Identification and Density Estimation of American Martens (Martes americana) Using a Novel Camera-Trap Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
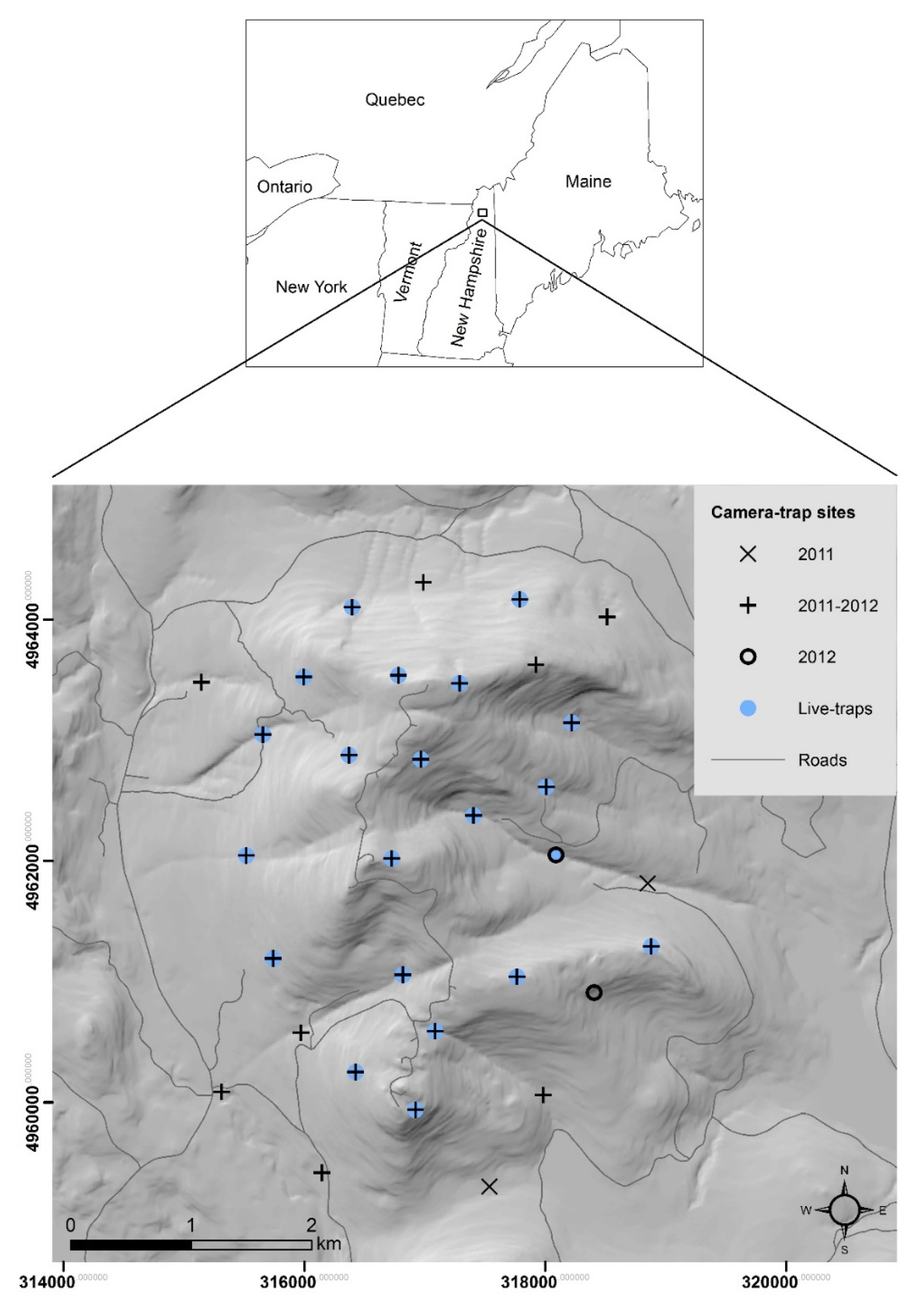
2.2. Live-Trapping
2.3. Camera-Trapping
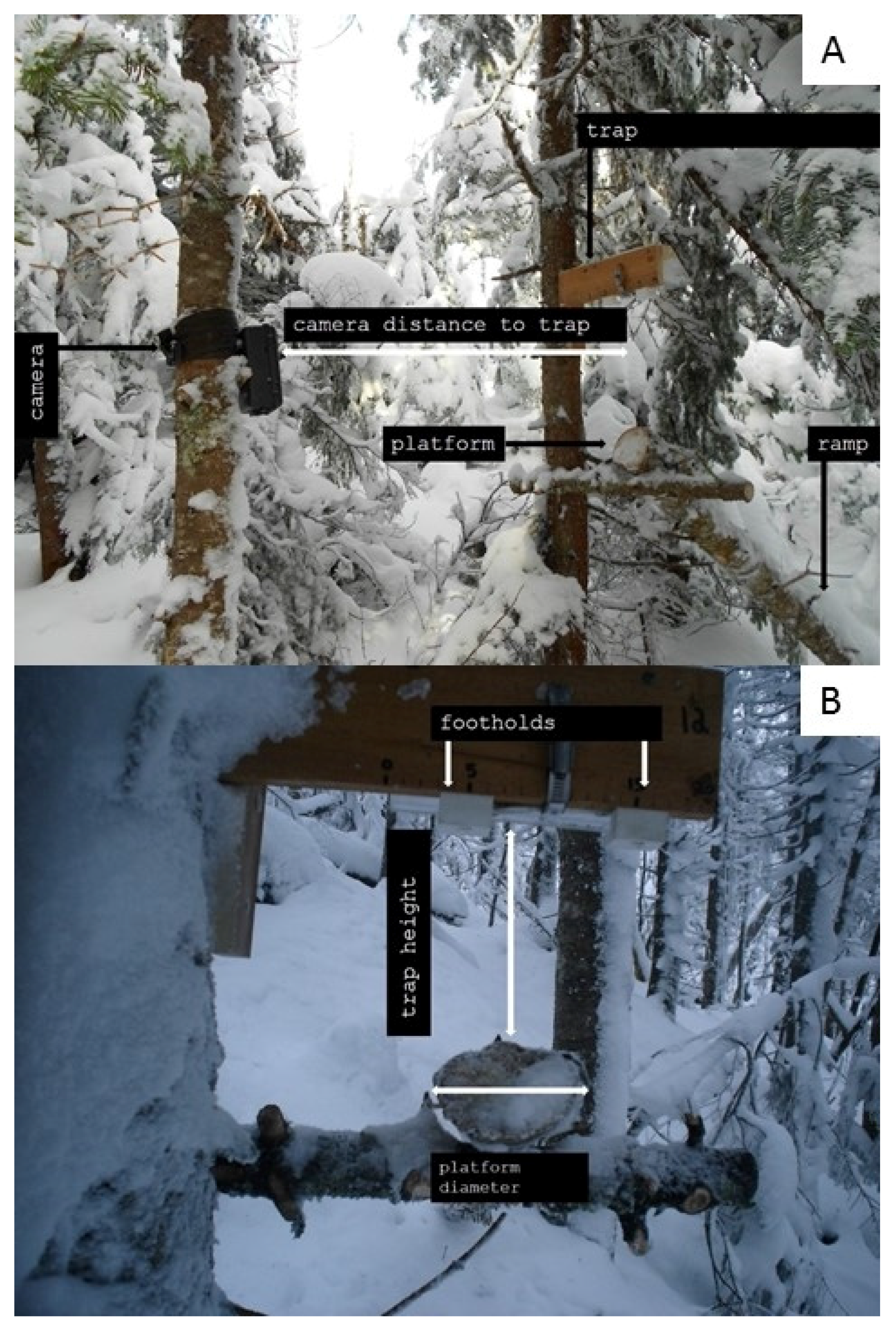

2.4. Ventral Scoring
2.5. Photographic Clusters
2.6. Identification of Un-Collared Martens
2.7. Identification Probability
2.8. Camera- and Live-Capture SCR
3. Results
3.1. Ventral Scoring
3.2. Photographic Clusters
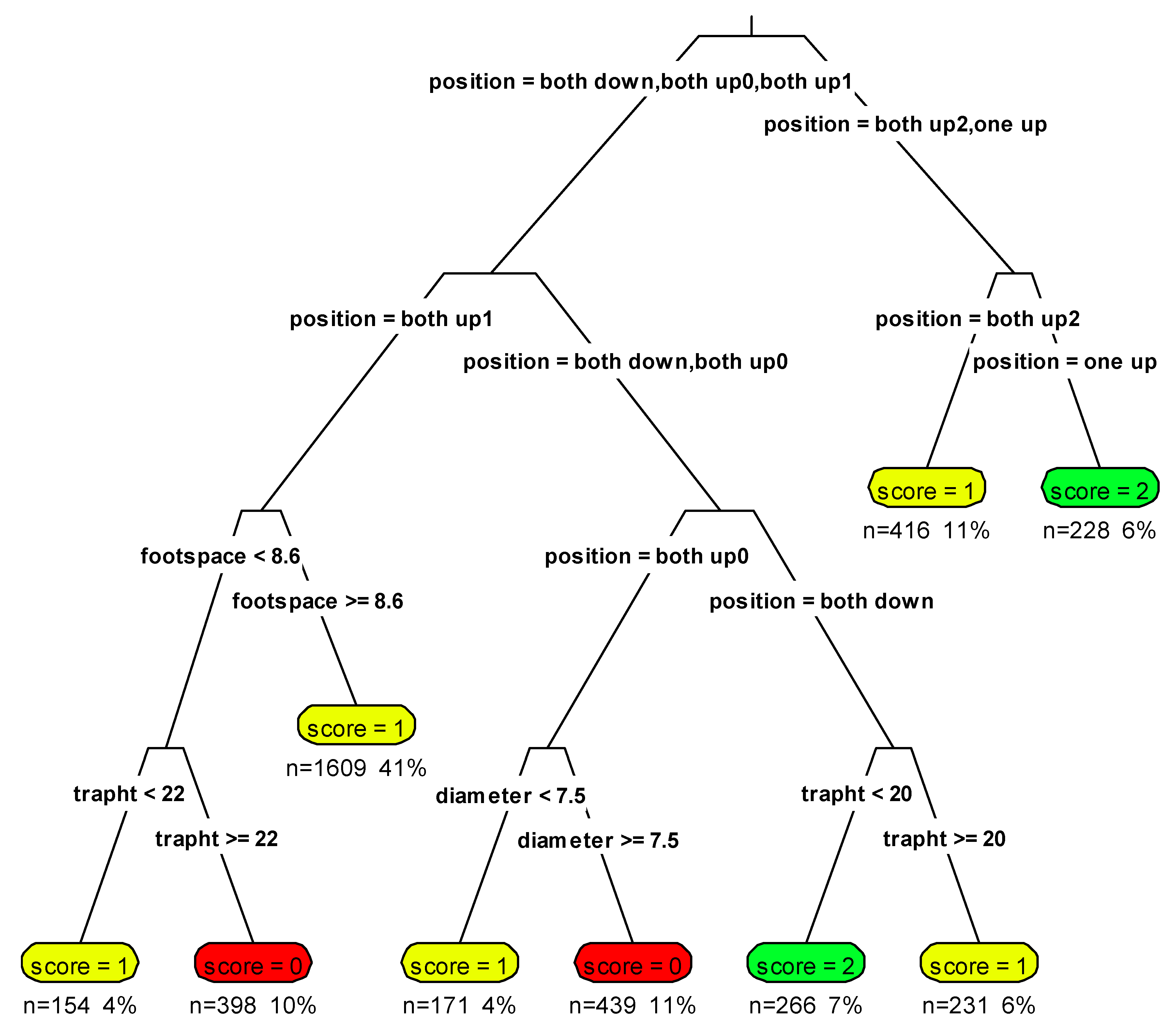
3.3. Identification of Un-Collared Martens
3.4. Identification Probability
3.5. Camera- and Live-Capture SCR
| Trapping Event a | D ± SE | CL | % Recap | TN | CPUE |
|---|---|---|---|---|---|
| Camera 2011 | 0.43 ± 0.12 | 0.25–0.75 | 100% (13 of 13) | 339 | 36 marten/100 TN |
| Camera 2012 | 0.60 ± 0.16 | 0.35–1.01 | 93% (14 of 15) | 224 | 38 marten/100 TN |
| Live-trap 2012 | 0.45 ± 0.24 | 0.16–1.22 | 66% (10 of 15) | 112 | 27 marten/100 TN |
| Trapping Event | Models a | k | AICc | ∆AICc | wi |
|---|---|---|---|---|---|
| Camera-trapping 2011 | modelbk | 4 | 793.1 | 0 | 1 |
| model0 | 3 | 822.2 | 29.1 | 0 | |
| modelb | 4 | 825.8 | 32.7 | 0 | |
| Camera-trapping 2012 | modelbk | 4 | 573.9 | 0 | 1 |
| model0 | 3 | 605.6 | 31.7 | 0 | |
| modelb | 4 | 606.3 | 32.5 | 0 | |
| Live-trapping 2012 | modelb | 4 | 224.4 | 0 | 0.51 |
| model0 | 3 | 225.8 | 1.4 | 0.25 | |
| modelbk | 4 | 225.9 | 1.6 | 0.23 |
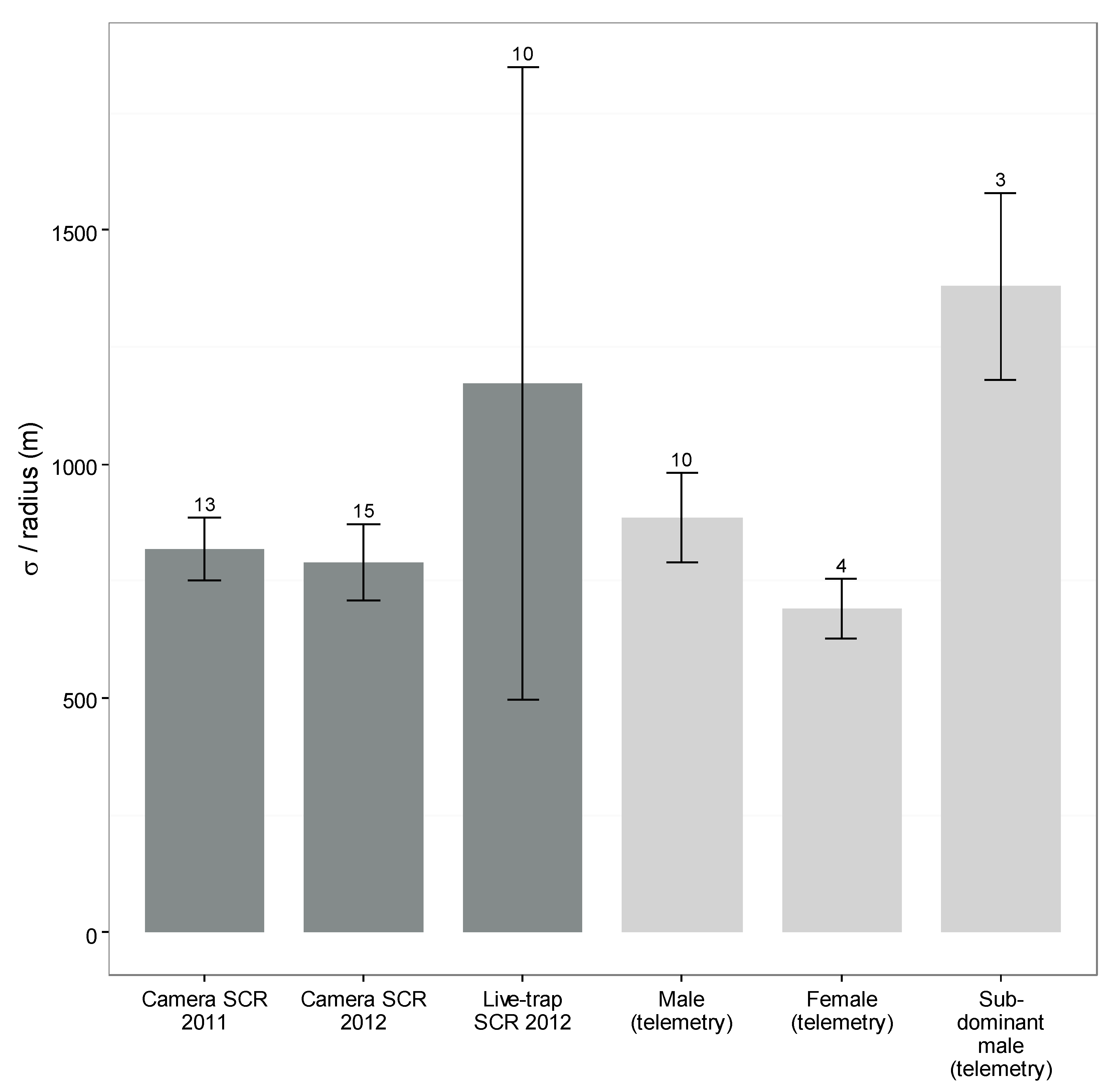
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Live-Trapping 2012 | Camera-Trapping 2012 | Camera-Trapping 2011 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Id a | S b | A c | rec | trap | Id a | S b | A c | rec | trap | Id a | S b | A c | rec | trap |
| F10 | F | J | 0 | 1 | Un1 | M | - | 1 | 2 | Un1 | F | - | 3 | 2 |
| F11 | F | A | 0 | 1 | Un2 | F | - | 2 | 2 | Un2 | M | - | 3 | 3 |
| F12 | F | A | 0 | 1 | Un3 | F | - | 1 | 1 | Un3 | F | - | 1 | 2 |
| F13 | F | A | 1 | 2 | Un4 | M | - | 5 | 5 | Un4 | F | - | 1 | 1 |
| F14 | F | A | 1 | 1 | Un5 | F | - | 1 | 2 | F4 | F | A | 5 | 2 |
| F15 | F | A | 0 | 1 | F14 ** | F | A | 0 | 1 | F5 | F | A | 7 | 5 |
| F5 | F | A | 1 | 1 | F5 | F | A | 1 | 2 | F7 ** | F | A | 11 | 6 |
| F9 | F | A | 2 | 1 | F9 | F | A | 8 | 2 | M1 | M | A | 11 | 4 |
