Abstract
The northern Ethiopian landscape is dotted with small patches of church forests that are religious centers for the Ethiopian Orthodox Tewahido Church (EOTC). These sacred groves are what remain of the once vast tropical Afromontane dry forest. Herein we review the landscape pattern of sacred groves in the Amhara region of Ethiopia, and their local scale nutrient status at two sites, Zahara and Debresena. A total of 1,488 sacred groves were inventoried within the study area, yielding an overall density of one sacred grove for every twenty square kilometers. Sacred groves averaged a little over five hectares and were separated from one another by more than two kilometers. At the local scale we found that soil carbon and nitrogen stocks have decreased significantly between the forest interior and the clearing indicating decreased soil fertility. Together our data indicate that these sacred groves are vulnerable to loss because of their small average size, isolation from seed sources, and decreasing soil status.
1. Introduction
Sacred groves, also known as church forests, fetish forests and sacred forests, are found all over the world including Ethiopia, Japan, Morocco, India, and Ghana [1,2,3]. These sites are often seats of religious and cultural ritual that have been maintained through community conservation and are refugia of biodiversity; in fact, there is a significant network of large “shadow” conservation sites that are protected because of their sacredness [4]. Recent work shows that many rare and endemic species are found only in sacred groves [4]. In turn, these forests provide essential ecosystem services that include both the material provisions (timber and non-timber forest products), non-material (spiritual value, cultural value), and support services (nutrient cycling, water storage, carbon storage, pollination) [5,6]. Globally, sacred natural sites are threatened by population growth, social inequity, poor or no governance, political corruption and government policies that encourage unsustainable land use practices [4].
In the Amhara region of northern Ethiopia, centuries of deforestation likely driven by the need for fuel and agricultural and grazing land has resulted in radical alteration of the natural Ethiopian landscape [7] (Figure 1). Yet even as deforestation has ravished the landscape, sacred sites have demonstrated remarkable resilience in the face of change. While the exact number is unknown, thousands of these church forests currently exist as islands in a sea of degraded land (Figure 2a) where they have become centers of forest conservation. Such forests also provide human services as they are the location of the Ethiopian Orthodox Tewahido Church (EOTC) in which an Orthodox priest, his monks and disciples live and maintain the church at it’s center [2]. Local followers (men, women and children) pray in the church and use the forest ecosystems for firewood, honey, freshwater and cattle grazing [8].
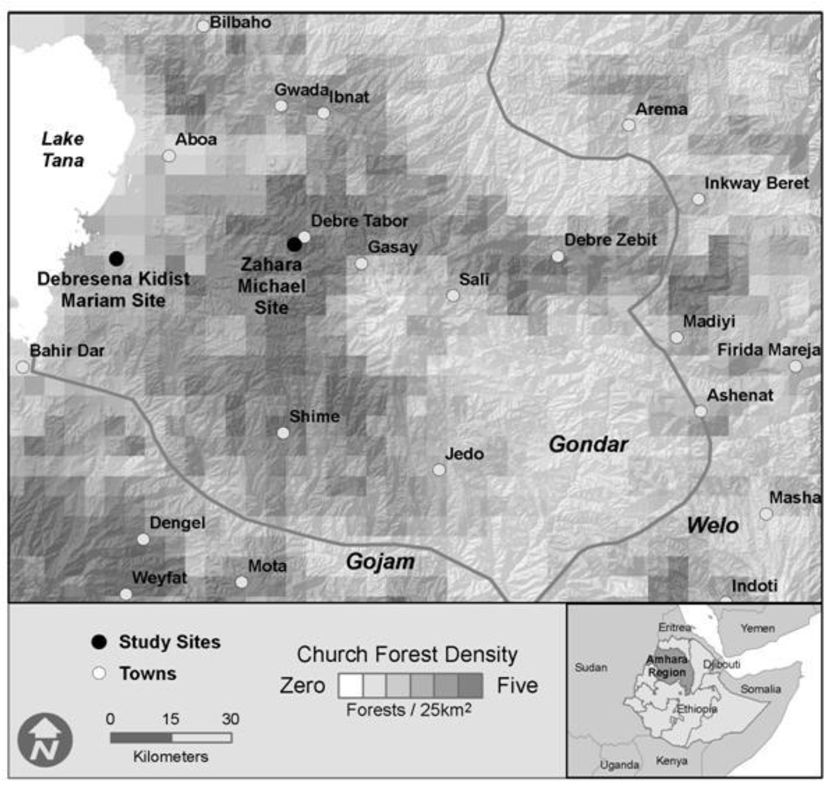
Figure 1.
Location of study sites and sacred grove density across study region, Ethiopia.
Figure 1.
Location of study sites and sacred grove density across study region, Ethiopia.
