Can Climate Change Trigger Massive Diversity Cascades in Terrestrial Ecosystems?
Abstract
:1. Introduction
2. Cascading Effects Involving Diversity Parameters
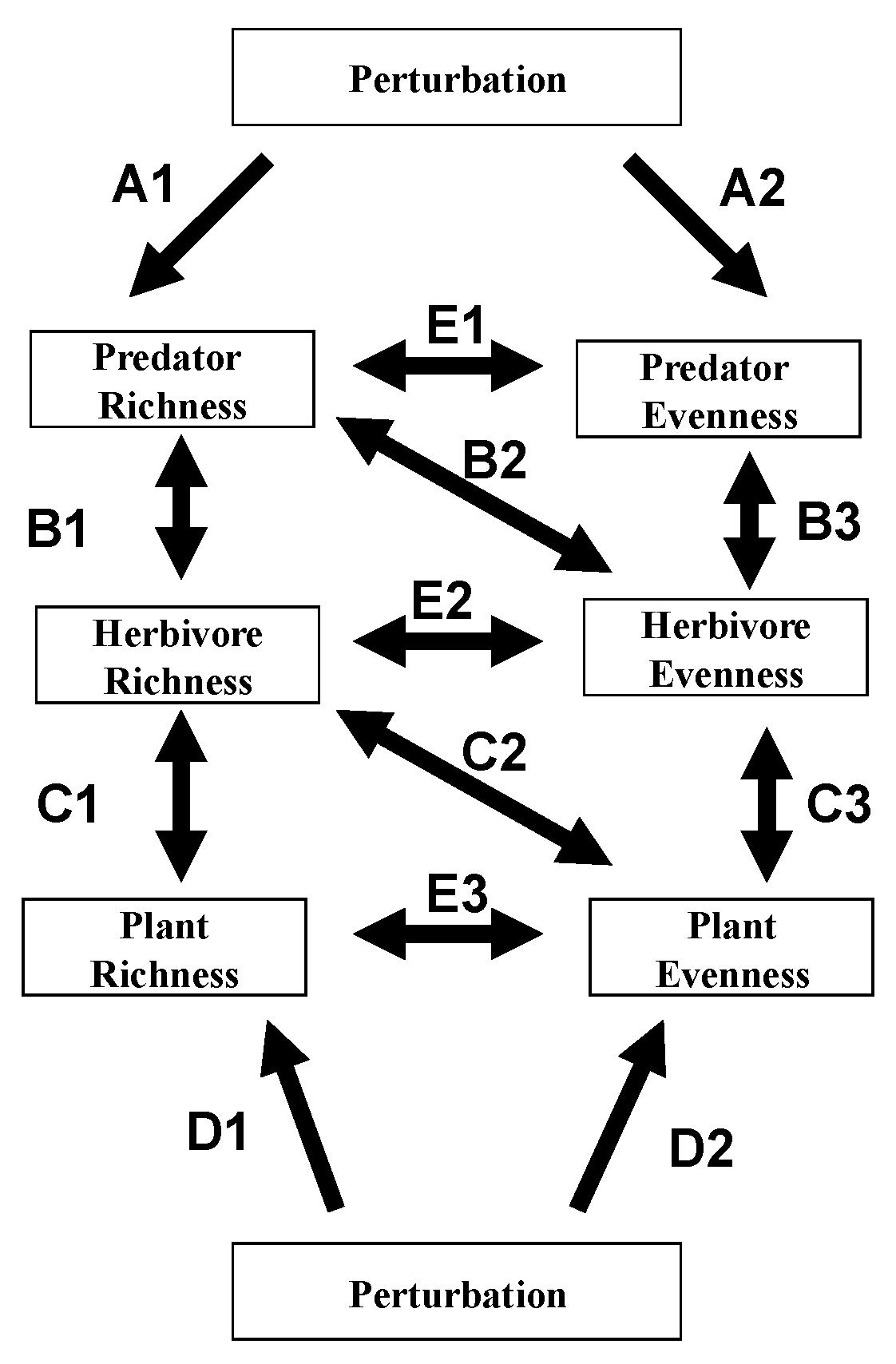
3. Cascading Effects in Tropical, Terrestrial Systems
4. Bottom-Up Diversity Cascades
5. Top-Down Diversity Cascades

6. Summary of Mechanisms
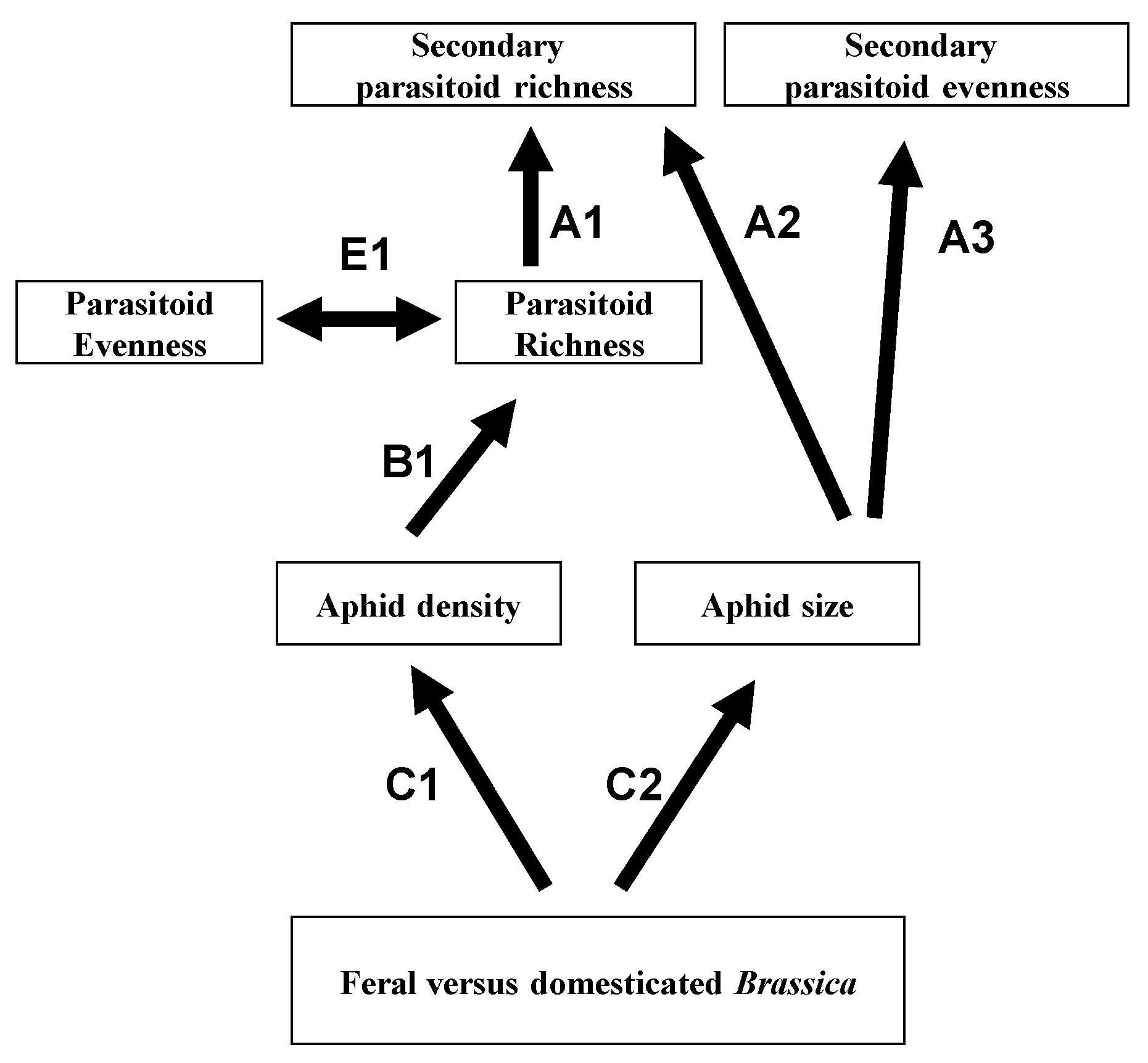
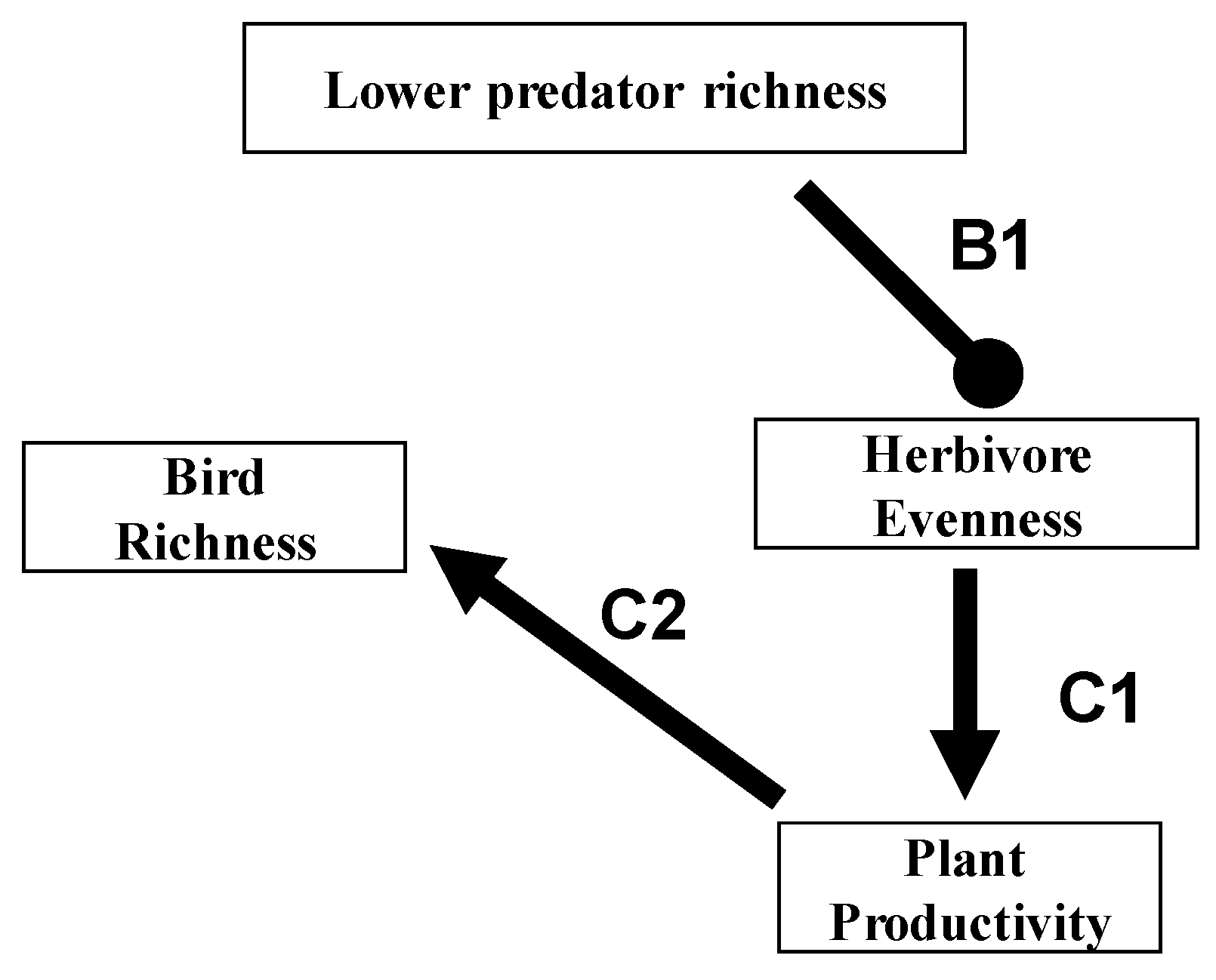
7. Relevance to Agriculture, Conservation and Emerging Infectious Disease
8. Climate Change and Biologically Significant Diversity Cascades
9. Future Directions
Hypotheses
10. Conclusions
Acknowledgments
Conflict of Interest
References
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar]
- Thompson, J.N. Conserving interaction biodiversity. In The Ecological Basis of Conservation: Heterogeneity, Ecosystems, and Biodiversity; Pickett, S.T.A., Ostfeld, R.S., Shachak, M., Likens, G.E., Eds.; Chapman & Hall: New York, NY, USA, 1997; pp. 285–293. [Google Scholar]
- Lewinsohn, T.M.; Roslin, T. Four ways towards tropical herbivore megadiversity. Ecol. Lett. 2008, 11, 398–416. [Google Scholar] [CrossRef]
- Carson, W.P.; Schnitzer, S.A. Tropical Forest Community Ecology; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Price, P.W.; Bouton, E.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weiss, A.E. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 1980, 11, 41–65. [Google Scholar]
- Tscharntke, T.; Hawkins, B.A. Multitrophic Level Interactions; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Singer, M.S.; Stireman, J.O. The tri-trophic niche concept and adaptive radiation of phytophagous insects. Ecol. Lett. 2005, 8, 1247–1255. [Google Scholar] [CrossRef]
- Abrams, P.; Menge, B.; Mittelbach, G.G.; Spiller, D.; Yodzis, P. The role of indirect effects in food webs. In Food Webs: Integration of Pattern and Dynamics; Polis, G.A., Winemiller, K.O., Eds.; Chapman & Hall: New York, NY, USA, 1995; pp. 371–395. [Google Scholar]
- Menge, B.A. Indirect effects in marine rocky intertidal interaction webs—Patterns and importance. Ecol. Monogr. 1995, 65, 21–74. [Google Scholar]
- Peckarsky, B.L.; Abrams, P.A.; Bolnick, D.I.; Dill, L.M.; Grabowski, J.H.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Preisser, E.L.; Schmitz, O.J.; et al. Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator-prey interactions. Ecology 2008, 89, 2416–2425. [Google Scholar] [CrossRef]
- Janzen, D.H. Deflowering of Central-America. Nat. Hist. 1974, 83, 48–53. [Google Scholar]
- Thompson, J.N. Evolutionary ecology and the conservation of biodiversity. Trends Ecol. Evol. 1996, 11, 300–303. [Google Scholar] [CrossRef]
- Del-Claro, K. Multitrophic relationships, conditional mutualisms, and the study of interaction biodiversity in tropical savannas. Neotrop. Entomol. 2004, 33, 665–672. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Tscharntke, T.; Lewis, O.T. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 2007, 445, 202–205. [Google Scholar] [CrossRef]
- Dyer, L.A.; Walla, T.R.; Greeney, H.F.; Stireman, J.O.; Hazen, R.F. Diversity of interactions: A metric for studies of biodiversity. Biotropica 2010, 42, 281–289. [Google Scholar] [CrossRef]
- Hairston, N.G.; Smith, F.E.; Slobodkin, L.B. Community structure, population control, and competition. Am. Nat. 1960, 94, 421–424. [Google Scholar]
- Paine, R.T. Food webs: Linkage, interaction strength and community infrastructure. J. Anim. Ecol. 1980, 49, 667–685. [Google Scholar] [CrossRef]
- Power, M.E. Effects of fish in river food webs. Science 1990, 250, 811–814. [Google Scholar]