| M1 * | M | A | 2 | 3 | M1 | M | A | 17 | 7 | M2 ** | M | A | 6 | 3 |
| M10 | M | A | 2 | 2 | M10 | M | A | 5 | 3 | M3 | M | A | 21 | 7 |
| M11 | M | A | 0 | 1 | M11 | M | A | 4 | 3 | M5 | M | A | 28 | 10 |
| M12 | M | A | 0 | 1 | M13 ** | M | A | 8 | 3 | M6 | M | J | 1 | 2 |
| M13 * | M | J | 4 | 4 | M15 ** | M | A | 8 | 2 | M7 | M | A | 13 | 5 |
| M14 | M | A | 1 | 1 | M5 | M | A | 8 | 6 | |||||
| M8 | M | A | 1 | 1 | M8 | M | A | 2 | 2 | |||||
References
- Seber, G.A.F. Estimation of Animal Abundance and Related Parameters, 2nd ed.; Macmillan: New York, NY, USA, 1982. [Google Scholar]
- Efford, M. Density estimation in live-trapping studies. Oikos 2004, 106, 598–610. [Google Scholar] [CrossRef]
- Jhala, Y.V.; Mukherjee, S.; Shah, N.; Chauhan, K.S.; Dave, C.V.; Meena, V.; Banerjee, K. Home range and habitat preference of female lions (Panthera leo persica) in Gir forests, India. Biodivers. Conserv. 2009, 18, 3383–3394. [Google Scholar] [CrossRef]
- O’Connell, A.F.; Nichols, J.D.; Karanth, K.U. Introduction. In Camera Traps in Animal Ecology: Methods and Analyses; O’Connell, A.F., Nichols, J.D., Karanth, K.U., Eds.; Springer Science and Business Media: New York, NY, USA, 2011; pp. 1–8. [Google Scholar]
- Pollock, K.H.; Nichols, J.D.; Brownie, C.; Hines, J.E. Statistical inference for capture-recapture experiments. Wildl. Monogr. 1990, 107, 3–97. [Google Scholar]
- Noyce, K.V.; Garshelis, D.L.; Coy, P.L. Differential vulnerability of black bears to trap and camera sampling and resulting biases in mark-recapture estimates. Ursus 2001, 12, 211–225. [Google Scholar]
- Karanth, K.U.; Nichols, J.D. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 1998, 79, 2852–2862. [Google Scholar] [CrossRef]
- Magoun, A.J.; Long, C.D.; Schwartz, M.K.; Pilgrim, K.L.; Lowell, R.E.; Valkenburg, P. Integrating motion-detection cameras and hair snags for wolverine identification. J. Wildl. Manag. 2011, 75, 731–739. [Google Scholar] [CrossRef]
- Negrões, N.; Sarmento, P.; Cruz, J.; Eira, C.; Revilla, E.; Fonseca, C.; Sollmann, R.; Torres, N.M.; Furtado, M.M.; Jacoma, A.T.A.; et al. Use of camera-trapping to estimate puma density and influencing factors in central Brazil. J. Wildl. Manag. 2010, 74, 1195–1203. [Google Scholar] [CrossRef]