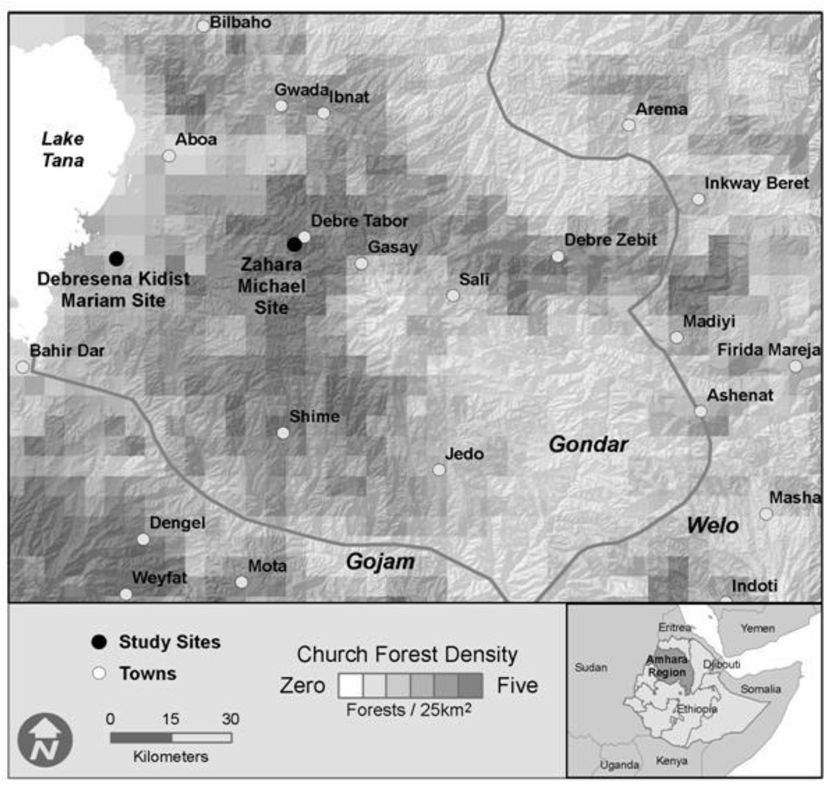
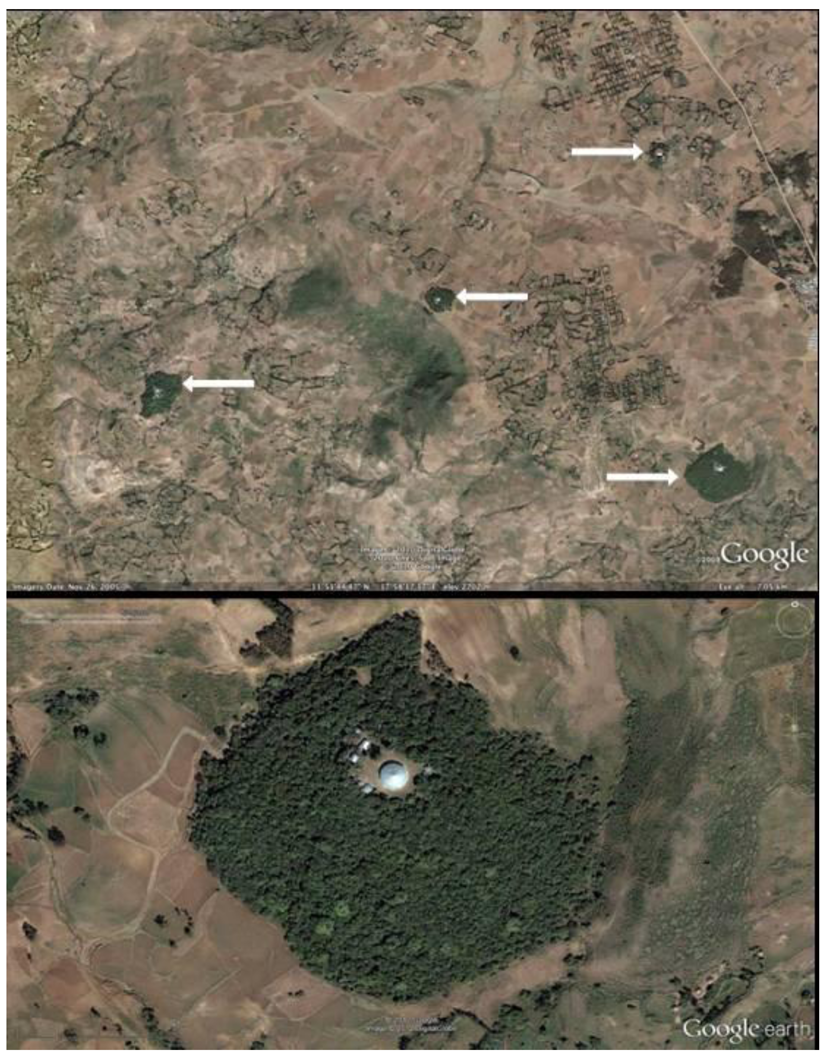
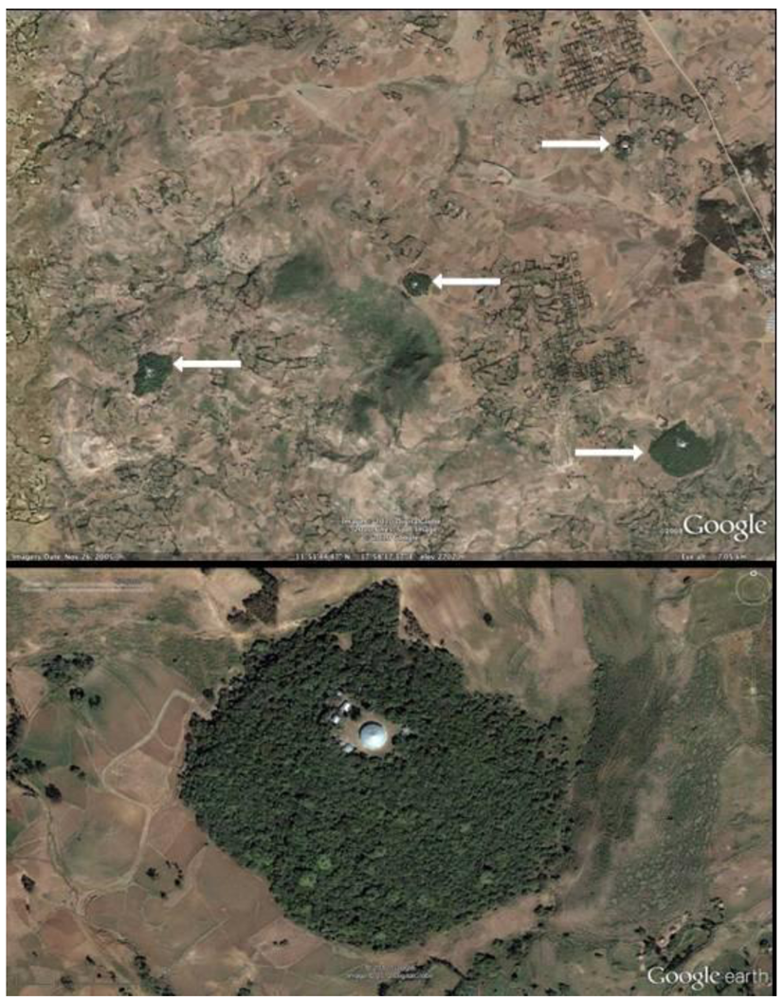
Figure 2.
(a) GoogleEarth image of study site Debresena (lower right) and other sacred groves. Arrows point to forest. (b) GoogleEarth image of one study forest, Debresena. The round Building in the center of the grove is the church.
Figure 2.
(a) GoogleEarth image of study site Debresena (lower right) and other sacred groves. Arrows point to forest. (b) GoogleEarth image of one study forest, Debresena. The round Building in the center of the grove is the church.
Deforestation, and land-use change in general, is one of the most serious threats to biodiversity and ecosystem function [9,10]. In tropical regions, in particular, deforestation has reduced extant forest globally by 50% over the last 200 years [11]. This forest change has impacts at multiple scales including local and regional weather patterns, carbon storage, biodiversity, and other ecosystem services [9,12,13,14]. The drivers of forest degradation by which we mean disturbances that range from deforestation to those that do not involve forest clearing, such as selective logging, harvesting of non-timber forest products, and cattle grazing—are largely linked to economically driven human activity [15]. Most notably, land degradation and clearing in tropical regions has been associated with population growth, road establishment, non-timber forest product harvesting, timber harvesting, fuel, grazing land establishment, and cultivation of oil palm and soybeans [15].
