Abstract
Among the diversity of secondary metabolites which are produced by plants as means of defence against herbivores and microbes, pyrrolizidine alkaloids (PAs) are common in Boraginaceae, Asteraceae and some other plant families. Pyrrolizidine alkaloids are infamous as toxic compounds which can alkylate DNA und thus cause mutations and even cancer in herbivores and humans. Almost all genera of the family Boraginaceae synthesize and store this type of alkaloids. This review reports the available information on the present status (literature up to early 2014) of the pyrrolizidine alkaloids in the Boraginaceae and summarizes the topics structure, distribution, chemistry, chemotaxonomic significance, and biological properties.
1. Introduction
Pyrrolizidine alkaloids (PAs) are common secondary metabolites in Boraginaceae, Fabaceae (tribe Crotalarieae) and Asteraceae (tribe Senecioneae) and serve as chemical defence compounds mainly against herbivores [1,2]. Several PA producing plants grow as weeds and therefore occur widely in agricultural production system throughout the world. They can enter the human food chain as a result of co-harvesting PA containing plants with edible grains. When bees visit areas, in which PA plants are abundant, PAs can be transferred into honey because nectar and pollen of PA plants contain alkaloids [3]. PA-contaminated human food can include cereals, milk, honey, eggs, and meat. In addition a PA-intake can occur when herbal teas and traditional medicines of the PA-containing plants are consumed [4,5,6,7,8,9,10,11,12,13,14,15,16,17].
Chronic health problems have been attributed to the presence of PAs in these products. The long-term toxicity is due to the conversion of pyrrolizidine alkaloids to the corresponding pyrrole derivatives which are highly reactive; they can alkylate DNA and have the ability to form DNA cross-linkage. As a consequence DNA replication is interrupted and mutations can occur, which can lead to liver and kidney cancer [6,18,19]. The ring nucleus (necine base) with a double bond in the 1:2 position is essential for genotoxic effects of theses alkaloids. PAs are bitter and modulate several neuroreceptors, including 5-HT receptors [20,21,22] which can induce immediate food avoidance in herbivores.
Many of the PAs have been shown to exhibit hepatotoxic, pulmotoxic, haemolytic, antimitotic, teratogenic, mutagenic and carcinogenic effects [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
Plants of the family Boraginaceae are famous for the production of PAs. These alkaloids occur as free necines (either the necine base heliotridine or retronecine) or as a mixture of free bases and their N-oxides. They can form single esters (monoester) at C-9 or C-7, open chain diesters at both C-7 and C-9 of the necine base, or in rare cases macrocyclic diesters linking C-7 with C-9 (Figure 1 and Figure 2).
Several reviews on the occurrence of PAs have been published already [23,24,25,26,27,28,29,30,31,32,35,37,38,39] In the present review, we have made a complete review of PAs in Boraginaceae and have added also older records which were not covered so far. Table 1 and Table 2 list the distribution of PAs in the Boraginaceae (a last comprehensive review was published by Hartmann and Witte [29]). GLC and GLC-MS data are tabulated in Table 3. For NMR data, we have only included NMR data of new PAs from the Boraginaceae in the time frame 1991–2013 for 13C (Table 4) and 1994–2013 for 1H (Table 5).
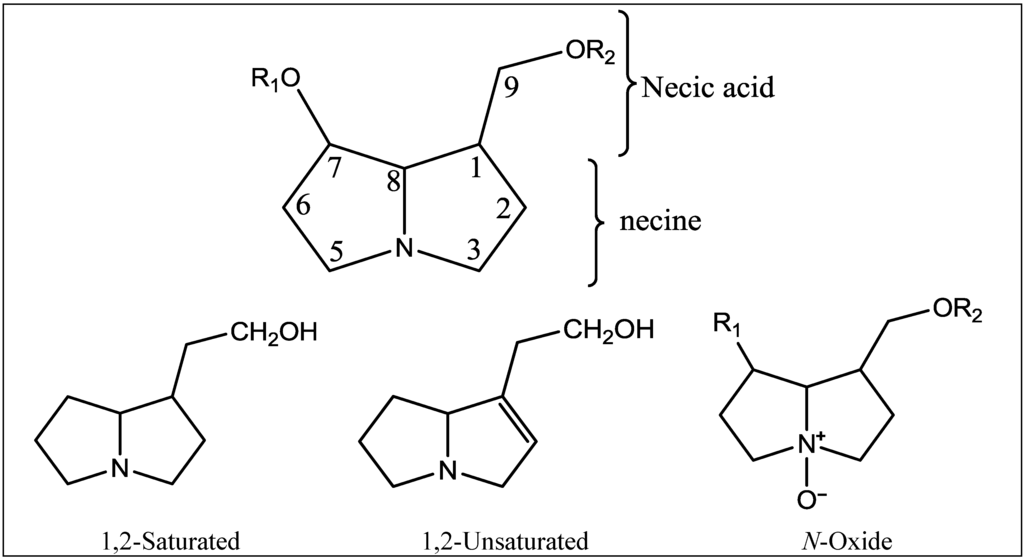
Figure 1.
Basic structures of pyrrolizidine alkaloids in Boraginaceae.
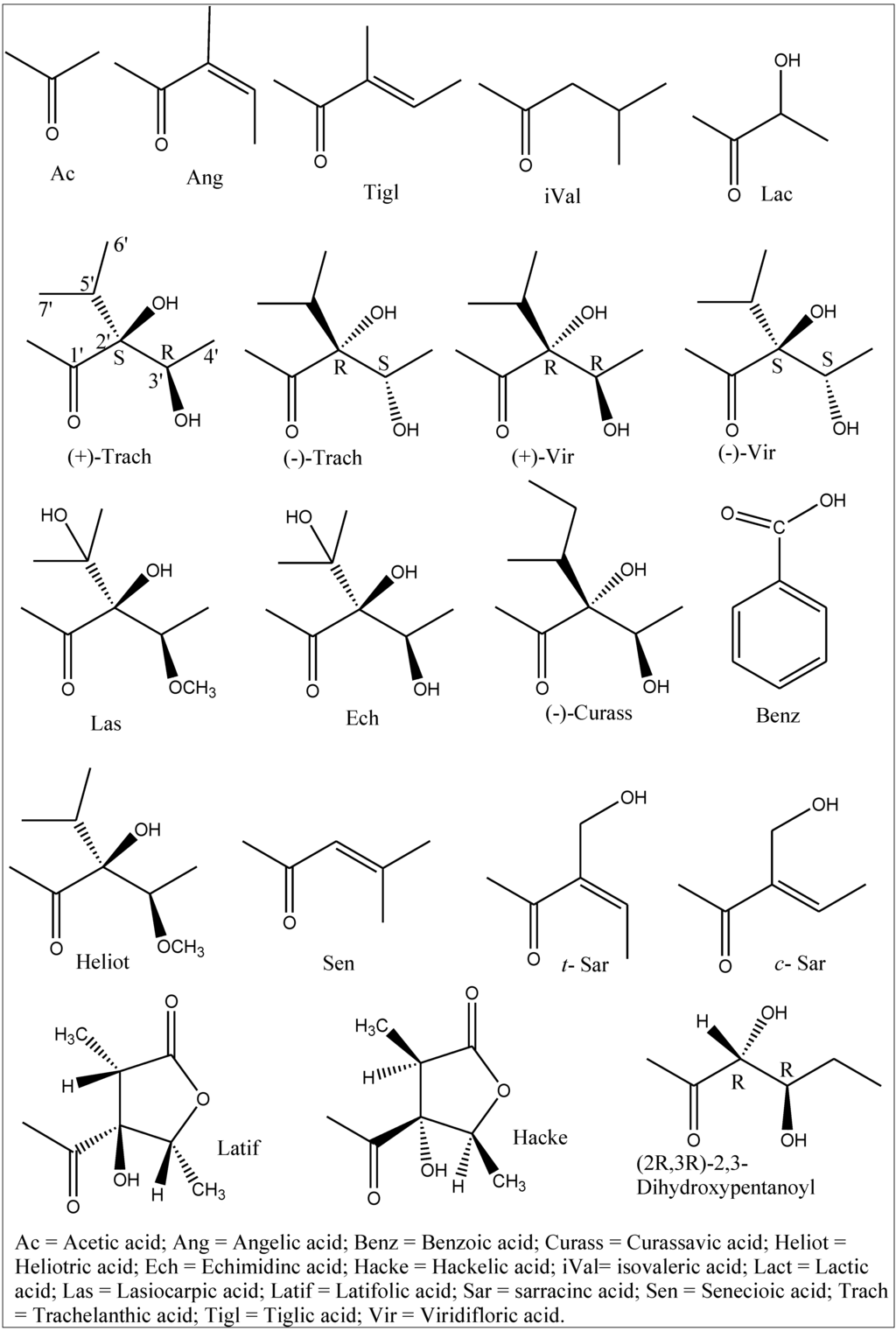
Figure 2.
Necic acids occurring in pyrrolizidine alkaloids of Boraginaceae.

Table 1.
List of plant species of the Boraginaceae containing PAs.
| Species | Pyrrolizidine alkaloids | References |
|---|---|---|
| Alkanna orientalis | 7-Angeloylretronecine, 9-angeloylretronecine, 7-tigloylretronecine, 9-tigloylretronecine, 7-seneioylretronecine, 9-senecioylretronecine, triangularine, dihydroxytriangularine, triangularicine, dihydroxytriangularicine, 7-angeloyl-9-(hydroxypropenoyl) retronecine, 7-tigloyl-9-(hydroxypropenoyl) retronecine, 7-angeloyl-9-(2,3-dihydroxypropanoyl) retronecine, 7-tigloyl-9-(2,3-dihydroxypropanoyl) retronecine. | [40,41] |
| A. tinctoria (A. tuberculata) | 7-Angeloylretronecine, 7-tigloylretronecine, 9-tigloylretronecine, triangularine, dihydroxytriangularine, triangularicine, dihydroxytriangularicine, 7-acetyl-9-sarracinoylretronecine, 7-angeloyl-9-(hydroxypropenoyl) retronecine, 7-tigloyl-9-(hydroxypropenoyl) retronecine, 7-angeloyl-9-(2,3-dihydroxypropanoyl) retronecine, 7-tigloyl-9-(2,3-dihydroxypropanoyl) retronecine. | [40,41] |
| Amsinckia carinata | Echiumine, furcatine, 3'-acetylfurcatine, intermedine, 7-acetylintermedine, lycopsamine, myoscorpine, supinine. | [42] |
| A. douglasiana | Amabiline, cynaustrline, intermedine, lycopsamine, tessellatine, 9-(3'-acetylviridifloryl)turniforcidine. | [42,43] |
| A. eastwoodiae | Amabiline, intermedine, 3'-acetylintermedine, echiumine, lindelofine, lycopsamine, supinine, tessellatine. | [42] |
| A. furacata | Furcatine, intermedine, 3'-acetylfurcatine, lycopsamine, supinine, tessellatine. | [42] |
| A. grandiflora | Amabiline, intermedine, lycopsamine, 3'-aetyllycopsamine, 7-acetyllycopsamine, tessellatine, 9-acetyltessellatine. | [42] |
| A. hispida | Echiumine, intermedine, lycopsamine. | [44] |
| A. intermedia | Echiumine, intermedine, lycopsamine, sincamidine. | [44] |
| A. lunaris | Lycopsamine, 3'-acetyllycopamine, intermedine, supinine, tesslatine, 9'-acetyltessellatine. | [42] |
| A. lycopsoides | Amabiline, echiumine, intermedine, 3'-acetylintermedine, lycopsamine, supinine, tessellatine. | [42,44] |
| A. lycopsoides × menziesii var. intermedia | Amabiline, intermedine, 3'-acetylintermedine, lycopsamine, supinine, tessellatine. | [42] |
| A. menziesii | Intermedine, lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine, 3',7-diacetyllycopsamine. | [45] |
| A. menziesii var. intermedia | Amabiline, cynaustraine (or steroisomer), echiumine, 3'-acetylechiumine, intermedine, 3'-acetylintermedine, 7-acetylintermedine, 3',7-diacetylintermedine, lindelfoline, lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine, myscorpine, 3'-acetylmyscorpine, supinine, symlandine, tessellatine. | [42,43] |
| A. retrosa | Amabiline, intermedine, 3'-acetylintermedine, lycopsamine, myscorpine, 3'-acetylmyscorpine, supinine, tessellatine. | [42] |
| A. spectabilis var. microcarpa | Intermedine, 3'-acetylintermedine, lindelofine, lycopsamine, myscorpine, supinine, tessellatine. | [42] |
| A. specabtilis var. spectabilis | Intermedine, 3'-actylintermedine, lindelofine, lycopsamine, supinine. | [42] |
| A. spectabilis var. nicolai | Intermedine, lindelofine, lycopsamine, tessellatine, trachelanthamine, supinine. | [42] |
| A. tessellata | Intermedine, lycopsamine, 3'-acetylintermedine, 3'-acetyllycopsamine, 7-acetylintermedine, 7-acetyllycopsamine, 3',7-diacetylintermedine, 3'-7-diacetyllycopsamine. | [46] |
| A. tessellata var. gloriosa | Amabiline, intermedine, lycopsamine, supinine, tessellatine, tracelanthamine, viridiflorine, 3'-acetylviridiflorine. | [42,43] |
| A. tessellata var. tessellata | Amabiline, intermedine, lycopsamine, 3'-acetyllycopsamine, tessellatine, 3'-acetyltessellatine, 9-acetyltessellatine, 9,3'-acetyltessellatine, 9-(3'-acetylviridifloryl)-turniforcidine. | [42] |
| A. vernicosa | Furcatine, intermedine, lycopsamine, supinine, 7-trachelanthyl retronecine. | [42] |
| Anchusa arvensis (=Lycopsis arvensis) | Echinatine, intermedine, 7-acetylintermedine, lycopsamine, 7-acetyllycopsamine, 3',7-diacetylintermedine (or its isomer 3',7-diaetyllycopsamine), supinine, 9-acetyltrachelanthamidine, 9-angeloyltrachelanthamidine. | [40,47] |
| A. hispida (=Gastrocotyle hispida) | 7-Angeloylheliotridine, intermedine, lyopsamine, 7-acetyllycopsamine, retronecine, trachelanthamine and its isomer. | [40] |
| A. milleri | Heliotridine, 7-angeloylheliotridine, rinderine, retronecine, supinine, viridiflorine, 9-curassavoyleliotridine, 7-acetyl-9-curassavoylheliotridine. | [40] |
| A. officinalis | Intermedine, curassavine, lycopsamine, 7-aceyllycopsamine. | [47,48,49] |
| A. strigosa | Heliotridine 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester, platyneine N-oxide 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester, retronecine 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester and its N-oxide, retronecine 2S-hydroxy-2S(1R-hydroxyethyl)-4-methyl-pentanoyl ester and its N-oxide, retronecine 2S-hydroxy-2S(1S-hydroxyethyl)-[1'S-hydroxyethyl)-4-methylpentanoyl]-4-methyl-pentanoyl ester, supinidine N-oxide 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester, trachelanthamidine 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester. | [50,51] |
| Arnebia decumbens | 7-angeloylrtronecine, 9-angeloylretronecine, 7-tigloylrtronecine, 9-tigloylretronecine, europine, heliotrine, lycopsamine, rinderine, supinine. | [52] |
| Arnebia euchroma | 7-angeloylrtronecine, 9-angeloylretronecine | [53] |
| A. hispidissima | Echimidine, monocrotaline. | [54] |
| Asperugo procumbens | Amabiline (or supinine), echinatine. | [47] |
| Borago officinalis | Amabiline, intermedine, 7-acetylintermedine, lycopsamine, 7-acetyllycopsamine, supinine, thesinine, thesinine-4'- O-β-D-glucoside. | [55,56] |
| Caccinia crassifolia | Supinine, Heliotridine or retronecine trachelanthate. | [57] |
| C. glauca | Retronecine 7:9 -dibenzoate. | [58] |
| Cerinthe glabra | Lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine, 3',7-diacetyllycopsamine, supinine, 7-hydroxymethyl butyryl-9-viridifloryl-retronecine (or isomer). | [59] |
| Cerinthe minor | Intermedine, 7-angeloylretronecine, 9-angeloyl-7-viridiflorylretronecine, lycopsamine. | [60,61] |
| Cordia myxa | macrophylline | [62] |
| C. sinensis | floridanine | [62] |
| Cryptantha cana | Intermdine, 3'-acetylintermedine, 7-acetylintemedine, lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine. | [63] |
| C. clevelandii | Intermdine, 3'-acetylintermedine, echiumine, 2'',3''-epoxyechiumine, thero-2',3' dihydroxyechiumine, erytho-3''-chloro-2''-hydroxyechiumine. | [63] |
| C. confertiflora | Amabiline, intermedine, lycopsamine, tessellatine, 9-acetyltessellatine. | [63] |
| C. crassipes | Lycopsamine, intermedine and their 3'-acetyllycopsamine, 3'-acetylintermedine, 7-acetyllycopsamine, 7-acetylintermedine, amabiline, echiumine, dihydroechumine, echimiplatine, lepanthine. | [64] |
| C. fendleri | 7-Angeloylretronecine, 9-angeloylretronecine, latifoline, neolatifoline. | [63] |
| C. flava | Intermedine, 3'-acetylintermedine, 7-acetylintemedine, lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine. | [63] |
| C. inequata | Echimidine, acetylechimidine, echiuplatine, methylechiuplatine, lycopsamine, intermedine, dihydroxyechiumine. | [65] |
| C. jamesii | Intermedine, lycopsamine, 3'- acetyllycopsamine, 7-aetyllyopsamine. | [66] |
| C. leiocarpa | 7-Angeloylretronecine, 9-angeloylretronecine, echiumine, intermedine, 3'-acetylintermedine, 2'',3''-epoxyechiumine, thero-2',3'-dihydroxyechiumine, erytho-3''-chloro-2''-hydroxyechiumine | [63] |
| C. thyrsiflora | Intermedine, 3'-acetylintermedine, 7-acetylintemedine, lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine | [63] |
| C. utahensis | Cryptanthine | [65] |
| C. virgata | Intermedine, 3'-acetylintermedine, 7-acetylintemedine, lycopsamine, 3'-acetyllycopsamine, 7-acetyllycopsamine | [63] |
| C. virginiensis | Amabiline, intermedine, lycopsamine, tessellatine. | [63] |
| Cynoglossum amabile | Amabiline, echinatine, 7-acetylechinatine, lycopsamine, rinderine, supinine. 9-angeloylretronecine, 9-angeloyl-7-viridiflorylretronecine, | [60,67,68] |
| C. australe | Cynausine, cynaustraline. | [68] |
| C. clandestinum | 9-Angeloyl-7-viridiflorylretronecine, trachelanhamine | [60] |
| C. columnae | N-oxides of echintine, rinderine, 3’-acetylrinderine, 7-tigloyl-9-(2-deoxy-2-methyl)echimidinyl heliotridine. | [69] |
| C. creticum | Cynoglossamine, echinatine, 3'-acetylechinatine, heliosupine, 3'-acetylheliosupine, 7-angeloylheliotridine, 7-angeloyl-9-(methylbutyryl) heliotridine, 7α-angeloyl-1-chloromethyl-1,2-dehydropyrrolizidine, 7-senecioylheliotridine, rinderine, 3'-acetylrenderine, supinine, trachelanthamine (or isomer). | [70,71,72] |
| C. furcatum (C. zeylanicum) | Echinatine, isoechinatine, neocoromandaline, cynaustraline, lactodine, viridinatine | [73,74,75,76] |
| C. germanicum | Echinatine, viridiflorine. | [77] |
| C. glochidiatum | Amabiline | [78] |
| C. lanceolatum | Cynaustine, cynaustraline. | [78] |
| C. latifolium | 7-Angeloylheliotridine, latifoline. | [79] |
| C. macrostylum | Echinatine, heliosupine. | [80] |
| C. montanum | Cynaustine, cynaustraline, echinatine, heliosupine. | [81] |
| C. nervosum | Heliotrine, echinatine, rinderine and their N-oxides. | [82] |
| C. officinale | Echinatine, 3'-acetylechinatine, 7-angeloylechinatine, heliosupine, 3'-acetylheliosupine, 7-angeloylheliotridine, 7-angeloyl-1-formyl-6,7-dihydro-5H-pyrrolizidine, 7-angeloyl-9-(2-methylbutyryl)heliotridine, 7-angeloyl-9-(2,3-dihydroxybutyryl) heliotridine, 7-tigloylheliotridine, rinderine, 7-angeloylrinderine, trachelanthamine, viridiflorine. | [67,83,84] |
| C. pictum | Echinatine, heliosupine, pictumine. | [85] |
| C. viridiforum | Heliosupine, viridiflorine. | [86] |
| Echium amoenum | Echimidine, echimidine isomer (tigloyl), 7-angeloylretronecine, 7-tigloylretronecine. | [87] |
| E. angustifolium | Echimidine | [88] |
| E. diffusum | Heliotridine or retronecine esters. | [28] |
| E. glomeratum | 7-Angeloylretronecine, 9-angeloylretronecine, (7S,8R)petranine, (7S,8S)petranine. | [89] |
| E. horridum | Echimidine, echimidine isomer (tigloyl), lycopsamine, 7-acetyllycopsamine, 7-angeloyllycopsamine, 7-tigloyllycopsamine, 7-angeloylretronecine, 7-tigloylretronecine, 7-angeloyl-9-(2-methylbutyryl)retronecine, 7-tigloyl-9-(2-methylbutyryl) retronecine, 7-angeloyl-9-(2, 3-dihydroxybutyryl)retronecine, 7-tigloyl-9-(2, 3-dihydroxybutyryl)retronecine, uplandicine. | [90] |
| E. humile | Echimidine, echihumiline, lycopsamine, 7-acetyllycopsamine, 7-senecioyllycopsamine, pycnanthine, 7-seneioylretronecine, 9-seneioylretronecine, 7-(2-methylutyryl)retronecine, 7-(methybutyryl)-9-(2,3-dihydroxybutyryl)retronecine, 7-(2-methybutyryl)-9-echimidinylretronecine. | [91] |
| E. hypertropicum | Echimidine, echihumiline, 7-(2-methylbutyryl)-9-echimidinylretronecine, 7-senecioylretronecine, 9-angeloylretronecine, lycopsamine, 7-acetyl-lycopsamine | [92] |
| E. italicum | Echimidine | [28] |
| E. lycopsis (=E. plantagineum) | Echimidine, echiumine, uplandicine, lycopsamine, intermedine, echimplatine, echiuplatine, 3'-acetylintermedine, 3'-acetyllycopsamine, 3'-acetylechiumine, 9-angeloylretronecine, leptanthine. | [4,93,94] |
| E. pininana | Echimidine, ehiupinine, 3'-aetylintermedine, hydroxymyoscorpine, myoscorpine. | [95] |
| E. rauwolfii | Echimidine, echimidine isomer (tigloyl), 7-acetyllycopsamine, 7-angeloyllycopsamine, 7-tigloyllycopsamine, 7-angeloylretronecine, 7-tigloylretronecine, 7-angeloyl-9-(2-methylbutyryl)retronecine, 7-tigloyl-9-(2-methylbutyryl)retronecine, 7-angeloyl-9-(2, 3-dihydroxybutyryl)retronecine, 7-tigloyl-9-(2, 3-dihydroxybutyryl)retronecine, uplandicine. | [90] |
| E. sericeum | Echimidine, symlandine (or symphytine). | [54] |
| E. setosum | Echimidine, echimidine isomer (tigloyl), 7-angeloylretronecine, 7-tigloylretronecine, 9-angeloylretronecine, 9-tigloylretronecine, 7-angeloyl-9-(2-methylbutryl) retronecine, 7-tigloyl-9-(2-methylbutryl) retronecine, 7-angeloyl-9-(2,3-dimethylbutryl) retronecine, 7-angeloyl-9-(2,3-dihydroxybutryl) retronecine, uplandicine. | [96] |
| E. simplex | 7-Angeloylretronecine, 9-angeloylretronecine. | [61] |
| E. stenosiphon Webb subsp. stenosiphon | Echimidine, 7-(2-methylbutyryl)-9-echimidinylretronecine | [92] |
| E. tuberculatum | Echimidine,7-angeloyl-9-viridiflorylretronecine, 7-viridiflorylretronecine. | [60] |
| E. vulgare | Asperumine, heliosupine, 3'-acetylheliosupine, Echinatine, echiuplantine, leptanthine, echimiplantine, echivulgarine, vulgarine, 7-O-acetylvulgarine, echimidine, echimidine isomer (tigloyl ?), 3'-acetylechimidine, 5'-acetylechimidine, echihumiline, retronecine, 7-angeloylretronecine, 9-angeloylretronecine, 7-tigloylretronecine, 9-tigloylretronecine, 7-(2-methylbutyryl) retronecine, 9-(2-methylbutyryl) retronecine, 7-angeloyl-9-(2-methylbutryl) retronecine, 7-tigloyl-9-(2-methylbutryl) retronecine, 7-angeloyl-9-(2, 3-di methylbutryl) retronecine, 7-tigloyl-9-(2, 3-dihydroxybutryl)retronecine, uplandicine | [11,96,97,98] |
| E. wildpretti | Echimidine and its N-oxide. | [99] |
| Ehretia aspera | Ehretinine | [100] |
| Eritrichium rupestre | 7-Angeloylretronecine, 7-angeloyl-9-viridiflorylretronecine, 7-viridiflorylretronecine | [60] |
| Hackelia californica | Hackelidine, longitubine, 7-acetylhackelidine, 9-latifolylretronecine, 7-acetyl-9-latifolylretronecine | [101,102] |
| H. floribunda | Latifoline and its N-oxide. | [103] |
| H. longituba | Latifoline, neolatifoline, longitubine, 7-angeloylretronecie, 9-angeloylretronecine. | [104] |
| Heliotropium acutifolium | Heliotrine | [105] |
| H. amplexicaule | Indicine | [29] |
| H. angiospermum | Subulacine, lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. arbainense | Europine, heliotrine, lasiocarpine. | [71] |
| H. arborescens (=H. peruvianum) | Indicine, 3'-acetylindicine, lasiocarpine. | [107] |
| H. arguzioides | Heliotrine, trichodesmine. | [28] |
| H. bacciferum | Europine, heliotrine, heleurine and their N-oxides, supinine. | [108,109] |
| H. bovei | Europine, 7-acetyleuropine, lasiocarpine, 5'-acetyllasiocarpine, lasiocarpine N-oxide, 5'-acetyllasiocarpine N-oxide. | [110] |
| H. bracteatum | Helibractinecine, retronecine, helibracteatinine, helibracteatine | [111,112] |
| H. bursiferum | 7-Angeloylretronecine. | [113] |
| H. circinatum | 7-angeloylheliotrine, echinatine, europine, heleurine, heliotrine, lasiocarpine. | [114] |
| H. confertifolium | lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. crassifolium | Ilamine, europine and their N-oxides. | [115] |
| H. curassavicum | Coromandaline, coromandalinine, curassavine, curassavinine, curassanecine, heliocurassavine, heliocurassavinine, heliocurassavicine, heliocoromandaline, heliovicine, 7-angeloylheliotridine, trachelanhamidine, retronecine, supinidine. | [106,116,117,118] |
| H. curassavicum var. argentium | 9-(3'-isovaleryl) viridifloryl retronecine, 9-(3'-acetyl) viridifloryl retronecine. | [119] |
| H. curassavicum var. curassavicum | 9-(3'-isovaleryl) viridifloryl retronecine, 9-(3'-acetyl) viridifloryl retronecine. | [119] |
| H. dasycarpum | heliotrine | [120] |
| H. digynum (H. luteum) | Europine, heliotrine, 7-angeloylheliotrine, lasiocarpine. | [121] |
| H. disciforme | Heliotrine, 2'-actylheliotrine, heliotrine N-oxide, heleurine, heliorine N-oxide. | [122] |
| H. dissitiflorum | Heliotrine, heliotrine N-oxide, europine, 5'-deoxylasiocarpine. | [123] |
| H. eichwaldii | Heliotrine, 7-angeloylheliotrine, lasiocarpine. | [124] |
| H. esfandiarii | Europine, europine N-oxide. | [125] |
| H. europaeum | Europine, acetyleuropine, heleurine, heliotrine, 7-angeloylheliotrine, lasiocarpine, 6-acetyllasiocarpine, heliotrine N-oxide, dehydroheliotrine, 5'-acetyllasiocarpine N-oxide, N-(dihydropyrrolizinomethyl)-heliotrine, supinine. | [126,127] |
| H. floridum | Floridine, floridinine, floridimine, heliovicine, 3'-acetyltrachelanthamine. | [128] |
| H. foliosisimum | lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. fruticosum | lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. hirsutissimum | Europine, heliotrine, heleurine, lasiocarpine, 3'-acetyllasiocarpine, 5'-acetyllasiocarpine, supinine, N-oxides of acetylasiocarpine, 3'-acetyleheliosupine. | [29,129] |
| H. indicum | Echinatine, helindicine, heliotrine, heleurine, indicine, acetylindicine, indicinine, lasiocarpine, lycopsamine, rinderine, supinine, lindelofidine, retronecine, supinidine, trachelanthamine. | [106,116,130,131,132] |
| H. keralense | Intermedine, isolycopsamine, retronesine. | [133] |
| H. lasiocarpum | Heliotrine, lasiocarpine. | [29] |
| H. marifolium | Europine, heliotrine, indicine, lasiocarpine. | [29] |
| H. maris mortui | Europine, lasiocarpine. | [29,71] |
| H. megalanthum | Lycopsamine, megalanthonine. | [134] |
| H. molle | subulacine | [29] |
| H. olgae | Heliotrine, incanine. | [135] |
| H. ovalifolium | Heliofoline, retronecine. | [136] |
| H. peruvianum | Rinderine | [29] |
| H. popovii subsp. gillianum | Heliotrine | [28] |
| H. procumbens | Lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. queretaroanum | Lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. racemosum | Lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. ramosissimum | Heliotrine | [28] |
| H. rotundifolium | Europine, 5'-acetyleuropine, heliotrine, lasiocarpine. | [137,138] |
| H. scabrum | Heliscabine, retronecine. | [139] |
| H. sessei | Lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| H. spathulatum | Amabiline, coromandaline, coromandalinine, heliovicine, curassavinine, curassavine, heliospathine, heliospathuline, lindelofidine, retronecine, supinidine, trachelanthamidine. | [116,140] |
| H. steudneri | Lycopsamine | [27] |
| H. strigosum | Strigosine, trachelanthamidine | [25,141] |
| H. suaveolens | Echinatine, europine, heliotrine, lasiocarpine. | [29] |
| H. subulatum | Subulacine; retronecine, heliotrine, 7-angeloylheliotridine | [142] |
| H. supinum | Echinatine, heliosupine, heliotrine, 7-angeloylheliotridine (and its trachelanthic and viridifloric esters), lasiocarpine, supinine. | [27,143] |
| H. ternatum | subulacine | [27] |
| H. transalpinum | Intermedine, indicine, lycopsamine, rinderine, 3'-acetylrinderine, supinine. | [144] |
| H. transalpinum var. transalpinum | Transalpinecine, subulacine. | [145] |
| H. transoxanum | Heliotrine | [105] |
| H. wigginsii | Lindelofidine, retronecine, supinidine, trachelanthamidine. | [106] |
| Lappula glochidiata | Echinatine | [146] |
| L. intermedia (Echinospermum intermedium) | Lasiocarpine | [147] |
| L. myosotis | Intermedine, lycopsamine , 7-acetylintermedine, 7-acetyllycopsamine. | [35,148] |
| L. spinocarpos | Amabiline, intermedine, 7-angeloylheiotridine, 9- heliotrinoylretronecine, lycopsamine, 7-acetyllycopsamine, retronecine, trachelanthamine, supinine, viridiflorine. | [40] |
| Lindelofia anchusoides (L. macrostyla) | Lindelofamine, lindelofine. | [28] |
| L. angustifolia | Amabiline, echinatine | [78] |
| L. longiflora | Echinatine and its N-oxide. | [149] |
| L. olgae | Viridiflorine | [150] |
| L. pterocarpa | Viridiflorine | [151] |
| L. spectabilis | Echinatine, 3'-acetylechinatine, monocorotaline. | [124] |
| L. stylosa | Echinatine, lindelofine, viridiflorine. | [135] |
| L. tschimganica | Echinatine, carategine, viridiflorine. | [152] |
| Lithospermum canesens | Canesine, canescenine, 3'-acetycanesine, 3'-acetylcanescenine, lycopsamine, 7-acetyllycopsamine, 7-acetylintermedine. | [153,154] |
| L. erythrorhizon | Intermedine, myoscorpine, hydroxymyoscorpine. | [155] |
| L. officinale | Lithosenine, acetyllithosenine. | [156] |
| L. purpureocoeruleum | Lycopsamine | [60] |
| Macrotomia echioides | Macrotomine | [28] |
| Mertensia bakeri | Lycopsamine | [157] |
| M. ciliata | Intermedine, lycopsamine. | [157] |
| Messerschmidia argentea | Indicine, 3'-acetylindicine, and their N-oxide. | [158] |
| M. sibirica | Lycopsamine, 9-angeloylretronecine. | [28] |
| Moltikiopsis ciliata (Lithospermum callosum) | Echinatine, heliotrine. | [108] |
| Myosotis scorpioides (=M. palustris) | Myoscorpine, symphytine, scorpioidine, 7-acetylscorpioidine. | [159] |
| M. sylvatica | Heliosupine, 3'-acetylheliosupine, 9-angeloylretronecine, trachelanthamine. | [25] |
| Neatostema apulum | Amabiline, lycopsamine and their N-oxides. | [160] |
| Nonnea lutea | 7-viridiflorylretronecine | [60] |
| N. setosa | 7-viridiflorylretronecine | [60] |
| Omphalodes verna | Isoretronocanol or its isomer. | [27] |
| Onosma alborosea | Intermedine, lycopsamine, 7-acetylintermedine, 7-acetylycopsamine. | [161] |
| O. alboroseum × sanguinolentum | 9-Angeloylretronecine, echimidine, lycopsamine, intermedine, 7-acetylintermedine, 7-acetylycopsamine. | [161,162] |
| O. arenaria | 7-Acetyllycopsamine, 5,6-diydro-7,9-dimethoxy-7H-pyrrolizine, 7-acetylretronecine, 7-acetyl-9-(2-methylbutryl) retronecine, 7-acetyl-9-(2,3-dimethylbutryl) retronecine, 7-acetyl-9-(2-hydroxy-3-methylbutryl) retronecine, 7-acetyl-9-(2,3-dihydroxybutryl) retronecine, 9-(butyryl-2-ene) supinidine, 3'-acetylsupinine, uplandicine. | [163] |
| O. arenaria subsp. pennina | Intermedine, lycopsamine, 7-acetylintermedine, 7-acetylycopsamine. | [161] |
| O. erecta | N-oxides of 7-O-acetylechinatine, viridinatine, stereoisomer, 7-epi-echimiplatine, onosmerectine. | [164] |
| O. hetrophyllum | Helioridine, 1-methylene-8α-pyrrolizidine. | [165] |
| O. leptantha | Echihumiline, 3’-acetylechihumiline, leptanthine and their N-oxides. | [166] |
| O. stellulatum | Echimidine, 7-viridiflorylretronecine, heliospathuline, leptanthine, lyopsamine and heir N-oxides, 7-acetylintermedine, dihydroechinatine, trahelanthamine, uplandicine. | [60,167] |
| Paracaryum himalayense | Viridiflorine | [151] |
| P. intermedium | 7-Angeloylheliotridine, 7-senecioylheliotridine, rinderine, 7-angeloylrindrine, 7-senecioylrinderine, viridiflorine. | [40] |
| P. regulosum | Echinatine, heliosupine, 7-angeloylheliotridine, rinderine, viridiflorine. | [40] |
| Paracynoglossum imeretinum | Echinatine, heliosupine. | [29] |
| Pulmonaria obscura | Intermedine, lycopsamine, 7-acetylintermedine, 7-acetylycopsamine. | [168] |
| Rindera austroechinata | Echinatine, rinderine, 7-angeloylheliotridine | [57,152] |
| R. baldschuanica | Echinatine, rinderine, trachelanthamine, turkestanine | [152] |
| R. cyclodonta | Echinatine | [120] |
| R. echinata | Echinatine, trachelanthamine | [120] |
| R. oblongifolia | Cerategine, echinatine, turkestanine. | [152] |
| R. umbellata | 7-Angeloyl-9-(+)-trachelanthyl heliotridine, lindelofine, punctanecine, 7-angeloyl heliotridane, 7-angeloyl heliotridine, heliosupine, 9-(+)-trachelanthyl-laburnine, echinatine. | [169] |
| Solenanthus circinnatus | Echinatine | [151] |
| S. coronatus | Echinatine | [135] |
| S. karateginus | Cerategine, ehinatine. | [151] |
| S. turkestanicus | Rinderine, turkestanine. | [135,150] |
| Symphytum aintabicum | Echimidine | [170] |
| S. asperum | Echimidine, symphytine, asperumine, ehinatine, heliosupine, acetylechimidine (or its isomer), aetyllyopsamine (or its isomer), symviridine | [171,172] |
| S. bohemium | Echimidine, lycopsamine, 7-acetyllycopsamine, symphytine. | [173] |
| S. caucasium | Asperumine, echimidine, echinatine, heliotrine, lasiocarpine. | [174] |
| S. consolidum | Echimidine, symphytine. | [175] |
| S. grandiflorum | Echimidine, lycopsamine, symphytine. | [176] |
| S. ibericum | Echimidine, lycopsamine, symphytine. | [176] |
| S. officinale | Lycopsamine, 7-acetyllycopsamine, symphytine, echimidine, echinatine, heliosupine, intermedine, 7-acetylintermedine, viridiflorine, symviridine. | [17,162,171,172,177,178,179] |
| S. orientale | Anadoline, echimidine, symphytine. | [28] |
| S. peregrinum | Intermedine, 7-acetylintermedine, lycopsamine, 7-acetyllycopsamine, symphytine. | [27] |
| S. sylvaticum subsp. sepulcrale var. sepulcrale | Echimidine N-oxide | [170] |
| S. tanaiense | Echimidine, lycopsamine, 7-acetyllycopsamine, symphytine. | [173] |
| S. tuberosum | 7-Angeloylretronecine; anadoline, echimidine, lycopsamine, 7-acetyllycopsamine, symphytine. | [28,162,176] |
| S × uplandicum | Echimidine, intermedine, 7-acetylintermedine lycopsamine, 7-acetyllycopsamine, symphytine, symlandine, symviridine, uplandicine. | [172,180] |
| Tournefortia sarmentosa | Supinine | [28] |
| T. sibirica | Turneforcine | [29] |
| T. sogdiana | Echinatine | [57] |
| Trahlenthus hissaricus | Trachelanthine, trachelanthamine, viridiforine. | [29] |
| T. korolkovii | Trachelanthine, trachelanthamidine, trachelanthamine. | [57,150] |
| Trichodesma africanum | Europine, intermedine, lycopsamine, trichodesmine, retronecine, viridiflorine | [40,71,181] |
| T. ehrenbergii | Senkirkine, supinine. | [54] |
| T. incanum | Inanine, trihodesmine. | [46] |
| T. zeylanicum | Supinine | [182] |
| Ulugbekia tschimganica | Uluganine | [183] |

Table 2.