- Goswami, V.R.; Lauretta, M.V.; Madhusudan, M.D.; Karanth, K.U. Optimizing individual identification and survey effort for photographic capture–recapture sampling of species with temporally variable morphological traits. Anim. Conserv. 2012, 15, 174–183. [Google Scholar] [CrossRef]
- Hebeisen, C.; Fattebert, J.; Baubet, E.; Fischer, C. Estimating wild boar (Sus scrofa) abundance and density using capture–resights in Canton of Geneva, Switzerland. Eur. J. Wildl. Res. 2008, 54, 391–401. [Google Scholar] [CrossRef]
- Alonso, R.S.; McClintock, B.T.; Lyren, L.M.; Boydston, E.E.; Crooks, K.R. Mark-recapture and mark-resight methods for estimating abundance with remote cameras: A carnivore case study. PLoS ONE 2015, 10, e0123032. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, P.M.; Burnham, K.P. Review of capture–recapture methods applicable to noninvasive genetic sampling. Mol. Ecol. 2005, 14, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Waits, L.P.; Paetkau, D. Noninvasive genetic sampling tools for wildlife biologists: A review of applications and recommendations for accurate data collection. J. Wildl. Manag. 2005, 69, 1419–1433. [Google Scholar] [CrossRef]
- Foster, R.J.; Harmsen, B.J. A critique of density estimation from camera-trap data. J. Wildl. Manag. 2012, 76, 224–236. [Google Scholar] [CrossRef]
- Powell, R.A.; Buskirk, S.W.; Zielinski, W.J. Fisher (Martes pennanti) and marten (Martes americana). In Wild Mammals of North America: Biology, Management, and Conservation, 2nd ed.; Feldhamer, G., Thompson, B.C., Chapman, J.A., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2003; pp. 635–649. [Google Scholar]
- Chapin, T.G.; Harrison, D.J.; Phillips, D.M. Seasonal habitat selection by marten in an untrapped forest preserve. J. Wildl. Manag. 1997, 61, 707–717. [Google Scholar] [CrossRef]
- Buskirk, S.W.; McDonald, L.L. Analysis of variability in home-range size of the American marten. J. Wildl. Manag. 1989, 53, 997–1004. [Google Scholar] [CrossRef]
- Yom-Tov, Y.; Yom-Tov, S.; Jarrell, G. Recent increase in body size of the American marten Martes americana in Alaska. Biol. J. Linn. Soc. 2008, 93, 701–707. [Google Scholar] [CrossRef]