Assessing forest degradation and land-use change over time is critical in conservation efforts to assess overall ecosystem status which can be done using basic landscape-scale inventory data (e.g., number of sacred groves, total area of forest) [16]. However, inventory information regarding the present status of the sacred grove mosaic in Ethiopia is lacking. Reports on sacred grove number vary from 1404 in the South Gondar Administrative Zone (SDAZ) alone to as many as 35,000 sacred groves in the vicinity of Lake Tana and the northern highlands [17]. Beyond basic inventory data, in highly fragmented landscapes similar to northern Ethiopia, the distance between forest patches and the number of patches per unit area are also important determinates of both forest health and resilience. Measures of isolation, such as the distance to each forest’s nearest neighbor has been established as useful information in regards to regeneration capacity [18,19]. Lehouk [20], for example, demonstrated that forest patch isolation was critically important in the context of fruiting plant regeneration in the afromontane forests of Kenya. Isolation measures were also found to relate to species richness in the afromontane forests of South Africa [21].
Measures of forest shape can be an effective way to infer “edge” effects—changes to forest character due to climatic changes moving from the edge to the interior, with the “edge” of the forest having greater light intensity, lower soil moisture, and increased wind effect. Such edge effects are known to degrade forests and can be felt up to 500 m into their interior [22]. Edge effects also impact soil and foliar nutrient status. The edge potentially receives more N deposition [23] which could translate to greater N status in both plant leaves and soils. In contrast, litter decay rates may decrease along the edge because of decreased soil moisture which feedbacks on decreased soil and foliar nutrient status. Plants that experience greater water stress, as seen in edges, exhibit greater water use efficiency in their enriched δ13C signals compared to non-edge plants [24]. Thus forest size is a large determinant of forest resilience, with smaller patches having a greater edge and thus more vulnerable to degradation [25]. In forest fragments in Brazil, small fragments (1–10 ha) had greater tree mortality than large fragments (100 ha) [26] and animal richness and abundance also declined, as did ecosystem services [27], with forest size. These edge effects and isolation can impact regeneration due to lack of seed sources [18]. In the sacred groves of Ethiopia in particular, soil compaction from livestock grazing which reduces seedling establishment [28,29]. In addition to aboveground impacts on forest structure and function, shifts from a forested landscape to grazing land can have differential effects on soil status. Cattle grazing increases soil compaction (measured as bulk density, g cm−3) which reduces air pockets and deters seedling germination and root depth [30]. Furthermore, with tree harvest followed by stable, undeviating grazing system, forest regeneration is hindered [28]. Studies by Murty et al. [31] showed a decrease in C and N in forest soil versus cultivated land soil with an average loss of about 24% and 15% respectively. The change in nutrient composition was afforded to changes in the quantity and quality of organic C inputs to the soil, nutrient inputs and deficits, and intensification of decomposition through soil disruption—all factors that can reduce forest regeneration. In contrast, many studies, have found the increases in C and N stocks with forest conversion likely due to land-use history [32]. Quantifying the extent of the edge effects and forest clearing on soil status and properties in and around small, vulnerable sacred groves can inform regeneration efforts in the region.
Increased drought exacerbated by climate change, and human population growth is putting more pressure on Ethiopian sacred groves [29]. In order to assess the status of sacred groves in the Amhara Region of Ethiopia we performed a two-scale analysis of sacred groves: (1) patterns of distribution and shape at the regional scale and; (2) nutrient status at the local scale with particular focus on soil status. At a regional scale we inventoried and analyzed the spatial pattern of sacred groves on the landscape. We hypothesized that larger forest fragments would have greater crown closure and shape complexity. At the local scale we analyzed the soil and foliar nutrient status of two small fragments that varied with elevation as representative of small groves in the region. We hypothesized that soil status, as measured by total C, N and C:N ratio would decrease with greater degradation (interior-edge-clearing) and that foliar nutrient status would similarly be higher in the interior than edge. Understanding forest status at both the regional and local scales allows us to develop a more complete view of the extent of forest degradation in the region.
2. Experimental Section
2.1. Study Sites
Landscape inventory data was collected over an approximately thirty thousand square kilometer area (upper-left 37°20″E, 12°20″N; lower right 39°10″E, 11°0″N) centered in the Gondar Administrative zone in the Amhara Regional State of Ethiopia (Figure 1). In August 2010, we collected soil and foliar samples from three habitats in each of two forest fragments: Zahara Michael (Zahara, 11°51″N, 37°59'E, 1934 m a.s.l.) and Debresena Kidist Mariam (Debresena, 11°48″N, 37°34'E, 2549 m.a.s.l.). Zahara measured 8.7 ha, while Debresena measured 10.5 ha [33]. Annual rainfall for both regions averages 700–800 mm and temperature ranges from 9.3–23.7 °C [33]. While our study was limited to only two sacred groves, we chose them as representative of the “small” groves in the region with varying elevations. Access to groves was negotiated by Wassie and granted by the church leader for each site. The two sites studied had similar basal area (50.8 and 46.4 m2 ha-1, Zahara and Debresena respectively) and rarefied richness of woody taxa (8, 17), but varied in overstorey rarefied richness, with Zahara having 22 species and Debresena 32 [8]. These sites also varied in dominant tree species, with Mimusops kummel A. DC. (Sapotaceae) in Zahara and Prunus africana (Hook f.) Kalkman (Rosaceae) at Debresena. Both sites exhibited cattle grazing and tree harvesting that was quantified [8].
Each sacred grove was surrounded by deforested, agricultural and grazing land (Figure 2b, Debresena). At time of study, groves had no delimited boundary. Both forests were disturbed in that they had worshipers, disciples, church leaders, and grazing animals entering and leaving the forests throughout the day. People tended to stay on well-worn paths leading to the church while grazing animals were seen throughout the forests.