- Carpenter, S.R.; Kitchell, J.F. The Trophic Cascade in Lakes; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Dyer, L.A.; Letourneau, D.K. Top-down and bottom-up diversity cascades in detrital vs. living food webs. Ecol. Lett. 2003, 6, 60–68. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Krivan, V.; Ovadia, O. Trophic cascades: The primacy of trait-mediated indirect interactions. Ecol. Lett. 2004, 7, 153–163. [Google Scholar] [CrossRef]
- Borrvall, C.; Ebenman, B.; Jonsson, T. Biodiversity lessens the risk of cascading extinction in model food webs. Ecol. Lett. 2000, 3, 131–136. [Google Scholar] [CrossRef]
- Borrvall, C.; Ebenman, B. Biodiversity and persistence of ecological communities in variable environments. Ecol. Complexity 2008, 5, 99–105. [Google Scholar] [CrossRef]
- Petchey, O.L.; Downing, A.L.; Mittelbach, G.G.; Persson, L.; Steiner, C.F.; Warren, P.H.; Woodward, G. Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 2004, 104, 467–478. [Google Scholar] [CrossRef]
- Thebault, E.; Huber, V.; Loreau, M. Cascading extinctions and ecosystem functioning: contrasting effects of diversity depending on food web structure. Oikos 2007, 116, 163–173. [Google Scholar] [CrossRef]
- Hillebrand, H.; Shurin, J.B. Biodiversity and aquatic food webs. In Aquatic Food Webs: An Ecosystem Approach; Belgrano, A., Scharler, U.M., Dunne, J., Ulanowicz, R.E., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 183–198. [Google Scholar]
- Dyer, L.A. The ecology of tri-trophic interactions in the tropics. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Pearson, C.V.; Dyer, L.A. Trophic diversity in two grassland ecosystems. J. Insect Sci. 2006. [Google Scholar] [CrossRef]
- Schmitz, O.J. Predators have large effects on ecosystem properties by changing plant diversity, not plant biomass. Ecology 2006, 87, 1432–1437. [Google Scholar] [CrossRef]
- Sinclair, A.R.E.; Mduma, S.; Brashares, J.S. Patterns of predation in a diverse predator-prey system. Nature 2003, 425, 288–290. [Google Scholar] [CrossRef]
- Abrams, P.A. Trait-initiated indirect effects due to changes in consumption rates in simple food webs. Ecology 2004, 85, 1029–1038. [Google Scholar] [CrossRef]
- Menge, B.A. Detection of direct versus indirect effects: Were experiments long enough? Am. Nat. 1997, 149, 801–823. [Google Scholar]
- Crowder, D.W.; Northfield, T.D.; Strand, M.R.; Snyder, W.E. Organic agriculture promotes evenness and natural pest control. Nature 2010, 466, 109–113. [Google Scholar] [CrossRef]
- Polis, G.A.; Strong, D.R. Food web complexity and community dynamics. Am. Nat. 1996, 147, 813–846. [Google Scholar]
- Menge, B.A. Top-down and bottom-up community regulation in marine rocky intertidal habitats. J. Exp. Mar. Biol. Ecol. 2000, 250, 257–289. [Google Scholar] [CrossRef]
- Halaj, J.; Wise, D.H. Terrestrial trophic cascades: How much do they trickle? Am. Nat. 2001, 157, 262–281. [Google Scholar]
- Shurin, J.B.; Borer, E.T.; Seabloom, E.W.; Anderson, K.; Blanchette, C.A.; Broitman, B.; Cooper, S.D.; Halpern, B.S. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 2002, 5, 785–791. [Google Scholar] [CrossRef]
- Shurin, J.B.; Gruner, D.S.; Hillebrand, H. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proc. R. Soc. B 2006, 273, 1–9. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Shurin, J.B.; Anderson, K.E.; Blanchette, C.A.; Broitman, B.; Cooper, S.D.; Halpern, B.S. What determines the strength of a trophic cascade? Ecology 2005, 86, 528–537. [Google Scholar]
- Hunter, M.D. Multiple approaches to estimating the relative importance of top-down and bottom-up forces on insect populations: Experiments, life tables, and time-series analysis. Basic Appl. Ecol. 2001, 2, 295–309. [Google Scholar] [CrossRef]