- Long, R.A.; Mackay, P. Noninvasive methods for surveying martens, sables, and fishers. In Biology and Conservation of Martens, Sables, and Fishers: A New Synthesis; Aubry, K.B., Zielinski, W.J., Raphael, M.G., Proulx, G., Buskirk, S.W., Eds.; Comstock Pub. Associates: Ithaca, NY, USA, 2012; pp. 320–342. [Google Scholar]
- Chapin, T.G.; Harrison, D.J.; Katnik, D.D. Influence of landscape pattern on habitat use by American marten in an industrial forest. Conserv. Biol. 1998, 12, 96–227. [Google Scholar] [CrossRef]
- Hargis, C.D.; Bissonette, J.; Turner, D.L. The influence of forest fragmentation and landscape pattern on American martens. J. Appl. Ecol. 1999, 36, 157–172. [Google Scholar] [CrossRef]
- Poole, K.G.; Porter, A.D.; Vries, A.D.; Maundrell, C.; Grindal, S.D.; St. Clair, C.C. Suitability of a young deciduous-dominated forest for American marten and the effects of forest removal. Can. J. Zool. 2004, 82, 423–435. [Google Scholar] [CrossRef]
- Carroll, C. Interacting effects of climate change, landscape conversion, and harvest on carnivore populations at the range margin: Marten and lynx in the northern Appalachians. Conserv. Biol. 2007, 21, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.J.; Safford, H.D.; Girvetz, E.H. Martens and fishers in a changing climate. In Biology and Conservation of Martens, Sables, and Fishers: A New Synthesis; Aubry, K.B., Zielinski, W.J., Raphael, M.G., Proulx, G., Buskirk, S.W., Eds.; Comstock Pub. Associates: Ithaca, NY, USA, 2012; pp. 371–397. [Google Scholar]
- De Bondi, N.; White, J.G.; Stevens, M.; Cooke, R. A comparison of the effectiveness of camera trapping and live trapping for sampling terrestrial small-mammal communities. Wildl. Res. 2010, 37, 456–465. [Google Scholar] [CrossRef]
- Villette, P.; Krebs, C.J.; Jung, T.S.; Boonstra, R. Can camera trapping provide accurate estimates of small mammal (Myodes rutilus and Peromyscus maniculatus) density in the boreal forest? J. Mammal. 2015. [Google Scholar] [CrossRef]
- Kays, R.W.; Slauson, K.M. Remote cameras. In Noninvasive Survey Methods for Carnivores; Long, R.A., MacKay, P., Zielinski, W.J., Ray, J.C., Eds.; Island Press: Washington, DC, USA, 2008; pp. 111–140. [Google Scholar]
