Crown-of-Thorns Starfish Larvae Can Feed on Organic Matter Released from Corals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of COTS Larvae
2.2. Collection of Coral Mucus
2.3. Feeding Experiments
2.4. Analysis
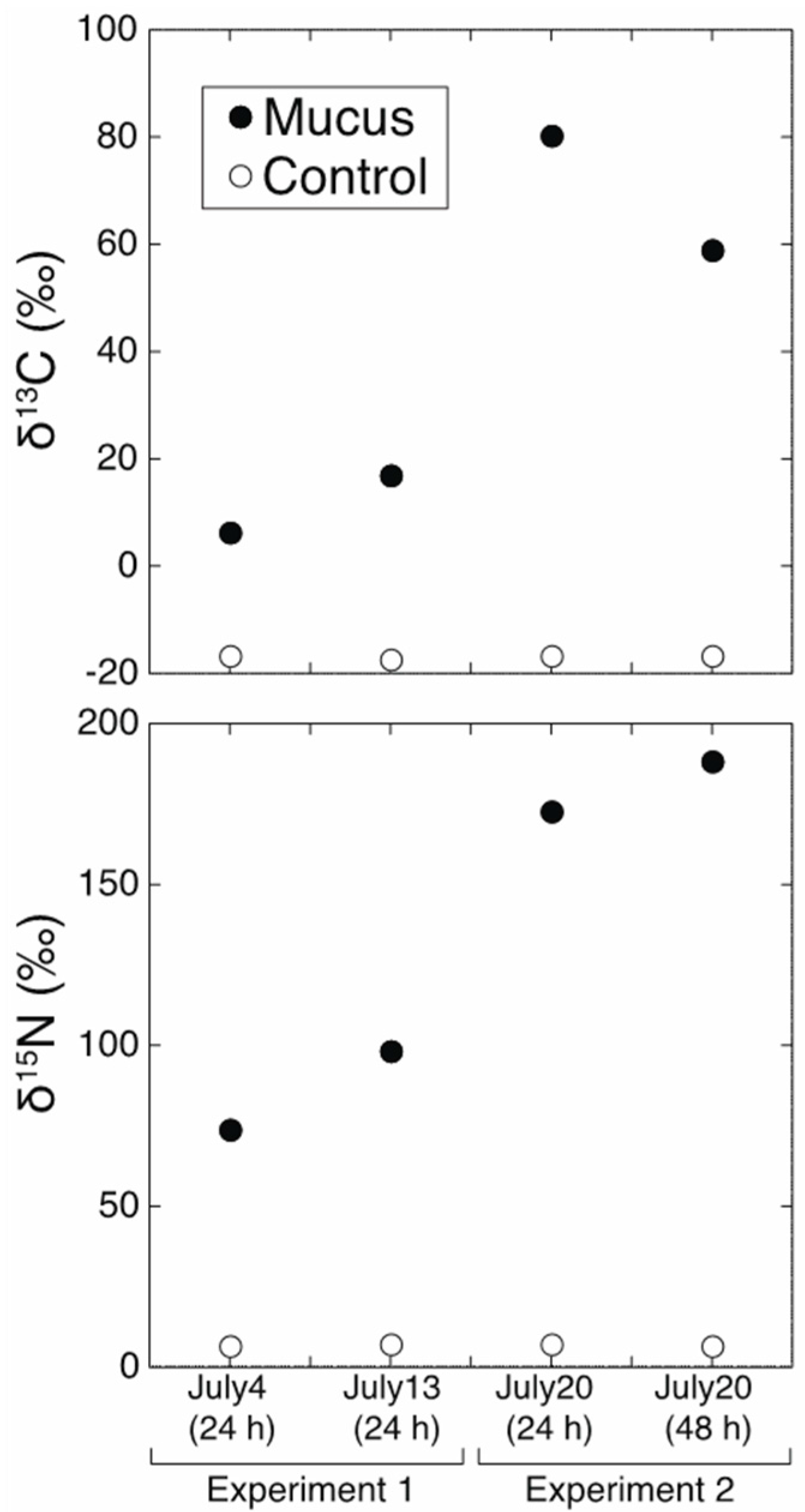
3. Results and Discussion
Acknowledgments
Author contributions
Conflicts of Interest
References
- Birkeland, C.; Lucas, J. Acanthaster Planci: Major Management Problem of Coral Reefs; CRC Press: Florida, FL, USA, 1990. [Google Scholar]
- Pratchett, M.; Caballes, C.; Rivera Posada, J.; Sweatman, H. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 133–200. [Google Scholar]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J. Quantitative studies of feeding and nutrition during larval stages of the coral reef asteroid Acanthaster planci (L.). J. Exp. Mar. Biol. Ecol. 1982, 65, 173–193. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval starvation to satiation: Influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Okaji, K.; Ayukai, T.; Lucas, J.S. Selective feeding by larvae of the crown-of-thorns starfish, Acanthaster planci (L.). Coral Reefs 1997, 16, 47–50. [Google Scholar] [CrossRef]
- Okaji, K. Feeding Ecology in the Early Life Stages of the Crown-of-Thorns Starfish, Acanthaster planci (L.). Ph.D. Thesis, James Cook University, Brisbane, Australia, June 1996. [Google Scholar]
- Ferrier-Pagès, C.; Gattuso, J.P. Biomass, production and grazing rates of pico- and nanoplankton in coral reef waters (Miyako Island, Japan). Microb. Ecol. 1998, 35, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Casareto, B.E.; Charpy, L.; Blanchot, J.; Suzuki, Y.; Kurosawa, K.; Ihikawa, Y. Photorophic prokaryotes in Bora Bay, Miyako Island, Okinawa, Japan. Proc. 10th Int. Coral Reef Symp. 2006, 31, 844–853. [Google Scholar]
- Tada, K.; Sakai, K.; Nakano, Y.; Takemura, A.; Montani, S. Size-fractionated phytoplankton biomass in coral reef waters off Sesoko Island, Okinawa, Japan. J. Plankton Res. 2001, 25, 991–997. [Google Scholar] [CrossRef]
- Kinjyo, K.; Yamakawa, E. Survey of chlorophyll distribution. In A Report on the Comprehensive Management Program of the Crown-of-Thorns Starfish Outbreaks in Okinawa, 2014; Okinawa Prefectural Government, Department of Environmental Affairs, Nature Conservation and Afforestation Promotion Division: Okinawa, Japan, 2014; pp. 99–112. [Google Scholar]
- Fukuoka, K.; Shimoda, T.; Abe, K. Community structure and abundance of copepods in summer on a fringing coral reef off Ishigaki Island, Ryukyu Islands, Japan. Plankt. Benthos Res. 2015, 10, 225–232. [Google Scholar] [CrossRef]