- Terborgh, J.; Lopez, L.; Nunez, P.; Rao, M.; Shahabuddin, G.; Orihuela, G.; Riveros, M.; Ascanio, R.; Adler, G.H.; Lambert, T.D.; et al. Ecological meltdown in predator-free forest fragments. Science 2001, 294, 1923–1926. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Hamback, P.A.; Beckerman, A.P. Trophic cascades in terrestrial systems: A review of the effects of carnivore removals on plants. Am. Nat. 2000, 155, 141–153. [Google Scholar] [CrossRef]
- Bell, T.; Neill, W.E.; Schluter, D. The effect of temporal scale on the outcome of trophic cascade experiments. Oecologia 2003, 134, 578–586. [Google Scholar]
- Knight, T.M.; Chase, J.M.; Hillebrand, H.; Holt, R.D. Predation on mutualists can reduce the strength of trophic cascades. Ecol. Lett. 2006, 9, 1173–1178. [Google Scholar] [CrossRef]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thebault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef]
- Reiss, J.; Bridle, J.R.; Montoya, J.M.; Woodward, G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 2009, 24, 505–514. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Lau, J.A.; Schoener, T.W.; Tiffin, P. Evolution in ecological field experiments: Implications for effect size. Ecol. Lett. 2008, 11, 199–207. [Google Scholar] [CrossRef]
- Oksanen, L.; Fretwell, S.; Arruda, J.; Niemela, P. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 1981, 118, 240–261. [Google Scholar]
- Hunter, M.D.; Price, P.W. Playing chutes and ladders: Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 1992, 73, 724–732. [Google Scholar]
- Fretwell, S.D. The regulation of plant communities by food chains exploiting them. Perspect. Biol. Med. 1977, 20, 169–185. [Google Scholar]
- Fretwell, S.D. Food chain dynamics: The central theory of ecology? Oikos 1987, 50, 291–301. [Google Scholar] [CrossRef]
- Oksanen, L. Trophic levels and trophic dynamics: A consensus emerging? Trends Ecol. Evol. 1991, 6, 58–60. [Google Scholar] [CrossRef]
- Hairston, J.; Hairston, S. Cause-effect relationships in energy flow, trophic structure, and interspecific interactions. Am. Nat. 1997, 142, 379–411. [Google Scholar]
- Rohde, K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos 1992, 65, 514–527. [Google Scholar] [CrossRef]
- Waide, R.B.; Willig, M.R.; Steiner, C.F.; Mittelbach, G.; Gough, L.; Dodson, S.I.; Juday, G.P.; Parmenter, R. The relationship between productivity and species richness. Annu. Rev. Ecol. Syst. 1999, 30, 257–300. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Steiner, C.F.; Scheiner, S.M.; Gross, K.L.; Reynolds, H.L.; Waide, R.B.; Willig, M.R.; Dodson, S.I.; Gough, L. What is the observed relationship between species richness and productivity? Ecology 2001, 82, 2381–2396. [Google Scholar]
- Arita, H.T.; Vazquez-Dominguez, E. The tropics: cradle, museum or casino? A dynamic null model for latitudinal gradients of species diversity. Ecol. Lett. 2008, 11, 653–663. [Google Scholar] [CrossRef]
- MacArthur, R.H. Species packing and competitive equilibrium for many species. Theor. Popul. Biol. 1970, 1, 1–11. [Google Scholar] [CrossRef]
- May, R.M. Stability and Complexity in Model Ecosystems; Princeton University Press: Princeton, NJ, USA, 1973. [Google Scholar]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Brandle, M.; Amarell, U.; Auge, H.; Klotz, S.; Brandl, R. Plant and insect diversity along a pollution gradient: Understanding species richness across trophic levels. Biodivers. Conserv. 2001, 10, 1497–1511. [Google Scholar] [CrossRef]