- Kelly, M.J.; Noss, A.J.; di Bitetti, M.S.; Maffei, L.; Arispe, R.L.; Paviolo, A.; de Angelo, C.D.; di Blanco, Y.E. Estimating puma densities from camera trapping across three study sites: Bolivia, Argentina, and Belize. J. Mammal. 2008, 89, 408–418. [Google Scholar] [CrossRef]
- Wellington, K.; Bottom, C.; Merrill, C.; Litvaitis, J.A. Identifying performance differences among trail cameras used to monitor forest mammals. Wildl. Soc. Bull. 2014, 38, 634–638. [Google Scholar] [CrossRef]
- Mendoza, E.; Martineau, P.R.; Brenner, E.; Dirzo, R. A novel method to improve individual animal identification based on camera-trapping data. J. Wildl. Manag. 2011, 75, 973–979. [Google Scholar] [CrossRef]
- Gerber, B.D.; Karpanty, S.M.; Kelly, M.J. Evaluating the potential biases in carnivore capture–recapture studies associated with the use of lure and varying density estimation techniques using photographic-sampling data of the Malagasy civet. Popul. Ecol. 2012, 54, 43–54. [Google Scholar] [CrossRef]
- Otis, D.L.; Burnham, K.P.; White, G.C.; Anderson, D.R. Statistical inference from capture data on closed animal populations. Wildl. Monogr. 1978, 62, 1–135. [Google Scholar]
- Link, W.A.; Yoshizaki, J.; Bailey, L.L.; Pollock, K.H. Uncovering a latent multinomial: Analysis of mark–recapture data with misidentification. Biometrics 2010, 66, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.B.; Royle, J.A. Spatially explicit models for inference about density in unmarked or partially marked populations. Ann. Appl. Stat. 2013, 7, 936–954. [Google Scholar] [CrossRef]
- Oliveira-Santos, L.G.R.; Zucco, C.A.; Antunes, P.C.; Crawshaw, P.G. Is it possible to individually identify mammals with no natural markings using camera-traps? A controlled case-study with lowland tapirs. Mamm. Biol. 2010, 75, 375–378. [Google Scholar] [CrossRef]
- Clare, J.D.; Anderson, E.M.; MacFarland, D.M. Predicting bobcat abundance at a landscape scale and evaluating occupancy as a density index in central Wisconsin. J. Wildl. Manag. 2015, 79, 469–480. [Google Scholar] [CrossRef]
- Kelly, M.J. Computer-aided photograph matching in studies using individual identification: An example from Serengeti cheetahs. J. Mammal. 2001, 82, 440–449. [Google Scholar] [CrossRef]
- Anderson, C.J.; Lobo, N.D.V.; Roth, J.D.; Waterman, J.M. Computer-aided photo-identification system with an application to polar bears based on whisker spot patterns. J. Mammal. 2010, 91, 1350–1359. [Google Scholar] [CrossRef]