2.2. Landscape Scale Inventory
We completed a comprehensive inventory of all sacred grove forests within the study area using Google Earth Pro software and Google’s vast imagery repository. Standard on-screen digitizing procedures (Avery and Berlin, 1992) were used to discretize the perimeter of all remaining sacred groves. The resolution of Google’s imagery was more than sufficient for inventory purposes (Figure 2a,b). Aerial photography was also used to estimate canopy closure using standard photogrammetric procedure (Paine and Kaiser, 2003). Each grove was categorized into five ordinal classes based on degree of closure: 1 (<25%), 2 (25–50%), 3 (50–75%), 4 (75–90%) and 5 (>90%). Following standard landscape ecology methods [34,35,36], the following variables were calculated for each sacred grove using GIS: total area (km2), mean elevation (m), distance to nearest neighbor, shape index (S = l /2√(α * π)), where l = perimeter and α = area) and isolation index (I = size of nearest neighbor patch / edge to edge distance to nearest neighbor). A shape index value of one indicates a perfectly circular forest, while greater values indicate increasing shape complexity. Isolation index values are inversely related to actual degree of isolation, relative to the forest matrix; large index values indicate less isolation. In addition, the study area was divided into 5 km × 5 km grid cells and the total number of forests per cell was calculated to help visualize and summarize sacred grove density across the landscape.
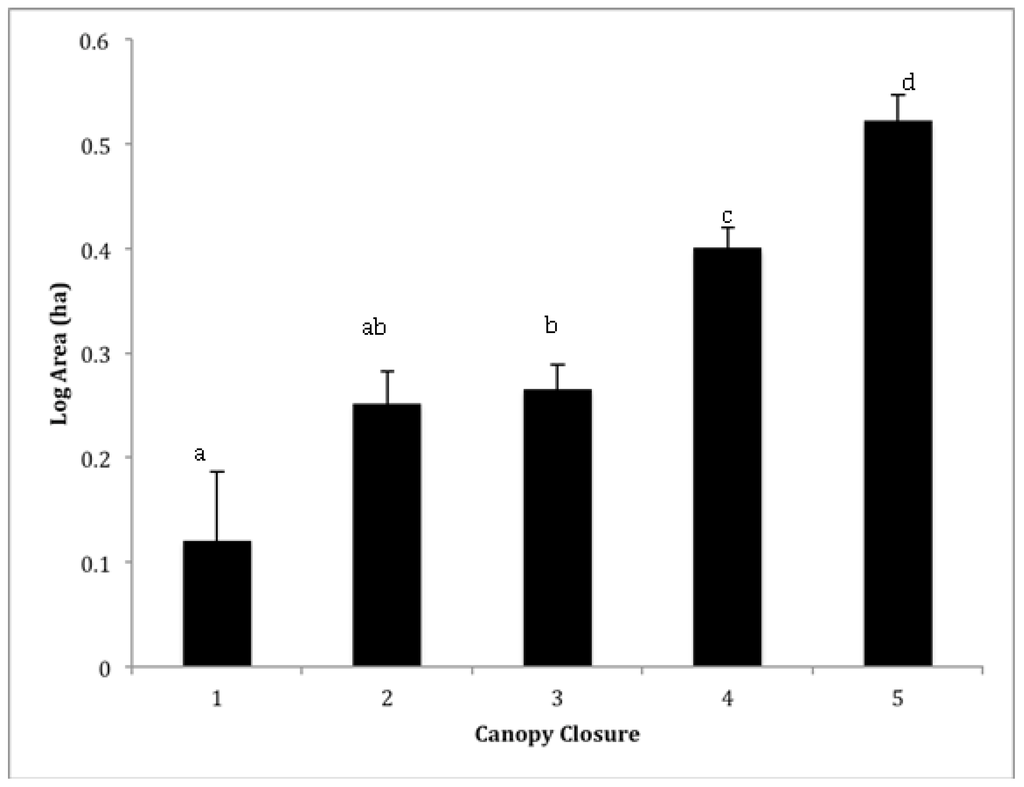
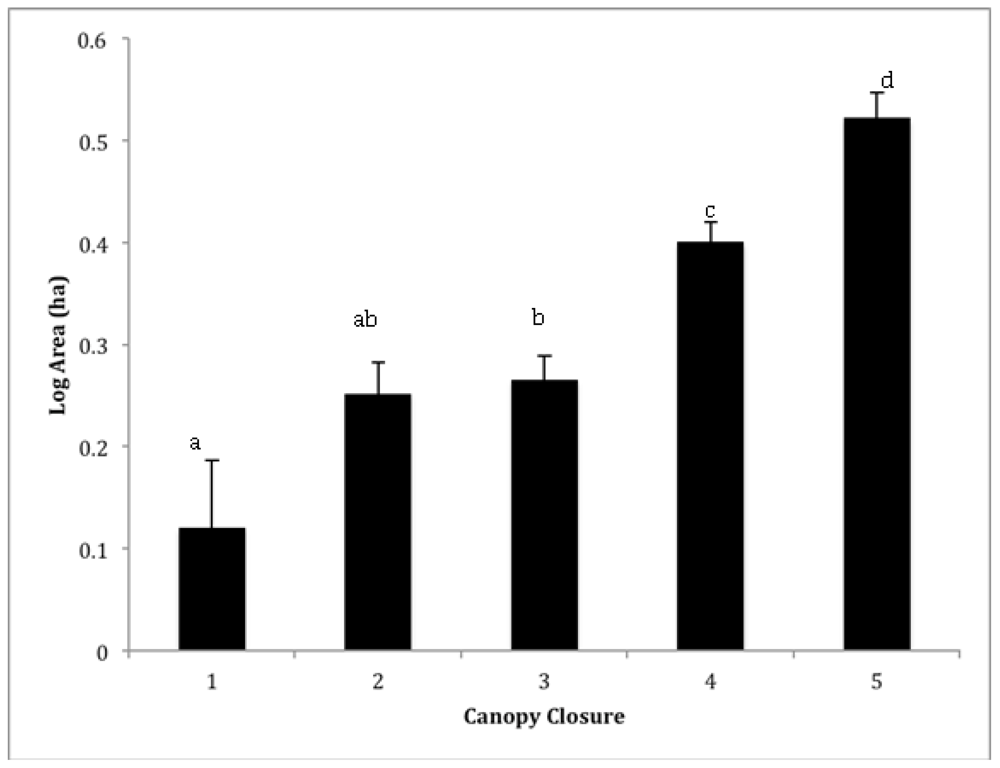
Figure 3.
Mean (±SE) log area (ha) of sacred groves within each canopy closure category: 1 (<25%), 2 (25–50%), 3 (50–75%), 4 (75–90%) and 5 (>90%). Lowercase letter indicate significant different among categories using a post-hoc Student’s t test.
Figure 3.
Mean (±SE) log area (ha) of sacred groves within each canopy closure category: 1 (<25%), 2 (25–50%), 3 (50–75%), 4 (75–90%) and 5 (>90%). Lowercase letter indicate significant different among categories using a post-hoc Student’s t test.