- Pearson, C.V.; Massad, T.J.; Dyer, L.A. Diversity cascades in alfalfa fields: From plant quality to agroecosystem diversity. Environ. Entomology 2008, 37, 947–955. [Google Scholar] [CrossRef]
- Schmied, A.; Fuhrer, E. The impact of ground beetle species (Coleoptera: Carabidae) in spruce stands, damaged by Pristiphora abietina (Hymenoptera: Tenthredinidae). Entomol. Gen. 1996, 21, 81–94. [Google Scholar]
- Gruner, D.S.; Taylor, A.D. Richness and species composition of arboreal arthropods affected by nutrients and predators: A press experiment. Oecologia 2006, 147, 714–724. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef]
- Crutsinger, G.M.; Collins, M.D.; Fordyce, J.A.; Gompert, Z.; Nice, C.C.; Sanders, N.J. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 2006, 313, 966–968. [Google Scholar] [CrossRef]
- Wimp, G.M.; Wooley, S.; Bangert, R.K.; Young, W.P.; Martinsen, G.D.; Keim, P.; Rehill, B.; Lindroth, R.L.; Whitham, T.G. Plant genetics predicts intra-annual variation in phytochemistry and arthropod community structure. Mole. Ecol. 2007, 16, 5057–5069. [Google Scholar] [CrossRef]
- Strong, D.R.; Lawton, J.H.; Southwood, T.R.E. Insects on Plants: Community Patterns and Mechanisms; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Rudgers, J.A.; Clay, K. An invasive plant-fungal mutualism reduces arthropod diversity. Ecol. Lett. 2008, 11, 831–840. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Plant quality and predation risk mediated by plant ontogeny: consequences for herbivores and plants. Oikos 2006, 115, 559–572. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Vellend, M. Biodiversity-ecosystem function research: Is it relevant to conservation? Annu. Rev. Ecol. Evol. Syst. 2005, 36, 267–294. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Jedlicka, J.A.; Bothwell, S.G.; Moreno, C.R. Effects of Natural Enemy Biodiversity on the Suppression of Arthropod Herbivores in Terrestrial Ecosystems. Ecol. Evol. Syst. 2009, 40, 573–592. [Google Scholar]
- Hochberg, M.E. Consequences for host population levels of increasing natural enemy species richness in classical biological control. Am. Nat. 1996, 147, 307–318. [Google Scholar]
- Denoth, M.; Frid, L.; Myers, J.H. Multiple agents in biological control: improving the odds? Biol. Control 2002, 24, 20–30. [Google Scholar] [CrossRef]
- Stireman, J.O.; Dyer, L.A.; Matlock, R.B. Top-down forces in managed versus unmanaged habitats. In Ecology of Predator-Prey Interactions; Barbosa, P., Castellanos, I., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 303–323. [Google Scholar]
- Otto, S.B.; Berlow, E.L.; Rank, N.E.; Smiley, J.; Brose, U. Predator diversity and identity drive interaction strength and trophic cascades in a food web. Ecology 2008, 89, 134–144. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Harvey, C.T.; Gross, K.; Ives, A.R. Biodiversity and biocontrol: Emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 2003, 6, 857–865. [Google Scholar] [CrossRef]
- Schmitz, O.J. Top predator control of plant biodiversity and productivity in an old-field ecosystem. Ecol. Lett. 2003, 6, 156–163. [Google Scholar] [CrossRef]
- Schmitz, O.J. Effects of predator hunting mode on grassland ecosystem function. Science 2008, 319, 952–954. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Dyer, L.A. Experimental test in lowland tropical forest shows top-down effects through four trophic levels. Ecology 1998, 79, 1678–1687. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Dyer, L.A. Density patterns of Piper ant-plants and associated arthropods: Top predator cascades in a terrestrial system? Biotropica 1998, 30, 162–169. [Google Scholar]