Alkaloid composition of investigated species of Boraginaceae.
| Compounds | Sources | References |
|---|---|---|
| 3'-Acetylcanesine | Lithospermum canesens | [153,154] |
| 3'-Acetylcanescenine | Lithospermum canesens | [153,154] |
| 7-Acetyl-9-curassavoylheliotridine | Anchusa milleri | [40] |
| 7-Acetyl-9-(2,3-dihydroxybutryl) retronecine | Onosma arenaria | [163] |
| 7-Acetyl-9-(2-dimethylbutryl) retronecine | Onosma arenaria | [163] |
| 3'-Acetylechinatine | Cynoglossum creticum | [70] |
| Cynoglossum officinale | [67] | |
| Lindelofia spectabilis | [124] | |
| Messerschmidia argentea | [158] | |
| 7-Acetylechinatine | Cynoglossum amabile | [67] |
| Cynoglossum officinale | [67,84] | |
| Onosma erecta | [69] | |
| 3'- Acetylechihumiline | Onosma leptantha | [166] |
| 3'-Acetylechiumine | Amsinckia menziessi var. intermedia | [42] |
| Cryptantha clevelandii | [63] | |
| 3'-Acetylechimidine | Echium vulgare | [96] |
| 5'-Acetylechimidine | Echium vulgare | [11] |
| 5'-Acetyleuropine | Heliotropium disciforme | [122] |
| Heliotropium rotundifloium | [138] | |
| 7-Acetyleuropine | Heliotropium bovi | [110] |
| 3'-Acetylfurcatine | Amsinckia carinata | [42] |
| Amsinckia furacata | [42] | |
| 3'-Acetylheliosupine | Cynoglossum creticum | [70] |
| Cynoglossum officinale | [67,83] | |
| Heliotropium hirsutissimum | [129] | |
| 7-Acetyl-9-(2-hydroxy-3-methylbutryl) retronecine | Onosma arenaria | [163] |
| 3'-Acetylindicine | Heliotropium arborescens (H. peruvianum) | [28] |
| Messerschmidia argentea | [158] | |
| 3'-Acetylintermedine | Amsinckia eastwoodiae | [42] |
| Amsinckia lycopsoides | [42] | |
| Amsinckia lycopsoides × menziesii var. intermedia | [42] | |
| Amsinckia menziesii var. intermedia | [43] | |
| Amsinckia retrosa | [42] | |
| Amsinckia spectabilis var. microcarpa | [42] | |
| Amsinckia spectabilis var. spectabilis | [42] | |
| Amsinckia tessellata | [46] | |
| Cryptantha cana | [63] | |
| Cryptantha crassipes | [64] | |
| Cryptantha clevelandii | [63] | |
| Cryptantha flava | [63] | |
| Cryptantha leiocarpa | [63] | |
| Cryptantha thyrsiflora | [63] | |
| Cryptantha virgata | [63] | |
| Echium pininana | [95] | |
| 7-Acetylintermedine | Amsinckia carinata | [42] |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia tessellata | [46] | |
| Anchusa arvensis | [40] | |
| Borago officinalis | [55] | |
| Cryptantha cana | [63] | |
| Cryptantha crassipes | [64] | |
| Cryptantha flava | [63] | |
| Cryptantha thyrsiflora | [63] | |
| Cryptantha virgata | [63] | |
| Lappula myostis | [35] | |
| Lithospermum canescens | [153,154] | |
| Onosma alborosea | [161] | |
| Onosma arenaria pennina | [161] | |
| Onosma stellulatum | [167] | |
| Pulmonaria obscura | [168] | |
| Symphytum officinale | [171,172,179] | |
| Symphytum peregrinum | [27] | |
| Symphytum × uplandicum | [180] | |
| 5'-Acetyllasiocarpine | Heliotropium hirsutissimum | [129] |
| 7-Acetyl-9-latifolylretronecine | Hackelia californica | [101] |
| 3'-Acetyllithosenine | Lithospermum officinale | [156] |
| 3'-Acetyllycopsamine | Amsinckia grandiflora | [42] |
| Amsinckia lunaris | [42] | |
| Amsinckia menziesii | [45] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia tessellata | [46] | |
| Amsinckia tessellata var. tessellata | [42] | |
| Cerinthe glabra | [40] | |
| Cryptantha cana | [63] | |
| Cryptantha crassipes | [64] | |
| Cryptantha flava | [63] | |
| Cryptantha jamesii | [66] | |
| Cryptantha thyrsiflora | [63] | |
| Echium lycopsis (E. plantagineum) | [93] | |
| 7-Acetyllycopsamine | Amsinckia grandiflora | [42] |
| Amsinckia menziesii | [45] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia tessellata | [46] | |
| Amsinckia tessellate var. tessellata | [42] | |
| Anchusa arvensis | [40] | |
| Anchusa hispida | [40] | |
| Anchusa officinalis | [48] | |
| Borago officinalis | [55] | |
| Cerinthe glabra | [40] | |
| Cryptantha cana | [63] | |
| Cryptantha crassipes | [64] | |
| Cryptantha flava | [63] | |
| Cryptantha jamesii | [66] | |
| Cryptantha thyrsiflora | [63] | |
| Cryptantha virgata | [63] | |
| Echium horridum | [90] | |
| Echium hypertropicum | [92] | |
| Echium humile | [91] | |
| Echium rauwolfi | [90] | |
| Lappula myostis | [35] | |
| Lappula spinocarpos | [40] | |
| Lithospermum canesens | [153,154] | |
| Onosma alborosea | [161] | |
| Onosma arenaria | [163] | |
| Onosma arenaria pennina | [161] | |
| Pulmonaria obscura | [168] | |
| Symphytum bohemium | [173] | |
| Symphytum officinale | [171,172,179] | |
| Symphytum peregrinum | [27] | |
| Symphytum tanaiense | [173] | |
| Symphytum tubertosum | [28] | |
| Symphytum × uplandicum | [180] | |
| 7-Acetyl-9-(2-methylbutyryl) retronecine | Onosma arenaria | [163] |
| 3'-Acetylmyscorpine | Amsinckia menziesii var. intermedia | [42] |
| Amsinckia retrosa | [42] | |
| 7-Acetylretronecine | Onosma arenaria | [163] |
| 3'-Acetylrinderine | Cynoglossum columnae | [69] |
| Heliotropium transplinum | [144] | |
| 7-Acetyl-9-sarracinoyl retronecine | Alkanna tinctoria | [40] |
| 7-Acetylscorpioidine | Myosotis scorpioides | [159] |
| 3'-Acetylsupinine | Onosma arenaria | [163] |
| 3'-Acetyltessellatine | Amsinckia tessellata var. tessellata | [42] |
| 9-Acetytessellatine | Amsinckia grandifkora | [42] |
| Amsinckia lunaris | [42] | |
| Amsinckia tessellata var. tessellata | [42] | |
| 3'-Acetyltrachelanthamine | Heliotropium floridum | [128] |
| 9-Acetyltrachelanthamine | Anchusa arvensis | [40] |
| 3'-Acetylviridiflorine | Amsinckia tessellata var. gloriosa | [42] |
| 9-(3'-Acetyl)viridiflory retronecine | Heliotropium curassavicum var. argentinum | [119] |
| Heliotropium curassavicum var. curassavicum | [119] | |
| 9-(3'-Acetylviridifloryl) turniforcidine | A. douglasiana | [42] |
| A. tessellata var. tessellata | [42] | |
| 7-Acetylvulgarine | Echium vulgare | [11] |
| Amabiline | Amsinckia douglasina | [42] |
| Amsinckia eastwoodiae | [42] | |
| Amsinckia grandiflora | [42] | |
| Amsinckia lycopsoides | [42] | |
| Amsinckia lycopsodes menziesii var. intermedium | [42] | |
| Amsinckia menziesii var intermedium | [42] | |
| Amsinckia retrosa | [42] | |
| Amsinckia tessellata var. gloriosa | [42] | |
| Amsinckia tessellata var. tessellata. | [42] | |
| Asperugo procumbens | [47] | |
| Borago officinalis | [55] | |
| Cryptantha confertiflora | [63] | |
| Cryptantha crassipes | [64] | |
| Cryptantha virginensis | [63] | |
| Cynoglossum amabile | [67,68] | |
| Cynoglossum glochidiatum | [78] | |
| Heliotropium spathulatum | [140] | |
| Lappula spinocarpos | [40] | |
| Lindelofia angustiflora | [78] | |
| Neatostema apulum | [160] | |
| Anadoline | Symphytum orientale | [28] |
| Symphytum tuberosum | [28] | |
| 7α-Angeloyl-1-chloromethy-1,2-dihydropyrrolizidine | Cynoglossum creticum | [70] |
| 7-Angeloyl-9-(2,3-dihydroxybutyryl)heliotridine | Cynoglossum officinale | [67] |
| 7-Angeloyl-9-(2,3-dihydroxybutyryl)retronecine | Echium horridum | [90] |
| Echium rauwolfii | [90] | |
| Echium setosum | [96] | |
| Onosma arenaria | [163] | |
| 7-Angeloyl-9-(2,3-dihydroxypropanoyl)retronecine | Alkanna orientalis | [40] |
| Alkanna tinctoria | [40] | |
| 7-Angeloyl-1-formyl-6,7-dihydro-5H-pyrrolizidine | Cynoglossum officinale | [67] |
| 7-Angeloyl-9-(hydroxypropenoyl)retronecine | Alkanna orientalis | [40] |
| Alkanna tinctoria | [40] | |
| 7-Angeloylechinatine | Cynoglossum officinale | [67] |
| 7-Angeloylheliotridine | Anchusa hispida (Gastrocotyle hispida) | [40] |
| Anchusa milleri | [40] | |
| Cynoglossum creticum | [70,72] | |
| Cynoglossum latifolium | [79] | |
| Cynoglossum officinale | [67,84] | |
| Heliotropium curassavicum | [117] | |
| Heliotropium supinum | [27,143] | |
| Lappula spinocarpos | [40] | |
| 7-Angeloylheliotridine | Paracaryum intermedium | [40] |
| Paracaryum regulosum | [40] | |
| Rindera austroechinata | [57] | |
| 7-Angeloylheliotrine | Heliotropium circinatum | [114] |
| Heliotropium digynum (H. luteum) | [121] | |
| Heliotropium eichwaldii | [124] | |
| Heliotropium europaeum | [127] | |
| Rindera umbellata | [169] | |
| 7-Angeloyllycopsamine | Echium horridum | [90] |
| Echium rauwolfii | [90] | |
| 7-Angeloyl-9-(2-methylbutyryl)heliotridine | Cynoglossum creticum | [70] |
| Cynoglossum offocinale | [67] | |
| 7-Angeloyl-9-(2-methylbutyryl)retronecine | Echium horridum | [90] |
| Echium rauwolfii | [90] | |
| 7-Angeloylretronecine | Alkanna orientalis | [40,41] |
| Alkanna tinctoria (A. tuberculata) | [40,184] | |
| Arnebia decumbens | [52] | |
| Arnebia euchroma | [53] | |
| Cerinthe minor | [60] | |
| Cryptantha fendleri | [63] | |
| Cryptantha leiocarpa | [63] | |
| Echium amoenum | [87] | |
| Echium glomeratum | [89] | |
| Echium horridum | [90] | |
| Echium rauwolfii | [90] | |
| Echium setosum | [96] | |
| Echium simplex | [162] | |
| Echium vulgare | [96] | |
| Eritrichium rupestre | [60] | |
| Hackelia longituba | [104] | |
| Heliotropium bursiferum | [113] | |
| Symphytum tuberosum | [162] | |
| 9-Angeloylretronecine | Alkanna orientalis | [40,41] |
| Arnebia decumbens | [52] | |
| Cryptantha fendleri | [63] | |
| Cryptantha leiocarpa | [63] | |
| Cynoglossum amabilie | [67] | |
| Echium glomaratum | [89] | |
| Echium hypertropicum | [92] | |
| Echium setosum | [96] | |
| Echium simplex | [162] | |
| Echium vulgare | [96] | |
| Hackelia longituba | [104] | |
| Messerchimidia sibrica | [28] | |
| Myosotis sylvatica | [25] | |
| 7-Angeloylrinderine | Cynoglossun officinale | [67] |
| 9-Angeloyltrachelanthamidine | Anchusa arvensis | [40] |
| 7-Angeloyl-9-(+)-trachelanthylheliotridine | Rindera umbellata | [169] |
| 9-Angeloyl-7-viridiflorylretronecine | Cerinthe minor | [60] |
| Cynoglossum amabile | [60] | |
| Cynoglossum clandestinum | [60] | |
| Echium tuberculatum | [60] | |
| Eritrichium rupestre | [60] | |
| Asperumine | Echium vulgare | [98] |
| Symphytum asperum | [172] | |
| Symphytum caucasium | [174] | |
| 9-(Butyryl-2-ene) supinidine | Onosma arenaria | [163] |
| Canescine | Lithospermum canescens | [153,154] |
| Canescenine | Lithospermum canescens | [153,154] |
| Carategine | Lindelofia tschimganica | [27] |
| Rindera oblongifolia | [152] | |
| Solanthus karateginus | [151] | |
| Coromandaline | Heliotropium curassavicum | [117,118] |
| Heliotropium spathulatum | [140] | |
| Coromandalinine | Heliotropium curassavicum | [117] |
| Heliotropium spathulatum | [140] | |
| Cryptanthine | Cryptantha utahensis | [65] |
| Curassanecine | Heliotropium curassavicum | [117] |
| Curassavine | Anchusa officinalis | [49] |
| Heliotropium curassavicum | [117] | |
| Heliotropium spathulatum | [140] | |
| Curassavinine | Heliotropium curassavicum | [117] |
| Heliotropium spathulatum | [140] | |
| 9-Curassavorylheliotridine | Anchusa milleri | [40] |
| Cynaustrine | Cynoglossum australe | [68] |
| Cynoglossum lanceolatum | [78] | |
| Cynoglossum montanum | [81] | |
| Cynaustraline | Amsinckia douglasiana | [42] |
| Cynoglossum australe | [68] | |
| Cynoglossum furcatum | [73] | |
| Cynoglossum lanceolatum | [78] | |
| Cynoglossum montanum | [81] | |
| Cynoglossamine | Cynoglossum creticum | [72] |
| Dehydroheliotrine | Heliotropium europaeum | [185] |
| 5-Deoxylasiocarpine | Heliotropium dissitiflorum | [123] |
| 3',7-Diacetylintermedine | Amsinckia menziesii var. intermedia | [42] |
| Amsinckia tessellata | [46] | |
| Anchusa arvensis | [40] | |
| 3',7-Diacetyllycopsamine | Amsinckia menziesii | [45] |
| Amsinckia tessellata | [46] | |
| 3',7-Diacetyllycopsamine | Anchusa arvensis | [40] |
| Cerinthe glabra | [59] | |
| 3',9-Diacetyltessellatine | Amsinckia tessellata var. tessellata | [42] |
| 5,6-Dihydro-7,9-dimethoxy-7H-pyrrolizine | Onosma arenaria | [163] |
| Dihydroechinatine | Onosma stellulatum | [167] |
| thero-2'',3''-Dihydroxyechiumine | Cryptantha clevelandiiCryptantha inequata | [63,65] |
| Cryptantha leiocarpa | [63] | |
| Dihydroxytriangularine | Alkanna orientalis | [40,41] |
| Alkanna tinctoria | [40,41] | |
| Dihydroxytriangularicine | Alkanna orientalis | [40] |
| Alkanna tinctoria | [40] | |
| Echihumiline | Echium hypertropicum | [92] |
| Echium humile | [91] | |
| Echium vulgare | [96] | |
| Onosma leptantha | [166] | |
| Echimidine | Arnebia hispidissima | [54] |
| Cryptantha inequata | [65] | |
| Echium amoenum | [87] | |
| Echium angustifolium | [88] | |
| Echium horridum | [90] | |
| Echium humile | [91] | |
| Echium hypertropicum | [92] | |
| Echium italicum | [28] | |
| Echium lycopsis (E. plantagineum) | [94] | |
| Echium pininana | [95] | |
| Echium rauwolfii | [90] | |
| Echium sericeum | [54] | |
| Echium. setosum | [96] | |
| Echium stenosiphon subsp. stenosiphon | [92] | |
| Echium tuberculatum | [60] | |
| Echium vulgare | [11,96] | |
| Echium wildpretti | [99] | |
| Onosma stellulatum | [60,167] | |
| Symphytum aintabicum | [170] | |
| Symphytum asperum | [171] | |
| Symphytum bohemium | [173] | |
| Symphytum caucasium | [174] | |
| Symphytum consolidum | [175] | |
| Symphytum sylvaticum | [170] | |
| Symphytum tuberosum | [162] | |
| Echimidine isomer (tigloyl) | Echium amoenum | [87] |
| Echium horridum | [90] | |
| Echimidine isomer (tigloyl) | Echium rauwolfii | [90] |
| Echium setosum | [96] | |
| Echium vulgare | [96] | |
| Echimiplatine | Cryptantha crassipes | [64] |
| Echium plantagineum | [93] | |
| Echium vulgare | [11] | |
| Echinatine | Asperugo procumbens | [47] |
| Cynoglossum amabile | [67,68] | |
| Cynoglossum columnae | [69] | |
| Cynoglossum creticum | [70,71,72] | |
| Cynoglossum furcatum (C. zeylanicum) | [74,75] | |
| Cynoglossum germanicum | [77] | |
| Cynoglossum macrostylum | [80] | |
| Cynoglossum montanum | [81] | |
| Cynoglossum nervosum | [82] | |
| Cynoglossum officinale | [67,84] | |
| Cynoglossum pictum | [85] | |
| Heliotropium circinatum | [114] | |
| Heliotropium indicum | [130] | |
| Heliotropium suaveolens | [29] | |
| Heliotropium supinum | [27,143] | |
| Lappula glochidiata | [146] | |
| Lindelofia longiflora | [149] | |
| Lindelofia spectabilis | [124] | |
| Lindelofia stylosa | [135] | |
| Moltikiopsis ciliata | [108] | |
| Paracaryum regulosum | [40] | |
| Paracynoglossum imeretium | [29] | |
| Rindera austroechinata | [57,152] | |
| Rindera baldschuanica | [152] | |
| Rindera cyclodonata | [120] | |
| Rindera echinata | [120] | |
| Rindera oblogifolia | [152] | |
| Rindera umbellata | [169] | |
| Solenanthus circinnatus | [151] | |
| Solenanthus coronatus | [135] | |
| Solenanthus karateginus | [151] | |
| Symphytum asperum | [172] | |
| Symphytum caucasium | [174] | |
| Symphytum officinale | [179] | |
| Tournefortia sogdiana | [57] | |
| Echiumine | Amsinckia carinata | [42] |
| Amsinckia eastwoodiae | [42] | |
| Amsinckia hispida | [44] | |
| Amsinckia intermedia | [44] | |
| Amsinckia lycopsoides | [44] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Cryptantha clevelandii | [63] | |
| Cryptantha crassipes | [64] | |
| Cryptantha leiocarpa | [63] | |
| Echium lycopsis | [94] | |
| Echiupine | Echium pininana | [95] |
| Echiuplatine | Cryptantha inequata | [65] |
| Echium plantagineum | [93] | |
| Echium vulgare | [11] | |
| Echivulgarine | Echium vulgare | [11] |
| Ehretinine | Ehretia aspera | [100] |
| 2'',3''-Epoxyechiumine | Cryptantha clevelandii | [63] |
| Cryptantha leiocarpa | [63] | |
| Erythro-2'',3''-chloro-2''-hydroxyechiumine | Cryptantha clevelandii | [63] |
| Cryptantha leiocarpa | [63] | |
| 7-Epi-echimiplatine | Onosma erecta | [69] |
| 1α-2α-Epoxy-1β-hydroxymethyl-8α-pyrrolizidine | Heliotropium transalpinum var. transalpinum | [145] |
| Europine | Heliotropium arbainense | [71] |
| Heliotropium bacciferum | [108,109] | |
| Heliotropium bovi | [110] | |
| Heliotropium circinatum | [114] | |
| Heliotropium crassifolium | [115] | |
| Heliotropium digynum (H.luteum) | [121] | |
| Heliotropium dissitiflorum | [123] | |
| Heliotropium esfandiarii | [125] | |
| Heliotropium europaeum | [126,127,186] | |
| Heliotropium hirsutissinum | [129] | |
| Heliotropium marifolium | [29] | |
| Heliotropium maris mortui | [71] | |
| Heliotropium rotundifolium | [137,138] | |
| Floridanine | Cordia sinensis | [62] |
| Floridimine | Heliotropium floridum | [128] |
| Floridine | Heliotropium floridum | [128] |
| Floridinine | Heliotropium floridum | [128] |
| Furcatine | Amsinckia carinata | [42] |
| Amsinckia furacata | [42] | |
| Amsinckia vernicosa | [42] | |
| Hackelidine | Hackelia californica | [102] |
| Heleurine | Heliotropium bacciferum | [109] |
| Heliotropium circinatum | [114] | |
| Heliotropium disciforme | [122] | |
| Heliotropium europaeum | [126,127,186] | |
| Heliotropium hirsutissimum | [129] | |
| Heliotropium indicum | [131] | |
| Helibracteatine | Heliotropium bracteatum | [112] |
| Helibractinecine | Heliotropium bracteatum | [111] |
| Helibracteatinecine | Heliotropium bracteatum | [112] |
| Helibracteatinine | Heliotropium bracteatum | [112] |
| Heliofoline | Heliotropium ovalifolium | [136] |
| Helindicine | Heliotropium indicum | [130] |
| Heliocoromandaline | Heliotropium curassavicum | [117] |
| Heliocurassavine | Heliotropium curassavicum | [117] |
| Heliocurassavicine | Heliotropium curassavicum | [117] |
| Heliocurassavinine | Heliotropium curassavicum | [117] |
| Heliospathine | Heliotropium spathulatum | [140] |
| Heliospathuline | Heliotropium spathulatum | [140] |
| Onosma stellulatum | [167] | |
| Heliosupine | Cynoglossium creticum | [70,71,72] |
| Cynoglossium macrostylum | [80] | |
| Cynoglossium montatum | [81] | |
| Cynoglossium officinale | [67,83,84] | |
| Cynoglossium pictum | [85] | |
| Cynoglossium viridiforum | [86] | |
| Heliotropium supinum | [27,143] | |
| Myosotis sylvatica | [25] | |
| Paracaryum regulosum | [90] | |
| Paracynoglossum imeretium | [29] | |
| Rindera umbellata | [169] | |
| Symphytum asperum | [171] | |
| Symphytum officinale | [179] | |
| Heliotridine | Anchusa milleri | [90] |
| Onosma heterophyllum | [165] | |
| Heliotridine 2S-hydroxy-2S(1S-hydroxyethyl0-4-methyl-pentanoyl ester | Anchusa strigosa | [50] |
| Heliotrine | Arenbia decumbens | [52] |
| Cynoglossum nervosum | [82] | |
| Heliotropium acutifolium | [105] | |
| H. arbainense | [71] | |
| H. bacciferum | [108,109] | |
| Heliotropium circinatum | [114] | |
| Heliotropium dasycarpum | [120] | |
| Heliotropium digynum | [121] | |
| Heliotropium disciforme | [122] | |
| Heliotropium dissitiflorum | [123] | |
| Heliotropium eichwaldii | [124] | |
| Heliotropium europaeum | [127,185,186] | |
| Heliotropium hirsutissimum | [129] | |
| Heliotropium indicum | [130,131] | |
| Heliotropium lasiocarpum | [29] | |
| Heliotrine | Heliotropium marifolium | [29] |
| Heliotropium olgae | [135] | |
| Heliotropium popovii subsp. gillianum | [28] | |
| Heliotropium rotundifolum | [137,138] | |
| Heliotropium suaveolens | [29] | |
| Heliotropium supinum | [27,143] | |
| Heliotropium transoxanum | [27] | |
| Molyikiopsis ciliate (Lithospermum callosum) | [108] | |
| Symphytum caucasium | [174] | |
| Heliovicine | Heliotropium spathulatum | [140] |
| Heliscabine | Heliotropium scabrum | [139] |
| Hydroxymyoscorpine | Echium pininana | [95] |
| Ilamine | Heliotropium crassifolium | [115] |
| Incanine | Heliotropium olgae | [29] |
| Trichodesma incanum | [46] | |
| Indicine | Heliotropium amplexicaule | [29] |
| Heliotropium arborescens (H. pruvianum) | [107] | |
| Heliotropium indicum | [130,131] | |
| Heliotropium marifolium | [29] | |
| Heliotropium transalpinum | [144] | |
| Messerschmidia argentea | [158] | |
| Indicinine | Heliotropium indicum | [130,131] |
| Isoechinatine | Cynoglossum furcatum (C. zeylanicum) | [75] |
| Isolycopsamine | Heliotropium keralense | [190] |
| Isoretronocanol (or its isomer) | Omphalodes verna | [27] |
| 9-(3'-Isovaleryl)viridiflory retronecine | Heliotropium curassavicum var. argentinum | [119] |
| Heliotropium curassavicum var. curassavicum | [119] | |
| Intermedine | Amsinckia carinata | [42] |
| Amsinckia douglasiana | [42] | |
| Amsinckia eastwoodiae | [42] | |
| Amsinckia furacata | [42] | |
| Amsinckia. grandiflora | [42] | |
| Amsinckia hispida | [44] | |
| Amsinckia intermedia | [44] | |
| Amsinckia lunaris | [42] | |
| Amsinckia lycopsoides | [42,44] | |
| Amsinckia lycopsoides × menziesii var. intermedia | [42] | |
| Amsinckia menziesii | [45] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia retrosa | [42] | |
| Intermedine | Amsinckia spectabilis var. microcarpa | [42] |
| Amsinckia spectabilis var. spectabilis | [42] | |
| Amsinckia spectabilis var. nicolai | [42] | |
| Amsinckia tessellata | [46] | |
| Amsinckia tessellata var. gloriosa | [42] | |
| Amsinckia tessellata var. tessellata | [42] | |
| Amsinckia vernicosa | [42] | |
| Anchusa arvensis (Lycopsis arvensis) | [40] | |
| Anchusa hispidia (Gastrocotyle hispidia) | [40] | |