- Allen, W.L.; Cuthill, I.C.; Scott-Samuel, N.E.; Baddeley, R. Why the leopard got its spots: Relating pattern development to ecology in felids. Proc. R. Soc. B Biol. Sci. 2010, 278, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Efford, M.G.; Borchers, D.L.; Byrom, A.E. Density estimation by spatially explicit capture–recapture: Likelihood-based methods. In Modeling Demographic Processes in Marked Populations; Thomson, D.L., Cooch, E.G., Conroy, M.J., Eds.; Springer US: New York, NY, USA, 2009; pp. 255–269. [Google Scholar]
- Royle, J.A.; Karanth, K.U.; Gopalaswamy, A.M.; Kumar, N.S. Bayesian inference in camera trapping studies for a class of spatial capture-recapture models. Ecology 2009, 90, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.R.; Anderson, D.R. Evaluation of two density estimators of small mammal population size. J. Mammal. 1985, 66, 13–21. [Google Scholar] [CrossRef]
- Obbard, M.E.; Howe, E.J.; Kyle, C.J. Empirical comparison of density estimators for large carnivores. J. Appl. Ecol. 2010, 47, 76–84. [Google Scholar] [CrossRef]
- Sollmann, R.; Gardner, B.; Belant, J.L. How does spatial study design influence density estimates from spatial capture-recapture models? PLoS ONE 2012, 7, e34575. [Google Scholar] [CrossRef] [PubMed]
- Ivan, J.S.; White, G.C.; Shenk, T.M. Using auxiliary telemetry information to estimate animal density from capture-recapture data. Ecology 2013, 94, 809–816. [Google Scholar] [CrossRef]
- Gardner, B.; Reppucci, J.; Lucherini, M.; Royle, J.A. Spatially explicit inference for open populations: Estimating demographic parameters from camera-trap studies. Ecology 2010, 91, 3376–3383. [Google Scholar] [CrossRef] [PubMed]
- Solberg, K.H.; Bellemain, E.; Drageset, O.M.; Taberlet, P.; Swenson, J.E. An evaluation of field and non-invasive genetic methods to estimate brown bear (Ursus arctos) population size. Biol. Conserv. 2006, 128, 158–168. [Google Scholar] [CrossRef]
- Davis, M.; Gratton, L.; Adams, J.; Goltz, J.; Stewart, C.; Buttrick, S.; Zinger, N.; Kavanagh, K.; Sims, M.; Mann, G. New England-Acadian Forests. Available online: http://worldwildlife.org/ecoregions/na0410/ (accessed on 21 November 2013).