- Letourneau, D.K.; Dyer, L.A.; Vega, G.C. Indirect effects of a top predator on a rain forest understory plant community. Ecology 2004, 85, 2144–2152. [Google Scholar] [CrossRef]
- Holt, R.D. Predation, apparent competition and the structure of prey communities. Theor. Popul. Biol. 1977, 12, 197–229. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutierrez, C.; Lopez, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- Losey, J.E.; Denno, R.F. Factors facilitating synergistic predation: The central role of synchrony. Ecol. Appl. 1999, 9, 378–386. [Google Scholar] [CrossRef]
- Losey, E.; Denno, R.F. Interspecific variation in the escape responses of aphids: Effect on risk of predation from foliar-foraging and ground-foraging predators. Oecologia 1998, 115, 245–252. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef]
- Ives, A.R.; Klug, J.L.; Gross, K. Stability and species richness in complex communities. Ecol. Lett. 2000, 3, 399–411. [Google Scholar] [CrossRef]
- Soluk, D.A.; Collins, N.C. Synergistic Interactions Between Fish and Stoneflies Facilitation and Interference Among Stream Predators. Oikos 1988, 52, 94–100. [Google Scholar] [CrossRef]
- Jonsson, M.; Malmqvist, B. Mechanisms behind positive diversity effects on ecosystem functioning: Testing the facilitation and interference hypotheses. Oecologia 2003, 134, 554–559. [Google Scholar]
- Cardinale, B.J.; Palmer, M.A.; Collins, S.L. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 2002, 415, 426–429. [Google Scholar] [CrossRef]
- Ives, A.R.; Murray, D.L. Can sublethal parasitism destabilize predator-prey population dynamics? A model of snowshoe hares, predators and parasites. J. Anim. Ecol. 1997, 66, 265–278. [Google Scholar] [CrossRef]
- Quicke, D.L.J. Parasitic Wasps; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Siemann, E.; Tilman, D.; Haarstad, J.; Ritchie, M. Experimental tests of the dependence of arthropod diversity on plant diversity. Am. Nat. 1998, 152, 738–750. [Google Scholar]
- Bukovinszky, T.; van Veen, F.J.F.; Jongema, Y.; Dicke, M. Direct and indirect effects of resource quality on food web structure. Science 2008, 319, 804–807. [Google Scholar] [CrossRef]
- Siemann, E. Experimental tests of effects of plant productivity and diversity on grassland arthropod diversity. Ecology 1998, 79, 2057–2070. [Google Scholar] [CrossRef]
- Feeley, K.J.; Terborgh, J.W. Direct versus indirect effects of habitat reduction on the loss of avian species from tropical forest fragments. Anim. Conserv. 2008, 11, 353–360. [Google Scholar] [CrossRef]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecol. Monogr. 1973, 43, 95–124. [Google Scholar] [CrossRef]
- Gurr, G.M.; Van Emden, H.F.; Wratten, S.D. Habitat manipulation and natural enemy efficiency implications for the control of pests. In Conservation Biological Control; Barbosa, P., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 155–184. [Google Scholar]
- Rosenheim, J.A.; Wilhoit, L.R.; Armer, C.A. Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 1993, 96, 439–449. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Species richness and parasitism in a fragmental landscape: Experiments and field studies with insects on Vicia sepium. Oecologia 2000, 122, 129–137. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Generalist predators disrupt biological control by a specialist parasitoid. Ecology 2001, 82, 705–716. [Google Scholar] [CrossRef]
- Snyder, W.E.; Wise, D.H. Contrasting trophic cascades generated by a community of generalist predators. Ecology 2001, 82, 1571–1583. [Google Scholar] [CrossRef]