| Anchusa officinalis | [48] | |
| Borago officinalis | [55] | |
| Cerinthe minor | [61] | |
| Cryptantha cana | [63] | |
| Cryptantha clevelandii | [63] | |
| Cryptantha confertiflora | [63] | |
| Cryptantha flava | [63] | |
| Cryptantha inequata | [65] | |
| Cryptantha jamesii | [66] | |
| Cryptantha leiocarpa | [63] | |
| Cryptantha thyrsiflora | [63] | |
| Cryptantha virgata | [63] | |
| Cryptantha virginiensis | [63] | |
| Heliotropium keralense | [133] | |
| Heliotropium transalpinum | [144] | |
| Lappula myostis | [35] | |
| Lappula spinocarpas | [40] | |
| Lithospermum erythrorhizon | [155] | |
| Mertensia ciliate | [157] | |
| Onosma alborosea | [161] | |
| Onosma arenaria pennina | [161] | |
| Pulmonaria obscura | [168] | |
| Symphytum peregrinum | [27] | |
| Symphytum × uplandicum | [180] | |
| Trichodesma africanum | [71] | |
| Lactodine | Cynoglossum furcatum | [76,187] |
| Lasiocarpine | Heliotropium arbainense | [71] |
| Heliotropium bovei | [110] | |
| Heliotropium circinatum | [114] | |
| Heliotropium digynum | [121] | |
| Heliotropium eichwaldii | [124] | |
| Heliotropium europaeum | [126,127,186] | |
| Heliotropium hirsutissimum | [129] | |
| Heliotropium indicum | [130,131] | |
| Heliotropium lasiocarpum | [29] | |
| Heliotropium marifolium | [29] | |
| Heliotropium maris mortui | [137] | |
| Heliotropium rotundifolium | [137,138] | |
| Heliotropium suaveolens | [29] | |
| Lappula intrmedia | [27] | |
| Symphytum caucasium | [174] | |
| Symphytum officinale | [177] | |
| Latifoline | Cryptantha fendleri | [63] |
| Cynoglossum latifolium | [79] | |
| Hackelia californica | [101] | |
| Hackelia floribunda | [103] | |
| Hackelia longituba | [104] | |
| Lindelofia stylosa | [27] | |
| 9-Latifolylretronecine | Hackelia californica | [101] |
| Leptanthine | Cryptantha crassipes | [64] |
| Echium plantagineum | [93] | |
| Echium vulgare | [11] | |
| Onosma leptantha | [166] | |
| Onosma stellulatum | [167] | |
| Lindelofine | Amsickia menzesii var. intermedia | [42] |
| Amsickia spectabilis var. microcarpa | [42] | |
| Amsickia spectabilis var. spectabilis | [42] | |
| Amsickia spectabilis var. nicola | [42] | |
| Lindelofia anchusoides (L. macrostyle) | [28] | |
| Rindera umbellata | [169] | |
| Lindelofamine | Lindelofia anchusoides (L. macrostyle) | [28] |
| Lindelofidine | Heliotropium angiospermum | [106] |
| Heliotropium confertifolum | [106] | |
| Heliotropium curassavicum | [106] | |
| Heliotropium foliosissimum | [106] | |
| Heliotropium fruticosum | [106] | |
| Heliotropium gregii | [106] | |
| Heliotropium indicum | [106,116] | |
| Heliotropium molle | [106] | |
| Heliotropium procumbens | [106] | |
| Heliotropium queretaroanum | [106] | |
| Heliotropium spathulatum | [116] | |
| Heliotropium sessei | [106] | |
| Heliotropium racemosum | [106] | |
| Heliotropium ternatum | [106] | |
| Heliotropium wigginsii | [106] | |
| Lithosenine | Lithospermum officinale | [156] |
| Longitubine | Hackelia californica | [101] |
| Hackelia logituba | [104] | |
| Lycopsamine | Amsinckia carinata | [42] |
| Amsinckia douglasiana | [42] | |
| Amsinckia eastwoodiae | [42] | |
| Amsinckia furacata | [42] | |
| Amsinckia grandiflora | [42] | |
| Amsinckia hispida | [44] | |
| Amsinckia intermedia | [44] | |
| Amsinckia lunaris | [42] | |
| Amsinckia lycopsoides | [42,44] | |
| Amsinckia lycopsoides × menziesii var. intermedia | [42] | |
| Amsinckia menziesii | [45] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia retrosa | [42] | |
| Amsinckia spectabilis var. microcarpa | [42] | |
| Amsinckia spectabilis var. spectabilis | [42] | |
| Amsinckia spectabilis var. nicolai | [42] | |
| Amsinckia tessellata | [46] | |
| Amsinckia tessellata var. gloriosa | [42] | |
| Amsinckia tessellata var. tessellata | [42] | |
| Amsinckia vernicosa | [42] | |
| Anchusa arvensis (Lycopsis arvensis) | [40] | |
| Anchusa hispidia (Gastrocotyle hispidia) | [40] | |
| Anchusa officinalis | [48] | |
| Arnebia decumbens | [52] | |
| Borago officinalis | [55] | |
| Cerinthe glabra | [59] | |
| Cerinthe minor | [61] | |
| Cryptantha cana | [63] | |
| Cryptantha confertiflora | [63] | |
| Cryptantha flava | [63] | |
| Cryptantha inequata | [65] | |
| Cryptantha jamesii | [66] | |
| Cryptantha thyrsiflora | [63] | |
| Cryptantha virgata | [63] | |
| Cryptantha virginiensis | [63] | |
| Cynoglossum amabile | [67] | |
| Echium hypertropicum | [92] | |
| Heliotropium transalpinum | [144] | |
| Heliotropium megalanthum | [134] | |
| Heliotropium steudneri | [27] | |
| Lappula myostis | [35] | |
| Lappula spinocarpas | [40] | |
| Lithospermum purpureocoeruleum | [60] | |
| Mertensia bakeri | [157] | |
| Mertensia ciliate | [157] | |
| Mertensia sibirica | [28] | |
| Neatostema apulum | [60] | |
| Onosma alborosea | [161] | |
| Onosma arenaria pennina | [161] | |
| Onosma stellulatum | [167] | |
| Pulmonaria obscura | [168] | |
| Symphytum bohemium | [173] | |
| Symphytum grandiflorum | [176] | |
| Symphytum ibericum | [176] | |
| Symphytum peregrinum | [27] | |
| Symphytum officinale | [171,177] | |
| Symphytum tanaiense | [173] | |
| Symphytum tuberosum | [162,176] | |
| Symphytum × uplandicum | [180] | |
| Trichodesma africanum | [71] | |
| Macrophylline | Cordia myxa | [62] |
| Macrotamine | Macrotomia echioides | [28] |
| Megalanthonine | Heliotropium megalanthum | [134] |
| Methyechiuplatine | Cryptantha inequata | [65] |
| 1-Methylene-8α-pyrrolizidine | Onosma heterophyllum | [165] |
| 7-(2-Methylbutyryl)retronecine | Echium humile | [91] |
| Echium vulgare | [96] | |
| 9-(2-Methylbutyryl)retronecine | Echium vulgare | [96] |
| 7-(2-Methylbutyryl)-9-(2,3-dihydroxybutyryl)retronecine | Echium humile | [91] |
| Echium vulgare | [96] | |
| 7-(2-Methylbutyryl)-9-echimidinyl retronecine | Echium humile | [91] |
| Echium hypertropicum | [92] | |
| Echium stenosiphon subsp. stenosiphon | [92] | |
| Echium vulgare | [96] | |
| Monocrotaline | Arnebia hispidissima | [54] |
| Lindelofia spectabilis | [124] | |
| Myoscorpine | Amsinckia carinata | [42] |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia retrosa | [42] | |
| Amsinckia spectabilis var. microcarpa | [42] | |
| Echium pininana | [95] | |
| Linelfolia erythrorhizon | [155] | |
| Myosotis scorpioides | [159] | |
| Neocoromandaline | Cynoglossum furcatum | [74] |
| Neolatifoline | Cryptantha fendleri | [63] |
| Hackelia logituba | [104] | |
| Onosmerectine | Onosma erecta | [69] |
| (7S,8R)Petranine | Echium glomeratum | [89] |
| (7S,8S)Petranine | Echium glomeratum | [89] |
| Pictumine | Cynoglossum pictum | [28] |
| Platynecine | Cryptantha leiocarpa | [63] |
| Platynecine N-oxide 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanosyl ester | Anchusa strigosa | [50,51] |
| Punctanecine | Rindera umbellata | [169] |
| Pycnanthine | Echium humile | [91] |
| Retronecine | Anchusa hispida (Gastrocotyle hispida) | [40] |
| Echium vulgare | [96] | |
| Heliotropium angiospermum | [106] | |
| Heliotropium confertifolum | [106] | |
| Heliotropium curassavicum | [106,116] | |
| Heliotropium foliosissimum | [106] | |
| Heliotropium fruticosum | [106] | |
| Heliotropium gregii | [106] | |
| Heliotropium indicum | [106,116] | |
| Heliotropium keralense | [133] | |
| Heliotropium molle | [106] | |
| Heliotropium ovalifolium | [136] | |
| Heliotropium procumbens | [106] | |
| Heliotropium queretaroanum | [106] | |
| Heliotropium racemosum | [106] | |
| Heliotropium scabrum | [139] | |
| Heliotropium spathulatum | [106,116] | |
| Heliotropium sessei | [106] | |
| Heliotropium ternatum | [106] | |
| Heliotropium wigginsii | [106] | |
| Lappula spinocarpos | [40] | |
| Trichodesma africanum | [40] | |
| Retronecine-7:9- dibenzoate | Caccinea glauca | [58] |
| Retronecine 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanosyl ester | Anchusa strigosa | [50,51] |
| Retronecine 2S-hydroxy-2S(1R-hydroxyethyl)-4-methyl-pentanosyl ester | Anchusa strigosa | [50,51] |
| Rinderine | Anchusa milleri | [40] |
| Arnebia decumbens | [52] | |
| Cynoglossum columnae | [69] | |
| Cynoglossum creticum | [70,72] | |
| Cynoglossum officinale | [67,84] | |
| Heliotropium indicum | [130,131] | |
| Heliotropium peruvianum | [29] | |
| Heliotropium transalpinum | [144] | |
| Paracaryum intermedium | [40] | |
| Paracaryum regulosum | [40] | |
| Rindera austroechinata | [57] | |
| Rindera baldschuanica | [152] | |
| Solanthus turkestanicus | [150] | |
| Scorpioidine | Myosotis scorpioides | [159] |
| 7-Senecioylhelotridine | Cynoglossum creticum | [70] |
| Paracarum intermedium | [40] | |
| 7-Senecioylretronecine | Alkanna orientalis | [40] |
| Echium hypertropicum | [92] | |
| Echium humile | [91] | |
| 9-Senecioylretronecine | Alkanna orientalis | [40] |
| Echium humile | [91] | |
| 7-Senecioylrinderine | Paracaryum intermedium | [40] |
| 7-Senecioyllycopsamine | Echium humile | [91] |
| Sincamidine | Amsinckia intermedia | [44] |
| Senkirkine | Trichodesma ehrenbergii | [54] |
| Strigosine | Heliotopium strigosum | [25,141] |
| Subulacine | Heliotopium angiospermum | [29] |
| Heliotopium molle | [29] | |
| Heliotopium subulacatum | [29] | |
| Heliotopium ternatum | [27] | |
| Heliotopium transalpinum var. transalpinum | [145] | |
| Supinine | Amsinckia carinata | [42] |
| Amsinckia eastwoodiae | [42] | |
| Amsinckia furacata | [42] | |
| Amsinckia lunaris | [42] | |
| Amsinckia lycopsoides | [42,44] | |
| Amsinckia lycopsoides × menziesii var. intermedia | [42] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia retrosa | [42] | |
| Amsinckia spectabilis var. microcarpa | [42] | |
| Amsinckia spectabilis var. spectabilis | [42] | |
| Amsinckia spectabilis var. nicolai | [42] | |
| Amsinckia tessellate var. gloriosa | [42] | |
| Amsinckia tessellate var. tessellate | [42] | |
| Anchusa arvensis (Lycopsis arvensis) | [40] | |
| Anchusa melleri | [40] | |
| Arnebia decumbens | [52] | |
| Borago officinalis | [55] | |
| Caccina crassifolia | [57] | |
| Cerinthe glabra | [59] | |
| Cynoglossum amabile | [67] | |
| Cynoglossum creticum | [70] | |
| Heliotropium bacciferum | [109] | |
| Heliotropium europaeum | [127] | |
| Heliotropium hirsutissimum | [129] | |
| Heliotropium indicum | [130,131] | |
| Heliotropium supinum | [27,143] | |
| Heliotropium transalpinum | [144] | |
| Lappula spinocarpos | [40] | |
| Tournefortia samentosa | [28] | |
| Trichodesma ehrenbergii | [54] | |
| Trichodesma zeylanicum | [182] | |
| Supinidine | Heliotropium angiospermum | [106] |
| Heliotropium confertifolum | [106] | |
| Heliotropium curassavicum | [106,116] | |
| Heliotropium foliosissimum | [106] | |
| Heliotropium fruticosum | [106] | |
| Heliotropium gregii | [106] | |
| Heliotropium indicum | [106,116] | |
| Heliotropium molle | [106] | |
| Heliotropium procumbens | [106] | |
| Heliotropium queretaroanum | [106] | |
| Heliotropium racemosum | [106] | |
| Heliotropium spathulatum | [106,116] | |
| Heliotropium sessei | [106] | |
| Heliotropium wigginsii | [106] | |
| Supinidine N-oxide 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester | Anchusa strigosa | [50,51] |
| Symlandine | Amsinckia menziesii var. inermedia | [42] |
| Echium sericeum | [54] | |
| Symphytum × uplandicum | [180] | |
| Symphytine | Myosotis scorpioides | [159] |
| Symphytum asperum | [171] | |
| Symphytum bohemium | [173] | |
| Symphytum consolidum | [175] | |
| Symphytum grandiflorum | [176] | |
| Symphytum ibericum | [176] | |
| Symphytum officinale | [171,177,178,179] | |
| Symphytum orientale | [28] | |
| Symphytum peregrinum | [27] | |
| Symphytum tanaiense | [173] | |
| Symphytum tuberosum | [28,176] | |
| Symphytum × uplandicum | [180] | |
| Symviridine | Symphytum asperum | [172] |
| Symphytum officinale | [172,177] | |
| Symphytum × uplandicum | [172] | |
| Tessellatine | Amsinckia douglasiana | [42] |
| Amsinckia eastwoodiae | [42] | |
| Amsinckia furacata | [42] | |
| Amsinckia grandiflora | [42] | |
| Amsinckia lunaris | [42] | |
| Amsinckia lycopsoides | [42,44] | |
| Amsinckia lycopsoides × menziesii var. intermedia | [42] | |
| Amsinckia menziesii var. intermedia | [42] | |
| Amsinckia retrosa | [42] | |
| Amsinckia spectabilis var. microcarpa | [42] | |
| Amsinckia spectabilis var. nicolai | [42] | |
| Amsinckia tessellate var. gloriosa | [42] | |
| Amsinckia tessellate var. tessellate | [42] | |
| Cryptantha confertiflora | [63] | |
| Cryptantha virginiensis | [63] | |
| Thesinine | Borago officinalis | [56] |
| Thesinine-4'-O-β-D-glucoside | Borago officinalis | [56] |
| 7-Tigloyl-9-(2-deoxy-2-methyl)echimidinylheliotridinEchium | Cynoglossum columnae | [69] |
| 7-Tigloyl-9-(2,3-dihydroxybutyryl)retronecine | Echium horridum | [90] |
| Echium rauwolfii | [90] | |
| Echium setosum | [96] | |
| Echium vulgare | [96] | |
| 7-Tigloyl-9-(2,3-dihydroxypropanoyl)retronecine | Alkanna orientalis | [40] |
| Alkanna tincotoria | [40] | |
| 7-Tigloylheliotridine | Cynoglossum officinale | [67] |
| 7-Tigloyllycopsamine | Echium horridum | [90] |
| Echium rauwolfii | [90] | |
| 7-Tigloyl-9-(2-methybutyryl)retronecine | Echium horridum | [90] |
| Echium rauwolfii | [90] | |
| Echium setosum | [96] | |
| Echium vulgare | [96] | |
| 7-Tigloylretronecine | Alkanna tinctoria | [40] |
| Arnebia decumbens | [40] | |
| Echium amoenum | [87] | |
| Echium setosum | [96] | |
| Echium vulgare | [96] | |
| 9-Tigloylretronecine | Alkanna orientalis | [40] |
| Alkanna tincotoria | [40] | |
| Arnebia decumbens | [52] | |
| Echium horridum | [90] | |
| Echium rauwolfii | [90] | |
| Echium setosum | [96] | |
| Echium vulgare | [96] | |
| Trachelanthamidine | Heliotropium angiospermum | [106] |
| Heliotropium confertifolum | [106] | |
| Heliotropium curassavicum | [106,116,118] | |
| Heliotropium foliosissimum | [106] | |
| Heliotropium fruticosum | [106] | |
| Heliotropium gregii | [106] | |
| Heliotropium indicum | [106,116] | |
| Heliotropium molle | [106] | |
| Heliotropium procumbens | [106] | |
| Heliotropium queretaroanum | [106] | |
| Heliotropium racemosum | [106] | |
| Heliotropium sessei | [106] | |
| Heliotropium spathulatum | [106,116] | |
| Heliotropium strigosa | [25] | |
| Heliotropium wigginsii | [106] | |
| Trachelanthamine | Anchusa hispida | [40] |
| Cynoglossum clandestinum | [60] | |
| Cynoglossum creticum | [70] | |
| Cynoglossum officinale | [67] | |
| Lappula spinocarpos | [40] | |
| Myosotis sylvatica | [25] | |
| Onosma stellulatum | [167] | |
| Rindera balaschuanica | [152] | |
| R. echinata | [120] | |
| Trachelanthus hissaricus | [29] | |
| Trachelanthus korolkovii | [57,150] | |
| Trachelanthine | Trachelanthus hissaricus | [29] |
| Trachelanthus korolkovii | [150] | |
| 7-Trachelanthyl-laburnine | Rindera umbellata | [169] |
| 7-Trachelanthylretronecine | Amsinckia vernicosa | [42] |
| Transalpinecine | Heliotropium transalpinum var. transalpinum | [145] |
| Triangularine | Alkanna orientalis | [40] |
| Alkanna tinctoria | [40] | |
| Triangularicine | Alkanna orientalis | [40] |
| Alkanna tinctoria | [40] | |
| Trichodesmine | Heliotropium arguzioides | [28] |
| Trichodesma africanum | [40,181] | |
| T. incanum | [46] | |
| Turkestanine | Rindera baldschuanica | [152] |
| Rindera oblongifolia | [152] | |
| Solenanthus turkestanicus | [135,150] | |
| Uplandicine | Echium rauwolfii | [90] |
| Echium setosum | [96] | |
| Echium vulgare | [96] | |
| Onosma arenaria | [163] | |
| Onosma stellulatum | [167] | |
| Symphytum × uplandicum | [180] | |
| Uluganine | Ulugbekia tshimganica | [183] |
| Viridantine | Cynoglossum furcatum | [76,187] |
| Onosma erecta | [69] | |
| Viridiflorine | Anchusa milleri | [40] |
| Cynoglossum germanicum | [77] | |
| Cynoglossum officinale | [40,84] | |
| Cynoglossum viridiforum | [97] | |
| Lappula spinocarpus | [40] | |
| Lindelofia olgae | [150] | |
| Lindelofia pterocarpa | [151] | |
| Lindelofia stylosa | [135] | |
| Lindelofia tschimganic | [152] | |
| Paracaryum intermedium | [40] | |
| Paracaryum regulosum | [40] | |
| Symphytum officinale | [179] | |
| Trachelanthus hissricus | [29] | |
| Trichodesma africanum | [40] | |
| 7-Viridiflorylretronecine | Echium tuberculatum | [60] |
| Eritrichium rupestre | [60] | |
| Nonnea lutea | [60] | |
| Nonnea setosa | [60] | |
| Onosma stellulatum | [60] | |
| Vulgarine | Echium vulgare | [11] |
2. Phytochemical Analysis of PAs
Various analytical techniques have been used for extraction, separation, identification and quantification of PAs. Recently, updated reviews were published [188,189] on the analysis of PAs in plants and foods along with different methods of preparation and extraction of PAs from different matrices including plants are their parts, such as seeds, pollen, but also from honey, body fluids, and insects. In addition to column chromatography and HPLC, droplet counter current chromatography (DCCC) has been used in preparative separation of pyrrolizidine alkaloids [190]. Advantages of this method include total sample recovery, good resolution and high reproducibility. A high-speed CCC was applied for preparative separation and purification of PAs from Amsinckia tessellata, Symphytum spp. and Trichodesma incanum [46]. For analytical purposes high-resolution capillary GLC alone or in combination with mass spectrometry is the method of choice for free PA bases [40,59,67,70,90,91,96,163,191,192,193,194,195]. HPLC and HPLC-MS are also helpful but less sensitive than GLC and GLC-MS [60,93,196,197,198,199]. In addition, the resolution is much lower than for GLC but in HPLC also PA-N-Oxides can be directly analysed. The present review describes the most common analytical tools for the analysis of PAs with an emphasis of mass spectrometry (mostly based on GLC-MS analyses).
2.1. Mass Spectrometry
Mass spectrometry is an important and sensitive tool for the identification and structural determination of PAs. The advantages of mass spectrometry are high sensitivity and possibilities of combination with liquid chromatographic methods (e.g., GLC or HPLC) for the analysis of complex mixtures which usually exist in natural sources. Mass spectral data of PAs have been documented in several publications so that a comprehensive database exists. With respect to PAs, the mass spectra provide the molecular mass, type and structure of necine nucleus (saturation at 1, 2-position), sites of hydroxylation and acylation of the hydroxyl groups (as monoester and/or diester). However, MS alone does not provide all necessary structural information (especially, stereochemistry) necessary for an unambiguous assignment of the structure to an unknown compound. There is no difference between the mass spectra of the epimeric pairs e.g., the spectrum of 7-angeloylretronecine is identical to that of 7-angeloylheliotridine, the OH-groups at C-7 are β and α–oriented, respectively. The same situation exists with the stereoisomer pair echimidine and heliosupine. Echinatine, rinderine (heliotridine bases), lycopsamine, intermedine and indicine (retronecine bases) esterified at C-9 with the stereoisomeric acids, (−)-viridifloric, (+)-trachelanthic, (−)-viridifloric, (+)-trachelanthic and (−)-trachelanthic exhibit almost identical mass spectra (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14 and Figure 15).
Mass spectra of PAs provide important informations about the structure of necine substituents. The 1,2-unsaturated necine esterified at C-9 with a free hydroxyl group at C-7 exhibit a base peak at m/z 138, whereas acylation of C-7 (uplandicine, 7-acetyllycopsamine) provide a base peak at m/z 180. PAs with 1,2-unsaturated necines but without a hydroxyl group at C-7 (as in supinidine type) with an esterification at C-9 results in a base peak m/z 122, while the saturated necine of the same type (trachelanthamidine, isoretroncanol, lindelofidine) shows a base peak at m/z 124. 1,2-unsaturated diester PAs esterified with angelic, tiglic or senecioic at C-7 shows a base peak at m/z 220 [40,42,59,67,70,90,91,96,163,193,200,201]. The fragmentation pathway of this type of PAs starts with the cleavage of the weak allylic ester bond at C-9.
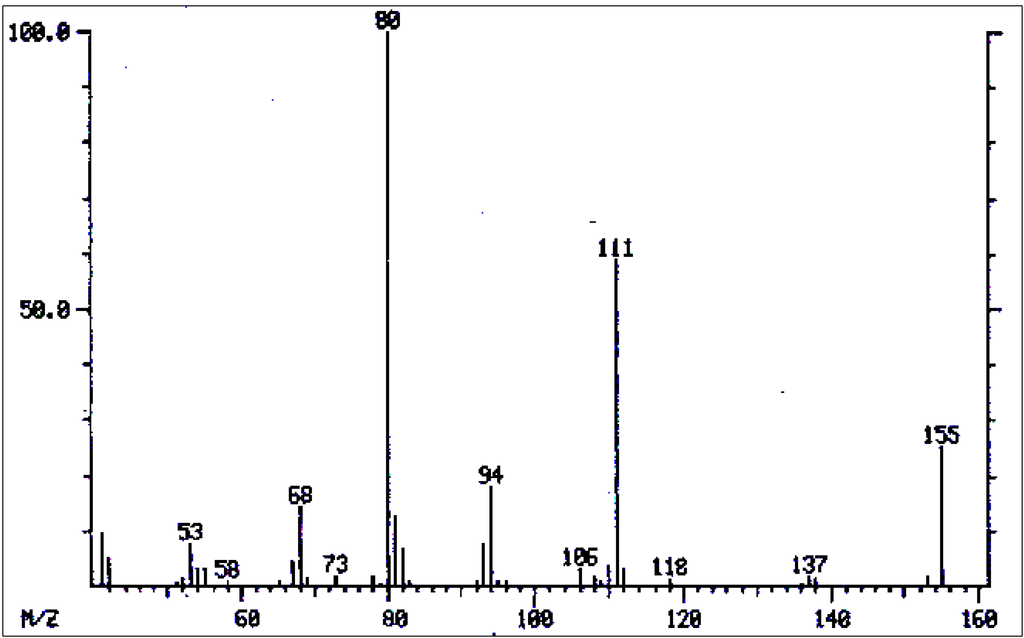
Figure 3.
Mass spectrum of retronecine/heliotridin.
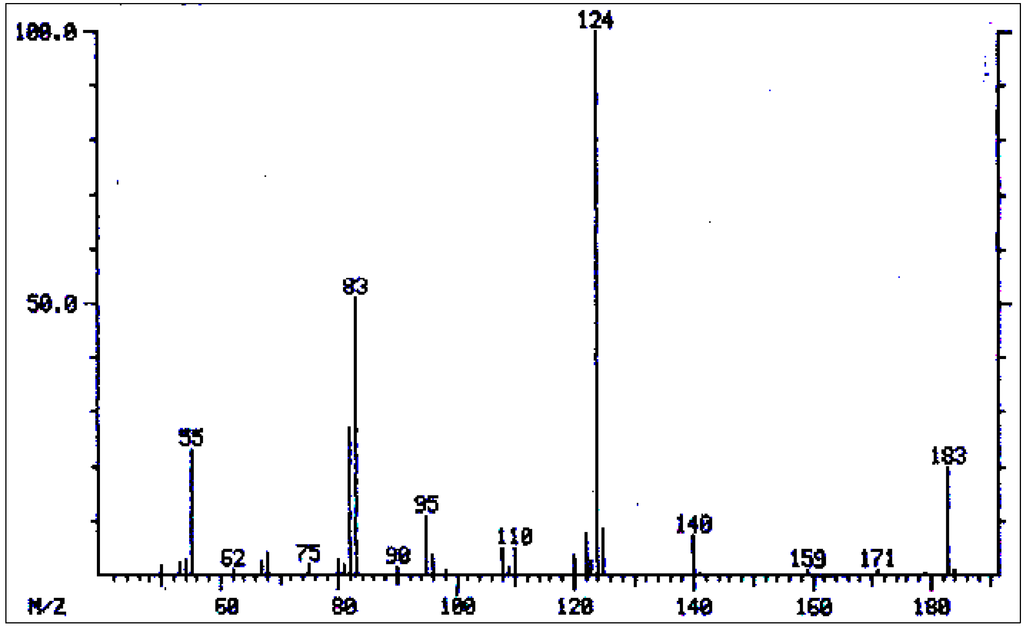
Figure 4.
Mass spectrum of 9-acetyltrachelanthamidine.
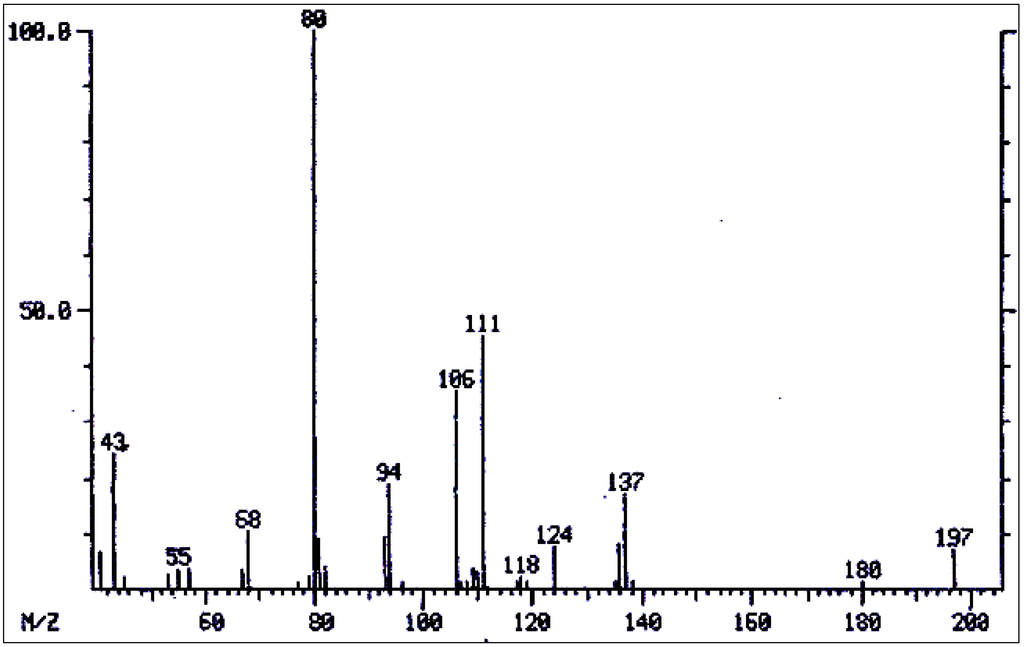
Figure 5.
Mass spectrum of 7-acetylretronecine/7-acetylheliotridine.
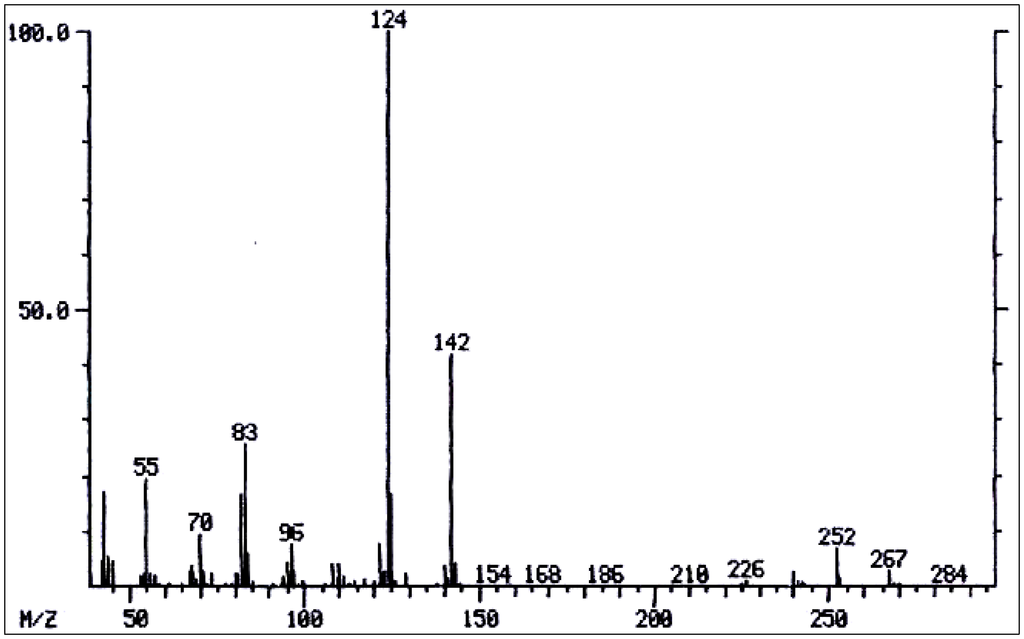
Figure 6.
Mass spectrum of viridiflorine/trachelanthamine.
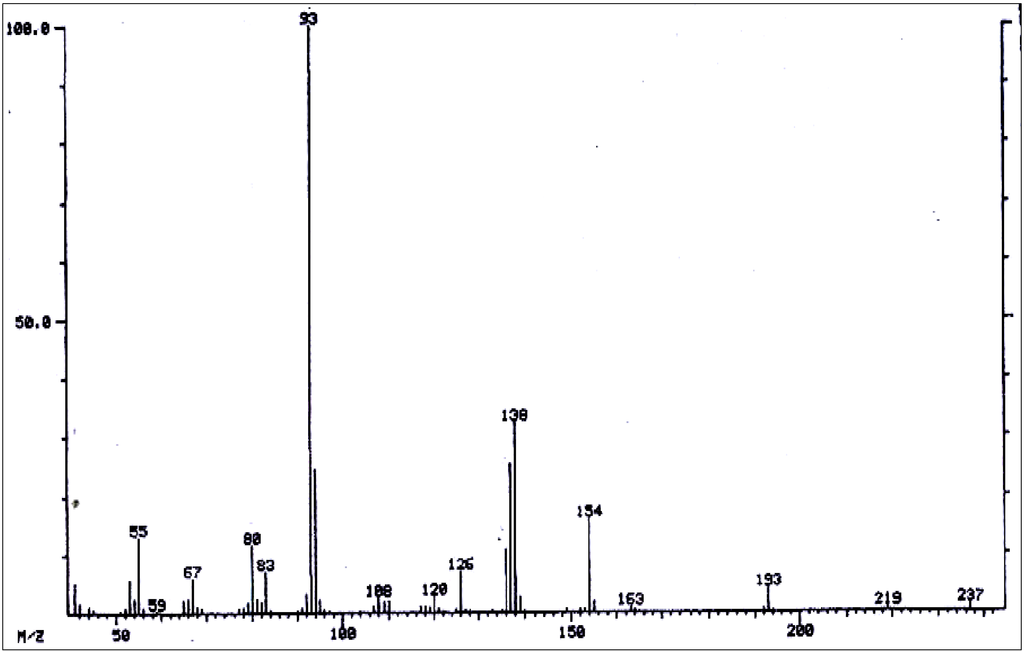
Figure 7.
Mass spectrum of 9-angeloyl or 9-tigloylretronecine 9-angeloyl or 9-tigloylheliotridine.
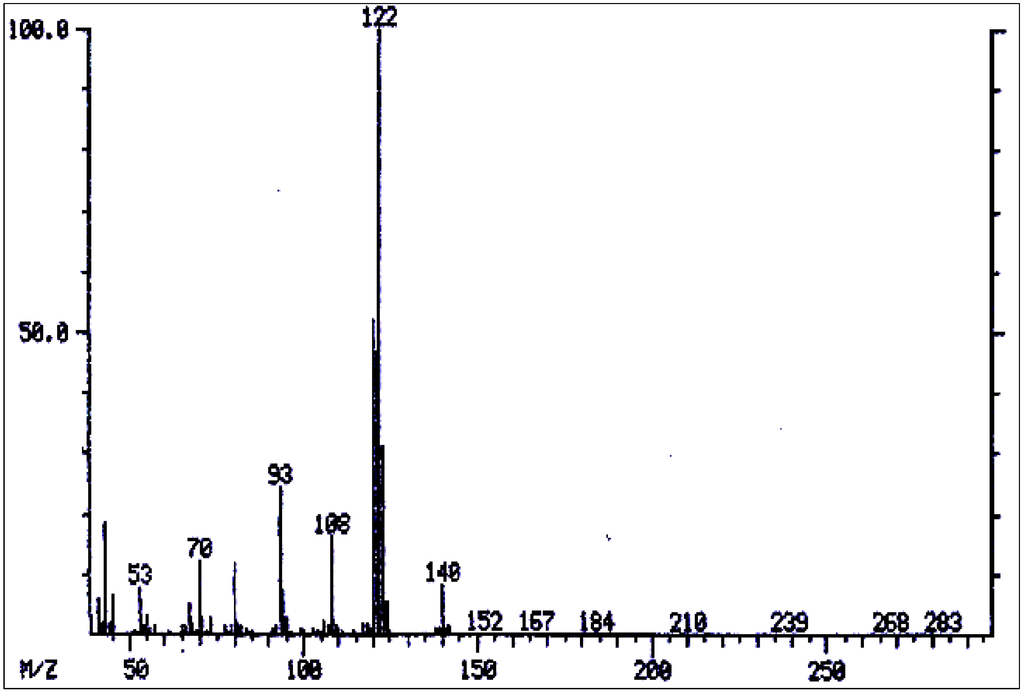
Figure 8.
Mass spectrum of amabiline.
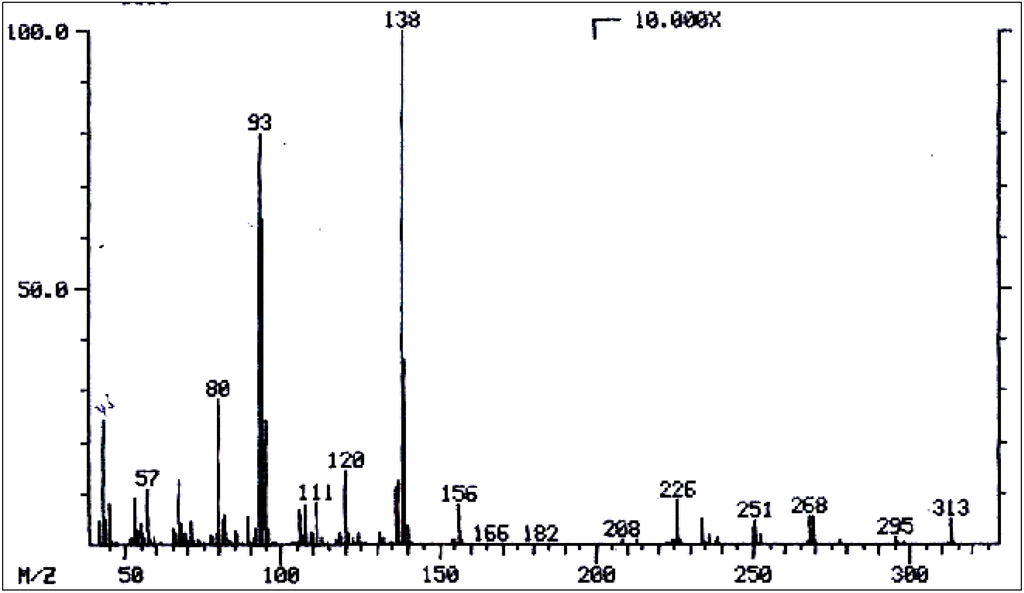
Figure 9.