2.3. Soil Sampling
Within each forest fragment (Zahara, Debresena), we volumetrically sampled 3 soil replicates within each of three habitats in the top 10 cm of the forest floor: forest interior, edge and clearing. Soils were homogenized within 24 hours, dried, and later shipped to Colgate University where they were ground, rolled and analyzed for total carbon (C), nitrogen (N) and the stable isotope δ13C on a Costech Analytical Elemental Analyzer (Valencia California) coupled to a Delta Plus Isotope Ratio mass spectrometer (Brenen, Germany) in the Colgate University Stable Isotope Laboratory. Soil bulk density (BD, g·cm−3; oven dried mass/sample volume), an indicator of soil compaction was also determined.
2.4. Foliar Sampling
Tree species composition varied between sites [8]. Mimusops kummel A. DC. (Sapotaceae) in Zahara and Prunus africana (Hook f.) Kalkman (Rosaceae) in Debresena. From each dominant tree we collected full sun leaves from three individuals in each habitat. Foliar samples were dried in the field and shipped to Colgate University. Samples were ground using a Wiley Mill (Thomas Scientific, Swedesboro, NJ), passed through a #40 screen and analyzed for total C, N, and δ13C as for soils.
Statistical Analyses: Relationships among landscape variables (e.g., canopy closure and proximity to nearest sacred grove) were analyzed using standard parametric statistics including Analysis of Variance (ANOVA) and linear regressions. Non-normal data were log transformed. A 2-way Analysis of variance (ANOVA) was used to examine the effects of Habitat, Site and a Habitat x Site interaction on soil properties and nutrients and a one-way ANOVA followed by a student’s t-test within site was used to determine differences among habitats within sites. A one-way ANOVA was used to determine differences in foliar stoichiometry between habitats only within sites because the plant families varied.
3. Results and Discussion
3.1. Landscape Inventory
A total of 1,488 forests were mapped within the entire study area, yielding an overall density of one sacred grove for every twenty square kilometers. However, sacred grove density was highest in a zone approximately 20 ~ 70 km from Lake Tana (Figure 1), in a corridor that extended from Debra Tabor to Debra Zebut in the eastern portion of Gondar, and in the area around the towns of Weyfat and Dengel in the Gojam Administrative Zone. There were relatively few sacred forests in the extreme southeast corner of Gondar. Sacred groves averaged 5.2 (±0.44) ha and were separated from one another by more than 2.10 km (± 0.03). The modal crown closure class was 75–95% (class 4), though a fifth had less than 50% closure (classes 1 and 2; Figure 3). Not surprisingly, smaller groves had significantly lower canopy closure than larger forests (Figure 3; ANOVA, F1468 = 20.64, p < 0.001). The shape of sacred groves varied from a low of 1.03 (nearly a perfect circle) to almost two and a half, averaging 1.21 (±0.00). Most sacred grove forests were found at a little less than two and a half kilometers above sea level (2334 ± 9m). Interestingly, the highest and lowest elevation forests were 3622 m and 1464 m respectively, but very little variance in elevation exists considering the entire matrix of sacred groves.
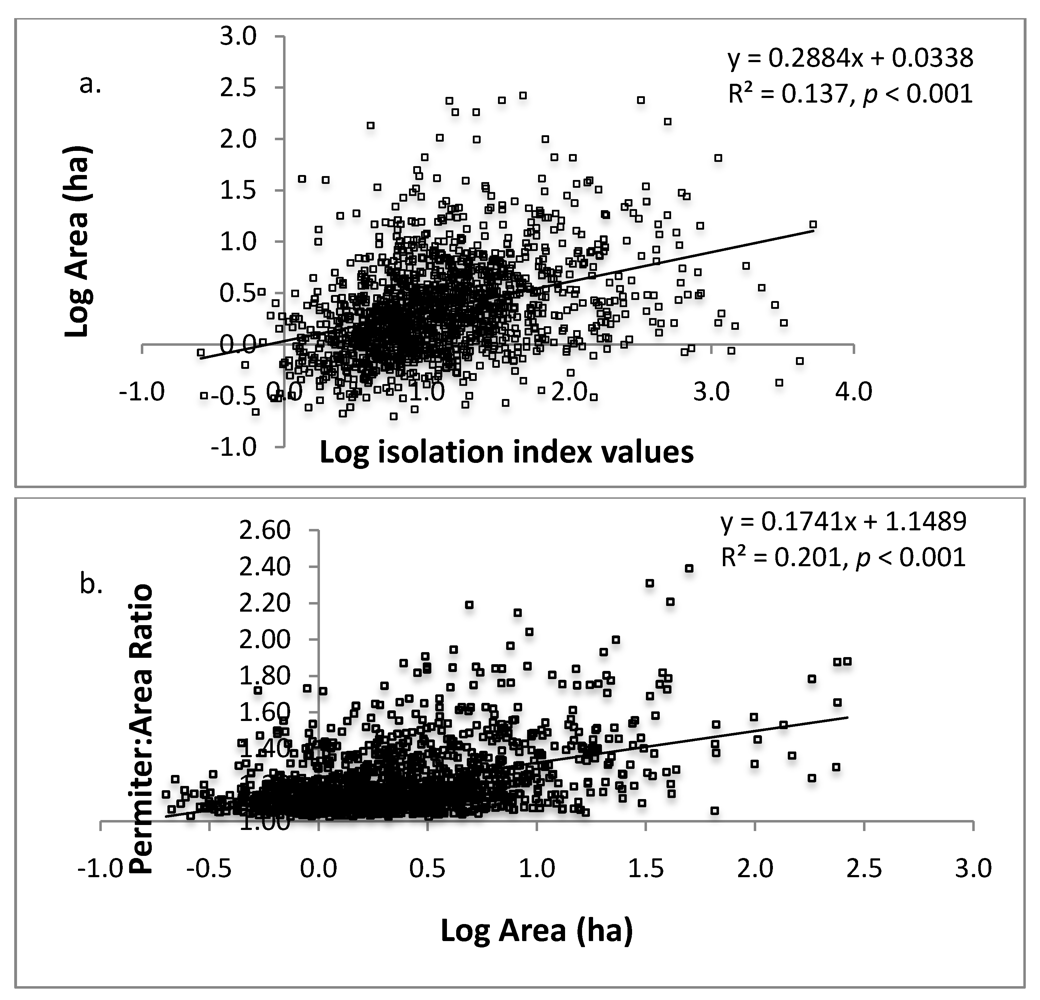
We found no linear relationship between grove area and distance to nearest neighbor (R2 = 0.048, p < 0.001), however, small groves were more isolated than larger groves according to isolation index results (Figure 4a). Interestingly, the shape of groves were more irregular (less circular) the larger they were (Figure 4b). The landscape inventory highlights the vulnerability of these forests given their average small size (5.2 ± 0.44 ha), and isolation from other groves (2.10 ± 0.03 km). In terms of area, small forests are more easily penetrated by edge effects, making the entire forest a large edge [25].