- Persad, A.B.; Hoy, M.A. Predation by Solenopsis invicta and Blattella asahinai on Toxoptera citricida parasitized by Lysiphlebus testaceipes and Lipolexis oregmae on citrus in Florida. Biol. Control 2004, 30, 531–537. [Google Scholar] [CrossRef]
- Philpott, S.M.; Maldonado, J.; Vandermeer, J.; Perfecto, I. Taking trophic cascades up a level: Behaviorally-modified effects of phorid flies on ants and ant prey in coffee agroecosystems. Oikos 2004, 105, 141–147. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Glik, T.E.; Goeriz, R.E.; Ramert, B. Linking a predator’s foraging behavior with its effects on herbivore population suppression. Ecology 2004, 85, 3362–3372. [Google Scholar] [CrossRef]
- Snyder, W.E.; Snyder, G.B.; Finke, D.L.; Straub, C.S. Predator biodiversity strengthens herbivore suppression. Ecol. Lett. 2006, 9, 789–796. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Bothwell, S.G. Comparison of organic and conventional farms: Challenging ecologists to make biodiversity functional. Front. Ecol. Environ. 2008, 6, 430–438. [Google Scholar] [CrossRef]
- Mills, N.J.; Kenis, M. A Study of the Parasitoid Complex of the European Fir Budworm, Choristoneura murinana (Lepidoptera, Tortricidae), and Its Relevance for Biological-Control of Related Hosts. Bull. Entomol. Res. 1991, 81, 429–436. [Google Scholar] [CrossRef]
- Compton, S.G.; Hawkins, B.A. Determinants of species richness in southern african fig wasp assemblages. Oecologia 1992, 91, 68–74. [Google Scholar]
- Russell, E.P. Enemies hypothesis—A review of the effect of vegetational diversity on predatory insects and parasitoids. Environ. Entomol. 1989, 18, 590–599. [Google Scholar]
- Letourneau, D.K. Plant-arthropod interactions in agroecosystems. In Agricultural ecology; Jackson, L.E., Ed.; Academic Press: New York, NY, USA, 1997; pp. 239–290. [Google Scholar]
- Ale, S.B.; Whelan, C.J. Reappraisal of the role of big, fierce predators! Biodivers. Conserv. 2008, 17, 685–690. [Google Scholar] [CrossRef]
- Keesing, F.; Holt, R.D.; Ostfeld, R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006, 9, 485–498. [Google Scholar] [CrossRef]
- Carlson, J.C.; Dyer, L.A.; Omlin, F.X.; Beier, J.C. Diversity cascades and malaria vectors. J. Med. Entomol. 2009, 46, 460–464. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Biodiversity and disease risk: The case of lyme disease. Conserv. Biol. 2000, 14, 722–728. [Google Scholar] [CrossRef]
- Logiudice, K.; Duerr, S.T.K.; Newhouse, M.J.; Schmidt, K.A.; Killilea, M.E.; Ostfeld, R.S. Impact of host community composition on lyme disease risk. Ecology 2008, 89, 2841–2849. [Google Scholar] [CrossRef]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Araujo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]
- Bannerman, J.A.; Gillespie, D.R.; Roitberg, B.D. The impacts of extreme and fluctuating temperatures on trait-mediated indirect aphid-parasitoid interactions. Ecol. Entomol. 2011, 36, 490–498. [Google Scholar] [CrossRef]
- Lurgi, M.; Lopez, B.C.; Montoya, J.M. Novel communities from climate change. Phil. Trans. R. Soc. B 2012, 367, 2913–2922. [Google Scholar] [CrossRef]
- Lurgi, M.; Lopez, B.C.; Montoya, J.M. Climate change impacts on body size and food web structure on mountain ecosystems. Phil. Trans. R. Soc. B 2012, 367, 3050–3057. [Google Scholar] [CrossRef]
- Gillespie, D.R.; Nasreen, A.; Moffat, C.E.; Clarke, P.; Roitberg, B.D. Effects of simulated heat waves on an experimental community of pepper plants, green peach aphids and two parasitoid species. Oikos 2012, 121, 149–159. [Google Scholar] [CrossRef]