Mass spectrum of 9-curassavoylheliotridine.
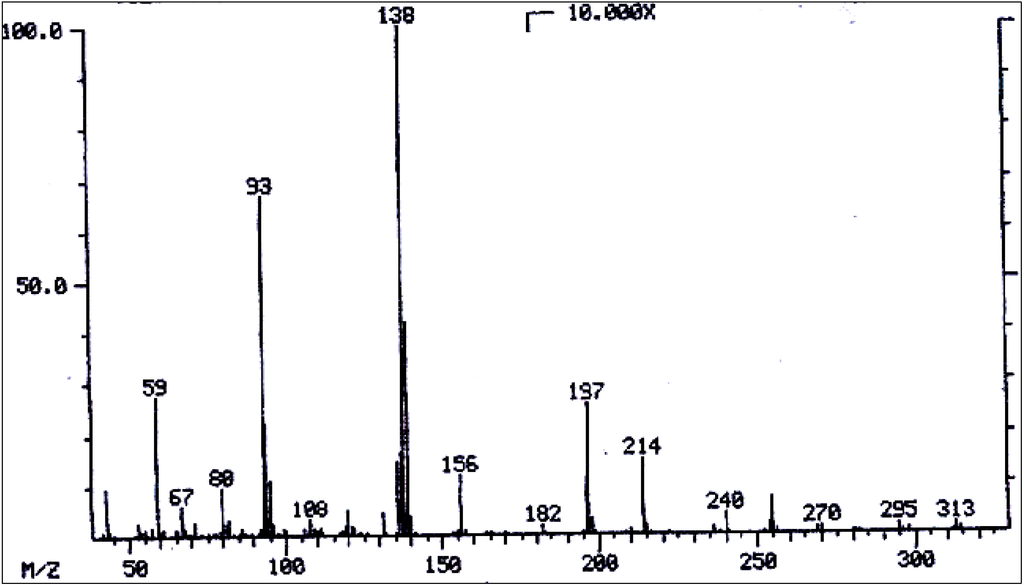
Figure 10.
Mass spectrum of heliotrine.
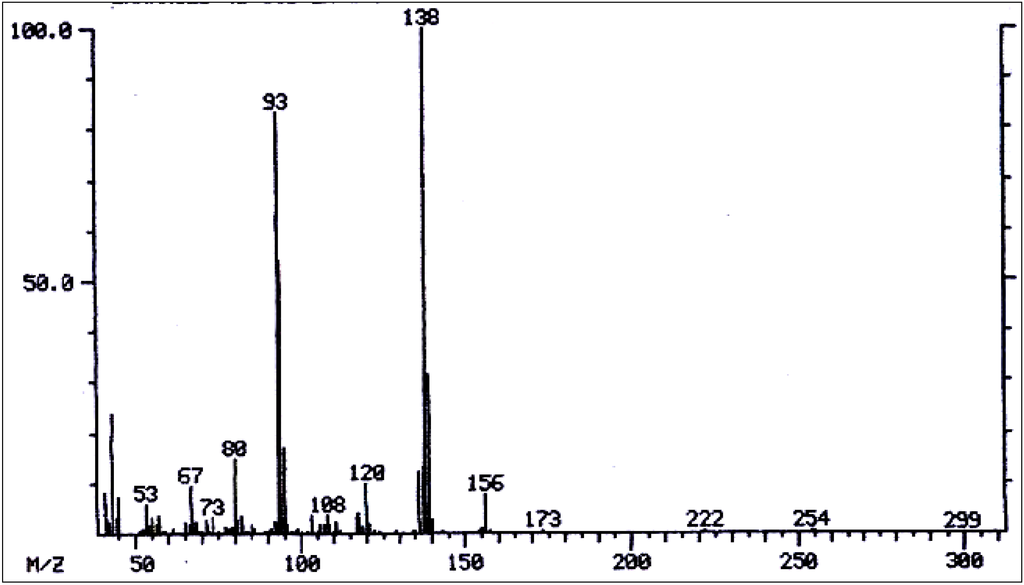
Figure 11.
Mass spectrum of lycopsamine/intermedine/indicine/echinatine/rinderine.
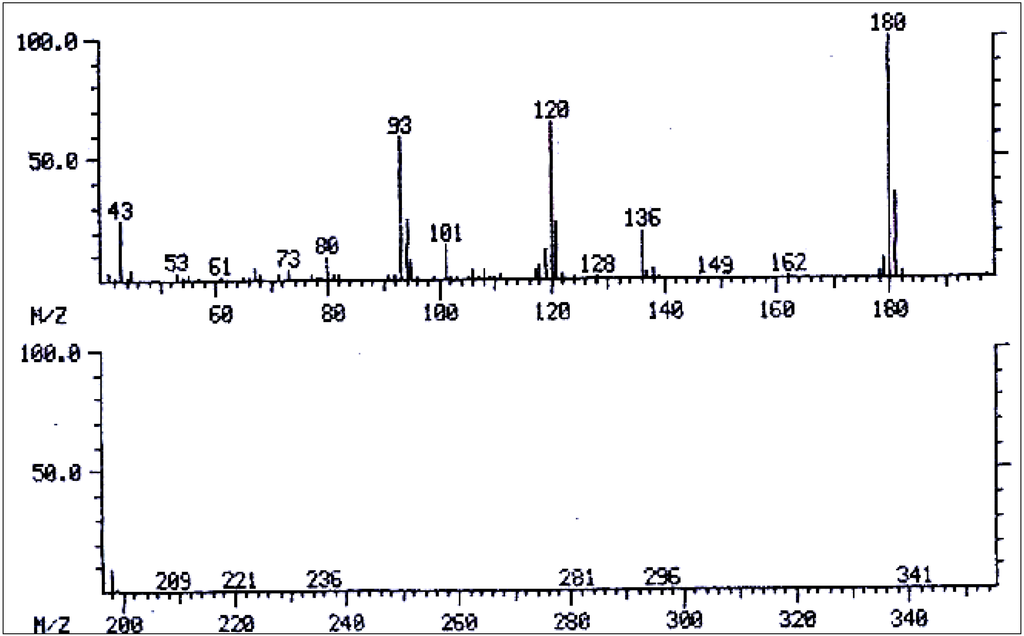
Figure 12.
Mass spectrum of 7-acetyllycopsamine/7-acetyl intermedine/7-acetyl indicine/ 7-acetyl echinatine/7-acetyl rinderine.
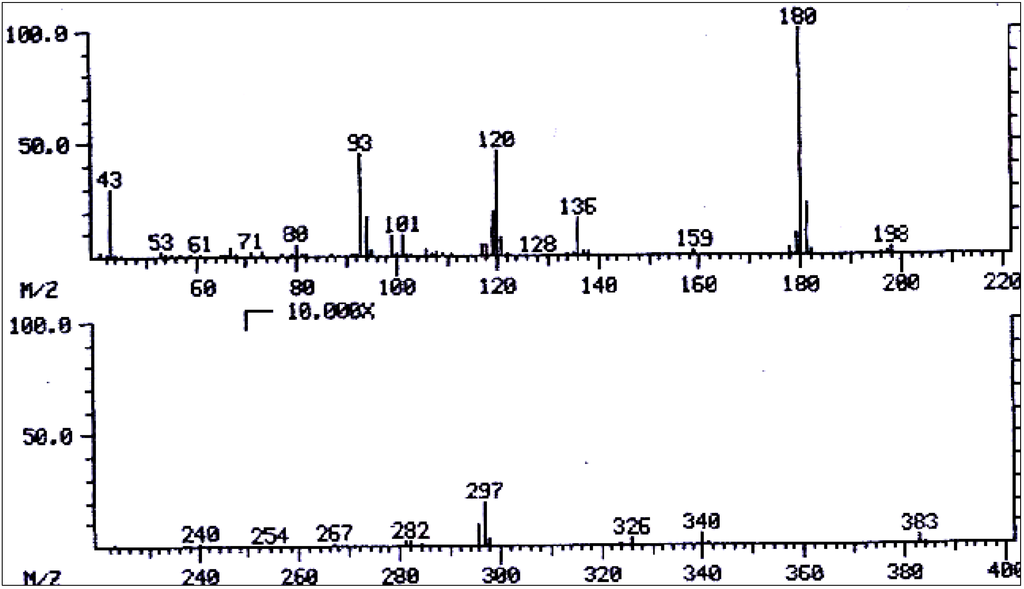
Figure 13.
Mass spectrum of 3',7-diacetyllycopsamine/3',7-diacetyl intermedine/3',7-diacetyl indicine/3',7-diacetyl echinatine/3',7-diacetyl rinderine.
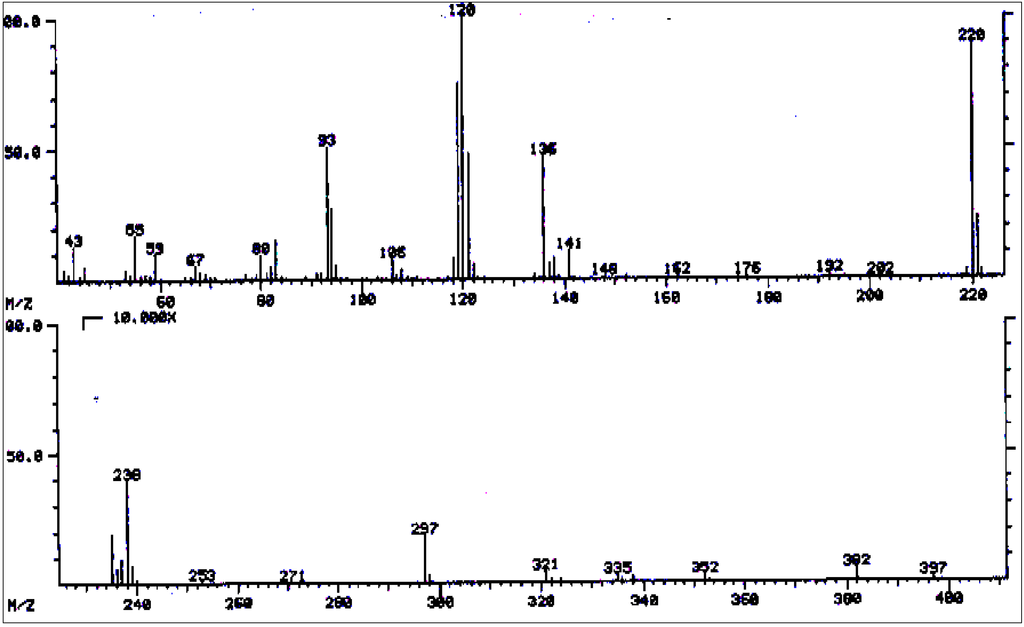
Figure 14.
Mass spectrum of echimidine/heliosupine.
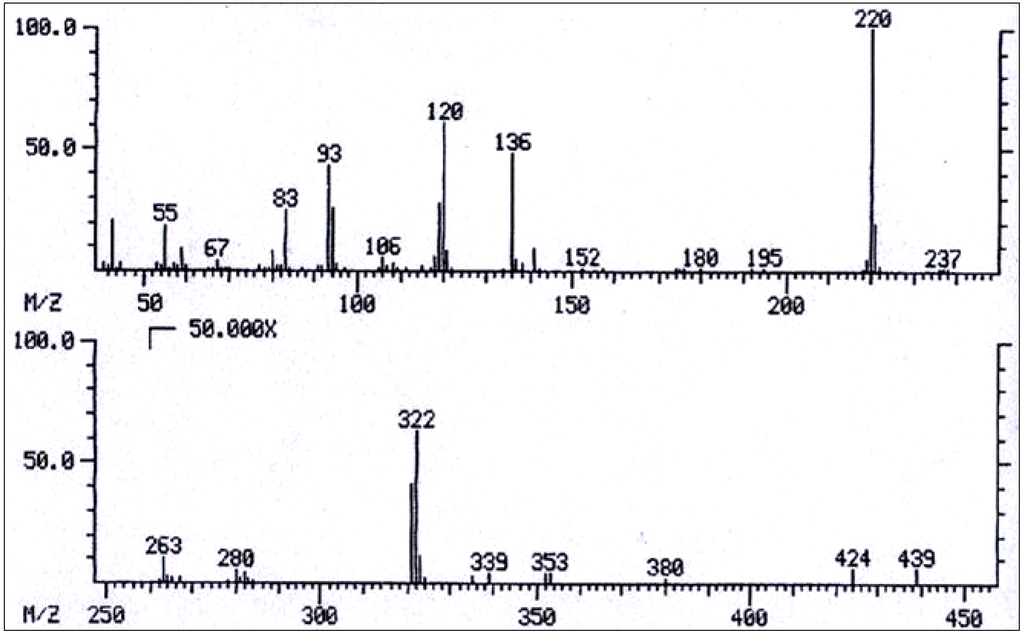
Figure 15.
Mass spectrum of 3'-acetylechimidine/3'-acetylheliosupine.
In our review we have tabulated the MS data useful for the identification and structural studies of PAs in Boraginaceae; Table 3 summarizes the mass spectral data (GLC-MS, LC-MS, direct inlet) of corresponding PAs.

Table 3.
Mass data of PAs (mostly derived from gas-liquid chromatography-mass spectrometry (GLC-MS) analyses) for all compounds identified in the Boraginaceae. Compounds are numbered as in Figure 16.
| No. | Alkaloid | RI | [M]+ | Characteristic ions m/z (relative abundance) | References |
|---|---|---|---|---|---|
| 1 | 3'-Acetylcanescine | - | 441 | 441(0.7), 426(2.6), 355(2.4), 255(6.3), 238(62.4), 220(18.3), 180(39), 136(47), 120(100), 93(74), 80(20). | [154] |
| 2 | 3'- Acetylcanescenine | - | 441 | 441(1.2), 426(2.4), 398(1.6), 355(1.2), 255(5.7), 238(58.2), 220(20.3), 180(44.3), 136(43.4), 120(100), 93(80.3), 80(22.1). | [154] |
| 3 | 7-Acetyl-9-curassavoylheliotridine | 2275 | 355 | 295(0.1), 268(0.2), 198(8), 181(36), 180(100), 136(22), 121(26), 120(84), 119(12), 95(15), 93(35), 80(17), 67(10), 57(12), 45(9), 43(48). | [40] |
| 4 | 7-Acetyl-9-(2,3-dihydroxybutryl) retronecine | 2092 | 299 | 239(3), 181(20), 180(100), 136(20), 120(59), 101(20), 94(32), 93(73), 80(12), 67(5), 55(5), 43(15). | [163] |
| 5 | 7-Acetyl-9-(2,3-dimethylbutryl) retronecine | 1947** | 295 | 235(8), 180(100), 136(20), 120(36), 101(30), 94(30), 93(65), 80(8), 43(42). | [163] |
| 6 | 3'-Acetylechinatine | 2220 | 341 | 326(0.1), 298(1), 255(2), 254(2), 181(2), 156(4), 139(21), 138(100), 137(10), 136(10), 120(6), 99(6), 94(20), 93(71), 80(8), 67(5), 43(22). | [84] |
| 7 | 7-Acetylechinatine | 2235 | 341 | 341(0.1), 281(2), 198(6), 181(39), 180(100), 136(18), 121(35), 120(70), 119(28), 101(9), 94(18), 93(55), 80(7), 43(33). | [67] |
| 8 | 3'-Acetylechiumine | 2245* | 423 | 423(2), 380(3), 338(3), 337(6), 336(3), 323(2), 280(3), 263(3), 256(2), 238(5), 237(6), 221(34), 220(100), 219(15), 159(5), 141(19), 138(12), 136(54), 121(20), 120(76), 119(34), 106(12), 94(42), 93(56), 83(27), 80(15), 59(3), 55(34), 53(17). | [42] |
| 9 | 3'-Acetylechimidine | 2640 | 439 | 439(0.1), 424(0.1), 322(1), 238(2), 221(25), 220(100), 219(3), 138(5), 137(6), 136(49), 121(8), 120(61), 119(28), 106(6(, 94(24), 93(46), 83(14), 59(11), 55(13). | [91] |
| 10 | 5'-Acetyleuropine | - | 371 | 156(18), 138(100), 94(36), 93(75), 59(74). | [138] |
| 11 | 7-Acetyleuropine | - | 371 | 371(2.7), 356(1), 311(3), 282(5), 180(100), 120(72), 93(65), 80(17), 59(95). | [110] |
| 12 | 3'-Acetylfurcatine | 3188* | 425 | 223(28), 222(100), 143(10), 136(36), 121(15), 120(82), 94(27), 93(61), 85(21), 80(7), 57(63). | [42] |
| 13 | 3'-Acetylheliosupine | 2640 | 439 | 424 (0.1), 321(1), 221 (28), 220(100), 141 (10), 138(4), 137(5), 136 (45), 121(14), 120 (89), 119(82), 106(7), 94 (21), 93(40), 83(11), 80(5), 59(8), 55(11), 43(22). | [67] |
| 14 | 7-Acetyl-9-(2-hydroxy-3-methylbutryl) retronecine | 2024** | 297 | 237(5), 180(100), 136(25), 120(52), 119(20), 101(25), 94(45), 93(95), 80(15), 43(35). | [163] |
| 15 | 3'-Acetylindicine | 2195 | 341 | 341(2), 255(2), 181(5), 138(100), 93(48), 43(10). | [158] |
| 16 | 3'-Acetylintermedine | 2255 | 341 | 341(5), 298 (4), 255 (16), 139(20),138(100), 137 (12),136 (12), 94 (30), 93 (71), 80 (10), 43 (21). | [40] |
| 17 | 7-Acetylintermedine | 2220 | 341 | 296(4), 281(3), 181(40), 180(100), 136(25), 121(30), 120(78), 119(15), 101(19), 95(10), 94(30), 93(73), 80(11), 43(40). | [40] |
| 18 | 5'-Acetyllasiocarpine | - | 453 | 363, 335, 321, 263, 220(100), 141, 136, 121, 120, 119, 106, 94, 93, 83, 80. | [186] |
| 19 | 3'-Acetyllithosenine | - | 457 | 457(0.78), 442(2), 339(1), 255(2), 238(100), 237(5), 222(10), 220(25), 138(30), 137(8), 136(49), 121(36), 120(75), 119(36), 95(9), 94(34), 93(57), 80(12). | [156] |
| 20 | 3'-Acetyllycopsamine | 2255 | 341 | 341(5), 298(4), 255(16), 139(20), 138(100), 137(12), 136(12), 94 (30), 93(71), 80 (10), 43(21). | [40] |
| 21 | 7-Acetyllycopsamine | 2230 | 341 | 341(1), 296(4), 281(3), 181(32), 180(100), 136(20), 121(35), 120(70), 119(11), 101 (19), 95(10), 94 (28), 93(61), 80(10), 43(36). | [91] |
| 22 | 7-Acetyl-9-(2-methylbutyryl) retronecine | 1914** | 281 | 221(12), 195(5), 180(100), 136(25), 120(42), 119(15), 101(35), 94(45), 93(80), 80(13), 67(100), 57(18), 53(5), 43(23). | [163] |
| 23 | 3'-Acetylmyscorpine | 3290* | 423 | 221(30), 220(100), 136(47), 120(67), 119(35), 94(39), 93(51), 83(32), 80(19), 55(39). | [42] |
| 24 | 7-Acetylretronecine | 1532** | 197 | 180(3), 137(12), 111(40), 106(30), 94(20), 80(100), 68(3), 43(30). | [163] |
| 25 | 3'-Acetylrinderine | 2222 | 341 | 326(0.1), 298(1), 255(1), 254(0.5), 181(5), 156(5), 139(30), 138(100), 137(17), 136(17), 120(10), 99(10), 95(9), 94(35), 93(95), 80(13), 67(9), 43(36). | [70] |
| 26 | 7-Acetyl-9-(sarracinoyl) retronecine | 2125 | 295 | 235(1), 197(32), 196(10), 181(8), 180(55), 179(15), 136(34), 121(12), 120(42), 119 (20), 101(19), 94(40), 93100), 80(15), 67(6), 53(9), 43(20). | [40] |
| 27 | 7-Acetylscorpioidine | - | 423 | 423(0.8), 181(23), 180(92), 179(17), 136(23), 121(10), 120(57), 119(45), 95(6), 94(35), 93(57), 83(100), 80(15), 55(39), 43(30). | [159] |
| 28 | 3'-Acetylsupinine | 2080** | 325 | 284(.02), 299(10), 136(10), 122(100), 120(48), 108(13), 101(13), 93(50), 80(13), 70(9), 53(6), 43(15). | [163] |
| 29 | 3'-Acetyltessellatine | 3064* | 341 | 341(41), 324(10), 323(10), 299(2), 280(8), 255(2), 254(4), 248(2), 238(2), 237(5), 236(17), 198(8), 181(4), 180(2), 156(7), 154(12), 138(22), 137(28), 136(27), 124(23), 120(63), 111(66), 108(30), 106(61), 99(17), 94(35), 93(14), 80(100), 55(10), 53(10). | [42] |
| 30 | 9-Acetytessellatine | 2962* | 341 | 341(7), 299(6), 282(11), 281(15), 248(3), 238(15), 237(16), 236(14), 198(18), 181(12), 180(10), 179(9), 153(13), 138(49), 136(45), 121(26), 120(100), 119(32), 108(11), 106(12), 94(52), 93(89), 80(28), 53(11). | [42] |
| 31 | 3'-Acetyltrachelanthamine | - | 327 | 327(1), 284(2), 240(6), 184(6), 142(27), 125(38), 124(100), 84(14), 83(59), 82(54). | [128] |
| 32 | 9-Acetyltrachelanthamidine | 1395 | 183 | 140(8), 125(8), 124(100), 110(5), 95(10), 83(50), 82 (27), 55(23). | [40] |
| 33 | 3'-Acetylviridiflorine | 2767* | 327 | 327(3), 284(6), 268(2), 267(2), 241(5), 240(9), 225(4), 184(4), 159(2), 142(21), 140(4), 125(17), 124(100), 83(14), 82(12), 55(19). | [42] |
| 34 | 9-(3'-Acetylviridifloryl) retronecine | - | 341 | 341(4.7), 255(3), 182(5), 157(4), 138(100), 120(10), 93(92), 85(4), 83(20), 80(16), 43(38). | [119] |
| 35 | 9-(3'-Acetyl)viridifloryl turneforcidine (or isomer) | 3050* | 343 | 343(5), 325(4), 300(12), 299(5), 257(14), 256(50), 212(13), 200(15), 197(14), 159(10), 158(36), 141(19), 140(30), 138(16), 122(21), 120(33), 117(10), 106(12), 96(44), 95(90), 83(11), 82(100), 69(17), 55(32). | [42] |
| 36 | 7- Acetylvulgarine | - | 439 | 440(M++ 1), 422(40), 380(15), 358(5), 340(65), 296(42), 282(85), 180(100). | [11] |
| 37 | Amabiline | 1985, 2652* | 283 | 383(1), 140(8), 123 (30), 122(100), 121(46), 120(51), 108(17), 93(25), 80(13), 70 (17), 53(7), 45(6), 43(19). | [67] |
| 38 | Anadoline | - | 381 | 381(6), 204(6), 167(6), 149(23), 139(22), 138(93), 137(45), 136(33), 120(17), 119(17), 118(13), 117(18), 111(12), 109(15), 100915), 97(17), 95(27), 94(55), 93(88), 85(25), 83(100), 82(17), 81(27), 80(27), 73(14), 71(37), 70(17), 67(27), 57(73), 56(17), 55(83), 54(13), 53(20). | [202] |
| 39 | 7α-Angeloyl-1-chloromethy-1,2-dihydropyrrolizidine | 1815 | 255 | 220(40), 172(15), 155(45), 136(23), 130(24), 129(32), 128(63), 121(11), 120(94), 119 (24), 106(30), 94 (100), 93(20), 83(20), 80(17), 67(8), 55(35). | [70] |
| 40 | 7-Angeloyl-9-(2,3-dihydroxybutyryl)heliotridine | 2333 | 339 | 339(1), 324(1), 294(1), 239(6), 222(25), 221(25), 220(65), 219(8), 138 (20), 137(10), 136(81), 121(24), 120(100), 119(85), 106(15), 94(50), 93(85), 83(24), 80(18), 75(2), 57(10), 55(25), 45(10). | [67] |
| 41 | 7-Angeloyl-9-(2,3-dihydroxybutyryl)retronecine | 2315 | 339 | 339(1), 239(5), 238(5), 237(5), 221(25), 220(99), 219(15), 141(20), 138(10), 137(11), 136 (100), 121(15), 120(83), 119(34), 106(10), 94(55), 93(95), 83(41), 80(20), 75(2), 57(10), 55(40), 45(10). | [96] |
| 42 | 7-Angeloyl-9-(2,3-dihydroxypropanoyl)retronecine | 2300 | 325 | 294(0.5), 237(5), 255(9), 221(10), 220(67), 219(16), 141(22), 138(5), 137(11), 136(100), 121(11), 120(57), 119(40), 106(10), 94(62), 93(95), 83(37), 80(17), 55(32). | [40] |
| 43 | 7-Angeloyl-1-formyl-6,7-dihydro-5H-pyrrolizidine | 1920 | 233 | 215(3), 150(100), 134(92), 133(35), 122(4), 106(15), 105(38), 104(15), 83(10), 79(16), 55(20). 46 | [67] |
| 44 | 7-Angeloyl-9-(hydroxypropenoyl)retronecine | 2053 | 307 | 221(13), 220(95), 207(24), 181(8), 141(30), 137(10), 136(100), 120(53), 119 (24), 106(10), 94(63), 93(77), 83(43), 80(21), 67(9), 55(41), 43 (26). | [40] |
| 45 | 7-Angeloylechinatine | 2467 | 381 | 336(2), 281(1), 238(11), 221(42), 220(86), 141(11), 138(11), 137(9), 136(55), 121(78), 120(100), 119(60), 117(4), 106(9), 99(3), 94 (30), 93(52), 83(17), 80(10), 55(17), 45(8), 43(21). | [67] |
| 46 | 7-Angeloylheliotridine | 1820 | 237 | 219(1), 154(2), 137(42), 136(20), 124(25), 111(35), 106(86), 94(25), 83(10), 80(100), 68(10), 55(20). | [70] |
| 47 | 7-Angeloylheliotine | - | 395 | 395(5), 295(7), 220(100), 136(57), 120(80), 119(70), 93(43), 83(34), 59(55), 43(18). | [127] |
| 48 | 7-Angeloyllycopsamine | 2460* | 381 | 381(0.1), 336(0.9), 281(0.5), 238(0.5), 220(100), 136(65), 121(40), 120(90), 94(40), 93(85), 83(50), 80(15), 55(48). | [90] |
| 49 | 7-Angeloyl-9-(2-methylbutyryl)heliotridine | 2180 | 321 | 321(0.5), 221(43), 220 (70), 195(5), 141(26), 138(4), 137(9), 136(85), 121(13), 120(100), 119(65), 106(13), 94(59), 93(71), 83 (23), 80(15), 67(4), 57(27), 55(30). | [67] |
| 50 | 7-Angeloyl-9-(2-methylbutyryl)retronecine | 2155 | 321 | 221(35), 220(100), 195(5), 141 (25), 138(3), 137(9), 136(90), 121(6), 120(53), 119(20), 106(8), 94(50), 93(70), 83(35), 80(15), 57(20), 55(35). | [96] |
| 51 | 7-Angeloylretronecine | 1787 | 237 | 237(2), 219 (3), 204(0.5), 191(1), 154(2), 138(5), 137(23), 136(18), 124(23), 111(38), 106(40), 94(20), 93(6), 83(11), 80(100), 55(22). | [96] |
| 52 | 9-Angeloylretronecine | 1797 | 237 | 237(1), 219(0.5), 193(3), 154(16), 138(32), 137(25), 136(10), 126(7), 120(2), 108(2), 94(25), 93(100), 83(8), 80(10), 55(13). | [96] |
| 53 | 7-Angeloylrinderine | 2465 | 381 | 381(0.1), 336(2), 281(1), 238(10), 221(34), 220(80), 141(11), 138(10), 137(9), 136(53), 121(70), 120(100), 119(59), 117(4), 106(10), 94(28), 93(50), 83(15), 80(10), 55(17), 45(8), 43(25). | [67] |
| 54 | 9-Angeloyltrachelamthamidine | 1700 | 223 | 140(1), 125 (30), 124(100), 123(20), 122(15), 110(4), 95(13), 83(45), 82(16), 70(6), 55(27). | [40] |
| 55 | 9-Angeloyl-7-viridiflorylretronecine | - | 381 | 220(8.3), 138(7.3), 137(9.8), 136(9.9), 120(28.6), 117(50), 106(29), 94(34), 93(23.1), 91(34.6), 79(40.8), 67(39.6), 64(81.8), 60(31), 58(57), 57(85), 55(100). | [60] |
| 56 | Asperumine | - | 397 | 380, 336, 220, 138, 120. | [203] |
| 57 | 9-(Butyryl-2-ene) supinidine | 1674** | 207 | 180(5), 159(7), 157(7), 122(100), 121(6), 120(23), 108(6), 93(15), 80(17), 71(25), 57(7), 53(11), 45(11), 43(14). | [163] |
| 58 | Canescine | - | 399 | 399(0.4), 384(4), 355(1), 338(0.3), 256(10), 238(66), 220(21), 180(11.6), 136(42), 120(100), 93(65), 80(20). | [154] |
| 59 | Canescenine | - | 399 | 399(0.1), 384(1.6), 355(0.4), 338(0.3), 256(9.6), 238(67.5), 220(20.6), 180(14.7), 136(45.1), 120(100), 93(61.6), 80(20.2). | [154] |
| 60 | Coromandaline | - | 285 | 285(2), 267(7), 241(3), 240(9), 142(65), 125(23), 124(100), 83(28), 82(18). | [140] |
| 61 | Coromandalinine | - | 283 | 283(0.8), 239(0.4), 238(0.3), 140(7), 123(27), 122(100), 121(35), 120(40), 108(13), 94(8), 93(20.5), | [140] |
| 62 | Cryptanthine | 353 | 354[M++1](1), 272(2), 254(3), 238(2), 138(2), 120(100), 118(2). | [65] | |
| 63 | Curassanecine | - | 157 | 126(14), 98(10), 83(100) 82(23). | [117] |
| 64 | Curassavine | - | 299 | 299(0.7), 281(1.4), 255(2.6), 254(5), 142(80), 125(27), 124(100). | [140] |
| 65 | Curassavinine | - | 297 | 297(0.3), 253(0.1), 252(0.1), 241(0.1), 239(0.1), 140(8), 123(31), 122(100), 121(44), 120(41), 108(13), 94(6.4), 93(20). | [140] |
| 66 | 9-Curassavoylheliotridine | 2190 | 313 | 295(2), 269(0.5), 268(0.5), 251(0.5), 226(0.9), 156(8), 139(35), 138(100), 120(15), 111(9), 106(8), 95(25), 94(64), 93(80), 80(28), 67(13), 57(11), 45(8), 43(25). | [40] |
| 67 | Cynaustine | - | 283 | C15H25NO4, 122(100). | [68] |
| 68 | Cynaustraline (or stereoisomer) | 2682* | 285 | 285(2), 267(4), 252(5), 242(1), 241(1), 240(3), 226(1), 142(34), 125(15), 124(100), 83(24), 82(14), 55(25). | [42] |
| 69 | Cynoglossamine | - | 445 | 147(100), 138(27), 119(17), 93(23). | [138] |
| 70 | 5-Deoxylasiocarpine | - | 395 | 396[M + H] + (2), 295(5.5), 363(1), 220(80), 120(83), 93(68), 83(32). | [123] |
| 71/72 | 3',7-Diacetylintermedine/3',7-Diacetyllycopsamine | 2340 | 383 | 340(5), 297(10), 296(10), 181(22), 180(100), 136(18), 121(8), 120(47), 119(20), 101(10), 99(10), 94(18), 93(47), 80(5), 43(30). | [40] |
| 73 | 3',9-Diacetyltessellatine | 3090* | 383 | 383(2), 342, 341(37)(7), 324(21), 323(10), 297(8), 296(6), 280(10), 238(2), 237(6), 236(18), 198(10), 181(7), 180(4), 179(8), 154(14), 153(22), 138(20), 136(49), 121(18), 120(100), 119(55), 108(12), 106(19), 99(13), 94(37), 93(82), 80(25), 53(11). | [42] |
| 74 | 5,6-Dihydro-7,9-dimethoxy-7H-pyrrolizine | 1415** | 181 | 150(90), 134(5), 120(100), 119(35), 106(12), 91(3), 79(5). | [163] |
| 75 | Dihydroechinatine | - | 301 | MS2, 284, 258, 240, 140, 122, 96. | [167] |
| 76 | thero-2'',3''-Dihydroxyechiumine | - | 415 | 415(0.5), 371(2), 254(56), 210(26), 166(5), 138(30), 136(37), 120(100), 93(70). | [63] |
| 77 | Dihydroxytriangularine | 2525 | 369 | 338(1), 324 (2), 269(30), 252(30), 237(40), 221(25), 220(100), 219(10), 141(20), 138(8), 137(10), 136(80), 121(20), 120(80), 119(30), 106 (8), 95 (9), 94 (45), 93(81), 83(39), 80(10), 55(28). | [40] |
| 78 | Dihydroxytriangularicine | 2525 | 369 | 338(0.1), 324 (0.1), 269(4), 252(4), 237(5), 221(24), 220(100), 219(3), 141(28), 138(6), 137(8), 136(67), 121(24), 120(75), 119(35), 106(8), 95(8), 94(47), 93(90), 83(41), 80(11), 55(24). | [40] |
| 79 | Echihumiline | 2578 | 397 | 397(0.1), 382 (0.2), 352 (0.1), 338(0.1), 321 (0.1), 297 (2), 238 (2), 221 (20), 220 (100), 219 (4), 138 (6), 137 (6), 136 (40), 121 (15), 120 (39), 119 (16), 106 (4), 94 (20), 93 (30), 83 (36), 80 (6), 59 (5), 55 (6). | [91] |
| 80 | Echimidine | 2560 | 397 | 397(0.1), 382 (0.1), 352 (0.1), 297(2), 221(21), 220(100), 219(5), 138(5), 137(6), 136(48), 121(26), 120(75), 119(30), 106(5), 94(30), 93 (61), 83(39), 80(10), 59(10), 55(25), 43(18). | [96] |
| 81 | Echimidine isomer (tigloyl) | 2580 | 397 | 397(0.1), 382 (0.1), 297(2), 238(3), 221(21), 220(100), 219(3), 138(5), 137(6), 136(48), 121(28), 120(62), 119(23), 106(5), 94(28), 93 (46), 83(30), 80(10), 59(10), 55(20), 43(18). | [96] |
| 82 | Echinatine | 2175 | 299 | 284(0.1), 254(1), 156(10), 139(30), 138 (100), 137(7), 136(4), 95(9), 94(25), 93 (46), 80(7), 67(5), 53(3), 43(12). | [70] |
| 83 | Echiumine | 3178* | 381 | 381(0.4), 338(1), 337(1), 336(1), 281(2), 255(1), 238(9), 237(2), 221(35), 220(100), 141(16), 138(8), 136(47), 121(32), 120(69), 119(20), 106(7), 94(39), 93(50), 83(28), 80(17), 59(1), 55(37), 53(9). | [42] |
| 84 | Echiuplatine | 381 | 383[M++1](3), 382(68), 365(1), 364(1), 322(2), 320(24), 300(5), 282(7), 280(1), 238(4), 220(51), 138(2), 120(100), 118(2). | [65] | |
| 85 | Echiupinine | - | 381 | 381(0.3), 337(1), 336(1), 282(0.2), 281(2), 212(38), 220(100), 136(43), 121(33), 120(55), 119(19), 118(18), 117(14), 103(16), 94(24), 93(47), | [95] |
| 86 | Echivulgarine | - | 479 | 480(M++ 1), 462(35), 418(10), 398(10), 380(90), 336(40), 322(100), 220(70). | [11] |
| 87 | Ehretinine | - | 275 | 275, 140(100), 123, 97. | [100] |
| 88 | 2'',3''-Epoxyechiumine | - | 397 | 254(9), 237(33), 236(100), 164(17), 157(4), 138(4), 136(19), 121(24), 120(25), 94(27), 93(44), 80(12), 71(4), 43(30). | [63] |
| 89 | Erythro-2'',3''-chloro-2''-hydroxyechiumine | - | 435 | 435(0.5), 274(37), 273(32), 272(92), 254(10), 236(13), 208(9), 138(10), 136(25), 121(26), 120(100), 94(32), 93(45), 80919), 71(19). | [63] |
| 90 | 1α-2α-Epoxy-1β-hydroxymethyl-8α-pyrrolizidine | - | 155 | 126(4), 124(13), 96(10), 80(4), 71(50, 70(100), 68(10), 67(5), 56(6), 55(65). | [106] |
| 91 | Europine | 2217 | 329 | 314(1), 256(5), 240(25), 239(20), 156(10), 139(20), 138(100), 120(5), 94(20), 93(50), 80(10), 67(4), 59(20), 43(5). | [52] |
| 92 | Floridanine | - | 441 | 426, 397, 382, 168, 152, 151(100), 150, 149, 123, 122, 110, 96, 94. | [62,204] |
| 93 | Floridimine | - | 301 | 301(1), 239(8), 226(7), 142(36), 124(100), 95(12), 94(10), 83(55), 55(23). | [128] |
| 94 | Floridine | - | 343 | 343(1), 328(2), 284(1), 240(2), 239(3), 225(10), 142(8), 124(100), 83(27). | [128] |
| 95 | Floridinine | - | 301 | 301(1), 242(5), 239(7), 226(5), 167(1), 142(46), 125(19), 124(100), 122(8), 110(6), 96(8), 95(7), 83(36), 82(24), 70(12), 59(12). | [128] |
| 96 | Furcatine | 3138* | 383 | 383(0.2), 339(1), 281(1), 240(6), 223(35), 222(100), 143(18), 136(33), 121(27), 120(75), 119(21), 94(44), 93(65), 85(13), 80(19), 74(8), 73(5), 60(1), 57(46). | [42] |
| 97 | Heleurine | 1970 | 297 | 239(1), 198(3), 181(6), 140(15), 131(6), 123(30), 122(100), 120(60), 119(70), 108(16), 93(17), 80(10), 70(11), 59(26). | [109] |
| 98 | Helibracteatine | - | 255 | 255(0.5), 238(6), 237(1), 224(9), 156(43.4), 155(45.5), 138(16.3), 122(2.3), 111(99.7), 99(31), 98(100), 82(35). | [112] |
| 99 | Helibractinecine | - | 173 | 173(22), 156(4), 155(11), 142(7), 129(10), 124(1), 112(5), 99(83), 98(83), 95(6), 83(7), 82(100). | [111] |
| 100 | Helibracteatinecine | - | 173 | 173(20), 156(4.5), 155(14), 142(3), 129(12), 124(1), 112(5), 99(85), 98(100), 82(83.5). | [112] |
| 101 | Helibracteatinine | - | 255 | 255(2), 238(3), 237(3.5), 224(9), 198(7), 181(60), 156(16), 155(93), 154(10), 124(12), 122(21), 100(23), 98(20), 83(23), 82(100). | [112] |
| 102 | Helifoline | - | 255 | 237(3), 156(5), 138(11), 137(3), 112(27), 111(100), 99(9), 98(42), 94(41), 83(9), 82(20), 80(24). | [136] |
| 103 | Helifolinecine | - | 173 | 155(9.5), 129(67), 124(3), 116(6), 112(8), 98(100), 82(59), 80(10). | [136] |
| 104 | Helindicine | - | 281 | 281(35), 207(100), 191(15), 149(37), 135(33), 109(34), 97(73), 95(55), 83(57), 81(46). | [130] |
| 105 | Heliocoromandaline | - | 285 | 285(1), 267(4), 252(4), 240(6), 142(41), 125(17), 124(100), 84(63), 83(20), 82(15). | [117] |
| 106 | Heliocurassavine | - | 299 | 299(1), 284(1), 281(1), 255(3), 254(10), 252(8), 243(7), 226(8), 142(48), 124(100), 83(56), 82(40). | [117] |
| 107 | Heliocurassavicine | - | 285 | 285(1), 267(3), 252(3), 240(3), 142(49), 125(22), 124(100), 84(63), 83(58), 82(41). | [117] |
| 108 | Heliocurassavinine | - | 285 | 285(1), 267(4), 252(4), 240(3), 142(55), 125(24), 124(100), 84(22), 83(48), 82(48), 81(36). | [117] |
| 109 | Heliospathine | - | 313 | 313(1.3), 269(2), 268(1), 156(12), 139(43), 138(100), 137(15), 120(11), 95(20), 94(57), 93(75), 80(10). | [140] |