As predicted, larger groves had greater crown closure (Figure 3) and we also found they tended to be less isolated than smaller groves (Figure 4a); however, the mean distance even for larger groves is still a chasm for many organisms and will decrease regenerative capacity of the groves [18,19]. Compounded with smaller size, small groves are clearly more vulnerable to changes in microclimate (increased temperature, decreased humidity) because of the loss of crown closure and greater degree of isolation. Small forests do, however, have generally more desirable shapes from an edge effect perspective (Figure 4b), the circular shape has a lower surface to volume ratio compared to the non-circular shape, and increased opportunity for new plants to establish themselves due to lower crown densities (Figure 3) [37]. These forest opening are caused by multiple factors including internal harvesting of trees and tree mortality due to increased edge effects [37] and is a common issue in small fragments. It is problematic because the positive feedback that results—increased tree loss increases internal forest temperature, wind penetration, and reduces structural habitat for organisms—which leads to further losses [38].
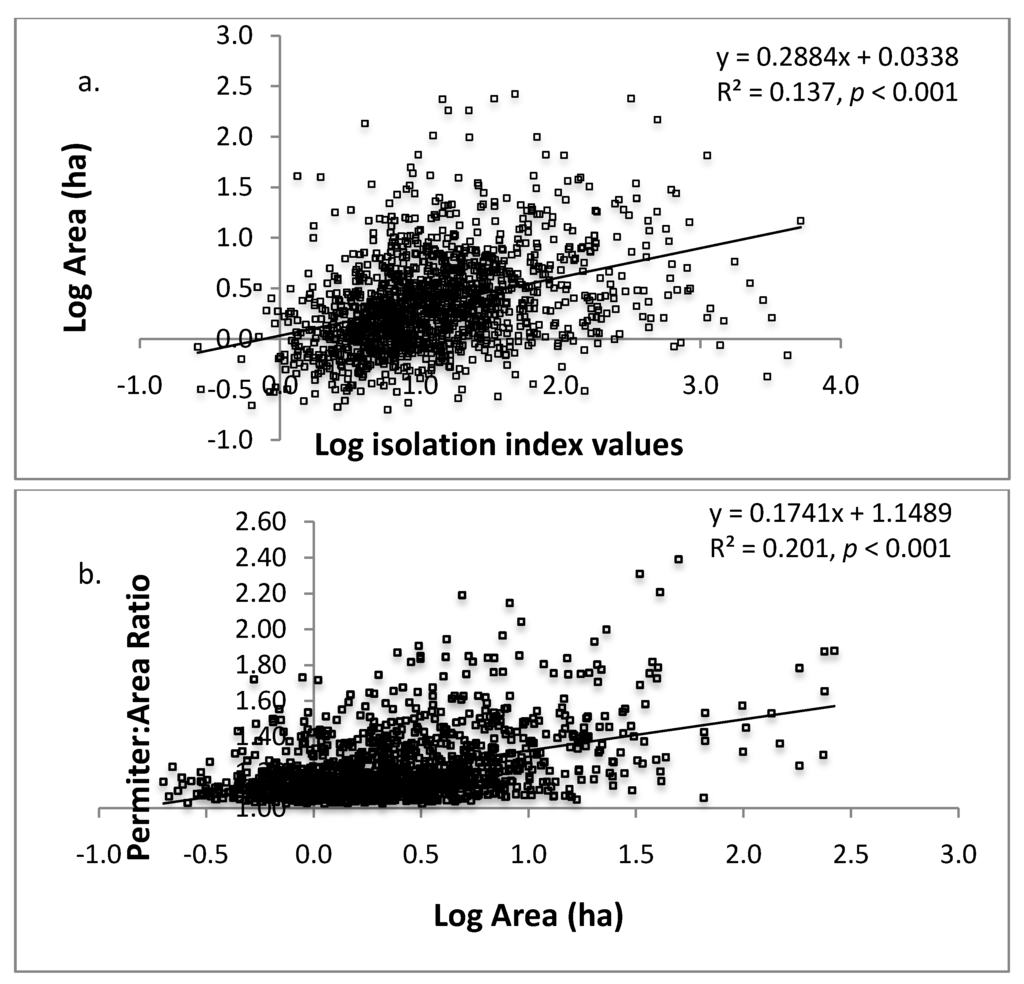
Figure 4.
Linear regressions of a) log isolation against log area (ha) of sacred grove; and b) log area (ha) against the perimeter:area ratio. The shape index measure the deviation from a circle (deviation = [perimeter / 2 * sqrt (area * π)]. Isolation index values are inversely related to actual degree of isolation; large index values indicate less isolation.
Figure 4.
Linear regressions of a) log isolation against log area (ha) of sacred grove; and b) log area (ha) against the perimeter:area ratio. The shape index measure the deviation from a circle (deviation = [perimeter / 2 * sqrt (area * π)]. Isolation index values are inversely related to actual degree of isolation; large index values indicate less isolation.
3.2. Soil & Foliar Properties and Nutrients
A total of three soils were collected from each habitat within each forest: forest interior, edge, and clearing. Soil BD varied significantly among habitats but not site and there was no site x habitat interaction (F17 = 15.21, p < 0.001; habitat, F3 = 37.98, p < 0.001; site F1 = 0.08, p = 0.786; site × habitat, F3 = 0.21, p = 0.979; Table 1). The BD increased with increasing disturbance with forest interior having the lowest and the clearing the highest indicating greater soil compaction (Table 1). Greater soil compaction, likely from cattle grazing, is often correlated with lower regeneration because roots have more trouble penetrating compacted soil [30,39]. Experimental germination studies conducted in sacred groves in Ethiopia showed a differential response depending on tree species, however. Wassie et al. [35] found that seedling germination was least successful in the clearing and varied in success from edge to interior with species. High light demanding species were more successful along the edge, whereas more shade tolerant species were more successful in the interior. While soil compaction can influence seedling establishment, it is clearly not the only factor as light and soil moisture also play a role.