- McNab, W.H.; Avers, P.E. Ecoregions and Subregions of the United States: Section Descriptions; USDA Forest Service Administrative Publication WO-WSA-5; USDA Forest Service: Washington, DC, USA, 1994. [Google Scholar]
- Sirén, A.P.; Pekins, P.J.; Ducey, M.J.; Kilborn, J.R. Spatial ecology and resource selection of a high elevation American marten population in the northeastern United States. Can. J. Zool. 2015. [Google Scholar] [CrossRef]
- Bull, E.L.; Heater, T.W.; Culver, F.G. Live-trapping and immobilizing American martens. Wildl. Soc. Bull. 1996, 24, 555–558. [Google Scholar]
- Poole, K.G.; Matson, G.M.; Strickland, M.A.; Magoun, A.J.; Graf, R.P.; Dix, L.M. Age and sex determination for American martens and fishers. In Martens, Fishers, and Sables: Biology and Conservation; Buskirk, S.W., Harestad, A.S., Raphael, M.G., Powell, R.A., Eds.; Cornell Univ. Press: Ithaca, NY, USA, 1994; pp. 204–223. [Google Scholar]
- Krohn, W.B.; Hoving, C.; Harrison, D.; Phillips, D.; Frost, H. Martes foot-loading and snowfall patterns in eastern North America: Implications to broad-scale distributions and interactions of mesocarnivores. In Martens and Fishers (Martes) in Human-Altered Environments; Harrison, D.J., Fuller, A.K., Proulx, G., Eds.; Springer Science and Business Media, Inc.: New York, NY, USA, 2004; pp. 115–131. [Google Scholar]
- Slauson, K.M.; Truex, R.L.; Zielinski, W.J. Determining the gender of American martens and fishers at track plate stations. Northwest Sci. 2008, 82, 185–198. [Google Scholar] [CrossRef]
- Dillon, A.; Kelly, M.J. Ocelot Leopardus pardalis in Belize: The impact of trap spacing and distance moved on density estimates. Oryx 2007, 41, 469–477. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth: Belmont, MA, USA, 1984. [Google Scholar]
- Galimberti, G.; Soffritti, G.; di Maso, M. Classification Trees for Ordinal Responses in R: The rpartScore Package. J. Stat. Softw. 2012, 47, 1–25. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 2 July 2015).
- Kendall, M.; Stuart, A. The Advanced Theory of Statistics; Macmillan: New York, NY, USA, 1979; Volume 2. [Google Scholar]
- Meyer, D.; Zeileis, A.; Hornik, K. Vcd: Visualizing Categorical Data. R Package Version 1.4-1. Available online: https://cran.r-project.org/web/packages/vcd (accessed on 15 July 2015).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1.1-7. Available online: https://cran.r-project.org/web/packages/lme4 (accessed on 10 November 2014).
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.0-1. Available online: https://cran.r-project.org/web/packages/AICcmodavg (accessed on 11 November 2014).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar]
- Efford, M.G. Secr: Spatially Explicit Capture-Recapture Models. R Package Version 2.7.0. Available online: https://cran.r-project.org/web/packages/secr (accessed on 5 September 2013).