- Olson, R. In situ culturing of larvae of the crown-of-thorns starfish Acanthaster planci. Mar. Ecol. Prog. Ser. 1985, 25, 207–210. [Google Scholar] [CrossRef]
- Olson, R. In situ culturing as a test of the larval starvation hypothesis for the crown-of-throns starfish, Acanthaster planci. Limnol. Oceanogr. 1987, 32, 895–904. [Google Scholar] [CrossRef]
- Nakamura, M.; Kumagai, N.H.; Sakai, K.; Okaji, K.; Ogasawara, K.; Mitarai, S. Spatial variability in recruitment of acroporid corals and predatory starfish along the Onna coast, Okinawa, Japan. Mar. Ecol. Prog. Ser. 2015, 540, 1–12. [Google Scholar] [CrossRef]
- Uthicke, S.; Doyle, J.; Duggan, S.; Yasuda, N.; McKinnon, A.D. Outbreak of coral-eating Crown-of-Thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 2015, 5, 16885. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Yasuda, N.; Ikehara, K.; Fukuoka, K.; Kameda, T.; Kai, S.; Nagai, S.; Watanabe, A.; Nakamura, T.; Kitazawa, S.; et al. Detection of a high-density brachiolaria-stage larval population of crown-of-thorns sea star (Acanthaster planci) in Sekisei Lagoon (Okinawa, Japan). Diversity 2016, 8, 9. [Google Scholar] [CrossRef]
- McKinnon, A.; Duggan, S.; De’ath, G. Mesozooplankton dynamics in nearshore waters of the Great Barrier Reef. Estuar. Coast. Shelf Sci. 2005, 63, 497–511. [Google Scholar] [CrossRef]
- Fabricius, K.; De’ath, G.; McCook, L.; Turak, E.; Williams, D.M. Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Pollut. Bull. 2005, 51, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Furnas, M.J.; Mitchell, A.W.; Gilmartin, M.; Revelante, N. Phytoplankton biomass and primary production in semi-enclosed reef lagoons of the central Great Barrier Reel Australia. Coral Reefs 1990, 9, 1–10. [Google Scholar] [CrossRef]
- Van Woesik, R.; Tomascik, T.; Blake, S. Coral assemblages and physico-chemical characteristics of the Whitsunday Islands: Evidence of recent community changes. Mar. Freshw. Res. 1999, 50, 427–440. [Google Scholar] [CrossRef]
- Le Borgne, R.; Rodier, M.; Le Bouteiller, A.; Kulbicki, M. Plankton biomass and production in an open atoll lagoon: Uvea, New Caledonia. J. Exp. Mar. Biol. Ecol. 1997, 212, 187–210. [Google Scholar] [CrossRef]
- Rochelle-Newall, E.J.; Torréton, J.P.; Mari, X.; Pringault, O. Phytoplankton-bacterioplankton coupling in a subtropical South Pacific coral reef lagoon. Aquat. Microb. Ecol. 2008, 50, 221–229. [Google Scholar] [CrossRef]