Soil pH was significantly higher in forested habitats compared to the clearing at both elevations with no site effect or site x habitat interaction (F17 = 6.07, p = 0.001; habitat, F3 = 13.31, p < 0.001; site F1 = 2.39, p = 0.148; site x habitat, F3 = 0.669, p = 0.530; Table 1). Studies across regions examining cleared forest soils lack predictable trends, sometimes showing increasing pH [40], decreasing pH [41] or variable pH [42]. The decrease in pH found in our sites indicates lower plant nutrient availability, namely Ca2+, Mg+, NH4+, and precipitation and loss of PO42−. Their loss could reduce regeneration potential, particularly when linked with higher BD. Consistent with variations in BD and pH across the gradient, C and N concentrations decreased with increasing degradation. As predicted; both C and N stocks were significantly lower in the surrounding grazing land than the forest interior; and there was a significant effect of site and a site x habitat interaction (C: F17 = 27.70; p < 0.001; habitat; F2 = 56.94; p < 0.001; site F1 = 11.62; p = 0.005; site x habitat; F2 = 6.49; p = 0.012; Figure 5a; N: F17 = 19.79; p < 0.0001; habitat; F2 = 36.19; p < 0.0001; site F1 = 12.79; p = 0.004; site x habitat; F2 = 6.88; p = 0.01; Figure 5b). The higher elevation Debresena site had higher C and N stock and showed a significant edge effect; possibly due a more recent deforestation on the perimeter. The C and N losses from soil impacts potential forest regeneration because of decreased soil fertility. Up to 72 % of the carbon in the top 10 cm on grazing land was lost to the atmosphere and to deeper soils and these losses were mirrored in nitrogen losses (Figure 5). How much of the C lost was released to the atmosphere is unclear; however, deforestation rates in the Amhara region are approximately 9.8% (8,278 ha) in the last five years alone [43]. Thus, if carbon losses are similar across the area, it could have regional and global impact on climate regulation [44].

Table 1.
Mean (+/-SE) of soil properties and foliar chemistry in soils collected from three habitats in or around two tropical dry montane forest fragments in northern Ethiopia (Site 1 = Zahara; Site 2 = Debresena).
| Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | Site 1 | Site 2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Habitat | N | Bulk Density (g·cm−3) | pH | C:N | δ13C | |||||
| Interior | 3 | 0.250a (0.025) | 0.233a (0.013) | 6.1a (0.35) | 6.13a (0.09) | 13.68ab (0.96) | 12.78ab (0.35) | -26.08ab (0.11) | -25.96a (0.06) | |
| Edge | 3 | 0.337b (0.033) | 0.330b (0.035) | 5.7a (0.23) | 6.00a (0.06) | 14.13a (0.50) | 12.35b (0.56) | -26.31b (0.01) | -26.15a (0.10) | |
| Clearing | 3 | 0.473d (0.017) | 0.466d (0.0305) | 4.93b (0.12) | 5.37b (0.19) | 11.31b (0.84) | 12.40ab (0.80) | -26.21ab (0.09) | -26.09a (0.05) | |
Different lower case letters indicate significant differences in a nutrient among habitats within a site and asterisks indicate significant differences between sites (p = <0.05) using one-way ANOVA and student’s t-test.
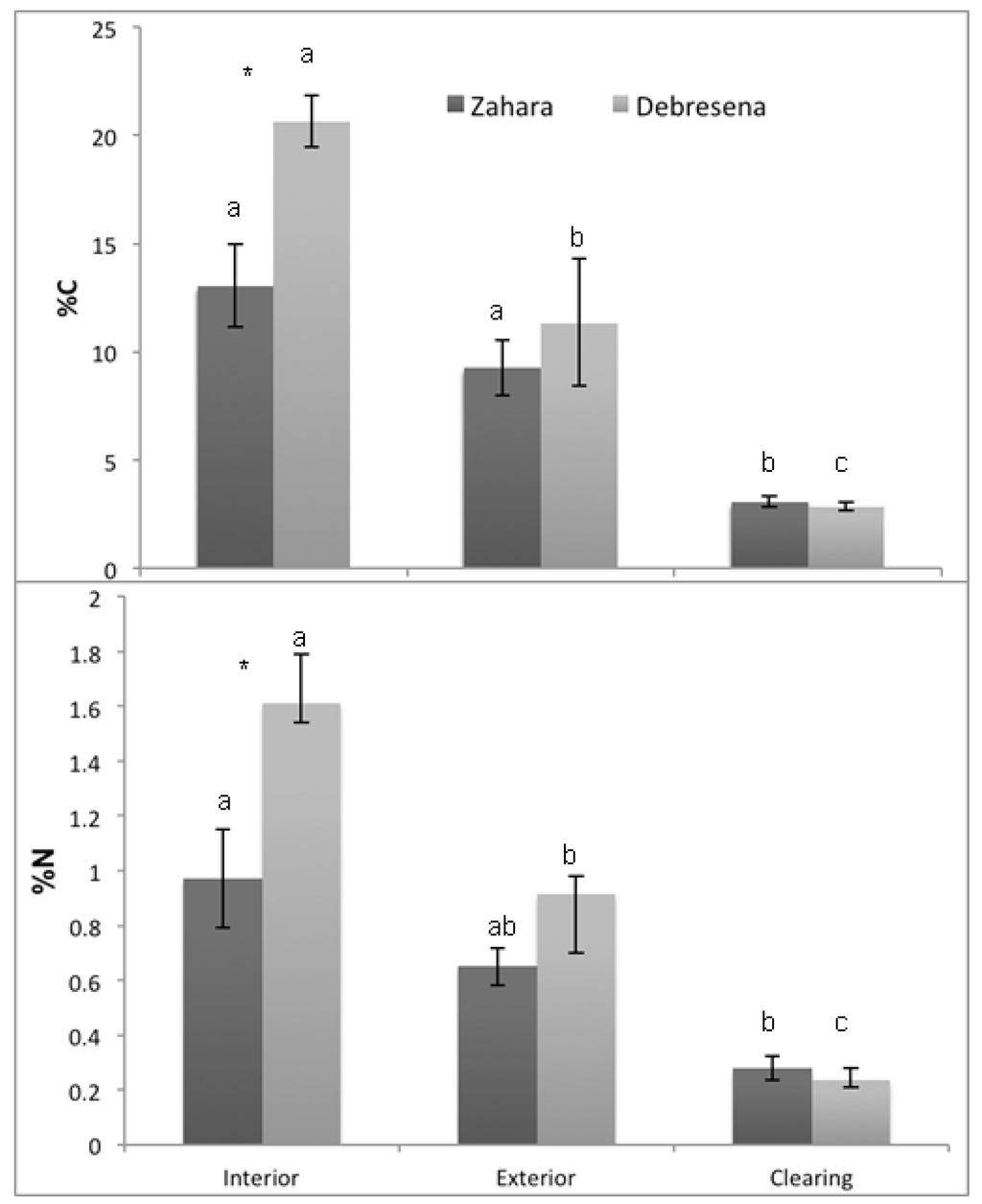
Figure 5.