- Noss, A.J.; Gardner, B.; Maffei, L.; Cuéllar, E.; Montaño, R.; Romero-Muñoz, A.; Sollmann, R.; O'Connell, A.F. Comparison of density estimation methods for mammal populations with camera traps in the Kaa-Iya del Gran Chaco landscape. Anim. Conserv. 2012, 15, 527–535. [Google Scholar] [CrossRef]
- Thompson, I.D.; Fryxell, J.; Harrison, D.J. Improved insights into use of habitat by American Martens. In Biology and Conservation of Martens, Sables, and Fishers: A New Synthesis; Aubry, K.B., Zielinski, W.J., Raphael, M.G., Proulx, G., Buskirk, S.W., Eds.; Comstock Pub. Associates: Ithaca, NY, USA, 2012; pp. 209–230. [Google Scholar]
- Borchers, D.L.; Efford, M.G. Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics 2008, 64, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Troscianko, J.; Stevens, M. Image calibration and analysis toolbox—A free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol. 2015. [Google Scholar] [CrossRef]
- Noss, A.J.; Cuéllar, R.L.; Barrientos, J.; Maffei, L.; Cuéllar, E.; Arispe, R.; Rúmiz, D.; Rivero, K. A camera trapping and radio telemetry study of lowland tapir (Tapirus terrestris) in Bolivian dry forests. Tapir Conserv. 2003, 12, 24–32. [Google Scholar]
- Gerber, B.; Karpanty, S.M.; Crawford, C.; Kotschwar, M.; Randrianantenaina, J. An assessment of carnivore relative abundance and density in the eastern rainforests of Madagascar using remotely-triggered camera traps. Oryx 2010, 44, 219–222. [Google Scholar] [CrossRef]
- Powell, R.A. Structure and spacing of Martes populations. In Martens, Fishers, and Sables: Biology and Conservation; Buskirk, S.W., Harestad, A.S., Raphael, M.G., Powell, R.A., Eds.; Cornell Univ. Press: Ithaca, NY, USA, 1994; pp. 101–121. [Google Scholar]
- Thompson, J.L. Density of Fisher on Managed Timberlands in North Coastal California. Ph.D. Thesis, Humboldt State University, Humboldt, CA, USA, 2008. [Google Scholar]
- Phillips, D.M.; Harrison, D.J.; Payer, D.C. Seasonal changes in home-range area and fidelity of martens. J. Mammal. 1998, 79, 180–190. [Google Scholar] [CrossRef]
- Mowat, G.; Paetkau, D. Estimating marten Martes americana population size using hair capture and genetic tagging. Wildl. Biol. 2002, 8, 201–209. [Google Scholar]
- Royle, J.A.; Magoun, A.J.; Gardner, B.; Valkenburg, P.; Lowell, R.E. Density estimation in a wolverine population using spatial capture–recapture models. J. Wildl. Manag. 2011, 75, 604–611. [Google Scholar] [CrossRef]
- Sollmann, R.; Gardner, B.; Chandler, R.B.; Shindle, D.B.; Onorato, D.P.; Royle, J.A.; O’Connell, A.F. Using multiple data sources provides density estimates for endangered Florida panther. J. Appl. Ecol. 2013, 50, 961–968. [Google Scholar] [CrossRef]
- Royle, J.A.; Chandler, R.B.; Sun, C.C.; Fuller, A.K. Integrating resource selection information with spatial capture–recapture. Methods Ecol. Evol. 2013, 4, 520–530. [Google Scholar] [CrossRef]
- Flynn, R.W.; Schumacher, T.V. Temporal changes in population dynamics of American martens. J. Wildl. Manag. 2009, 73, 1269–1281. [Google Scholar] [CrossRef]
- Wegge, P.; Pokheral, C.P.; Jnawali, S.R. Effects of trapping effort and trap shyness on estimates of tiger abundance from camera trap studies. Anim. Conserv. 2004, 7, 251–256. [Google Scholar] [CrossRef]
- Jackson, R.M.; Roe, J.D.; Wangchuk, R.; Hunter, D.O. Estimating Snow Leopard Population Abundance Using Photography and Capture-Recapture Techniques. Wildl. Soc. Bull. 2006, 34, 772–781. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D.; Kumar, N.S. Estimating tiger abundance from camera trap data: Field surveys and analytical issues. In Camera Traps in Animal Ecology: Methods and Analyses; O’Connell, A.F., Nichols, J.D., Karanth, K.U., Eds.; Springer Science and Business Media: New York, NY, USA, 2011; pp. 97–117. [Google Scholar]
- Raphael, M.G. Techniques for monitoring populations of fishers and American martens. In Martens, Fishers, and Sables: Biology and Conservation; Buskirk, S.W., Harestad, A.S., Raphael, M.G., Powell, R.A., Eds.; Cornell Univ. Press: Ithaca, NY, USA, 1994; pp. 224–240. [Google Scholar]
- Soutiere, E.C. Effects of timber harvesting on marten in Maine. J. Wildl. Manag. 1979, 43, 850–860. [Google Scholar] [CrossRef]
- Thompson, I.D.; Colgan, P.W. Numerical responses of martens to a food shortage in northcentral Ontario. J. Wildl. Manag. 1987, 51, 824–835. [Google Scholar] [CrossRef]
- Pauli, J.N.; Whiteman, J.P.; Marcot, B.G.; McClean, T.M.; Ben-David, M. DNA-based approach to aging martens (Martes americana and M. caurina). J. Mammal. 2011, 92, 500–510. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirén, A.P.K.; Pekins, P.J.; Abdu, P.L.; Ducey, M.J. Identification and Density Estimation of American Martens (Martes americana) Using a Novel Camera-Trap Method. Diversity 2016, 8, 3. https://doi.org/10.3390/d8010003
Sirén APK, Pekins PJ, Abdu PL, Ducey MJ. Identification and Density Estimation of American Martens (Martes americana) Using a Novel Camera-Trap Method. Diversity. 2016; 8(1):3. https://doi.org/10.3390/d8010003
Chicago/Turabian StyleSirén, Alexej P. K., Peter J. Pekins, Peter L. Abdu, and Mark J. Ducey. 2016. "Identification and Density Estimation of American Martens (Martes americana) Using a Novel Camera-Trap Method" Diversity 8, no. 1: 3. https://doi.org/10.3390/d8010003
APA StyleSirén, A. P. K., Pekins, P. J., Abdu, P. L., & Ducey, M. J. (2016). Identification and Density Estimation of American Martens (Martes americana) Using a Novel Camera-Trap Method. Diversity, 8(1), 3. https://doi.org/10.3390/d8010003