- Torréton, J.P.; Rochelle-Newall, E.; Pringault, O.; Jacquet, S.; Faure, V.; Briand, E. Variability of primary and bacterial production in a coral reef lagoon (New Caledonia). Mar. Pollut. Bull. 2010, 61, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Charpy, L.; Dufour, P.; Garcia, N. Particulate organic matter in sixteen Tuamotu atoll lagoons (French Polynesia). Mar. Ecol. Ser. 1997, 151, 55–65. [Google Scholar] [CrossRef]
- Sakka, A.; Legendre, L. Carbon budget of the planktonic food web in an atoll lagoon (Takapoto, French Polynesia). J. Plankton Res. 2002, 24, 301–320. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Furla, P. Pico- and nanoplankton biomass and production in the two largest atoll lagoons of French Polynesia. Mar. Ecol. Prog. Ser. 2001, 211, 63–76. [Google Scholar] [CrossRef]
- Charpy, L.; Rodier, M.; Fournier, J.; Langlade, M.J.; Gaertner-Mazouni, N. Physical and chemical control of the phytoplankton of Ahe lagoon, French Polynesia. Mar. Pollut. Bull. 2012, 65, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Yoshida, T.; Othman, B.; Toda, T. Biomass and estimated production rates of metazoan zooplankton community in a tropical coral reef of Malaysia. Mar. Ecol. 2014, 35, 112–131. [Google Scholar] [CrossRef]
- Nakajima, R.; Tsuchiya, K.; Nakatomi, N.; Yoshida, T.; Tada, Y.; Konno, F.; Toda, T.; Kuwahara, V.S.; Hamasaki, K.; Othman, B.H.R.; et al. Enrichment of microbial abundance in the sea-surface microlayer over a coral reef : Implications for biogeochemical cycles in reef ecosystems. Mar. Ecol. Prog. Ser. 2013, 490, 11–22. [Google Scholar] [CrossRef]
- Wild, C.; Huettel, M.; Klueter, A.; Kremb, S.G. Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 2004, 428, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.E.; Bythell, J.C. Perspectives on mucus secretion in reef corals. Mar. Ecol. Prog. Ser. 2005, 296, 291–309. [Google Scholar] [CrossRef]
- Wild, C.; Naumann, M.; Niggl, W.; Haas, A. Carbohydrate composition of mucus released by scleractinian warm- and cold-water reef corals. Aquat. Biol. 2010, 10, 41–45. [Google Scholar] [CrossRef]
- Huettel, M.; Wild, C.; Gonelli, S. Mucus trap in coral reefs: Formation and temporal evolution of particle aggregates caused by coral mucus. Mar. Ecol. Prog. Ser. 2006, 307, 69–84. [Google Scholar] [CrossRef]
- Naumann, M.S.; Richter, C.; El-Zibdah, M.; Wild, C. Coral mucus as an efficient trap for picoplanktonic cyanobacteria: Implications for pelagic-benthic coupling in the reef ecosystem. Mar. Ecol. Prog. Ser. 2009, 385, 65–76. [Google Scholar] [CrossRef]
- Hata, H.; Kudo, S.; Yamano, H.; Kurano, N.; Kayanne, H. Organic carbon flux in Shiraho coral reef (Ishigaki Island, Japan). Mar. Ecol. Prog. Ser. 2002, 232, 129–140. [Google Scholar] [CrossRef]