- De Sassi, C.; Tylianakis, J.M. Climate change disproportionately increases herbivore over plant or parasitoid biomass. PloS One 2012, 7, e40557. [Google Scholar] [CrossRef]
- Brose, U.; Dunne, J.A.; Montoya, J.M.; Petchey, O.L.; Schneider, F.D.; Jacob, U. Climate change in size-structured ecosystems Introduction. Phil. Trans. R. Soc. B 2012, 367, 2903–2912. [Google Scholar] [CrossRef]
- Harrington, R.; Woiwod, I.; Sparks, T. Climate change and trophic interactions. Trends Ecol. Evol. 1999, 14, 146–149. [Google Scholar] [CrossRef]
- Kratina, P.; Greig, H.S.; Thompson, P.L.; Carvalho-Pereira, T.S.A.; Shurin, J.B. Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 2012, 93, 1421–1430. [Google Scholar] [CrossRef]
- Petchey, O.L.; McPhearson, P.T.; Casey, T.M.; Morin, P.J. Environmental warming alters food-web structure and ecosystem function. Nature 1999, 402, 69–72. [Google Scholar] [CrossRef]
- Traill, L.W.; Lim, M.L.M.; Sodhi, N.S.; Bradshaw, C.J.A. Mechanisms driving change: Altered species interactions and ecosystem function through global warming. J. Anim. Ecol. 2010, 79, 937–947. [Google Scholar] [CrossRef]
- Harmon, J.P.; Moran, N.A.; Ives, A.R. Species response to environmental change: Impacts of food web interactions and evolution. Science 2009, 323, 1347–1350. [Google Scholar] [CrossRef]
- Hoekman, D. Turning up the heat: Temperature influences the relative importance of top-down and bottom-up effects. Ecology 2010, 91, 2819–2825. [Google Scholar] [CrossRef]
- Merrill, R.M.; Gutierrez, D.; Lewis, O.T.; Gutierrez, J.; Diez, S.B.; Wilson, R.J. Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J. Anim. Ecol. 2008, 77, 145–155. [Google Scholar] [CrossRef]
- Voigt, W.; Perner, J.; Jones, T.H. Using functional groups to investigate community response to environmental changes: Two grassland case studies. Global Change Biol. 2007, 13, 1710–1721. [Google Scholar] [CrossRef]
- Binzer, A.; Guill, C.; Brose, U.; Rall, B.C. The dynamics of food chains under climate change and nutrient enrichment. Phil. Trans. R. Soc. B 2012, 367, 2935–2944. [Google Scholar] [CrossRef]
- De Sassi, C.; Lewis, O.T.; Tylianakis, J.M. Plant-mediated and nonadditive effects of two global change drivers on an insect herbivore community. Ecology 2012, 93, 1892–1901. [Google Scholar] [CrossRef]
- Jeffs, C.T.; Lewis, O.T. Effects of climate warming on hostparasitoid interactions. Ecol. Entomol. 2013, 38, 209–218. [Google Scholar] [CrossRef]
- Newman, J.A. Climate change and cereal aphids: the relative effects of increasing CO2 and temperature on aphid population dynamics. Global Change Biol. 2004, 10, 5–15. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: A metaanalysis. Global Change Biol. 2006, 12, 27–41. [Google Scholar] [CrossRef]
- Shurin, J.B.; Clasen, J.L.; Greig, H.S.; Kratina, P.; Thompson, P.L. Warming shifts top-down and bottom-up control of pond food web structure and function. Phil. Trans. R. Soc. B 2012, 367, 3008–3017. [Google Scholar] [CrossRef]
- O’Connor, M.I.; Gilbert, B.; Brown, C.J. Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. Am. Nat. 2011, 178, 626–638. [Google Scholar] [CrossRef]
- Petchey, O.L.; Brose, U.; Rall, B.C. Predicting the effects of temperature on food web connectance. Phil. Trans. R. Soc. B 2010, 365, 2081–2091. [Google Scholar] [CrossRef]
- De Sassi, C.; Staniczenko, P.P.A.; Tylianakis, J.M. Warming and nitrogen affect size structuring and density dependence in a host-parasitoid food web. Phil. Trans. R. Soc. B 2012, 367, 3033–3041. [Google Scholar] [CrossRef]