| 110 | Heliospathuline | - | 299 | 299(14.7), 282(6), 281(16.5), 238(18), 237(19), 236(20), 156(38), 139(30), 137(36), 136(20), 124(19), 120(60), 111(100), 108(51), 106(63), 94(41), 93(13), 80(78), 45(12). | [140] |
| 111 | Heliosupine | 2553 | 397 | 382(0.1), 352(0.1), 297(2), 238(4), 221(29), 220(100), 141(11), 138(10), 137(8), 136(50), 121(40), 120(95), 119(70), 106(10), 94(26), 93(52), 83 (12), 80(10), 59(10), 55(15), 43(15). | [70] |
| 112 | Heliotridine | 1447 | 155 | 138(2), 111(55), 94(18), 80(100), 68(15). | [40] |
| 113 | Heliotridine 2S-hydroxy-2S(1S-hydroxyethyl-4-methyl-pentanoyl ester | - | 313 | MS2: 269, 270, 224, 156, 138, 120, 94. | [50] |
| 114 | Heliotrine | 2100 | 313 | 255(0.3), 214(0.5), 197(1), 156(5), 139(22), 138(100), 136(13), 120(7), 94(41), 93(83), 80(40), 59(85). | [52] |
| 115 | Heliovicine | - | 285 | 267(5), 252(5), 240(4), 226(3), 175(1), 142(35), 124(100), 96(11), 95(9), 83(25), 82(20), 55(33). | [128] |
| 116 | Heliscabine | - | 255 | 255(4), 156(42), 138(12), 111(100), 99(33), 98(92), 82(77), 80(10). | [139] |
| 117 | Hydroxymyoscorpine | - | 397 | 397(0.2), 382(0.5), 338(0.1), 297(3.6), 221(21.3), 220(100), 136(40), 121(16), 120(41), 119(16.6), 94(18.4), 93(36). | [95] |
| 118 | 7-(Hydroxy-methylbutyryl)-9-viridifloryl retronecine | 2560** | 399 | 384(2), 354(0.4), 296(0.3), 282(2), 256(6), 239(21), 238(55), 138(15), 136(21), 121(35), 120(100), 108(2), 101(5), 95(10), 94(20), 93(42), 83(5), 80(6), 73(5), 67(3), 59(12), 55(0.5), 43(25). | [59] |
| 119 | Ilamine | - | 313 | 314(M+ +1, 18), 224(6), 139(3), 122(52), 120(25), 93(20), 80(8), 59(100). | [115] |
| 120 | Incanine | - | 337 | 294(2), 250(4), 222(10), 206(8), 155(5), 136(100), 120(77), 119(85), 94(42), 93(56), 80(26), 53(17), 43(69). | [46] |
| 121 | Indicine | 2126 | 299 | 255(4), 156(11), 138(100), 93(95). | [158] |
| 122 | Isoechinatine (9-(+)-viridiflorylheliotridine) | - | 299 | 299(11), 156(10), 139(33), 138(100), 137(14), 94(32), 93(68), 80(18). | [75,138] |
| 123 | Isolycopsamine | - | 299 | 281(12.3), 236(10.3), 138(67), 137(42), 136(34), 124(21), 121(15), 120(70), 119(15), 118(15), 117(14), 111(68), 110(21), 109(11), 108(49), 106(81), 95(17), 94(43), 93(28), 80(100). | [133] |
| 124 | Isoretronocanol enantiomer | 1883* | 141 | 141(20), 140(9), 124(13), 110(12), 108(11), 83(100), 82(56), 70(10), 68(11), 55(48). | [42] |
| 125 | 9-(3'-Isovaleryl)viridifloryl retronecine | - | 383 | 383(0.2), 255(3), 240(3), 223(5), 20194), 138(100), 120(10), 93(66), 85(28), 80(10), 57(19), 43(10), | [119] |
| 126 | Intermedine | 2133 | 299 | 156(9), 139(35), 138(100), 137(13), 136(13), 120(10), 95(15), 94(50), 93(80), 80(14), 67(9), 45(7), 43(18). | [40] |
| 127 | Lactodine | - | 227 | 227(9), 138(32), 137(41), 124(15), 111(60), 106(21), 94(44), 80(100). | [76,187] |
| 128 | Lasiocarpine | - | 411 | 396(2), 311(4), 279(5), 221(43), 220(11), 137(20), 136(49), 124(22), 120(74), 119(42), 106(15), 95(49), 93(23), 93(33), 83(39). | [71] |
| 129 | Latifoline | - | 393 | 393(6), 293(9), 221(10), 220(74), 219(35), 137(11), 136(100), 120(49), 119(42), 118(8), 94(33), 93(75), 83(29), 80(17), 67(9). | [103,104] |
| 130 | 9-Latifolylretronecine | - | 311 | 312(M+ +1, 40), 195(40), 161(20), 119(100). | [101] |
| 131 | Lepanthine | - | 315 | MS2: 298, 156, 138, 120, 94. | [166,167] |
| 132 | Lindelofine (or steroisomer) | 2678* | 285 | 285(5), 267(5), 252(6), 242(1), 241(1), 240(1), 226(1), 142(30), 125(21), 124(100), 83(26), 82(15), 55(30). | [42] |
| 133 | Lithosenine | - | 415 | 415(0.2), 400(10), 297(8), 256(3), 238(100), 237(15), 222(16), 220(24), 138(30), 137(8), 136(49), 121(36), 120(75), 119(36), 95(9), 94(34), 93(57), 80(12) | [156] |
| 134 | Longitubine | - | 353 | 353(29), 293(12), 181(29), 180(78), 136(75), 120(69), 119(62), 118(15), 106(10), 101(22), 94(58), 93(100), 80(32), 67(17). | [104] |
| 135 | Lycopsamine | 2145 | 299 | 299(0.5), 254(1), 156(8), 139(31), 138 (100), 137(12), 136(12), 120(10), 108(4), 95(15), 94 (55), 93(84), 80(14), 67(10), 45(8), 43(20). | [91] |
| 136 | Macrophylline | - | 239 | 98, 83(100), 55. | [62] |
| 137 | Megalanthonine | - | 301 | 301(2), 283(2), 256(11), 240(8), 212(19), 158(86), 141(15), 140(43), 138(14), 124(10), 122(18), 114(13), 97(18), 96(44), 95(62), 83(15), 82(100), 55(32). | [134] |
| 138 | 1-Methylene-8α-pyrrolizidine | 1274 | 139 | 139(15), 95(100). | [205] |
| 139 | 7-(2-Methylbutyryl)retronecine | 1760 | 239 | 239(20), 222(2), 154(20), 138(12), 137(12), 136(28), 124(37), 120(20), 111(28), 108(15), 106(28), 94(30), 93(20), 80 (100), 68(10), 57(19). | [96] |
| 140 | 9-(2-Methylbutyryl)retronecine | 1795 | 239 | 239(2), 195(5), 154(3), 138(98), 137(8), 136(5), 120(6), 108(5), 94(60), 93(100), 80 (27), 67(15), 57(30). | [96] |
| 141 | 7-(2-Methylbutyryl)-9-(2,3-dihydroxybutyryl)retronecine | 2285 | 341 | 341(1), 239(10), 223(20), 222(100), 143(18), 138(11), 137(7), 136(50), 121(17), 120(80), 119(30), 106(8), 94(40), 93(83), 85(15), 80(15), 57(30), 45(9). | [91] |
| 142 | 7-(2-Methylbutyryl)-9-echimidinyl retronecine | 2512 | 399 | 399(0.1), 384(0.5), 354(0.5), 297(1), 223(20), 222(100), 221(5), 143(15), 138(8), 137(8), 136(40), 121(30), 120(95), 119(30), 106(9), 94(31), 93 (70), 85(15), 80(15), 67(8), 59(15), 57(28), 45(8), 43(24). | [91] |
| 143 | Monocrotaline | 2268 | 325 | 236(35), 136(46), 120(100), 119(64), 93(36), 80(12), 43(69). | [195] |
| 144 | Myoscorpine | 3212* | 381 | 381(0.2), 338(1), 337(2), 336(2), 281(2), 255(1), 238(9), 237(3), 221(33), 220(100), 141(22), 138(8), 136(46), 121(34), 120(71), 119(22), 106(8), 94(36), 93(55), 83(39), 80(19), 59(4), 55(42), 53(11). | [42] |
| 145 | Neolatifoline | - | 393 | 393(7), 293(11), 221(16), 220(85), 219(31), 137(13), 136(100), 120(43), 119(48), 118(15), 94(39), 93(79), 83(32), 80(16), 67(8). | [104] |
| 146 | (7S,8R)Petranine | - | 285 | ESIMS (positive mode) m/z 286.11966 | [89] |
| 147 | (7S,8S)Petranine | - | 285 | ESIMS (positive mode) m/z 286.12162 | [89] |
| 148 | Platynecine | - | 157 | 113(18), 82(100), 68(18), 67(4), 55(27). | [106] |
| 149 | Platynecine N-Oxide 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanosyl ester | - | 331 | 314, 288, 174, 156, 112. | [50] |
| 150 | Pycnanthine | 2793 | 397 | 397(0.8), 382(0.4), 354(2), 352(2), 281(3), 254(12), 237(32), 236(100), 235(7), 138(26), 137(14), 136(74), 121(46), 120(77), 119(20), 99(20), 94(50), 93(58), 80(20), 71(20), 58(15), 45(20), 44(22), 43(20). | [91] |
| 151 | Punctanecine | - | 383 | 383(0.2), 368(0.5), 338(3), 296(2), 283(3), 266(4), 240(25), 222(19), 155(13), 140(45), 139(40), 137(73), 123(25), 122(48), 120(14), 96(23), 95(26), 83(27), 82(100). | [169,206] |
| 152 | Retronecine | 1430 2190* | 155 | 155(26), 138(2), 111(55), 94(15), 80(100), 68(15). | [40] |
| 153 | Retronecine dibenzoate | 2785 | 363 | 363(0), 258(10), 241(20), 136(43), 119(55), 105(100), 94(60), 93(78), 77(35). | [207] |
| 154 | Retronecine 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanosyl ester | - | 313 | MS2: 396, 270, 224, 156, 138. | [50] |
| 155 | Retronecine 2S-hydroxy-2S(1S-hydroxyethyl)-[1'S-hydroxyethyl)-4-methylpentanoyl]-4-methyl-pentanoyl ester, | - | 471 | ESIMS: m/z 472 [M + H]+ , for C24H41NO8 | [51] |
| 156 | Rinderine | 2155 | 299 | 284(0.1), 254(0.6), 156(9), 139(35), 138(100), 137(10), 136(10), 120(5), 95 (12), 94(23), 93(70), 80(12), 67(7), 53(5), 43(16). | [70] |
| 157 | Scorpioidine | - | 381 | 381(7), 199(7), 139(11), 138(69), 137(28), 136(13), 120(9), 101(8), 95(4), 94(21), 93(41), 83(100), 80(9), 67(6), 57(6), 55(34), 53(6), 43(10). | [159] |
| 158 | 7-Senecioylhelotridine | 1870 | 237 | 137(35), 136(18), 124(20), 111(35), 106(90), 94(21), 83(15), 80(100), 68(8), 55(18). | [70] |
| 159 | 7-Senecioylretronecine | 1816 | 237 | 237(4), 154(4), 137(30), 136(16), 124(25), 111(37), 106(40), 94(21), 83(23), 80(100), 68 (8), 55(15). | [91] |
| 160 | 9-Senecioylretronecine | 1833 | 237 | 237(1), 193(2), 155(11), 154(10), 138(23), 137(28), 136(15), 126(9), 109(5), 94(25), 93(100), 83(20), 80(16), 67(6), 55(11). | [91] |
| 161 | 7-Senecioylrinderine | 2515 | 381 | 336(0.1), 221(28), 220(61), 138(10), 137(15), 136(50), 121(75), 120(100), 119(85), 106(23), 95(15), 94(48), 93(72), 83(27), 80(30), 55(40), 43(40). | [40] |
| 162 | 7-Senecioyllycopsamine | 2497 | 381 | 336(0.5), 281(1), 238(5), 221(28), 220(100), 219(4), 141(15), 138(14), 137(12), 136(80), 121(38), 120(70), 119(20), 94(38), 93(61), 83 (76), 80(11), 55(15), 45(8), 43(28). | [91] |
| 163 | Sincamidine | - | 313 | C16H27NO5(similar to heliotrine) | [44] |
| 164 | Senkirkine | 2460 | 365 | 365(1), 266(10), 250(6), 222(9), 168(24), 153(41), 80(38), 43(100). | [54,195] |
| 165 | Subulacine | - | 155 | 155(17), 126(4), 124(13), 96(10), 80(4), 71(5), 70(100), 68(10), 56(6), 55(65). | [106,208] |
| 166 | Supinine | 1978 | 283 | 283(0.2), 140(6), 123(25), 122(100), 121(40), 120(49), 108(11), 93(20), 80(8), 70(7), 53(5), 45(4), 43(12). | [70] |
| 167 | Supinidine | 1887* | 139 | 139(40), 138(12), 122(32), 120(14), 111(15), 110(18), 108(30), 94(13), 80(100), 70(4), 68(17), 55(14), 53(17). | [42] |
| 168 | Supinidine N-oxide 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester | - | 313 | MS2: 296, 270, 224, 156, 138. | [50] |
| 169 | Symlandine | 3194* | 381 | 381(0.3), 338(1), 337(2), 336(2), 281(2), 255(1), 238(9), 237(3), 221(30), 220(100), 141(19), 138(10), 136(47), 121(35), 120(72), 119(21), 106(9), 94(36), 93(52), 83(31), 80(17), 59(3), 55(40), 53(12). | [42] |
| 170 | Symphytine | 3240* | 381 | 381(0.4), 338(1), 337(1), 336(3), 281(2), 255(1), 238(9), 237(2), 221(33), 220(100), 141(21), 138(7), 136(44), 121(33), 120(70), 119(20), 106(7), 94(36), 93(52), 83(33), 80(18), 59(3), 55(38), 53(12). | [42] |
| 171 | Symviridine | - | 381 | 381(0.3), 337(0.1), 336(0.1), 281(0.2), 221(34.5), 220(100), 136(44.2), 121(22), 120(50), 119(11), 118(15), 117(7.6), 103(10), 95(23.5), 93(44.5). | [172] |
| 172 | Tessellatine | 2930* | 299 | 299(7), 281(7), 256(5), 255(3), 248(4), 238(11), 237(9), 236(11), 156(19), 139(25), 138(64), 137(23), 124(16), 120(40), 111(45), 108(29), 106(39), 94(54), 93(40), 80(100), 53(17). | [42] |
| 173 | 7-Tigloyl-9-(2,3-dihydroxybutyryl)retronecine | 2325 | 339 | 239(5), 238(5), 237(8), 221(22), 220(90), 219 (20), 141(20), 138(10), 137(11), 136(100), 121(15), 120(80), 119(35), 106(10), 94(58), 93(90), 83(46), 80(20), 75(2), 57(9), 55(40), 45(10) . | [96] |
| 174 | 7-Tigloyl-9-(2,3-dihydroxypropanoyl)retronecine | 2320 | 325 | 237(8), 225(12), 221(12), 220(70), 141(27), 138(5), 137(12), 136(93), 121(8), 120(55), 119(48), 106(10), 94(59), 93(100), 83(55), 80(18), 55(24). | [40] |
| 175 | 7-Tigloylheliotridine | 1873 | 237 | 237(0.5), 137(55), 136(15), 124(19), 120(5), 111(41), 107(10), 106(100), 94(20), 86(6), 83(12), 80(95), 68(5), 55(19). | [67] |
| 176 | 7-Tigloyl-9-( hydroxypropenoyl) retronecine | 2073 | 307 | 221(14), 220(100), 207(27), 181(10), 141(42), 137(10), 136(95), 120(53), 119(28), 106(10), 94(63), 93(84), 83(50), 80(20), 67(9), 55(37), 43(24). | [40] |
| 177 | 7-Tigloyllycopsamine | 2473* | 381 | 336(1), 281(1.5), 238(1), 221(20), 220(100), 136(60), 121(30), 120(80), 94(40), 93(70), 83(50), 80(18), 55(40). | [90] |
| 178 | 7-Tigloyl-9-(2-methybutyryl)retronecine | 2170 | 321 | 221(36), 220(100), 195(5), 141(32), 138(2), 137(10), 136(82), 121(6), 120(53), 119(25), 106(10), 94(53), 93(80), 83(40), 80(15), 57(20), 55(33). | [96] |
| 179 | 7-Tigloylretronecine | 1816 | 237 | 237(3), 219 (0.5), 154(2), 138(3), 137(29), 136 (15), 124(25), 120(5), 111(44), 106(50), 94(20), 93(6), 83(15), 80 (100), 55(22). | [96] |
| 180 | 9-Tigloylretronecine | 1843 | 237 | 193(5), 154(15), 138(20), 137(26), 136(13), 126(7), 119(5), 109(4), 94(23), 93(100), 83(10), 80(12), 55(18). | [96] |
| 181 | Trachelanthamidine | 1853* | 141 | 141(24), 140(10), 124(15), 110(9), 108(7), 83(100), 82(62), 70(8), 68(8), 55(45). | [42] |
| 182 | Trachelanthamine | 1970 | 285 | 267(5), 252(4), 240(4), 142(50), 125(18), 124(100), 110(4), 96(6), 83(20), 82(11), 70(6), 55(14), 43(12). | [70] |
| 183 | Trachelanthamidine 2S-hydroxy-2S(1S-hydroxyethyl)-4-methyl-pentanoyl ester | - | 299 | ESIMS: m/z 300 [M + H]+, 142, 89, 83, 82, 55. | [51] |
| 184 | 7-Trachelanthyl retronecine | 2899* | 299 | 299(12), 281(6), 236(11), 156(25), 138(50), 120(47), 111(45), 108(29), 106(40), 94(40), 93(320, 80(100). | [42] |
| 185 | Transalpinecine | - | 173 | 98, 83, 70. | [145] |
| 186 | Triangularine | 2375 | 335 | 237(23), 236(6), 235(4), 221(5), 220(30), 219(18), 141(12), 137(12), 136(100), 121(28), 120(64), 119(35), 95(12), 94(60), 93(98), 83(58), 80(15), 55(30). | [40] |
| 187 | Triangularicine | 2394 | 335 | 237(30), 236 (6), 235(3), 221(5), 220(31), 219(10), 141(18), 137(10), 136(83), 121(27), 120(51), 119(40), 95(10), 94(55), 93(100), 83(60), 80(15), 55(25). | [40] |
| 188 | Trichodesmine | 2388 | 353 | 353(1), 264(56), 222(5), 136(36), 120(100), 93(23), 81(16), 43(40). | [195] |
| 189 | Turneforcidine | - | 157 | 113(20), 82(100), 68(21), 67(6), 55(32). | [106] |
| 190 | Uluganine | 399 | 399(2), 384(8), 356(1), 355(3), 354(2). | [183] | |
| 191 | Uplandicine | 2337 | 357 | 357(4), 342(5), 297(23), 281(4), 256(4), 207 (7), 206(52), 181(80), 180 (100), 179 (43), 136(75), 121(49), 120(85), 119 (50), 101(23), 94(55), 93(74), 80(28), 73(74), 59(23), 45(20), 44(69), 43(48). | [163] |
| 192 | Viridinatine | - | 443 | 282(17), 138(41), 137(86), 124(32), 111(70), 106(42), 94(44), 80(100). | [76,187] |
| 193 | Viridiflorine | 1983 | 285 | 284(0.3), 267(5), 252(5), 240(5), 142 (70), 125 (23), 124 (100), 117(3), 110 (5), 83 (35), 82 (22), 70 (15), 55(25), 43(21). | [67] |
| 194 | 9-Viridifloryl turneforcidine (or isomer) | 2394* | 301 | 301(0.3), 283(8), 257(8), 256(14), 212(18), 159(21), 158(68), 141(17), 140(50), 138(32), 122(24), 121(12), 120(31), 117(10), 106(13), 96(43), 95(81), 83(20), 82(100), 69(22), 55(40). | [42] |
| 195 | Vulgarine | - | 397 | 398[M+ 1] +, 380(18), 336(5), 316(5), 298(40), 254(15), 240(52), 138(100), 120(14). | [11] |
The RI reported for DB1 capillary column; * RI for DB-1701 capillary column; ** RI for OV1 capillary column; - not determined.

Figure 16.
Pyrrolizidine alkaloid structures in Boraginaceae. Compounds are numbered as in Table 3.
2.1.1. Gas Liquid Chromatography-Mass Spectrometry (GLC-MS)
High resolution Gas-liquid chromatography coupled with mass spectrometry (GLC-MS) has become a valuable and highly sensitive means for separation, convenient identification and quantification of complex PA mixtures, even in minute quantities or in diasteroisomeric forms [40,59,67,70,90,91,96,163,191,192,193,194,195]. The combination of molecular weight, group mass fragmentation pattern and Kovats retention indices, enables an unequivocal identification of most of PA tertiary bases even in trace amounts or of closely related isomers. High resolution gas chromatography-mass spectrometry (HRGC-MS) using the SIM mode was applied to detect trace amounts of 1,2-unsaturated PAs in 55 commercially available pollen products [16]. The detection limit of the overall procedure and the reliable quantitation limit were 0.003 μg g −1 to 0.01 μg g −1. GLC and GLC-MS cannot detect N-oxides, because they are not volatile. PA N-oxides need to be reduced to the free base (for example by reduction with zinc in HCl) as an extra step in the sample preparation to make them suitable for GLC analysis.
2.1.2. Liquid Chromatography-Mass Spectrometry (LC-MS)
Coupling of liquid chromatography (LC) with mass spectrometry (MS) allows a simultaneous determination of PAs and their corresponding N-oxides in a single chromatographic run without the requirement of a reduction step [50]. Quantitative analysis of PA mixtures (e.g., from comfrey, Symphytum officinale) was carried out efficiently using electrospray liquid chromatography-mass spectrometry (LC-MS). The method is based on HPLC coupled to an ion-trap and orbitrap MS with electrospray ionization interface [177]. Plant samples from 9 Boraginaceous species have been screened with gradient HPLC equipped with diode array and electron impact mass spectrometry [60]. Several saturated and 1,2-unsaturated PAs (mainly as N-oxides) were detected using HPLC-ESI-MS in fresh pollen collected from flowers of the PAs producing plants Echium vulgare and E. plantagineum and /or from bee pollen from bees that had foraged on PA plants [12]. The same method has also been used to determine the PA profile in E. plantagineum [93], Cryptantha crassipes [64], and Anchusa strigosa [50]. PAs (N-oxides and free bases) occurring in small amounts could be efficiently analyzed using an RP-HPLC ion trap MS method with an atmospheric pressure chemical ionization (APCA) interface [167].
Most PAs in plants are present as N-oxides or their tertiary bases. The N-oxides of this class are polar, water soluble, non-volatile and thermally labile. Thus, the application of LC-MS to the analysis of PA N-oxides has become a widely applied method [64,177,199,209,210], for example, in honey samples.
2.2. Nuclear Magnetic Resonance (NMR)
For the determination of the type of necine, the sites of ester attachment and the stereochemical orientation of necic acids, NMR methods are essential. An excellent comprehensive review of 1H NMR spectral data of PAs (more than 350 compounds) has been published by Logie et al. [211], in which the most useful shift values for the different types of PAs have been documented. Roeder [212] reported the 13C NMR data of 136 PAs and updated his data in a book chapter of Rizk [28]. After that time, more NMR data have been published for new PAs which are documented in this review.
2.3. Enzyme-Linked Immunoassay
A highly sensitive and specific competitive enzyme-linked immunosorbent assay (ELISA) can be used for detection and quantification of PAs [213,214,215,216]. The limit of detection of this ELISA method was 1.9, 10, 18, 20 and 60 ng for lasiocarpine, lasiocarpine N-oxide, heliotrine, and heliourine N-oxide, respectively [217]. These data suggest that this technique can be an excellent tool to diagnose poisoned animals and identify PA contaminated food items.
3. Tissue Culture
Several Boraginaceae species were subjected to in vitro cultivation and their PA contents have been studied [153,218,219]. PA profiles of Hairy root cultures of Cynoglossum officinale and Symphytum officinale have been established [220]. In these cultures, all PAs are genuinely present as N-oxides. Recently, Abd El-Mawla [221] reported the influence of methyl jasmonate, quercetin and salicylic acid as elicitors of PA production in the hairy root cultures of Echium rauwolfii.
4. Variation of PAs between Plant Organs and Developmental Stages
Distributions of PAs vary within different plant organs. In Heliotropium indicum a tissue-specific PA analysis revealed the presence of PAs in all tissues: highest levels were detected in the inflorescences which contained more than 70% of total plant alkaloids [116,220]; the alkaloid contents of stems, leaves and roots were 3, 7 and 19%, respectively. Young leaves, young inflorescences or seedlings showed high alkaloid levels, reaching 5%–6% dry matter in H. spanthulatum [116]. The total alkaloid contents of the root and rhizome of Pulmonaria obscura lay between 0.026 and 0.158 mg/g dry weight, whereas leaves and inflorescences accumulated only trace amounts of PAs (below 0.4 ng/mg dry weight) [168]. Extreme differences were found in PA levels between different leaves of Cynoglossum officinale [219]. The youngest leaves of the rosette plants had significantly higher PA contents than the oldest leaves (190 times). Alkaloid profiles and contents (total PAs and free bases) in the different plant organs of Paracaryum rugulosum, P. intermedium, Anchusa arvensis, Anchusa milleri, Alkanna orientalis, Alkanna tuberculata, Lappula spinocarpos and Trichodesma africana have been reported [40].

Table 4.