- Nakajima, R.; Tanaka, Y. The role of coral mucus in the material cycle in reef ecosystems: Biogeochemical and ecological perspectives. J. Jpn. Coral Reef Soc. 2014, 16, 3–27. [Google Scholar] [CrossRef]
- Richman, S.; Loya, Y.; Slobodkin, L. The rate of mucus production by corals and its assimilation by the coral reef copepod Acartia negligens. Limnol. Oceanogr. 1975, 20, 918–923. [Google Scholar] [CrossRef]
- Gottfried, M.; Roman, M.R. Ingestion and incorporation of coral-mucus detritus by reef zooplankton. Mar. Biol. 1983, 72, 211–218. [Google Scholar] [CrossRef]
- Naumann, M.S.; Haas, A.; Struck, U.; Mayr, C.; el-Zibdah, M.; Wild, C. Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs 2010, 29, 649–659. [Google Scholar] [CrossRef]
- Crossland, C. In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. Coral Reefs 1987, 6, 35–42. [Google Scholar] [CrossRef]
- Vogler, C.; Benzie, J.; Lessios, H.; Barber, P.; Wörheide, G. A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol. Lett. 2008, 4, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Haszprunar, G.; Spies, M. An integrative approach to the taxonomy of the crown-of-thorns starfish species group (Asteroidea: Acanthaster): A review of names and comparison to recent molecular data. Zootaxa 2014, 3841, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.S.; Mayr, C.; Struck, U.; Wild, C. Coral mucus stable isotope composition and labeling: Experimental evidence for mucus uptake by epizoic acoelomorph worms. Mar. Biol. 2010, 157, 2521–2531. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Ayukai, T. Assessment of the Role of Dissolved Organic Matter and Bacteria in the Nutrition of Crown-of-Thorns Starfish Larva; Australian Institute of Marine Science: Townsville, Australia, 1992. [Google Scholar]
- Hoegh-Gulberg, O. Is Acanthaster planci able to utilise dissolved organic matter (DOM) to satisfy the energetic requirements of larval development? In The Possible Causes and Consequences of Outbreaks of the Crown-of-Thorns Starfish; Great Barrier Reef Marine Park Authority Workshop: Townsville, Australia, 1992; pp. 37–54. [Google Scholar]
- Wyatt, A.S.J.; Lowe, R.J.; Humphries, S.; Waite, A.M. Particulate nutrient fluxes over a fringing coral reef: Source—Sink dynamics inferred from carbon to nitrogen ratios and stable isotopes. Limnol. Oceanogr. 2013, 58, 409–427. [Google Scholar] [CrossRef]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval phenotypic plasticity in the boom-and-bust crown-of-thorns seastar, Acanthaster planci. Mar. Ecol. Prog. Ser. 2015, 539, 179–189. [Google Scholar] [CrossRef]
