coast | Red Sea |
| Outbreak ability | Yes | yes | Yes | yes |
| number of arms | max. 23 | max. 23 | max. 23 | max. 13–14 |
| Specific color | “electric-blue“ | light blue to rusty | gray-green to gray-purple, bulls-eye appearance | gray-green to gray-purple, bulls-eye appearance |
| Harmfulness to humans | harmful | harmful | very harmful | less harmful |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haszprunar, G.; Vogler, C.; Wörheide, G. Persistent Gaps of Knowledge for Naming and Distinguishing Multiple Species of Crown-of-Thorns-Seastar in the Acanthaster planci Species Complex. Diversity 2017, 9, 22. https://doi.org/10.3390/d9020022
Haszprunar G, Vogler C, Wörheide G. Persistent Gaps of Knowledge for Naming and Distinguishing Multiple Species of Crown-of-Thorns-Seastar in the Acanthaster planci Species Complex. Diversity. 2017; 9(2):22. https://doi.org/10.3390/d9020022
Chicago/Turabian StyleHaszprunar, Gerhard, Catherine Vogler, and Gert Wörheide. 2017. "Persistent Gaps of Knowledge for Naming and Distinguishing Multiple Species of Crown-of-Thorns-Seastar in the Acanthaster planci Species Complex" Diversity 9, no. 2: 22. https://doi.org/10.3390/d9020022
APA StyleHaszprunar, G., Vogler, C., & Wörheide, G. (2017). Persistent Gaps of Knowledge for Naming and Distinguishing Multiple Species of Crown-of-Thorns-Seastar in the Acanthaster planci Species Complex. Diversity, 9(2), 22. https://doi.org/10.3390/d9020022