13C NMR data of new pyrrolizidine alkaloids (PAs) in the time frame 1991–2013.
| Alkaloid | C-1 | C-2 | C-3 | C-5 | C-6 | C-7 | C-8 | C-9 | C-1' | C-2' | C-3' | C-4' | C-5' | C-6' | C-7' | C-1'' | C-2'' | C-3'' | C-4'' | C-5'' | C-6'' | solvent | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Necine Base | |||||||||||||||||||||||
| Helibractinecine | 82.8 | 38.3 | 53.4 | 52.5 | 36.2 | 73.7 | 71.2 | 68.6 | CDCL3-CD3OD | [111] | |||||||||||||
| Helibracteatinecine | 82.6 | 34.1 | 53.0 | 52.9 | 33.3 | 79.7 | 73.0 | 67.2 | CDCL3-CD3OD | [112] | |||||||||||||
| Turneforcidine | 40.8 | 32.6 | 56.2 | 52.8 | 37.5 | 72.0 | 73.7 | 65.6 | CD3OD-ND3 | [222] | |||||||||||||
| Transalpinecine | 82.3 | 84.4 | 59.2 | 55.9 | 28.0 | 24.6 | 72.3 | 63.9 | CD3OD | [145] | |||||||||||||
| Subulacine | 70.9 | 63.4 | 54.0 | 58.4 | 27.1 | 26.2 | 67.9 | 60.2 | CD3OD | [145] | |||||||||||||
| 1α-2α-epoxy-1β-hydroxymethyl-8α-pyrrolizidine | 83.7 | 65.2 | 62.3 | 56.2 | 27.9 | 25.0 | 72.1 | 64.0 | CD3OD | [145] | |||||||||||||
| Tertiary base and N-oxide alkaloids | |||||||||||||||||||||||
| 3'-Acetylcanescine | 133.0 | 127.5 | 62.6 | 53.6 | 34.4 | 74.4 | 75.4 | 62.3 | 175.1 | 84.3 | 72.4-(CO:170.4), (Me: 21.2) | 17.2 | 33.1 | 17.2 | 17.1 | 171.7 | 46.9 | 69.1 | 29.4 | 29.2 | CDCL3/D6-DMSO | [154] | |
| 3'-Acetylcanescenine | 133.0 | 127.5 | 62.6 | 53.6 | 34.4 | 74.4 | 75.4 | 62.3 | 175.1 | 84.3 | 73.7-(CO:170.4), (Me: 21.3) | 16.0 | 33.1 | 17.8 | 14.2 | 171.7 | 46.9 | 69.1 | 29.4 | 29.2 | CDCL3/D6-DMSO | [154] | |
| 3’-Acetylechihumiline N-oxide | 133.0 | 124.9 | 79.4 | 70.5 | 33.9 | 73.3 | 95.6 | 63.0 | 174.7 | 85.6 | 74.8-(Me: 21.6) | 61.1 | 74.0 | 27.3 | 26.4 | 166.8 | 116.2 | 161.9 | 28.0 | 21.0 | CD3OD | [166] | |
| 7-Acetylechinatine N-oxide | 133.0 | 124.3 | 79.0 | 70.1 | 33.4 | 73.8 | 95.0 | 62.1 | 175.0 | 85.2 | 72.4 | 18.2 | 34.0 | 18.5 | 16.7 | 171.5 | 21.1 | CD3OD | [69] | ||||
| 7-Acetyleuropine | 135.0 | 128.3 | 62.3 | 54.4 | 30.6 | 76.9 | 78.8 | 62.6 | 174.2 | 84.0 | 79.2- (OMe:56.8) | 13.3 | 73.4 | 26.7 | 24.9 | 171.3 | 21.5 | CDCL3 | [110] | ||||
| 3'-Acetylindicine | 132.3 | 130.7 | 62.8 | 53.0 | 36.1 | 71.1 | 78.5 | 63.3 | 174.5 | 81.6 | 71.8-(CO:170.0), (Me 21.1) | 14.3 | 32.3 | 17.2 | 16.3 | CDCL3 | [158] | ||||||
| 3'-Acetylindicine N-Oxide | 132.4 | 121.9 | 78.0 | 69.6 | 34.7 | 69.6 | 95.8 | 62.1 | 173.8 | 81.9 | 72.4-(CO:170.3), (Me: 21.2) | 14.0 | 33.0 | 17.3 | 16.8 | CDCL3 | [158] | ||||||
| 3'-Acetyllithonine | 132.5 | 128.2 | 62.2 | 53.0 | 33.8 | 73.5 | 74.8 | 61.5 | 172.3 | 82.9 | 72.1-(CO:169.0), (Me: 20.5) | 14.7 | 72.4 | 24.8 | 25.9 | 170.5 | 47.3 | 68.1 | 28.8 | 28.9 | CDCL3 | [156] | |
| 3'-Acetylrinderine | 135.0 | 128.4 | 61.9 | 54.2 | 34.0 | 75.2 | 80.4 | 62.4 | 174.3 | 81.8 | 72.5-(CO:110.2), (Me 13.9) | 21.1 | 33.1 | 16.8 | 17.2 | CDCL3 | [70] | ||||||
| 3'-Acetyltrachelanthamine | 44.7 | 30.4 | 54.1 | 54.9 | 25.6 | 31.5 | 67.8 | 67.9 | 174.3 | 81.6 | 72.2 -(CO: 169.7), (Me: 21.1) | 13.9 | 32.8 | 16.7 | 17.2 | CDCL3 | [128] | ||||||
| Canescine | 133.0 | 127.5 | 62.6 | 53.6 | 34.4 | 74.4 | 75.4 | 62.3 | 175.1 | 84.3 | 69.5 | 17.2 | 33.1 | 17.2 | 17.1 | 171.7 | 46.9 | 69.1 | 29.4 | 29.2 | CDCL3/D6-DMSO | [154] | |
| Canescenine | 133.0 | 127.5 | 62.6 | 53.6 | 34.4 | 74.4 | 75.4 | 62.3 | 175.1 | 84.3 | 71.1 | 16.0 | 33.1 | 17.8 | 14.2 | 171.7 | 46.9 | 69.1 | 29.4 | 29.2 | CDCL3/D6-DMSO | [154] | |
| Cryptanthine | 133.9 | 125.8 | 62.9 | 53.9 | 34.7 | 75.4 | 75.6 | 60.7 | 167.7 | 127.4 | 139.3 | 16.0 | 20.6 | 175.4 | 77.4 | 71.4 | 16.7 | 22.6 | [65] | ||||
| Echihumiline | 132.8 | 127.6 | 62.0 | 53.7 | 34.2 | 73.7 | 76.1 | 62.0 | 174.3 | 83.1 | 69.8 | 18.5 | 72.4 | 26.0 | 24.9 | 165.5 | 115.3 | 159.0 | 27.7 | 20.4 | CDCL3 | [91] | |
| Echimidine isomer | 133.3 | 129 | 63.2 | 54.1 | 34.8 | 74.2 | 76 | 62.9 | 174.7 | 83.7 | 70.0 | 18.9 | 73.8 | 26.5 | 25.2 | 167.5 | 128.9 | 138.2 | 14.8 | 12.3 | CDCL3 | [87] | |
| Echiupinine | 133.0 | 128.1 | 62.3 | 53.7 | 34.3 | 72.8 | 75.6 | 62.2 | 175.1 | 82.9 | 68.7 | 17.2 | 32.9 | 17.1 | 16.9 | 167.1 | 115.6 | 158.1 | 27.5 | 20.3 | CDCL3 | [95] | |
| Echiupinine N-oxide | 131.8 | 128.1 | 78.2 | 69.1 | 34.3 | 71.4 | 93.7 | 60.2 | 174.9 | 84.0 | 69.4 | 16.9 | 33.2 | 17.5 | 17.2 | 166.2 | 114.5 | 160.3 | 27.6 | 20.6 | CDCL3 | [95] | |
| 7-Epi-echimiplatine N-oxide | 132.4 | 125.9 | 79.7 | 70.2 | 35.8 | 71.4 | 96.7 | 63.6 | 176.1 | 86.9 | 71.1 | 19.0 | 75.6 | 26.3 | 27.0 | D2O | [164] | ||||||
| 2'',3''-Epoxyechiumine | noise | 128.5 | 62.7 | 53.6 | 34.4 | 75.3 | 75.4 | 62.5 | 175.1 | 82.9 | 69.4 | 17.1 | 33.0 | 16.9 | 17.3 | noise | 77.2 | 60.0 | 13.6 | 19.3 | CDCL3 | [63] | |
| Erythro-3''-chloro-2''-hydroxyechiumine | 132.6 | 127.8 | 62.4 | 53.7 | 34.5 | 75.4 | 75.8 | 62.3 | 173.6 | 83.0 | 69.4 | 16.9 | 33.0 | 17.1 | 17.3 | 175.1 | 77.2 | 62.8 | 17.9 | 23.0 | CDCL3 | [63] | |
| Floridine | 44.2 | 31.2 | 54.0 | 54.0 | 25.4 | 29.5 | 67.6 | 66.2 | 173.4 | 83.4 | 72.7 -(CO: 169.6), (Me: 21.2) | 15.1 | 73.5 | 26.2 | 24.9 | CDCL3 | [128] | ||||||
| Floridinine | 43.3 | 28.6 | 53.8 | 54.0 | 24.6 | 30.6 | 68.4 | 66.2 | 174.6 | 82.5 | 69.8 | 18.6 | 73.9 | 25.0 | 26.0 | CDCL3 | [128] | ||||||
| Floridimine | 44.3 | 28.7 | 54.2 | 54.3 | 25.3 | 30.6 | 67.6 | 63.2 | 174.7 | 84.1 | 69.9 | 18.6 | 73.8 | 26.0 | 24.9 | CDCL3 | [128] | ||||||
| Helindicine | 134.2 | 125.3 | 62.5 | 55.5 | 36.7 | 70.5 | 80.5 | 61.7 | 175.6 | 84.5 | 70.5 | 17.3 | 34.2 | 17.2 | 17.6 | CD3OD | [130] | ||||||
| Heliotridine 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester | 134.0 | 123.5 | 62.0 | 55.9 | 35.0 | 70.8 | 79.4 | 62.2 | ? | ? | 45.0 | 25.2 | 23.1 | 24.4 | 73.9-(8':17.4) | CD3OD | [50] | ||||||
| Heliscabine | 81.1 | 38.4 | 52.8 | 52.0 | 36.0 | 72.8 | 69.8 | 70.0 | 168.1 | 127.2 | 139.7 | 16.0 | 20.6 | CDCL3 | [139] | ||||||||
| Heliospathine | 133.1 | 128.9 | 62.4 | 53.9 | 36.1 | 70.5 | 78.2 | 62.2 | 174.5 | 84.4 | 71.2 | 17.4 | 38.9 | 24.7 | 12.1-(8':11.9) | CDCL3 | [140] | ||||||
| Heliospathuline | 139.1 | 124.2 | 62.4 | 53.9 | 34.8 | 76.5 | 76.2 | 59.3 | 174.3 | 83.8 | 72.4 | 16.5 | 31.9 | 15.6 (7'':17.2) | CDCL3 | [140] | |||||||
| Hydroxymyoscorpine | 132.8 | 128.5 | 62.8 | 53.7 | 34.3 | 73.8 | 75.7 | 62.5 | 174.2 | 83.0 | 69.6 | 18.4 | 73.5 | 25.9 | 24.8 | 167.1 | 128.6 | 137.8 | 14.4 | 11.9 | CDCL3 | [95] | |
| Isoechinatine (9-(+)-viridiflorylheliotridine) | 136.1 | 126.3 | 61.7 | 54.3 | 33.8 | 74.7 | 79.8 | 61.9 | 174.3 | 83.9 | 71.6 | 17.5 | 32.2 | 17.8 | 15.9 | CDCL3 | [72,75] | ||||||
| Iso-lycopsamine | 139.1 | 123.2 | 63.0 | 53.4 | 34.9 | 76.4 | 76.5 | 59.5 | 174.1 | 82.8 | 69.4 | 16.5 | 33.38 | 17.2 (7'':12.27) | CDCL3 | [133] | |||||||
| Lactodine | 138.1 | 123.2 | 63.8 | 54.1 | 35.1 | 69.4 | 76.5 | 58.9 | 173.3 | 68.4 | 16.3 | CDCL3 | [187] | ||||||||||
| Leptanthine | 133.7 | 124.6 | 61.7 | 54.9 | 36.3 | 70.0 | 79.9 | 61.6 | 174.8 | 85.1 | 70.2 | 18.0 | 74.2 | 26.0 | 25.4 | CD3OD | [166] | ||||||
| Lithosenine N-oxide | 133.7 | 124.0 | 78.0 | 69.7 | 36.3 | 71.2 | 96.8 | 62.5 | 175.5 | 86.1 | 71.2 | 19.1 | 75.2 | 27.0 | 26.3 | CD3OD | [166] | ||||||
| Lithosenine | 133.0 | 126.9 | 62.2 | 53.1 | 33.8 | 73.4 | 74.8 | 61.4 | 173.5 | 83.4 | 68.9 | 18.0 | 72.5 | 24.7 | 25.9 | 170.3 | 47.5 | 68.1 | 28.9 | 29.0 | CDCL3 | [156] | |
| Megalanthonine | 36.0 | 31.2 | 51.1 | 54.6 | 36.8 | 69.5 | 72.8 | 66.2 | 174.5 | 84.1 | 72.0 | 17.0 | 32.0 | 15.6 | 17.8 | CDCL3 | [134] | ||||||
| Methylechiuplatine | 133.5 | 128.0 | 62.9 | 54.0 | 34.7 | 73.6 | 76.1 | 61.3 | 171.3 | 44.9 | 69.7 | 44.8 | 172.2 | 51.8:OMe | 27.3 | 166.9 | 127.7 | 139.2 | 15.9 | 20.6 | CDCL3 | [65] | |
| Myoscorpine | 133.1 | 128.1 | 62.7 | 53.8 | 34.4 | 73.7 | 75.8 | 62.2 | 175.1 | 82.9 | 69.3 | 17.2 | 32.9 | 17.1 | 16.9 | 167.0 | 128.5 | 137.8 | 14.4 | 11.9 | CDCL3 | [95] | |
| Myoscorpine N-oxide | 131.8 | 124.0 | 7 8.4 | 69.3 | 34.3 | 72.4 | 93.8 | 60.1 | 14.9 | 84.0 | 69.4 | 16.9 | 33.1 | 17.5 | 17.2 | 166.2 | 127.9 | 139.0 | 14.6 | 12.0 | CDCL3 | [95] | |
| Onosmerectine N-oxide | 133.5 | 123.8 | 78.6 | 69.7 | 35.7 | 70.7 | 96.7 | 62.5 | 175.3 | 85.7 | 74.6 | 70.8 | 18.7 | 26.6 | 25.9 | CD3OD | [164] | ||||||
| (7S, 8R)-Petranine | 134.4 | 121.1 | 70.9 | 63.4 | 34.7 | 70.2 | 87.7 | 59.8 | 167.5 | 126.9 | 140.3 | 16.1 | 20.6 | 68.8 | CDCL3 | [89] | |||||||
| (7S, 8S)-Petranine | 134.6 | 121.3 | 71.2 | 63.6 | 34.8 | 70.6 | 88.1 | 59.9 | 167.0 | 127.0 | 149.9 | 16.3 | 20.8 | 69.1 | CDCL3 | [89] | |||||||
| Platynecine N-oxide 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester | 37.3 | 30.6 | 73.0 | 70.6 | 35.8 | 70.1 | 91.5 | 67.3 | ? | ? | 44.5 | 25.3 | 23.3 | 24.8 | 73.9-(8':17.4) | CD3OD | [50] | ||||||
| Pycnanthine | 132.8 | 126.0 | 62.2 | 53.9 | 34.2 | 72.4 | 75.8 | 61.7 | 174.4 | 83.6 | 71.3 | 15.8 | 32.0 | 17.7 | 17.0 | 165.5 | 112.4 | 160.1 | 66.8 | 30.3 | CDCL3 | [91] | |
| Retronecine 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester | 133.2 | 123.8 | 62.0 | 54.0 | 36.7 | 70.0 | 80.0 | 61.0 | 175.4 | 82.3 | 45.2 | 25.3 | 23.3 | 24.6 | 73.9-(8':17.6) | CD3OD | [51] | ||||||
| Retronecine N-oxide 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester | 133.9 | 123.4 | 78.8 | 70.0 | 35.7 | 70.6 | 97.2 | 62.4 | 175.9 | 82.0 | 45.0 | 25.2 | 23.3 | 24.6 | 73.6-(8':17.7) | CD3OD | [51] | ||||||
| Retronecine N-oxide 2S-hydroxy-2S-(1R-hydroxyethyl)-4-methyl-pentanoyl ester | 134.0 | 123.4 | 78.8 | 70.0 | 35.8 | 70.6 | 97.0 | 62.0 | 175.5 | 81.0 | 44.0 | 25.8 | 23.9 | 24.3 | 74.0-(8':16.5) | CD3OD | [51] | ||||||
| Retronecine 2S-hydroxy-2S-(1S-hydroxyethyl)-2S-[(1'S-hydroxyethyl)-4-methylpentanoyl]-4-methyl-pentanoyl ester | 135.4 | 124.0 | 62.0 | 54.9 | 36.6 | 70.4 | 79.8 | 61.7 | 175.7 | 82.2 | 43.6 | 25.2 | 24.0 | 24.7 | 73.4-(8': 7.4) | 180.0 | 81.3 | 45.0 | 25.2 | 23.1 | 24.7 (7'':73.6) (8'':17.0) | CD3OD | [51] |
| Symviridine | 133 | 127.8 | 62.7 | 53.8 | 34.3 | 72.7 | 75.8 | 62.3 | 174.4 | 83.4 | 70.7 | 17.1 | 32.9 | 17.7 | 16.0 | 166.9 | 115.5 | 158.2 | 27.5 | 20.3 | CDCL3 | [172] | |
| Supinidine N-oxide 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester | 137.7 | 123.0 | 76.9 | 71.1 | 25.2 | 28.3 | 90.2 | 61.2 | 176.2 | 82.0 | 45.2 | 25.2 | 23.5 | 24.6 | 73.3-(8':17.6) | CD3OD | [51] | ||||||
| Tessellatine | 138.6 | 124.5 | 63.0 | 53.4 | 34.7 | 75.7 | 76.0 | 59.6 | 173.1 | 83.5 | 71.4 | 16.9 | 31.9 | 17.6 (7'':15.7) | CDCL3 | [42] | |||||||
| Threo-2'',3''-dihydroxyechiumine | 132.9 | 125.0 | 63.3 | 54.0 | 34.8 | 74.9 | 75.8 | 63.0 | 174.9 | 83.2 | 69.8 | 16.9 | 33.2 | 17.2 | 17.2 | 175.4 | 78.7 | 71.5 | 16.5 | 22.1 | CDCL3 | [63] | |
| Trachelanthamidine 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester | 48.0 | 29.7 | 55.9 | 55.8 | 25.6 | 31.3 | 71.9 | 63.4 | 181.6 | 81.8 | 44.1 | 25.0 | 24.0 | 25.0 | 73.9-(8':17.4) | CD3OD | [51] | ||||||
| Uplandicine | 132.7 | 127.7 | 62.1 | 53.4 | 34.0 | 73.6 | 75.5 | 62.2 | 174.2 | 83.2 | 69.6 | 18.5 | 73.6 | 24.7 | 25.9 | 170.2 | 21.1 | - | - | - | CDCL3 | [163] | |
| Viridiflorine | 44.5 | 31.5 | 54.5 | 54.2 | 25.5 | 30.0 | 67.8 | 67.2 | 174.4 | 83.6 | 71.0 | 17.2 | 32.1 | 17.9 | 15.9 | [42] | |||||||
| Viridinatine | 139.3 | 124.2 | 64.2 | 54.2 | 36.7 | 77.3 | 77.3 | 59.4 | 174.1 | 82.8 | 68.4 | 16.2 | 33.5 | 17.2 | 17.3 | 176.2 | 83.2 | 69.1 | 16.8 | 34.12 | 18.1 (7'':18.9) | CDCL3 | [187] |
| Viridinatine N-oxide stereoisomer | 132.5 | 125.4 | 79.5 | 70.1 | 35.8 | 71.3 | 96.6 | 63.3 | 176.6 | 85.9 | 70.9 | 17.6 | 34.6 | 18.9 | 18.4 | 176.1 | 86.2 | 72.3 | 18.6 | 34.2 | 18, 17.2 | ||

Table 5.
1H NMR data of new PAs of in the time frame 1995–2013.
| Alkaloid | H-1 | H-2 | H-3 | H-5 | H-6 | H-7 | H-8 | H-9 | H-3' | H-4' | H-5' | H-6' | C-7' | H-2'' | H-3'' | H-4'' | H-5'' | solvent | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Necine Base | |||||||||||||||||||
| Helibractinecine [C8H15NO3] | 1.94 | α 3.13 β 2.93 | α 3.07 β 2.91 | 2.03 | 4.50 | 3.34 | α 3.60 β 3.66 | CDCL3-CD3OD | [111] | ||||||||||
| Helibracteatinecine [C8H15NO3] | 1.63 | α 3.26 β 2.55 | α 3.26 β 2.52 | α 1.84 β 2.11 | 3.94 | 3.12 | α 3.79 β 3.84 | CDCL3-CD3OD | [112] | ||||||||||
| Turneforcidine [C8H15NO2] | 2.53 | α 1.68 β 2.10 | α 3.08 β 2.55 | α 3.03 β 2.70 | α 2.00 β 1.99 | 4.18 | 3.16 | α 3.57 β 3.53 | CD3OD-ND3 | [222] | |||||||||
| Transalpinecine [C8H15NO3] | 4.18 | α 3.86 β 3.38 | α 3.06 β 3.36 | α 1.95 β 2.16 | α 1.88 β 2.66 | 3.99 | α 3.90 β 3.61 | CD3OD | [145] | ||||||||||
| Subulacine [C8H15NO2] | 3.95 | α 3.79 β 3.38 | α 2.97 3.62 | α 1.96 2.24 | α 2.19 2.26 | 4.45 | α 3.97 β 3.79 | CD3OD | [145] | ||||||||||
| 1α-2α-epoxy-1β-hydroxymethyl-8α-pyrrolizidine [C8H15NO2] | 4.59 | α 3.81 β 3.89 | α 3.23 β 3.50 | α 2.08 β 2.23 | α 2.00 β 2.30 | 4.31 | α 3.93 β 3.69 | CD3OD | [145] | ||||||||||
| Tertiary base and N-oxide alkaloids | |||||||||||||||||||
| 3'-Acetylcanescine [C22H35NO8] | 5.83 | α 3.95 β 3.38 | α 3.33 β 2.65 | 2.09 | 5.38 | 4.35 | α 4.79 β 4.67 | 5.21-(COMe: 2.00) | 1.19 | 2.00 | 0.94 | 0.91 | 2.45 | 1.26 | 1.26 | CDCL3/D6-DMSO | [154] | ||
| 3'-Acetylcanescenine [C22H35NO8] | 5.83 | α 3.95 β 3.38 | α 3.33 β 2.65 | 2.09 | 5.38 | 4.35 | α 4.74 β 4.73 | 5.17 -(COMe: 2.03) | 1.19 | 2.00 | 0.94 | 0.91 | 2.45 | 1.26 | 1.26 | CDCL3/D6-DMSO | [154] | ||
| 3’-Acetylechihumiline N-oxide [C22H33NO9] | 6.03 | α 4.37 β 4.69 | 3.90 – 3.75 (m) | α 2.20 β 2.78 | 5.76 | 4.83 | 4.72 | 5.46 -(COMe: 1.98) | 1.34 | 1.25 | 1.35 | 5.67 | - | 1.92 | 2.18 | CD3OD | [166] | ||
| 3'-Acetylechinatine [C17H27NO6] | 5.76 | α 3.37 β 3.92 | α 2.68 β 3.30 | α 1.91 β 1.98 | 4.17 | 3.96 | α 4.78 β 4.96 | 5.21-(COMe: 2.04) | 1.29 | 2.01 | 0.88 | 0.96 | CDCL3 | [70] | |||||
| 7-Acetylechinatine N-oxide | 6.00 | α 4.70 β 4.38 | α 3.83 β 3.79 | α 2.74 β 2.23 | 5.69 | 4.88 | 4.78 | 3.96 | 1.42 | 2.17 | 0.90 | 0.93 | 2.06 | CD3OD | [164] | ||||
| 7-Acetyleuropine [C18H29NO7] | 5.81 | α 4.02 β 3.38 | α 3.28 β 2.86 | 1.93 | 5.09 | 4.16 | α 4.93 β 4.87 | 3.81-(OMe: 3.27) | 1.27 | 1.19 | 1.31 | 2.06 | CDCL3 | [110] | |||||
| 3'-Acetylindicine [C17H27NO6] | 5.92 | α 3.96 β 3.45 | α 3.29 β 2.76 | α 1.98 β 2.02 | 4.30 | 4.21 | 4.80 | 5.20 -(COMe: 2.04) | 1.23 | 2.09 | 0.95 | 0.92 | CDCL3 | [158] | |||||
| 3'-Acetylindicine N-Oxide [C17H27NO7] | 5.79 | 4.45 | 3.73-3.80 | α 2.01 β 2.62 | 4.70 | 4.66 | 4.83 | 5.23 -(COMe: 2.03) | 1.24 | 2.05 | 0.97 | 0.91 | CDCL3 | [158] | |||||
| 3'-Acetyllithosenine [C22H35NO9] | 5.75 | α 3.77 β 3.29 | α 3.17 β 2.54 | 1.96 | 5.23 | 4.16 | α 4.68 β 4.59 | 5.28 -(COMe: 1.87) | 1.13 | 1.25 | 1.11 | 2.33 | 1.17 | 1.17 | CDCL3 | [156] | |||
| 3'-Acetylrinderine [C17H27NO6] | 5.76 | α 3.37 β 3.92 | α 2.68 β 3.30 | α 1.91 β 1.98 | 4.21 | 3.96 | α 4.66 β 4.93 | 5.28 -(COMe: 2.02) | 1.25 | 2.01 | 0.92 | 0.98 | CDCL3 | [70] | |||||
| 3'-Acetyltrachelanthamine [C17H29NO5] | 2.09 | α 1.70 β 2.02 | α 3.28 β 2.60 | α 3.07 β 2.65 | α 1.89 β 1.84 | α 2.05 β 1.63 | 3.38 | 4.16 | 5.22 -(COMe: 1.97) | 1.23 | 2.02 | 0.90 | 0.96 | CDCL3 | [128] | ||||
| Canescine [C20H33NO7] | 5.83 | α 3.95 β 3.38 | α 3.33 β 2.65 | 2.09 | 5.38 | 4.35 | α 4.79 β 4.67 | 4.07 | 1.19 | 2.00 | 0.94 | 0.91 | 2.45 | 1.26 | 1.26 | CDCL3/ D6-DMSO | [154] | ||
| Canescenine [C20H33NO7] | 5.83 | α 3.95 β 3.38 | α 3.33 β 2.65 | 2.09 | 5.38 | 4.35 | α 4.74 β 4.73 | 3.89 | 1.19 | 2.00 | 0.94 | 0.91 | 2.45 | 1.26 | 1.26 | CDCL3/ D6-DMSO | [154] | ||
| Cryptanthine | 5.74 | α 4.0 β 3.37 | α 3.40 β 2.61 | 2.12 | 5.3 | 4.39 | α 4.84 β 4.64 | - | 6.1 | 1.98 | 1.89 | 3.8 | 1.17 | 1.24 | CDCL3 | [65] | |||
| 5'-Deoxylasiocarpine [C21H33NO6] | 5.8 | α 3.50 β 4.18 | α 3.00 β 3.40 | 1.8 | 5.12 | 4.37 | 4.88 | 3.7 -(OMe: 3.22) | 1.21 | 2.20 | 0.95 | 0.94 | 6.13 | 1.95 | 1.82 | CDCL3 | [123] | ||
| Echihumiline [C20H31NO7] | 5.87 | α 3.35 β 3.72 | α 2.83 β 2.83 | 2.09 | 5.48 | 4.13 | α 4.65 β 4.96 | 4.21 | 1.26 | - | 1.24 | 1.31 | 5.59 | - | 1.90 | 2.15 | CDCL3 | [91] | |
| Echihumiline N-oxide [C20H31NO8] | 5.97 | α 4.54 β 4.77 | α 3.73 β 4.13 | α 2.24 β 2.89 | 5.77 | 5.45 | α 4.71 β 4.94 | 4.22 | 1.27 | - | 1.25 | 1.30 | 5.57 | - | 1.92 | 2.17 | CDCL3 | [91] | |
| Echimidine isomer (tigloyl) [C20H31NO7] | 5.81 | α 3.34 β 3.83 | α 2.60 β 3.27 | 2.05 | 5.37 | 4.32 | α 4.60 β 4.86 | 4.12 | 1.21 | 1.17 | 1.26 | - | 6.05 | 1.91 | 1.76 | CDCL3 | [87] | ||
| 7-Epi-echimiplatine N-oxide [C15H25NO7] | 5.89 | α 4.42 β 4.21 | α 3.74 β 3.59 | α 2.47 β 2.01 | 4.67 | 4.55 | 4.78 | 4.18 | 1.14 | 1.16 | 1.21 | D2O | [164] | ||||||
| Floridine [C17H29NO6] | 2.23 | α 2.15 β 2.15 | α 3.75 β 2.77 | α 3.48 β 2.65 | 2.04 | α 2.20 β 1.70 | 4.01 | α 4.16 β 4.27 | 5.45 -(COMe: 1.98) | 1.36 | - | 1.25 | 1.40 | CDCL3 | [128] | ||||
| Floridinine [C15H27NO5] | 2.36 | α 2.08 β 2.20 | α 3.51 β 2.66 | α 3.24 β 2.85 | α 2.0 β 1.94 | α 2.15 β 1.63 | 3.70 | α 4.20 β 4.53 | 4.19 | 1.27 | - | 1.28 | 0.96 | CDCL3 | [128] | ||||
| Floridimine [C15H27NO5] | 2.30 | α 2.17 β 2.28 | α 3.89 β 2.83 | α 3.59 β 2.89 | 2.11 | α 2.26 β 1.80 | 4.30 | α 4.35 β 4.45 | 4.26 | 1.32 | - | 1.28 | 1.33 | CDCL3 | [128] | ||||
| Helibracteatine [C13H21NO4] | 1.85 | α 3.22 β 2.54 | α 3.22 β 2.54 | α 1.85 β 2.05 | 4.08 | 3.30 | α 4.35 β 4.46 | 6.10 | 1.99 | 1.91 | CDCL3 + D2O | [112] | |||||||
| Helibracteatinine [C13H21NO4] | α 1.80 β 2.10 | α 3.35 β 2.78 | α 3.20 β 2.60 | 1.80 | 5.09 | 3.30 | α 3.64 β 3.84 | 6.12 | 1.98 | 1.88 | CDCL3 + D2O | [112] | |||||||
| Helindicine [C15H23NO4] | 5.94 | α 4.40 β 3.92 | α 3.97 β 3.34 | 2.15 | 4.63 | 4.90 | α 4.98 β 4.80 | 4.05 | 1.17 | 2.03 | 0.95 | 0.93 | CD3OD | [130] | |||||
| Heliotridine 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester [C16H27NO5] | 5.95 | 3.70 4.18 | 3.10 2.64 | 2.10 | 4.52 | 4.60 | 4.85 | 1.74 1.79 | 1.82 | 0.87 | 0.99 | 3.78-(8':1.15) | CD3OD | [50] | |||||
| Heliscabine [C13H21NO4] | 1.86 | α 3.42 β 3.05 | α 3.30 β 3.05 | 2.16 | 4.66 | 3.64 | α 4.25 β 4.35 | 6.10 | 1.99 | 1.89 | CDCL3 | [139] | |||||||
| Ilamine [C16H27NO5] | 5.81 | 3.45 4.10 | 2.62 3.35 | 1.86 | 1.66 2.10 | 4.43 | 4.78 | 3.81 | 1.28 | 1.31 | 1.20 (8-OMe: 3.30) | CDCL3 | [115] | ||||||
| Ilamine N-oxide [C16H27NO6] | 5.81 | α 3.43 β 4.55 | α 3.48 β 3.79 | α 1.99 β 1.89 | 2.50 | 4.81 | α 4.85 β 4.69 | 3.80 | 1.26 | 1.29 | 1.22-(8-OMe:3.30) | CDCL3 | [115] | ||||||
| Isoechinatine (9-(+)-Viridiflorylheliotridine) [C15H25NO5] | 5.66 | 3.30 3.83 | 2.55 3.22 | 1.80 1.92 | 4.13 | 3.97 | 4.70 5.00 | 3.92 | 1.24 | 2.13 | 0.89 | 0.85 | CDCL3 | [72,75] | |||||
| Leptanthine [C15H25NO6] | 5.90 | α 3.90 β 4.40 | 3.90 | 2.20 | 4.65 | 4.95 | 4.90 | 4.20 – Me 1.98 | 1.29 | 1.28 | 1.32 | [166] | |||||||
| Leptanthine N-oxide [C15H25NO7] | 6.05 | α 4.65 β 4.85 | 4.1 – 4.0 (m) | α 2.23 β 2.63 | 4.83 | 5.20 | α 4.92 β 4.98 | 4.22 – Me 1.98 | 1.28 | 1.25 | 1.31 | [166] | |||||||
| Lactodine [C11H17NO4] | 5.68 | α 3.90 β 3.34 | α 3.26 β 2.59 | α 1.93 β 1.87 | 4.10 | 4.02 | α 5.01 β 4.89 | 4.20 | 1.39 | CDCL3 + D2O | [187] | ||||||||
| Lithosenine [C20H33NO8] | 5.78 | α 3.77 β 3.29 | α 3.16 β 2.57 | 1.96 | 5.21 | 4.20 | α 4.66 β 4.52 | 4.03 | 1.13 | 1.17 | 1.11 | 2.33 | 1.16 | 1.16 | CDCL3 | [156] | |||
| Megalanthonine [C15H27NO5] | 2.78 | α 1.89 β 2.15 | α 3.26 β 2.61 | α 3.21 β 2.73 | α 2.03 β 2.14 | 4.29 | 3.48 | α 4.09 β 4.46 | 2.64 | - | 2.64 | 3.70 | 1.34 | 6.80 | 1.96 | 1.80 | CDCL3 | [134] | |
| Methylechiuplatine [C20H29NO7] | 5.77 | α 3.97 β 3.37 | α 3.32 β 2.64 | 2.09 | 5.40 | 4.33 | α 4.68 β 4.62 | [65] | |||||||||||
| Neo coramandaline [C15H27NO4] | 1.9 | α 1.85 β 1.55 | α 3.20 β 2.50 | α 3.05 β 2.64 | α 1.85 β 1.55 | α 1.90 β 1.35 | 3.56 | α 4.30 β 4.20 | 3.98 | 1.23 | 2.14 | 0.88 | 0.92 | CDCL3 | [74] | ||||
| Onosmerectine N-oxide [C15H25NO7] | 5.96 | α 4.66 β 4.45 | 3.87 | α 2.58 β 2.12 | 4.75 | 4.85 | α 4.92 β 4.88 | 4.22 | 1.26 | 1.31 | 1.27 | CD3O | [164] | ||||||
| (7S, 8R)-Petranine [C14H20CINO3] | 5.82 | α 4.55 β 4.77 | α 4.09 β 4.35 | α 2.48 β 2.70 | 4.92 | 5.36 | 4.78 | 6.18 | 2.00 | 1.90 | α5.42 β 5.75 | CDCL3 | [89] | ||||||
| (7S, 8S)-Petranine [C14H20CINO3] | 5.83 | α 4.54 β 4.78 | α 4.09 β 4.35 | α 2.51 β 2.69 | 4.94 | 5.38 | 4.88 | 6.20 | 2.02 | 1.90 | α5.71 β 5.39 | CDCL3 | [89] | ||||||
| Platynecine N-oxide 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester [C16H29NO6] | 2.22 | α 1.90 β 2.33 | α 3.30 β 3.89 | α 3.61 β 3.87 | α 2.07 β 2.14 | 4.52 | 3.84 | α 4.29 β 3.85 | α 1.76 β 1.70 | 1.82 | 0.87 | 0.98 | 3.78-(8':1.15) | CD3OD | [50] | ||||
| Pycnanthine [C20H31NO7] | 5.83 | α 3.55 β 4.06 | α 2.80 β 3.50 | 2.15 | 5.47 | 4.12 | α 4.65 β 4.75 | 3.99 | 1.26 | 2.15 | 0.93 | 0.85 | 5.92 | - | 3.65 | 2.06 | CDCL3 | [91] | |
| Retronecine 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester [C16H27NO5] | 5.96 | α 3.98 β 4.46 | α 3.49 β 3.87 | 2.19 | 4.64 | 4.90 | 4.95 | 1.75 | 1.83 | 0.88 | 0.99 | 3.788'-1.17 | CD3OD | [51] | |||||
| Retronecine N-oxide 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester [C16H27NO6] | 5.93 | α 4.36 β 4.65 | α 3.70 β 3.82 | α 2.63 β 2.08 | 4.72 | 4.68 | 4.85 | α 1.73 β 1.80 | 1.82 | 0.88 | 0.98 | 3.76-(8':1.16) | CD3OD | [51] | |||||
| Retronecine N-oxide 2S-hydroxy-2S-(1R-hydroxyethyl)-4-methyl-pentanoyl ester [C16H27NO6] | 5.95 | α 4.36 β 4.60 | α 3.70 β 3.85 | α 2.60 β 2.08 | 4.72 | 4.69 | 4.82 | α 1.66 β 1.47 | 1.74 | 0.88 | 1.00 | 3.91-(8':1.22) | CD3OD | [51] | |||||
| Retronecine 2S-hydroxy-2S-(1S-hydroxyethyl)-2S-[(1'S-hydroxyethyl)-4-methylpentanoyl]-4-methyl-pentanoyl ester [C24H41NO8] | 5.95 | α 3.96 β 4.39 | α 3.29 β 3.89 | 2.18 | 4.61 | 4.91 | 4.94 | α 1.79 β 1.82 | 1.80 | 0.95 | 0.99 | 3.70-(8':1.16) | 1.79 | 1.85 | α 1.81 β 0.87 | 3.77-(6'':1.14) | CD3OD | [51] | |
| Symviridine [C20H31NO6] | 5.84 | α4.02 β3.43 | α 3.40 β 2.68 | 2.10 | 5.41 | 4.48 | α 4.78 β 4.66 | 3.97 | 1.22 | 2.13 | 0.86 | 0.92 | 5.57 | - | 1.88 | 2.12 | CDCL3 | [172] | |
| Supinidine N-oxide 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester [C16H27NO4] | 5.90 | α 4.36 β 4.56 | α 3.61 β 3.67 | α 2.08 β 2.38 | 2.50 2.03 | 4.66 | 4.77 | α 1.73 β 1.80 | 1.83 | 0.88 | 0.98 | CD3OD | [51] | ||||||
| Trachelanthamidine 2S-hydroxy-2S-(1S-hydroxyethyl)-4-methyl-pentanoyl ester [C16H29NO4] | 2.28 | 1.90 2.27 | α 3.22 β 3.48 | α 3.74 β 3.12 | 2.08 2.19 | 2.25 1.97 | 3.99 | 3.69 3.63 | 1.77 1.69 | 1.83 | 0.94 | 1.00 | 3.77-(8':1.15) | CD3OD | [51] | ||||
| Uplandicine [C17H27NO7] | 5.83 | α 3.38 β 3.93 | α 2.70 β 3.35 | 2.06 | 5.34 | 4.43 | α 4.62 β 4.87 | 4.14 | 1.22 | - | 1.26 | 1.19 | 1.98 | CDCL3 | [163] | ||||
| Viridinatine [C22H37NO8] | 5.71 | α 3.80 β 3.30 | α 3.16 β 2.19 | α 1.92 β 1.85 | 5.48 | 4.10 | α 4.98 β 4.76 | 3.90 | 1.28 | 2.15 | 0.86 | 0.93 | - | 3.95 | 1.32 | 2.14 (6'':0.90) (7'':1.00) | CDCL3 + D2O | [187] | |
| Viridinatine N-oxide stereoisomer [C22H37NO9] | 5.86 | α 4.51 β 4.22 | α 3.73 β 3.61 | α 2.45 β 2.00 | 4.67 | 5.57 | 4.75 | 4.04 | 1.04 | 1.89 | 0.75–0.78 | - | 3.93 | 1.10 | 2.05, 0.81 | D2O | [164] | ||
5. Biological Activity of PAs
5.1. Antimicrobial Activity
The growth of bacterial species, mostly human pathogens such as E. coli, S. pneumoniae, Bacillus subtilis, B. anthracis and Staphylococcus aureus were inhibited by different pure PAs and PA extracts [90,110]. Successive root bark extracts of Cordia gilletii (Boraginaceae) were tested for their antimicrobial activity against 10 strains of bacteria and 1 strain of fungi by broth microdilution and agar diffusion methods. The methanol extract showed direct antimicrobial activity against all tested microorganisms with minimum inhibitory concentrations (MIC) ranging between 125 and 1,000 µg/ml. [223]. Joosten and van Veen [224] discussed the impact of plant-produced PAs on plant-associated microorganisms, their detoxification by microorganisms and the ecological consequences of this activity.