| Site | Chl-a (µg·L−1) | Reference |
|---|---|---|
| Miyako Island (Okinawa, Japan) | 0.10–0.15 | [10] |
| Miyako Island (Okinawa, Japan) | 0.1–0.4 | [11] |
| Sesoko Island (Okinawa, Japan) | 0.11–0.77 (0.45) | [12] |
| West coast of Okinawa Island (Japan) | <0.05–1.79 (0.17) | [13] |
| Ishigaki Island (Okinawa, Japan) | 0.09–0.55 | [14] |
| Princess Charlotte Bay (GBR, Australia) | 0.06–0.28 (0.16) | [20] |
| Princess Charlotte Bay (GBR, Australia) | 0.40 | [21] |
| Cairns-Innisfail sector (GBR, Australia) | 0.03–0.64 (0.25) | [20] |
| Wet Tropics (GBR, Australia) | 0.70 | [21] |
| Central GBR (Australia) | 0.19–0.72 (0.38) | [22] |
| Whitsunday Islands (GBR, Australia) | 0.31–1.21 (0.79) | [23] |
| Uvea Atoll (New Caledonia) | 0.23 | [24] |
| The Southwest lagoon (New Caledonia) | 0.25–2.14 (0.60) | [25] |
| Maître Island (New Caledonia) | 0.26–0.42 (0.30) | [26] |
| Tikehau Atoll (Tuamotu, French Polynesia) | 0.17 | [27] |
| Takapoto Atoll (Tuamotu, French Polynesia) | 0.23 | [27] |
| Takapoto Atoll (Tuamotu, French Polynesia) | 0.21–0.23 (0.22) | [28] |
| Fakarava/Rangiroa Atolls (French Polynesia) | 0.008–0.25 | [29] |
| Ahe Atoll (French Polynesia) | 0.08–0.85 (0.34) | [30] |
| Tioman Island (Malaysia) | 0.20–0.24 (0.22) | [31] |
| Bidong Island (Malaysia) | 0.28–0.30 (0.29) | [32] |
| Collection Day (mm/dd/yy) | δ13C (‰) | δ15N (‰) | C/N |
|---|---|---|---|
| 07/18/2015 | 288.9 | 1562.5 | 6.7 |
| 463.7 | 1783.9 | 7.7 | |
| 322.9 | 1703.7 | 7.2 | |
| 358.5 ± 53.5 | 1683.4 ± 64.7 | ||
| 07/24/2015 | 690.6 | 2091.3 | 7.8 |
| 688.1 | 1869.4 | 7.2 | |
| 743.3 | 1990.6 | 7.3 | |
| 707.4 ± 18.0 | 1983.8 ± 64.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, R.; Nakatomi, N.; Kurihara, H.; Fox, M.D.; Smith, J.E.; Okaji, K. Crown-of-Thorns Starfish Larvae Can Feed on Organic Matter Released from Corals. Diversity 2016, 8, 18. https://doi.org/10.3390/d8040018
Nakajima R, Nakatomi N, Kurihara H, Fox MD, Smith JE, Okaji K. Crown-of-Thorns Starfish Larvae Can Feed on Organic Matter Released from Corals. Diversity. 2016; 8(4):18. https://doi.org/10.3390/d8040018
Chicago/Turabian StyleNakajima, Ryota, Nobuyuki Nakatomi, Haruko Kurihara, Michael D. Fox, Jennifer E. Smith, and Ken Okaji. 2016. "Crown-of-Thorns Starfish Larvae Can Feed on Organic Matter Released from Corals" Diversity 8, no. 4: 18. https://doi.org/10.3390/d8040018
APA StyleNakajima, R., Nakatomi, N., Kurihara, H., Fox, M. D., Smith, J. E., & Okaji, K. (2016). Crown-of-Thorns Starfish Larvae Can Feed on Organic Matter Released from Corals. Diversity, 8(4), 18. https://doi.org/10.3390/d8040018