- Hoover, J.K.; Newman, J.A. Tritrophic interactions in the context of climate change: A model of grasses, cereal Aphids and their parasitoids. Global Change Biol. 2004, 10, 1197–1208. [Google Scholar] [CrossRef]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Stireman, J.O.; Dyer, L.A.; Janzen, D.H.; Singer, M.S.; Li, J.T.; Marquis, R.J.; Ricklefs, R.E.; Gentry, G.L.; Hallwachs, W.; Coley, P.D.; et al. Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. USA 2005, 102, 17384–17387. [Google Scholar] [CrossRef]
- Dyer, L.A.; Richards, L.A.; Short, S.A.; Dodson, C.D. Effects of CO2 and temperature on tritrophic interactions. PloS One 2013, 8, e62528. [Google Scholar]
- Massad, T.J.; Dyer, L.A. A meta-analysis of the effects of global environmental change on plant-herbivore interactions. Arthropod-Plant Int. 2010, 4, 181–188. [Google Scholar]
- Holt, R.D.; Polis, G.A. A theoretical framework for intraguild predation. Am. Nat. 1997, 149, 745–764. [Google Scholar]
- Ostfeld, R.S.; Holt, R.D. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front. Ecol. Environ. 2004, 2, 13–20. [Google Scholar] [CrossRef]
- Novotny, V.; Basset, Y. Review—Host specificity of insect herbivores in tropical forests. Proc. R. Soc. B 2005, 272, 1083–1090. [Google Scholar] [CrossRef]
- Schoener, T.W.; Spiller, D.A. Devastation of prey diversity by experimentally introduced predators in the field. Nature 1996, 381, 691–694. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boutin, S.; Boonstra, R.; Sinclair, A.R.E.; Smith, J.N.M.; Dale, M.R.T.; Martin, K.; Turkington, R. Impact of food and predation on the snowshoe hare cycle. Science 1995, 269, 1112–1115. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R.; Boutin, S.; Sinclair, A.R.E. What drives the 10-year cycle of snowshoe haves? Bioscience 2001, 51, 25–35. [Google Scholar]
- Bascompte, J.; Jordano, P.; Melian, C.J.; Olesen, J.M. The nested assembly of plant-animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Ann. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Dyer, L.A.; Singer, M.S.; Lill, J.T.; Stireman, J.O.; Gentry, G.L.; Marquis, R.J.; Ricklefs, R.E.; Greeney, H.F.; Wagner, D.L.; Morais, H.C.; et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 2007, 448, 696–699. [Google Scholar] [CrossRef]
- Staniczenko, P.P.A.; Kopp, J.C.; Allesina, S. The ghost of nestedness in ecological networks. Nat. Commun. 2013. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Cornell, H.V.; Hochberg, M.E. Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology 1997, 78, 2145–2152. [Google Scholar] [CrossRef]
- Dyer, L.A.; Letourneau, D.K. Relative strengths of top-down and bottom-up forces in a tropical forest community. Oecologia 1999, 119, 265–274. [Google Scholar] [CrossRef]
- O’Dowd, D.J.; Green, P.T.; Lake, P.S. Invasional 'meltdown' on an oceanic island. Ecol. Lett. 2003, 6, 812–817. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dyer, L.A.; Letourneau, D.K. Can Climate Change Trigger Massive Diversity Cascades in Terrestrial Ecosystems? Diversity 2013, 5, 479-504. https://doi.org/10.3390/d5030479
Dyer LA, Letourneau DK. Can Climate Change Trigger Massive Diversity Cascades in Terrestrial Ecosystems? Diversity. 2013; 5(3):479-504. https://doi.org/10.3390/d5030479
Chicago/Turabian StyleDyer, Lee A., and Deborah K. Letourneau. 2013. "Can Climate Change Trigger Massive Diversity Cascades in Terrestrial Ecosystems?" Diversity 5, no. 3: 479-504. https://doi.org/10.3390/d5030479
APA StyleDyer, L. A., & Letourneau, D. K. (2013). Can Climate Change Trigger Massive Diversity Cascades in Terrestrial Ecosystems? Diversity, 5(3), 479-504. https://doi.org/10.3390/d5030479