5.2. Sequestration in Insects and Antifeedant Properties
Several insects are PA specialists which not only tolerate PAs of their host plants but use them for their own chemical defence against predators, as morphogens and even as pheromones [101,144,225,226,227,228,229,230,231]. Defensive PAs can be obtained as larvae from food plants and as adults via nectar. PA sequestering insects often show aposematic colouration. Several moths and butterflies use unsaturated PAs to produce dihydropyrrolizidines with pheromone activity [110]. In some moths PAs are morphogens inducing the formation of large coremata in males, which function as pheromone emitting organs. A recent review published by Macel [232], threw light on the dual role PAs in plant-insect interactions.
The antifeedant activity of six PAs isolated from Anchusa strigosa towards the generalist herbivore Spodoptera exigua and the cabbage specialist Pieris brassicae was evaluated [50]. 1,2-Unsaturated PAs (e.g., retronecine 2S-hydroxy (1S-hydroxyethyl)-4-methyl-pentanoyl ester and its N-oxide) reduced feeding by P. brassicae by 52 and 68%, respectively compared to control. However, the alkaloids lacking to 1,2-double bond (trachelanthamidine ester and platynecine ester) showed no deterrent activity.
5.3. Biological Importance
Toxicity of 1,2-unsaturated PAs as hepatotoxic, pulmotoxic, hemolytic, antimitotic, teratogenic, mutagenic and carcinogenic natural products for humans and livestock is well documented [23,24,25,26,27,28,29,30,31,32,35,36]. The potential PA contamination of food and feeding stuff has attracted recurrent attention. It is evident, that humans should not ingest food or herbal teas that contain PAs. However, some saturated PAs have interesting pharmacological and biological effects, e.g., spasmolytic, antihistaminic, anti-HIV and antiviral activities and as glucosidase inhibitor [233,234,235]. Solanecio angulatus and Crotalaria phillipsiae (PA containing plants from the families Asteraceae and Fabaceae) serve as sources of novel trypanocidal compounds [236]. The crude ethanol and hexane extracts of Heliotropium subulatum showed significant antiviral activity to Coxsackie, Poliomyelitis and Measles at 500 and 100 μg/mL, respectively, while heliotrine and 7-angeloylheliotrine demonstrated activity against Poliomyelitis and Vesicular stomatitis at concentration of 10 μg /mL [237]. PAs can inhibit the specific binding of radioligands to muscarinic acetylcholine and serotonin receptors and to a lesser degree to adrenergic receptors [20]. This neuromodulatory effect might be responsible for the avoidance of PA plants by many herbivores.
6. Chemotaxonomic Information of PAs in Boraginaceae
The distribution of PAs is documented in Table 1 and Table 2. A comparison of phytochemical occurrences of alkaloids with regard to recent results in the molecular phylogeny of the Boraginaceae was not intended in this review, but will be part of a separate publication. It has been shown previously that the occurrence of PAs in the plant kingdom does not necessarily mirror phylogenetic relatedness because the genes which encode the enzymes of alkaloid biosynthesis can be switched on or off [2,238].
6.1. Genus Amsinckia
PA profiles of twenty species and varieties of the genus Amsinckia have been investigated. All identified alkaloids were from retronecine, (+)- and (−)-supinidine, (+)-isoretrenocanol and trachelanthamidine types [42,43,44,46,104].
6.2. Genus Cynoglossum
Plants belongs to this genus contain alkaloids mainly derived from heliotridine type, in addition to (+)- and (−)-supinidine and trachelanthamidine bases [60,67,68,69,70,71,72,73,74,75,76,77,78,80,81,82,83,84,85,86,239].
6.3. Genus Echium
PAs of about 15 species from this genus have been investigated which accumulate PAs from retronecine type [11,28,29,60,87,89,90,91,92,93,96,99]. However, in earlier papers [47,97,98], the structure of major compounds in Echium vulgare were wrongly assigned to heliosupine and 3'-acetylheliosupine by means of paper chromatography and/or mass spectrometry. These compounds belong to heliotridine types. Recent publications revised the structures to the stereoisomeric analogue, echimidine and 3'-acetylechimidine (retronecine type), based on NMR and other advanced spectroscopic techniques [11,96,229].
6.4. Genus Heliotropium
Biosynthetic studies on necines that have been incorporated into PAs as well as the isolated and/or identified alkaloids in the genus Heliotropium indicated that it contains retronecine, (−)-trachelanthamidine, (−)-supindine, (−)-isoretronecanol, laburnine, lindelofidine, platynecine and subulacine nucleus, in addition heliotridine type PAs [28,29]. In addition to the isolation of the open-chained necines, a cyclic diester PA, e.g., helindicine has been recorded in H. indicum [130], incanine in H. olgae and trichodesmine in H. arguzioides [29].
6.5. Genus Paracaryum and Paracynoglossum
Only PAs from the heliotridine and (−)-trachelanthamidine type have been reported to be accumulated in these species [28,29,40,151].
6.6. Genus Rindera
All the previously examined Rindera species contain heliotridine–type PAs [120,152,169]. Echinatine is considered as a chemotaxonomical marker in this genus.
6.7. Genus Symphytum
Eleven Symphytum species (S. aintabicum, S. bohemium, S. consolidum, S. grandiflorum, S. ibericum, S. orientale, S. peregrinum, S. sylvaticum subsp. sepulcrale var. sepulcrale, S. tanaiense, S. tuberosum, S. × uplandicum) have been documented to synthesis pyrrolizidine alkaloids of retronecine type [17,29,170,177]. However, both retronecine and heliotridine type alkaloids were identified in S. asperum [171], S. caucasium [174], and S. officinale [29].
6.8. Other Boraginaceae Species
Species of genus Alkanna [40,41,184], Cryptantha [63,64,65,66], Mertensia [157], and Messerschmidia [158] produce only PAs of the retronecine type. Whereas Onosma heterophyllum and O. erecta [164] accumulate only heliotridine type PAs, other species of this genus synthesise retronecine type PAs [60,161,163,166,167] in addition to, supinidine [163] and trachelanthamidine type [167].
7. Conclusions
Our knowledge on PAs in Boraginaceae has improved substantially during the last decades, especially as advanced analytical methods, such as GLC, HPLC in combination with mass spectrometry have been widely applied. However, information on the biochemistry and physiology of PAs in plants and their function in plant insect interactions is still limited. More research has addressed pharmacological and toxicological properties of PAs in food plants and herbal medicines; public awareness is rightly concerned with PAs in food items and their place in the food chain. This review summarizes the state of our existing knowledge.
Acknowledgments
The authors thank Sameh Soliman and Ahmed El-Kadeem, Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University for providing some published papers.
Author Contributions
This review was planned by both authors. Assem El-Shazly collected the information and drafted the manuscript which was interpreted and edited by Michael Wink. Both authors finally approved the published version.
Conflicts of Interest
Michael Wink is Editor-in-Chief of Diversity.
References
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Detzel, A.; Wink, M. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Edgar, J.A.; Smith, L.W. Pyrrolizidine alkaloids in Honey from Echium plantagineum L. J. Agric. Food Chem. 1981, 29, 958–960. [Google Scholar] [CrossRef]
- Hartmann, T. Chemical ecology of pyrrolizidine alkaloids. Planta 1999, 207, 483–495. [Google Scholar] [CrossRef]
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.A.; Seawright, A.A. Pyrrolizidine alkaloids in human diet. Mutation Res. 1999, 443, 53–67. [Google Scholar] [CrossRef]
- Edgar, J.A.; Smith, L.W. Transfer of Pyrrolizidine Alkaloids into Eggs: Food Safety Implications. ACS Symp. Ser. 1999, 754, 118–128. [Google Scholar] [CrossRef]
- Edgar, J.A.; Roeder, E.; Molyneux, R.J. Honey from plants containing pyrrolizidine alkaloids: A potential threat to health. J. Agric. Food Chem. 2002, 50, 2719–2730. [Google Scholar] [CrossRef]
- Fu, P.P.; Yang, Y.; Xia, Q.; Chou, M.W.; Cui, Y.Y.; Lin, A.G. Pyrrolizidine alkaloids-tumorigenic components in Chinese herbal medicines and dietary supplements. J. Food Drug Anal. 2002, 10, 198–211. [Google Scholar]
- Azadbakht, M.; Talavaki, M. Qualitative and quantitative determination of pyrrolizidine alkaloids of wheat and flour contaminated with Senecio in Mazandaran province farm. Int. J. Pharm. Res. 2003, 2, 179–183. [Google Scholar]
- Boppré, M.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloids of Echium vulgare honey found in pure pollen. J. Agric. Food Chem. 2005, 53, 594–600. [Google Scholar] [CrossRef]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, W.O. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implication for commercial processing of bee pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef]
- Sharma, R.A.; Singh, B.; Singh, D.; Chandrawat, P. Ethnomedicinal, pharmacological properties and chemistry of some medicinal plants of Borginaceae in India. J. Med. Plant Res. 2009, 3, 1153–1175. [Google Scholar]
- European Food Safety Authority. Opinion of the scientific panel on contamination in the food chain on a request from the European Commission related to pyrrolizidine alkaloids as undesirable substances in animal feed. EFSA J. 2007, 447, 1–51. [Google Scholar]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; van Egmond, H.P.; Omar, M.F.; Mofleh, J. An outbreak of hepatic veno-occlusive disease in western Afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J. Toxicol. 2010. [Google Scholar] [CrossRef]
- Kempf, M.; Heil, S.; Haβlauer, I.; Schmidt, L.; von der Ohe, K.; Theuring, C.; Reinhard, A.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in pollen and pollen products. Mol. Nutr. Food Res. 2010, 54, 292–300. [Google Scholar] [CrossRef]
- Staiger, C. Comfrey: A Clinical Overview. Phytother. Res. 2012, 26, 1441–1448. [Google Scholar]
- Cooper, R.A.; Huxtable, R.J. The relationship between reactivity of metabolites of pyrrolizidine alkaloids and extrahepatic toxicity. Proc. West Pharmacol. Soc. 1999, 42, 13–16. [Google Scholar]
- Kim, H.Y.; Stermitz, F.R.; Li, J.K.; Coulombe, R.A. Comparative DNA cross-linkage by activated pyrrolizidine alkaloids. Food Chem. Toxicol. 1999, 37, 619–625. [Google Scholar] [CrossRef]
- Schmeller, T.; El-Shazly, A.; Wink, M. Allelochemical activities of pyrrolizidine alkaloids: Interaction with neuroreceptors and acetylcholine related enzymes. J. Chem. Ecol. 1997, 23, 399–416. [Google Scholar] [CrossRef]
- Wink, M.; Roberts, M.F. Compartmentation of alkaloid synthesis, transport and storage. In Alkaloids, Biochemistry, Ecology and Medicinal Application; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA and London, UK, 1998; Chapter 10; pp. 239–269. [Google Scholar]
- Wink, M.; Schmeller, T.; Latz-Brüning, B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA and other molecular targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Bull, L.B.; Culvenor, C.C.J.; Dick, A.T. The Pyrrolizidine Alkaloids; North Holland: Amsterdam, The Netherlands, 1968. [Google Scholar]
- McLean, E.K. The toxic activities of pyrrolizidine (Senecio) alkaloids. Pharmacol. Rev. 1970, 22, 429–476. [Google Scholar]
- Smith, L.W.; Culvenor, C.C.J. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar]
- Robins, D.J. The pyrrolizidine alkaloids. Fortschr. Chem. Org. Naturst. 1982, 41, 115–203. [Google Scholar]
- Mattocks, A.R. Chemistry and Toxicology of Pyrrolizidine Alkaloids; Academic Press: London, UK and New York, NY, USA, 1986. [Google Scholar]
- Rizk, A.M. Naturally Occurring Pyrrolizidine Alkaloids; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Hartmann, T.; Witte, L. Chemistry, biology and chemoecology of pyrrolizidine alkaloids. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Elsevier Science Ltd.: Kidlington, UK, 1995; Vol. 9, Charpter 4; pp. 155–233. [Google Scholar]
- Roeder, E. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 1995, 50, 83–98. [Google Scholar]
- Roeder, E. Analysis of pyrrolizidine alkaloids. Curr. Org. Chem. 1999, 3, 557–576. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal plants in China containing pyrrolizidine alkaloids. Pharmazie 2000, 55, 711–725. [Google Scholar]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat Prod. Commun. 2008, 3, 1205–1216. [Google Scholar]
- Wink, M.; van Wyk, B.E. Mind-altering and Poisonous Plants of the World; Briza Publications: Pretoria, South Africa, 2008. [Google Scholar]
- Roeder, E.; Wiedenfeld, H. Pyrrolizidine alkaloids in medicinal plants of Mongolia, Nepal and Tibet. Pharmazie 2009, 64, 699–716. [Google Scholar]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids: Toxicity and problems. Food Addit. Contam. 2011, 28, 282–292. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, P.P.; Lin, G. Hepatotoxicity of naturally occurring pyrrolizidine alkaloids. Asian J. Pharmacodyn. Pharmacokinet. 2006, 6, 187–192. [Google Scholar]
- Dreger, M.; Stanisławska, M.; Krajewska-Patan, A.; Mielcarek, S.; Mikołajczak, L.P.; Buchwald, W. Pyrrolizidine alkaloids–chemistry, biosynthesis, pathway, toxicity, safety and perspectives of medicinal usage. Herba Polonica 2009, 55, 127–147. [Google Scholar]
- Roeder, E.; Wiedenfeld, H. Plants containing pyrrolizidine alkaloids used in the Traditional Indian Medicine—including Ayurveda. Pharmazie 2013, 68, 83–92. [Google Scholar]
- El-Shazly, A.; El-Domiaty, M.; Witte, L.; Wink, M. Pyrrolizidine alkaloids in members of the Boraginaceae from Sinia (Egypt). Biochem. Syst. Ecol. 1998, 26, 619–639. [Google Scholar] [CrossRef]
- Roeder, E.; Sarg, T.; El-Dahmy, S.; Abdel-Ghani, A. Pyrrolizidine alkaloids from Alkanna orientalis. Fitoterapia 1992, 63, 405–408. [Google Scholar]
- Kelley, R.B.; Seiber, J.N. Pyrrolizidine alkaloid chemosystematics in Amsinckia. Phytochemistry 1992, 31, 2369–2387. [Google Scholar] [CrossRef]
- Kelley, R.B.; Seiber, J.N. Pyrrolizidine alkaloid from Amsinckia. Phytochemistry 1992, 31, 2513–2518. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Smith, L.W. Alkaloids of Amsinckia species: A. intermedia, A. hispida and A. lycoposoides. Aust. J. Chem. 1966, 19, 1955–1964. [Google Scholar] [CrossRef]
- Roitman, J.N. Pyrrolizidine alkaloids of Amsinckia menziesii. Aust. J. Chem. 1983, 36, 769–778. [Google Scholar] [CrossRef]
- Cooper, R.A.; Bowers, R.J.; Beckham, C.J.; Huxtable, R.J. Preparative separation of pyrrolizidine alkaloids by high-speed counter-current chromatography. J. Chromatogr. A 1996, 732, 43–50. [Google Scholar] [CrossRef]
- Pedersen, E. Pyrrolizidine alkaloids in Danish species of the family Boraginaceae. Arch. Pharm. Chem. Sci. Ed. 1975, 3, 55–64. [Google Scholar]
- Broch-Due, A.I.; Aasen, A.J. Alkaloids of Anchusa officinalis L. Identification of pyrrolizidine alkaloid lycopsamine. Acta Chem. Scand. Ser. B. 1980, 34, 75–77. [Google Scholar] [CrossRef]
- Hendriks, H.; Bruins, A.P.; Huizing, H.J. Detection of curassavine and some related pyrrolizidine alkaloids in an Anchusa officinalis strain with means of positive ion and negative ion chemical ionization GC-MS. Biomed. Environ. Mass Spctrom. 1988, 17, 129–132. [Google Scholar] [CrossRef]
- Siciliano, T.; de Leo, M.; Bader, A.; de Tommasi, N.; Vrieling, K.; Braca, A.; Morelli, I. Pyrrolizidine alkaloids from Anchusa strigosa and their antifeedant activity. Phytochemistry 2005, 66, 1593–1600. [Google Scholar] [CrossRef]
- Braca, A.; Bader, A.; Siciliano, T.; Morelli, I.; Tommasi, N. New pyrrolizidine alkaloid and glycosides from Anchusa strigosa. Planta Med. 2003, 69, 835–841. [Google Scholar] [CrossRef]
- El-Dahmy, S.; Adel Ghani, A. Alkaloids of Arnebia decumbens Vent. Az. J. Pharm. Sci. 1995, 15, 24–34. [Google Scholar]
- Roeder, E.; Rengel-Mayer, B. Pyrrolizidine alkaloids from Arnebia euchroma. Planta Med. 1993, 59, 192. [Google Scholar]
- Wassel, G.; El-Menshawi, B.; Saeed, A.; Mahran, G. Toxic pyrrolizidine alkaloids of certain Boraginaceae. Acta Pharm. Suec. 1987, 24, 199–204. [Google Scholar]
- Langer, T.; Franz, C. Determination of pyrrolizidine alkaloids in commercial samples of borage seed oil products by GC-MS. Sci. Pharm. 1997, 65, 321–328. [Google Scholar]
- Herrmann, M.; Joppe, H.; Schmaus, G. Thesinine-4'-O-β-D-glucoside the first glycosylated plant pyrrolizidine alkaloid from Borago officinalis. Phytochemistry 2002, 60, 399–402. [Google Scholar] [CrossRef]
- Telezhenetskaya, M.V.; Matkarmov, A.D.; Khadzhibekov, S.N.; Yunusov, S.Y. Alkaloids of some Central Asian plants. Khim. Prir. Soedin 1987, 3, 663–664. [Google Scholar]
- Siddiqi, M.A.; Suri, K.A.; Suri, O.P.; Atal, C.K. A new pyrrolizidine alkaloid from Caccinia glauca. Pytochemistry 1978, 17, 2049–2050. [Google Scholar] [CrossRef]
- El-Shazly, A. Pyrrolizidine alkaloids of Cerinthe glabra Miller (Boraginaceae). Zagazig. J. Pharm. Sci. 2004, 13, 1–5. [Google Scholar]
- Mroczek, T.; Baj, S.; Chrobok, A.; Glowniak, K. Screening for pyrrolizidine alkaloids in plant materials by electron ionization RP-HPLC-MS with thermabeam interface. Bio. Med. Chromatogr. 2004, 18, 745–751. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H.; Kaus, K.J. Das Pyrrolizidinalkaloid Intermedin aus Cerinthe minor L. Sci. Pharm. 1990, 58, 9–13. (In German) [Google Scholar]
- Wassel, G.; El-Menshawi, B.; Saeed, A.; Mahran, G.; Reisch, J. New sources of pyrrolizidine alkaloids: Genus Cordia (Ehretiaceae) and Schismus (Gramineae). Sci. Pharm. 1987, 55, 163–166. [Google Scholar]
- Stermiz, F.; Pass, M.A.; Kelley, R.B.; Liddell, J.R. Pyrrolizidine alkaloids from Cryptantha species. Phytochemistry 1993, 33, 383–387. [Google Scholar] [CrossRef]
- Williams, M.T.; Warnock, B.J.; Betz, J.M.; Beck, J.J.; Gardner, D.R.; Lee, S.T.; Molyneux, R.J.; Colegate, S.M. Detection of high levels of pyrrolizidine-N-oxides in the endangered plant Cryptantha crassipes (Terlingua creek cat’s-eye) using HPLC-ESI-MS. Phytochem. Anal. 2011, 22, 532–540. [Google Scholar] [CrossRef]
- Colegate, S.M.; Gardner, D.R.; Davis, T.Z.; Betz, J.M.; Panter, K.E. Dehydropyrrolizidine alkaloids in two Cryptantha species: Including two new open chain diesters one of which is amphoteric. Phytochem. Anal. 2013, 24, 201–212. [Google Scholar] [CrossRef]
- Beck, J.J.; Stermitz, F.R. Pyrrolizidine alkaloids from Brickellia grandiflora and Cryptantha jamesii. Biochem. Syst. Ecol. 2002, 30, 1079–1081. [Google Scholar] [CrossRef]
- El-Shazly, A.; Sarg, T.; Ateya, A.; Abdel-Aziz, E.; Witte, L.; Wink, M. Pyrrolizidine alkaloids of Cynoglossum officinale and Cynoglossum amabile (Family Boraginaceae). Biochem. Syst. Ecol. 1996, 24, 415–421. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Smith, L.W. Alkaloids of Cynoglossum australe and C. amabile. Aust. J. Chem. 1967, 20, 2499–2503. [Google Scholar] [CrossRef]
- Damianakos, H.; Jeziorek, M.; Pietrosiuk, A.; Sykłowska-Baranek, K.; Chinou, I. Isolation of pyrrolizidine alkaloids from Cynoglossum columnae Ten. (Boraginaceae). Planta Med. 2013. [Google Scholar] [CrossRef]
- El-Shazly, A.; Sarg, T.; Witte, L.; Wink, M. Pyrrolizidine alkaloids from Cynoglossum creticum. Phytochemistry 1996, 42, 1217–1221. [Google Scholar] [CrossRef]
- Zalkow, L.H.; Bonetii, S.; Gelbaum, L.T.; Gordon, M.M.; Patil, B.B.; Shani, A.; van Derveer, I.D. Pyrrolizidine alkaloid from middle eastern plans. J. Nat. Prod. 1979, 42, 603–614. [Google Scholar] [CrossRef]
- Asibal, C.F.; Glinski, J.A.; Gelbaum, L.T.; Zalkow, L.H. Pyrrolizidine alkaloids from Cynoglossum creticum. Synthesis of pyrrolizidine alkaloids echinatine, rinderine and analogues. J. Nat. Prod. 1989, 52, 109–118. [Google Scholar] [CrossRef]
- Chen, Z.W.; Tang, J.; Li, J.; Zhang, Q.; Zhao, X.; Hu, J. Constituents of Cynoglossum zeylanicum. Zhongguo Yaoke Daxue Xuebao (J. Chin. Pharm.) 1987, 18, 51–53. (In Chinese) [Google Scholar]
- Ravi, S.; Lakshmanan, A.J. Coromandaline, a pyrrolizidine alkaloid from Cynoglossum furcatum. Ind. J. Chem. 2000, 39B, 80–82. [Google Scholar]
- Ravicumar, R.; Lakshmanan, A.J. Isoechinatine, a pyrrolizidine alkaloid from Cynoglossum furcatum. Ind. J. Chem. 2004, 43B, 406–409. [Google Scholar]
- Ravi, S.; Ravikumar, R.; Lakshmanan, A.J. Pyrrolizidine alkaloids from Cynoglossum furcatum. J. Asian Nat. Prod. Res. 2008, 10, 307–310. [Google Scholar] [CrossRef]
- Mattoks, A.R.; Pigott, C.D. Pyrrolizidine alkaloids from Cynoglossum germanicum. Phytochemistry 1990, 29, 2871–2872. [Google Scholar] [CrossRef]
- Suri, K.A.; Sawhney, R.S.; Atal, C.K. Pyrrolizidine alkaloids from Cynoglossum lanceolatum, C. glochidiatum and Lindelofia angustifolia. Indian J. Pharm. 1975, 37, 69–70. [Google Scholar]
- Crowely, H.C.; Culvenor, C.C.J. Alkaloids of Cynoglossum latifolium, latifoline and 7-angeloylretronecine. Aust. J. Chem. 1962, 15, 139–144. [Google Scholar] [CrossRef]
- Kelly, H.A.; Robins, D.J. Pyrrolizidine alkaloids from Cynoglossum macrostylum. Fitoterapia 1992, 63, 91. [Google Scholar]
- Guner, N. Pyrrolizidine alkaloids from Cynoglossum montanum. Marmara Univ. Eczacilik Derg. 1987, 3, 57–60. [Google Scholar]
- Hagan, D.; Robins, D.J. Pyrrolizidine alkaloids from Cynoglossum nervosum. Fitoterapia 1991, 62, 186–187. [Google Scholar]
- Resch, J.F.; Meinwald, J. A revised structure for acetylheliosupine. Pytochemistry 1982, 21, 2430–2431. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Witte, L.; Theuring, C.; Hartmann, T. Distribution, biosynthesis and turnover of pyrrolizidine alkaloids in Cynoglossum officinale. Phyochemistry 1995, 39, 287–292. [Google Scholar] [CrossRef]
- Man’ko, I.V.; Marchenko, L.G. Pictumine, a new alkaloid from Cynoglossum pictum. Khim. Prir. Soedin. 1972, 5, 655–656. [Google Scholar]
- Man’ko, I.V. Alkaloids of Cynoglossum amabile and C. viridiflorum. Rastit. Resur. 1972, 8, 243–246. [Google Scholar]
- Mehrabani, M.; Ghannadi, A.; Sajjadi, E.; Ghassemi, N.; Shams-Ardakani, M. Toxic pyrrolizidine alkaloids of Echium amoenum Fisch. & Mey. DARU J. Pharm. Sci. 2006, 14, 122–127. [Google Scholar]
- Sarg, T.; El-Dahmy, S.; Abdel-Aziz, E.; Abdel-Ghani, A.; Roeder, E. Pyrrolizidine alkaloids from Echium angustifolium. Fitoterapia 1992, 63, 466–468. [Google Scholar]
- Alali, F.Q.; Tahboub, Y.R.; Ibrahim, E.S.; Qandil, A.; Tawaha, K.; Burgess, J.P; Sy, A.; Nakanishi, Y.; Kroll, D.J.; Oberlies, N.H. Pyrrolizidine alkaloids from Echium glomeratum (Boraginaceae). Phytochemistry 2008, 69, 2341–2346. [Google Scholar] [CrossRef]
- El-Shazly, A.; Abdel-All, M.; Tei, A.; Wink, M. Pyrrolizidine alkaloids from Echium rauwolfii and Echium horridum (Boraginaceae). Z. Naturforsch. 1999, 54c, 295–300. [Google Scholar]
- El-Shazly, A.; Sarg, T.; Ateya, A.; Abdel-Aziz, E.; El-Dahmy, S.; Witte, L.; Wink, M. Pyrrolizidine and tetrahydroisoquinoline alkaloids from Echium humile. Phytochemistry 1996, 42, 225–230. [Google Scholar] [CrossRef]
- Carvalho, J.C.B.; Almeida, H.D.S.; Lobo, J.F.R.; Ferreira, J.L.P.; Oliveira, A.P.; Leandro Rocha, L. Pyrrolizidine alkaloids in two endemic capeverdian Echium species. Biochem. Syst. Ecol. 2013, 50, 1–6. [Google Scholar]
- Colegate, S.M.; Edgar, J.A.; Knill, A.M.; Lee, S.T. Solid-phase extraction and HPLC-MS profiling of pyrrolizidine alkaloids and their N-oxides: A case study of Echium plantagineum. Phytochem. Anal. 2005, 16, 108–119. [Google Scholar] [CrossRef]
- Culvenor, C.C.J. The alkaloids of Echium plantagineum L. Aust. J. Chem. 1956, 9, 512–520. [Google Scholar] [CrossRef]
- Roeder, E.; Liu, K.; Bourauel, T. Pyrrolizidine alkaloids from Echium pininana. Phytochemistry 1991, 30, 3107–3110. [Google Scholar] [CrossRef]
- El-Shazly, A.; Sarg, T.; Ateya, A.; Abdel-Aziz, E.; El-Dahmy, S.; Witte, L.; Wink, M. Pyrrolizidine alkaloids from Echium setosum and Echium vulgare. J. Nat. Prod. 1996, 59, 310–313. [Google Scholar] [CrossRef]
- Man’ko, I.V. Alkaloids of Cynoglossum officinale and Echium vulgare and standard preparation from Cynoglossum officinale. Farm. Zh. (Kiev) 1964, 19, 22–26. [Google Scholar]
- Karimov, A.; Telezhenetskaya, M.V.; Lutfullin, K.; Yunusov, S.Y. Alkaloids of Echium vulgaris and Berberis oblonga. Khim. Prir. Soedin. 1975, 11, 433–434. [Google Scholar]
- Dominguez, D.M.; Reina, M.; Santos-Guerra, A.; Santana, O.; Agullo, T.; Lopez-Balbo, C.; Gonzalez-Coloma, A. Pyrrolizidine alkaloids from Canarian endemic plants and their biological effects. Biochem. Syst. Ecol. 2008, 36, 153–166. [Google Scholar] [CrossRef]
- Suri, O.P.; Jamwal, R.S.; Suri, K.A.; Atal, C.K. Ehretinine, a noval pyrrolizidine alkaloid from Ehretia aspera. Phytochemistry 1980, 19, 1273–1274. [Google Scholar] [CrossRef]
- L’Empereur, K.M.; Li, Y.; Stermitz, F. Pyrrolizidine alkloids from Hackelia californica and Gnophaela latipennis, and H. californica-hasted arctiid moth. J. Nat. Prod. 1989, 52, 360–366. [Google Scholar] [CrossRef]
- Li, Y. Two new pyrrolizidine alkaloids from Hackelia californica (Gray) Johnston (Boraginaceae). Huaxue Xuebao 1990, 48, 415–418. [Google Scholar]
- Hagglund, K.M.; L’Empereur, K.M.; Roby, M.K.; Stermitz, F.R. Latifoline and latifoline N-oxide from Hackelia floribunda. J. Nat. Prod. 1985, 48, 638–639. [Google Scholar] [CrossRef]
- Roitman, J.N. Longitubine and neolatifoline, new pyrrolizidine alkaloids from Hackelia longituba. Aust. J. Chem. 1988, 41, 1827–1833. [Google Scholar]
- Akramov, S.T.; Shadmanov, Z.; Samatov, A.; Yunusov, S.Y. Alkaloids of Senecio jacobaea, Heliotropium acutiflorum and H. transoxanum. Khim. Prir. Soedin. 1966, 4, 258. [Google Scholar]
- Birecka, H.; Frohlich, M.W.; Glickman, M. Free and esterified necines in Heliotropium species from Mexico and Texas. Phytochemistry 1983, 22, 1167–1171. [Google Scholar] [CrossRef]
- Marquez, V.C. Chromatographic separation of Bulensia retamo, Heliotropium arborescens and Cestrum auriculatum. Bol. Soc. Quim. Peru. 1961, 27, 161–172. [Google Scholar]
- Rizk, A.M.; Hammouda, F.M.; Roeder, E.; Wiedenfeld, H.; Ismail, S.I.; Hassan, N.M. Occurrence of pyrrolizidine alkaloids in Heliotropium bacciferum Forssk. Sci. Pharm. 1988, 56, 105–110. [Google Scholar]
- Farrag, N.M.; Adel-Aziz, E.M.; El-Shafae, A.M.; Ateya, A.M.; El-Domiaty, M.M. Pyrrolizidine alkaloids of Heliotropium bacciferum Frossk from Egypt. Int. J. Pharmacogn. 1996, 34, 374–377. [Google Scholar] [CrossRef]
- Reina, M.; Mericli, A.H.; Cabbera, R.; Gonzalez-Coloma, A. Pyrrolizidine alkaloid from Heliotropium bovei. Phytochemistry 1995, 38, 355–358. [Google Scholar] [CrossRef]
- Lakshmanan, A.J.; Shanmugasundaram, S. Helibractinecine, a pyrrolizidine alkaloid from Heliotropium bracteatum. Phytochemistry 1994, 36, 245–248. [Google Scholar] [CrossRef]
- Lakshmanan, A.J.; Shanmugasundaram, S. Ester alkaloid from Heliotropium bracteatum. Phytochemistry 1995, 40, 291–294. [Google Scholar] [CrossRef]
- Marquina, G.; Laguna, A.; Velez, H.; Ripperger, H. 9-Angeloylretronecine N-oxide from Heliotropium bursiferum. Pharmazie 1988, 34, 55–56. [Google Scholar]
- Eröksüz, H.; Eröksüz, Y.; Ozer, H.; Ceribasi, A.O.; Tosun, F.; Tamer, U.; Kizilay, C.A. Toxicity of dietery Heliotropium circinatum to rats. Vet. Hum. Toxicol. 2003, 45, 198–201. [Google Scholar]
- Farsam, H.N.; Yassa, N.; Sarkhail, P.; Shafiee, A. New pyrrolizidine alkaloids from Heliotropium crassifolium. Planta Med. 2000, 66, 389–391. [Google Scholar] [CrossRef]
- Catalfamo, J.L.; Martin, W.B.; Birecka, H. Accumulation of alkaloids and their necines in Heliotropium curassavicum, H. spathulatum and H. indicum. Phytochemistry 1982, 21, 2669–2675. [Google Scholar] [CrossRef]
- Mohanraj, S.; Subramanian, P.S. Curassavine, an alkaloid from Heliotropium curassavicum Linn. with a C8 necic acid skeleton. J. Chem. Soc. Chem. Comm. 1978. [Google Scholar] [CrossRef]
- Subramanian, P.S.; Mohanraj, S.; Cockrum, P.A.; Culvenor, C.C.J.; Edgar, J.A.; Farhn, J.L.; Smith, L.W. The alkaloids of Heliotropium curassavicum. Aust. J. Chem. 1980, 33, 1357–1363. [Google Scholar] [CrossRef]
- Davicino, J.G.; Pestchanker, M.J.; Giodano, O.S. Pyrrolizidine alkaloids from Heliotropium curassavicum. Phytochemistry 1988, 27, 960–962. [Google Scholar] [CrossRef]
- Akramov, S.T.; Kiyamitdinova, F.; Yunusov, S.Y. Alkaloids of Rindera cyclodonata, Rinderia echinata and Heliotropium dasycarpum. Khim. Prir. Soedin. 1967, 3, 288–289. [Google Scholar]
- Hammouda, F.M.; Rizk, A.M.; Ismail, S.I.; Atteya, S.Z.; Ghaleb, H.A.; Madkour, M.K.; Pohland, A.E.; Wood, G. Poisonous plants containing edible ones and toxic substances in plant foods. Part 3, Pyrrolizidine alkaloids from Heliotropium digynum Forssk (H. luteum Poir.). Pharmazie 1984, 39, 703–705. [Google Scholar]
- Farsam, H.; Yassa, N.; Shafiee, A.; Amanlou, M. Pyrrolizidine alkaloids from Heliotropium disciforme. Pharm. Pharmacol. Lett. 1998, 8, 79–80. [Google Scholar]
- Shafiee, A.; Salimi, M.; Farsam, H.; Yassa, N. Pyrrolizidine alkaloids from Heliotropium dissitiflorum Boiss. DARU J. Pharm. Sci. 2002, 10, 168–170. [Google Scholar]
- Suri, O.P.; Sawhney, R.S.; Atal, C.K. Pyrrolizidine alkaloids from Heliotropium eichwaldii and Lindelofia spectabilis. Indian J. Chem. 1975, 13, 505–506. [Google Scholar]
- Yassa, N.H.; Farsam, H.; Shafiee, A.; Rustaiyan, A. Pyrrolizidine alkaloids from Heliotropium esfandiarii. Planta Med. 1996, 62, 583–584. [Google Scholar] [CrossRef]
- Yassa, N.H.; Farsam, H.; Rustaiyan, A.; Shafiee, A. Alkaloids of Boraginaceae II [1], pyrrolizidine alkaloids of Heliotropium europaeum L. population Garmsar. J. Sci. Islamic Repub. Iran 1999, 10, 39–42. [Google Scholar]
- Tosun, F.; Tamer, U. Determination of pyrrolizidine alkaloids in the seeds of Heliotropium europaeum by GC-MS. J. Fac. Pharm. Ankara 2004, 33, 7–9. [Google Scholar]
- Reina, M.; Gonzalez-Coloma, A.; Gutierrez, C.; Cabbera, R.; Henriquez, J.; Villarroel, L. Bioactive saturated pyrrolizidine alkaloid from Heliotropium floridum. Phytochemistry 1997, 46, 845–853. [Google Scholar]
- Constantinidis, T.; Harvala, C.; Skaltsounis, A.L. Pyrrolizidine N-oxide alkaloids of Heliotropium hirsutissimum. Phytochemistry 1993, 32, 1335–1337. [Google Scholar] [CrossRef]
- Souza, J.S.N.; Machado, L.L.; Pessoa, O.D.L.; Braz-Filho, R.; Overk, C.R.; Yao, P.; Cordell, G.A.; Lemos, T.L.G. Pyrrolizidine alkaloids from Heliotropium indicum. J. Braz. Chem. Soc. 2005, 16, 1410–1414. [Google Scholar] [CrossRef]
- Singh, J.P.; Pandey, D.P.; Singh, A.; Singh, R. Alkaloids of Heliotropium indicum. J. Ind. Chem. Soc. 2005, 82, 175–176. [Google Scholar]
- Dash, G.K.; Abdullah, M.S. A review on Heliotropium indicum L. (Boraginaceae). Int. J. Pharm. Sci. Res. 2013, 4, 1253–1258. [Google Scholar]
- Ravi, S.; Lakshmanan, A.J.; Herz, W. Isolycopsamine, a pyrrolizidine alkaloid from Heliotropium keralense. Phytochemistry 1990, 29, 361–364. [Google Scholar] [CrossRef]
- Reina, M.; Gonzalez-Coloma, A.; Gutierrez, C.; Cabbera, R.; Henriquez, J.; Villarroel, L. Pyrrolizidine alkaloids from Heliotropium megalanthum. J. Nat. Prod. 1998, 61, 1418–1420. [Google Scholar] [CrossRef]
- Kiyamitdinova, F.; Akramov, S.T.; Yunusov, S.Y. Alkaloids from the family Boraginaceae. Khim. Prir. Soedin. 1967, 3, 411–412. [Google Scholar]
- Mohanraj, S.; Kulanthaivel, P.; Subramanian, P.S.; Herz, W. Helifoline, a pyrrolizidine alkaloid from Heliotropium ovalifolium. Phytochemistry 1981, 20, 1991–1995. [Google Scholar] [CrossRef]
- Zalkow, L.H.; Gelbaum, L.; Keinan, E. Isolation of the pyrrolizidine alkaloid europine N-oxide from Heliotropium maris-mortui and H. rotundifolium. Phytochemistry 1978, 17, 172. [Google Scholar] [CrossRef]
- Asibal, C.F.; Gelaum, L.T.; Zalkow, L.H. Pyrrolizidine alkaloids from Heliotropium rotundifolium. J. Nat. Prod. 1989, 52, 726–731. [Google Scholar]
- Lakshmanan, A.J.; Shanmugasundaram, S. Heliscabine, a pyrrolizidine ester alkaloid from Heliotropium scabrum. Phytochemistry 1995, 39, 473–475. [Google Scholar] [CrossRef]
- Roeder, E.; Breitmaier, E.; Birecka, H.; Frohlich, M.W.; Badzies-Crombach, A. Pyrrolizidine alkaloids of Heliotropium spathulatum. Phytochemistry 1991, 30, 1703–1706. [Google Scholar] [CrossRef]
- Mattocks, A.R. Strigosine, the major alkaloid of Heliotropium strigosum. J. Chem. Soc. 1964, 1974–1977. [Google Scholar] [CrossRef]
- Singh, B.; Sahu, P.M.; Jain, S.C.; Singh, S. Antineoplastic and antiviral screening of pyrrolizidine alkaloids from Heliotropium subulatum. Pharm. Biol. 2002, 40, 581–586. [Google Scholar] [CrossRef]
- Crowley, H.C.; Culvenor, C.C.J. The alkaloids of Heliotropium supinum L., with observations on viridifloric acid. Aust. J. Chem. 1959, 12, 694–705. [Google Scholar] [CrossRef]
- Trigo, J.R.; Witte, L.; Brown, K.S.; Hartmann, T.; Barata, L. pyrrolizidine alkaloids in the acrtiid moth hyalurga syma. J. Chem. Ecol. 1993, 4, 669–679. [Google Scholar]
- Medina, J.C.M.; Gauze, G.F.; Vidott, G.J.; Sarragiotto, M.H.; Basso, E.A.; Peixoto, J.L.B. Structural characterization of saturated pyrrolizidine alkaloids from Heliotropium transalpinum var. transalpinum Vell by NMR spectroscopy and theoretical calculation. Tetrahedron Lett. 2009, 50, 2640–2642. [Google Scholar]
- Suri, K.A.; Suri, O.P.; Dhar, K L.; Atal, C.K. Chemical composition of Lappula glochidiata and Crotalaria anagyroides. Indian J. Chem. 1978, 16B, 78. [Google Scholar]
- Man’ko, I.V.; Vasil’kov, P.N. Lappula intermedia alkaloids. Tr. Leninger. Khim.-Farm. Inst. 1968, 26, 166. [Google Scholar]
- Wiedenfeld, H.; Amarsanaa, B.; Altanchimeg, D.; Narantuya, S. Pyrrolizidine alkaloid containing plants in Mongolian traditional medicine: Lappula myosotis Moench. Sci. Pharm. 2005, 73, 139–145. [Google Scholar]
- Kelly, H.A.; Robins, D.J. Pyrrolizidine alkaloids from Lindelofia longiflora. Fitoterapia 1990, 61, 89–90. [Google Scholar]
- Akramov, S.T.; Kiyamitdinova, F.; Yunusov, S.Y. Alkaloids of Solenanthus turkestanicus, Lindelofia olgae, and Trachelanthus korolkovi. Dokl. Akad. Nauk Uzb. SSR 1962, 19, 29. [Google Scholar]
- Akramov, S.T.; Kiyamitdinova, F.; Yunusov, S.Y. Alkaloids of Solenanthus circinatus, Paracaryum himalayense and Lindelofia pterocarpa. Dokl. Akad. Nauk Uzb. SSR 1964, 21, 28. [Google Scholar]
- Akramov, S.T.; Kiyamitdinova, F.; Yunusov, S.Y. Study of Rindera and Lindelofia. Dokl. Akad. Nauk Uzb. SSR 1965, 22, 35–38. [Google Scholar]
- Pietrosiuk, A.; Syklowska-Baranek, K.; Wiedenfeld, H.; Wolinowska, R.; Furmanowa, M.; Jaroszyk, E. The shikonin derivatives and pyrrolizidine alkaloids in hairy root cultures of Lithospermum canescens (Michx.) Lehm. Plant Cell Rep. 2006, 25, 1052–1058. [Google Scholar] [CrossRef]
- Wiedenfeld, H.; Pietrosiuk, A.; Furmanowa, M.; Roeder, E. Pyrrolizidine alkaloids from Lithospermum canescens Lehm. Z. Naturforsch. 2003, 58c, 173–176. [Google Scholar]
- Roeder, E.; Renge, B. Pyrrolizidine alkaloids from Lithospermum erythrorhizon. Phytochemistry 1990, 29, 690–693. [Google Scholar] [CrossRef]
- Krenn, L.; Wiedenfeld, H.; Roeder, E. Pyrrolizidine alkaloids from Lithospermum officinale. Phytochemistry 1994, 37, 275–277. [Google Scholar] [CrossRef]
- Li, Y.; Stermitz, F.R. Pyrrolizidine alkaloids from Mertensia species of Colorado. J. Nat. Prod. 1988, 51, 1289–1290. [Google Scholar] [CrossRef]
- Ogihara, K.; Miyagi, Y.; Higa, M.; Yogi, S. Pyrrolizidine alkaloids from Messerschmidia argentea. Phytochemistry 1997, 44, 545–547. [Google Scholar] [CrossRef]
- Resch, J.F.; Rosberger, D.F.; Meinwald, J.; Appling, J.W. Biologically active pyrrolizidine alkaloids from the true forget-me-not, Myosotis scorpioides. J. Nat. Prod. 1982, 45, 358–362. [Google Scholar] [CrossRef]
- Roeder, E.; Bourauel, T. Pyrrolizidine alkaloids from Neatostema apulum. Phytochemistry 1992, 31, 3613–3615. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H.; Kroeger, R.; Teppner, H. Pyrrolizidine alkaloids of three taxa of Onosma (Boraginaceae-Lithospermeae). Phyton 1993, 33, 41–49. [Google Scholar]
- Roeder, E.; Wiedenfeld, H.; Kersten, R.; Kröger, R. Determination of open chain pyrrolizidine alkaloids by capillary gas chromatography. Planta Med. 1990, 56, 522. [Google Scholar]
- El-Shazly, A.; Adel-Ghani, A.; Wink, M. Pyrrolizidine Alkaloids from Onosma arenaria Waldst. and Kit (Boraginaceae). Biochem. Syst. Ecol. 2003, 31, 477–485. [Google Scholar] [CrossRef]
- Damianakos, H.; Sotiroudis, G.; Chinou, I. Pyrrolizidine alkaloids from Onosma erecta. J. Nat. Prod. 2013, 76, 1892–1835. [Google Scholar]
- Mellidis, A.S.; Papageorgiou, V.P. Pyrrolizidine alkaloids of the plant Onosma heterophylla. Chem. Chron. 1988, 17, 67–73. [Google Scholar]
- Kretsi, O.; Aligiannis, N.; Skaltsounis, A.L.; Chinou, I.B. Pyrrolizidine alkaloids from Onosma leptantha. Helv. Chim. Acta 2003, 86, 3136–3140. [Google Scholar] [CrossRef]
- Mroczek, T.; Ndjoko, K.; Glowniak, K.; Hostetmann, K. On-line structure characterization of pyrrolizidine alkaloids in Onosma stellulatum and Emilia coccinea by liquid chromatography-ion-trap mass spectrometry. J. Chromatogr. A. 2004, 1056, 91–97. [Google Scholar]
- Haberer, W.; Witte, L.; Hartmann, T.; Dobler, S. Pyrrolizidine alkaloids in Pulmonaria obscura. Planta Med. 2002, 68, 480–482. [Google Scholar] [CrossRef]
- Mandić, B.M.; Simić, M.R.; Vučković, I.M.; Vujisić, L.V.; Novaković, M.M.; Snežana, S.; Trifunović, S.S.; Snežana, D.; Nikolić-Mandić, S.D.; Vele, V.; Tešević, V.V.; Vajs, V.V.; Milosavljević, S.M. Pyrrolizidine alkaloids and fatty acids from the endemic plant species Rindera umbellata and the effect of lindelofine-N-oxide on tubulin polymerization. Molecules 2013, 18, 10694–10706. [Google Scholar] [CrossRef]
- Kurucu, S.; Kartal, M.; Choudary, M.I.; Topcu, G. Pyrrolizidine Alkaloids from Symphytum sylvaticum Boiss. subsp. sepulcrale. (Boiss. & Bal.) Greuter & Burdet var. sepulcrale and Symphytum aintabicum Hub.-Mor. & Wickens. Turk J Chem. 2002, 26, 195–199. [Google Scholar]
- Huizing, H.J.; Gadella, T.W.G.; Kliphuis, E. Chemotaxonomical investigation of the Symphytum officinale polyploidy complex and S. asperum (Boraginaceae): The pyrrolizidine alkaloids. Plant Syst. Evol. 1982, 140, 279–292. [Google Scholar] [CrossRef]
- Roeder, E.; Bourauel, T.; Neuberger, V. Symviridine, a new pyrrolizidine alkaloids from Symphytum species. Phytochemistry 1992, 31, 4041–4042. [Google Scholar] [CrossRef]
- Jaarsma, T.A.; Lohmanns, E.; Gadella, T.W.J.; Malingre, T.M. Chemotaxonomy of the Symphytum officinale agg. (Boraginaceae). Plant Syst. Evol. 1989, 167, 113–127. [Google Scholar] [CrossRef]
- Mel’kumova, Z.V.; Telzhenetskaya, M.V.; Yunusov, S.Y.; Man’ko, I.V. Refinement of the structure of asperumine. Khim. Prir. Soed. 1974, 4, 478–480. [Google Scholar]
- Huizing, H.J.; Malinerg, T.M. Purification and separation of pyrrolizidine alkaloids from Boraginaceae on a polystyrene-divinylbenzene resin. J. Chromatogr. 1979, 176, 274–279. [Google Scholar] [CrossRef]
- Jaarsma, T.A.; Lohmanns, E.; Hendriks, H.; Gadella, T.W.J.; Malingre, T.M. Chemo-and karyotaxonomic studies on some rhizomatous species of the genus Symphytum (Boraginaceae). Plant Syst. Evol. 1990, 169, 31–39. [Google Scholar] [CrossRef]
- Liu, F.; Wan, S.Y.; Jiang, Z.; Li, S.F.; Ong, E.S.; Osorio, J.C. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography-electrospray ionisation mass spectrometry. Talanta 2009, 15, 916–923. [Google Scholar]
- Furuya, T.; Araki, K. Studies on constituents of crude drugs. 1. Alkaloids of Symphytum officinale Linn. Chem. Pharm. Bull. 1968, 16, 2512–2516. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H.; Stengl, P. 13C-NMR Daten der stereoisomeren Alkaloide aus Symphytum officinale L. Arc. Pharm. (Weinheim) 1982, 315, 87–88. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Edgar, J.A.; Frahn, J.L.; Smith, L.W. The alkaloids of Symphytum × uplandicum (Russian comfrey). Aust. J. Chem. 1980, 33, 1105–1113. [Google Scholar] [CrossRef]
- Omar, M.; Defeo, J.; Youngken, H.W. Chemical and toxicity studies of Trichodesma africanum L. J. Nat. Prod. 1983, 46, 153–156. [Google Scholar] [CrossRef]
- O’Kelly, J.; Sargeant, K. Supinine from the seeds of Trichodesma zeylanicum R. Br. J. Chem. Soc. 1961, 484. [Google Scholar]
- Khassanova, M.A.; Abdullaev, U.A.; Telezhenetskaya, M.V.; Yunusov, S.Yu. Structure of uluganine. Khim. Prir. Soedin. 1974, 6, 809–810. [Google Scholar]
- Roeder, E.; Wiedenfeld, H.; Schraut, R. Pyrrolizidine alkaloids from Alkanna tinctoria. Phytochemistry 1984, 23, 2125–2126. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Smith, L.W. Aquaternary N-dihydropyrrolizinomethyl derivative of heliotrine from Heliotropium Europaeum. Tetrahedron Lett. 1969, 41, 3603–3606. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Johns, S.R.; Smith, L.W. Acetyllasiocarpine, an alkaloids of Heliotropium europaeum. Aust. J. Chem. 1975, 28, 2319–2322. [Google Scholar] [CrossRef]
- Ravi, S.; Ravikumar, R.; Lakshmanan, A.J. Pyrrolizidine alkaloids from Cynoglossum furcatum. Nig. J. Nat. Prod. Med. 2007, 11, 87–89. [Google Scholar]
- Crews, C.; Berthiller, F.; Krska, R. Update on analytical methods for toxic pyrrolizidine alkaloids. Anal. Bioanal. Chem. 2010, 396, 327–338. [Google Scholar] [CrossRef]
- Crews, C. Methods for Analysis of Pyrrolizidine Alkaloids in Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer-Verlag: Berlin & Heidelberg, Germany, 2013; pp. 1049–1068. [Google Scholar]
- Zalkow, L.H.; Asibal, C.F.; Glinski, J.A.; Bonetti, S.J.; Gelbaum, L.T.; van Derveer, D.; Powis, G. Macrocyclic pyrrolizidine alkaloids from Senecio anonymus. Separation of a complex alkaloid extract using droplet counter-current chromatography. J. Nat. Prod. 1988, 51, 690–702. [Google Scholar]
- Bicchi, C.; Caniato, R.; Tabacchi, R.; Tsoupras, G. Capillary gas chromatography/positive and negative ion chemical ionization mass spectrometry on pyrrolizidine alkaloids of Senecio inaequidens using ammonia and hydroxyl ions as the reagent species. J. Nat. Prod. 1989, 52, 32–41. [Google Scholar]
- Stelljes, M.E.; Kelley, R.B.; Molyneux, R.J.; Seiber, J.N. GC-MS determination of pyrrolizidine alkaloids in four Senecio species. J. Nat. Prod. 1991, 54, 759–773. [Google Scholar]
- Witte, L.; Rubiolo, P.; Bicchi, C.; Hartmann, T. Comparative analysis of pyrrolizidine alkaloids from natural sources by gas chromatography-mass spectrometry. Phytochemistry 1993, 32, 187–196. [Google Scholar]
- El-Shazly, A. Pyrrolizidine alkaloid profiles of some Senecio species from Egypt. Z. Naturforsch. 2002, 57c, 429–433. [Google Scholar]
- Asres, K.; Sporer, F.; Wink, M. Patterns of pyrrolizidine alkaloids in 12 Ethiopian Crotalaria species. Biochem. Syst. Ecol. 2004, 32, 915–930. [Google Scholar] [CrossRef]
- Crews, C.; Startin, J.R.; Clarke, P.A. Determination of pyrrolizidine alkaloids in honey from selected sites by solid phase extraction and HPLC-MS. Food Addit. Contam. 1997, 14, 419–428. [Google Scholar] [CrossRef]
- Lin, G.; Zhou, K.Y.; Zhao, X.G.; Wang, Z.T.; But, P.P. Determination of hepatotoxic pyrrolizidine alkaloids by on-line high performance liquid chromatography mass spectrometry with an electrospray interface. Rapid Commun. Mass Spectrom. 1998, 12, 1445–1456. [Google Scholar]
- Zhang, F.; Wang, C.H.; Wang, W.; Chen, L.X.; Ma, H.Y.; Zhang, C.F.; Zhang, M.; Bligh, S.W.; Wang, Z.T. Quantitative analysis by HPLC-MS2 of the pyrrolizidine alkaloid adonifoline in Senecio scandens. Phytochem. Anal. 2008, 19, 25–31. [Google Scholar] [CrossRef]
- Ruan, J.; Li, N.; Xia, Q.; Fu, P.P.; Peng, S.; Ye, Y.; Lin, G. Characteristic ion clusters as determinants for the identification of pyrrolizidine alkaloid N-oxides in pyrrolizidine alkaloid–containing natural products using HPLC-MS analysis. J. Mass Spectrom. 2012, 47, 331–337. [Google Scholar] [CrossRef]
- Neuner-Jehle, N.; Nesvadba, H.; Spiteller, G. Anwendung der Massenspektrometric zur Strukturaufklärung von Alkaloiden, 6. Mitt. Mh. Chem. 1965, 96, 321–338. (In German) [Google Scholar]
- Pedersen, E.; Larsen, E. Mass spectrometry of some pyrrolizidine alkaloids. Org. Mass Spectrom 1970, 4, 249–256. [Google Scholar] [CrossRef]
- Culvenor, C.C.J.; Edgar, J.A.; Frahn, J.L.; Smith, L.W.; Ulubelen, A.; Doganca, S. The structure of anadaloine. Aust. J. Chem. 1975, 28, 173–178. [Google Scholar] [CrossRef]
- Wuilloud, J.C.A.; Gratz, S.R.; Gamble, B.M.; Wolnik, K.A. Simultaneous analysis of hepatotoxic pyrrolizidine alkaloids and N-oxides in comfrey root by LC-ion trap mass spectrometry. Analyst 2004, 129, 150–156. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H.; Hoeing, A. Pyrrolizidinalkaloide aus Senecio aureus. Planta Med. 1983, 49, 57–59. [Google Scholar]
- Flores, A.S.; de Azevedo Tozzi, A.M.G.; Trigo, J.R. Pyrrolizidine alkaloid profiles in Crotalaria species from Brazil: Chemotaxonomic significance. Biochem. Syst. Ecol. 2009, 37, 459–469. [Google Scholar]
- Mead, E.W.; Looker, M.; Gardner, D.R.; Stermitz, F.R. Pyrrolizidine alkaloids of Liatris punctata and its root parasite, Castilleja integra. Phytochemistry 1992, 31, 3255–3257. [Google Scholar] [CrossRef]
- Tei, A. Identifizierung und Strukturaufklärung von Alkaloiden und anderen Sekundärstoffen mit Hilfe von GC-MS und Kernresonanzspektroskopie. Ph.D. Thesis, Ruprecht-Karls-Universität Heidelberg, Heidelberg, Germany, 2000. [Google Scholar]
- Stermiz, F.R.; L’Empereur, K.M. Identity of “subulasine N-oxide” with 1β,2β-epoxy-1α-hydroxymethyl-8α-pyrrolizidine. Tetrahedron Lett. 1988, 29, 4943–4944. [Google Scholar] [CrossRef]
- Griffin, C.T.; Danaher, M.; Elliott, C.T.; Kennedy, D.G.; Furey, A. Detection of pyrrolizidine alkaloids in commercial honey using liquid chromatography–ion trap mass spectrometry. Food Chem. 2013, 136, 1577–1583. [Google Scholar] [CrossRef]
- Martinello, M.; Cristofoli, C.; Gallina, A.; Mutinelli, F. Easy and rapid method for the quantitative determination of pyrrolizidine alkaloids in honey by ultra performance liquid chromatography-mass spectrometry: An evaluation in commercial honey. Food Control 2014, 37, 146–152. [Google Scholar] [CrossRef]
- Logie, C.G.; Grue, M.R.; Liddell, J.R. Proton NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1994, 37, 43–109. [Google Scholar] [CrossRef]
- Roeder, E. Carbon-13 NMR spectroscopy of pyrrolizidine alkaloids. Phytochemistry 1990, 29, 11–29. [Google Scholar] [CrossRef]
- Bober, M.A.; Milco, L.A.; Miller, R.B.; Mount, M.; Wicks, B.; Kurth, M.J. A competitive enzyme-linked immunosorbent assay (ELISA), to detect retronecine and monocrotaline in vitro. Toxicon. 2004, 27, 1059–1064. [Google Scholar]
- Roeder, E.; Pflueger, T. Analysis of pyrrolizidine alkaloids: a competitive enzyme-linked immunoassay (ELISA) for the quantitative determination of some toxic pyrrolizidine alkaloids. Nat. Toxins. 1995, 3, 305–309. [Google Scholar] [CrossRef]
- Lee, S.T.; Schoch, T.K.; Stegelmeier, B.L.; Gardner, D.R.; Than, K.A.; Molyneus, R.J. Development of enzyme-linked immunosorbent assays for the hepatotoxic riddelliine and reddelliine N-oxide. J. Agric. Food Chem. 2001, 49, 4144–4151. [Google Scholar] [CrossRef]
- Cavallaro, V.; Than, K.A.; Colegate, S.M.; Edgar, J.A. An indirect competitive ELISA for pyrrolizidine alkaloids of Heliotropium europaeum. In Poisonous Plants and Related Toxins; Acamovic, T., Stewart, C.S., Pennycott, T.W., Eds.; CABI publishing: Wallingford, UK, 2004; pp. 114–119. [Google Scholar]
- Lee, S.T.; Knill, A.; Michalewicz, A.; Stevens, V.; Colegate, S.M. Heliotropium europaeum alkaloids: A quaternary pyrrolizidine alkaloids approach to elisa development. In Poisonous Plant Global Research and Solution; United States Department of Agriculture: Washington, DC, USA, 2007; Chapter 81; pp. 476–480. [Google Scholar]
- Von Borstel, K.; Hartmann, T. Selective uptake of pyrrolizidine N-oxides by cell suspension cultures from pyrrolizidine alkaloid producing plants. Plant Cell Rep. 1986, 5, 39–42. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Verpoorte, R.; Meijden, E.V.D. Extreme differences in pyrrolizidine alkaloid levels between leaves of Cynoglossum officinale. Phytochemistry 1994, 37, 1013–1016. [Google Scholar] [CrossRef]
- Frölich, C.; Ober, D.; Hartmann, T. Tissue distribution, core biosynthesis and diversification of pyrrolizidine alkaloids of the lycopsamine type in three Boraginaceae species. Phytochemistry 2007, 68, 1026–1037. [Google Scholar] [CrossRef]
- Abd El-Mawla, A.M. Effect of cretin elicitors on production of pyrrolizidine alkaloids in hairy root cultures of Echium rauwolfii. Pharmazie 2010, 65, 224–226. [Google Scholar]
- Marin-Loaiza, J.C.; Ernst, L.; Beuerle, T.; THeuring, C.; Cespedes, C.L.; Hartmann, T. Pyrrolizidine alkaloids of the endemic Mexican genus Pittocaulon and assignment of stereoisomeric 1,2-saturated necine bases. Phytochemistry 2008, 69, 154–167. [Google Scholar] [CrossRef]
- Okusa, P.N.; Penge, O.; Devleeschouwer, M.; Duez, P. Direct and indirect antimicrobial and antioxidant activity of Cordia dilletii De Wild (Boraginaceae). J. Ethanopharmacol. 2007, 112, 476–481. [Google Scholar] [CrossRef]
- Joosten, L.; van Veen, J.A. Defensive properties of pyrrolizidine alkaloids against microorganisms. Phytochem. Rev. 2011, 10, 127–136. [Google Scholar] [CrossRef]
- Wink, M. Allelochemical properties and raison d’être of alkaloids. In The Alkaloids; Cordell, G., Ed.; Academic Press: New York, NY, USA, 1993; vol. 43, pp. 1–118. [Google Scholar]
- Boppre, M. Insects pharmacophagously utilizing defensive plant chemicals (pyrrolizidine alkaloids). Naturwissenschaften 1986, 73, 17–26. [Google Scholar] [CrossRef]
- Boppre, M. Lepidoptera and pyrrolizidine alkaloids. Exemplification of complexity in chemical ecology. J. Chem. Ecol. 1990, 16, 165–185. [Google Scholar] [CrossRef]
- Von Nickisch-Rosenegk, E.; Wink, M. Sequestration of pyrrolizidine alkaloids in several arctiid moths (Lepidoptera: Arctiidae). J. Chem. Ecol. 1993, 19, 1889–1903. [Google Scholar] [CrossRef]
- Dobler, S.; Haberer, W.; Witte, L.; Hartmann, T. Selective sequestration of pyrrolizidine alkaloids from diverse host plants by Longitarsus flea beetles. J. Chem. Ecol. 2000, 5, 1281–1298. [Google Scholar]
- Honda, Y.; Honda, K.; Omura, H. Major components in the hairpencil secretion of a butterfly, Euploea mulciber (Lepidoptera, Danaidae): Their origin and male behavioral responses to pyrrolizidine alkaloids. J. Insect Physiol. 2006, 52, 1043–1053. [Google Scholar] [CrossRef]
- Beuerle, T.; Theuring, C.; Klewer, N.; Schulz, S.; Hartmann, T. Absolute configuration of the creatonotines and callimorphines, two classes of arctiid-specific pyrrolizidine alkaloids. Insect Biochem. Mol. Biol. 2007, 37, 80–89. [Google Scholar] [CrossRef]
- Macel, M. Attract and deter: A dual role for pyrrolizidine alkaloids in plant-insect interaction. Phytochem. Rev. 2011, 10, 75–82. [Google Scholar] [CrossRef]
- Denmark, S.E.; Hurd, A.R. Synthesis of (+)-casuarine. J. Org. Chem. 2000, 65, 2875–2886. [Google Scholar] [CrossRef]
- Asano, N.; Ikeda, K.; Kasahara, M.; Arai, Y.; Kizu, H. Glycosidase-inhibiting pyrrolidines and pyrrolizidines with long side chain in Scilla peruviana. J. Nat. Prod. 2004, 67, 846–850. [Google Scholar] [CrossRef]
- Garcia-Moreno, M.I.; Rodriguez-Lucena, D.; Mellet, C.O.; Garcia Fernandez, J.M. Pseudoamide-type pyrrolidine and pyrrolizidine glycomimetics and their inhibitory activities against glycosidases. J. Org. Chem. 2004, 69, 3578–3581. [Google Scholar] [CrossRef]
- Nibret, E.; Sporer, F.; Asres, K.; Wink, M. Antitrypanosomal and cytotoxic activity of pyrrolizidine alkaloid-producing plants of Ethiopia. J. Pharm. Pharmacol. 2009, 61, 801–808. [Google Scholar] [CrossRef]
- Singh, B.; Sahu, P.M.; Singh, S. Antimicrobial activity of alkaloids from Heliotropium subulatum. Fitoterapia 2002, 73, 153–155. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Pfister, J.A.; Molyneux, R.J.; Baker, D.C. Pyrrolizidine alkaloid content of hounds tongue (Cynoglossum officinale L.). J. Range Managem. 1992, 45, 254–256. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).