Mean (±SE) a) percent carbon (%C) and b) percent nitrogen (%N) per hectare in forest floor soils of three habitat types (clearing, edge and forest interior) in two sacred groves (Zahara, Debresena) in northern Ethiopia. Lowercase letters indicate significant differences between habitats within fragment using student’s t-test and asterisks.
Figure 5.
Mean (±SE) a) percent carbon (%C) and b) percent nitrogen (%N) per hectare in forest floor soils of three habitat types (clearing, edge and forest interior) in two sacred groves (Zahara, Debresena) in northern Ethiopia. Lowercase letters indicate significant differences between habitats within fragment using student’s t-test and asterisks.
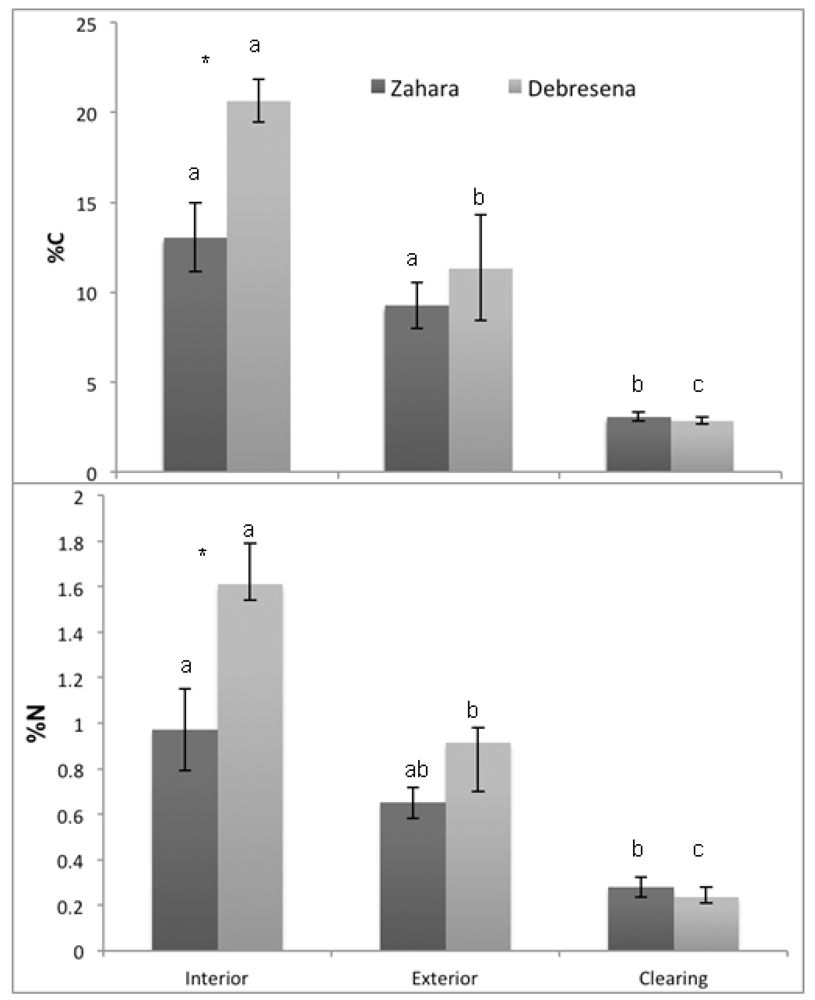
“*” indicate significant differences between sites within habitats.
Foliar samples were collected from three Mimusops and three Prunus within each habitat at each of their elevations, Zahara and Debresena, respectively. Foliar chemistry only varied significantly in %N between habitats in Prunus (F5 = 7.65, p = 0.049) with %N significantly higher in the forest edge compared to the forest interior (Table 2). Interestingly, there was no effect of edge on %C or δ13C signatures (Table 2) the latter indicating either little to no water stress at the edge for the dominant tree species at each site or that the microclimatic effects of edges have penetrated the entire forest.
4. Conclusions
The tropical dry montane forests of Ethiopia are biodiversity hotspots in the Amhara region. Our findings indicate that the sacred groves in our 30,000 km2 study area are vulnerable to loss because the majority of the groves are small ~5.2 (± 0.44) ha and isolated from each other by ~2.10 km (± 0.03). Small forests are more susceptible to edge effects than large forests [26] and their isolation disconnects them from seed sources [26]. Our findings at the local scale support the regional scale data in that the nutrient status and bulk density of forest floor soils in our two ~10 ha groves decreased from the interior → edge → clearing (Figure 5), indicating lower nutrient availability for plants and more compacted soil, both limiting seedling germination and establishment potential. We question these forests resilience to further perturbations [45,46]. The study area contains ~ 84,466 ha of forest, approximately 0.53% of the total area of the region; deforestation rates were estimated at 9.8% loss (8,278 ha) in the last five years alone [43]. Decreasing deforestation in the region and increasing forest cover is essential to minimize the loss of these forests.

Table 2.
The mean (±1 SE) percent nitrogen (%N), carbon (%C), C:N ratio, and the stable isotope C of foliar samples collected from trees (Mimusops and Prunus) from the edge and interior of two forest fragments in northern Ethiopia. A one-way ANOVA was conducted within sites between edge and interior.
| %N | Anova | %C | Anova | C:N | Anova | δ13C | Anova | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | N | Edge | Interior | F | p | Edge | Interior | F | p | Edge | Interior | F | p | Edge | Interior | F | p |
| Mimusops | 6 | 1.91 + (0.14) | 1.54 + (0.06) | 5.60 | 0.07 | 47.90 + (0.67) | 45.47 + (0.83) | 5.23 | 0.084 | 25.42 (1.85) | 29.60 (0.59) | 4.63 | 0.098 | −29.70 (0.97) | −29.84 (0.19) | 0.02 | 0.891 |
| Prunus | 6 | 2.07* (0.16) | 1.56* (0.09) | 7.86 | 0.049 | 46.90 (0.81) | 44.61 (0.47) | 5.91 | 0.072 | 22.92+ (1.83) | 28.72+ (1.61) | 5.63 | 0.077 | −27.76 (0.87) | −29.17 (0.39) | 2.21 | 0.211 |
Acknowledgments
This work was supported by a grant to CC from Picker Interdisciplinary Institute, Colgate University; Stable Isotope Laboratory, Colgate University (NSF EAR-0216179); and a Colgate University Natural Sciences Summer Fellowship to JH. It was also support by a grant to ML from National Geographic Conservation Trust Advisory Board (Grant #C173-09) and TREE Foundation for partial funding. We also thank the Cardelús Lab Group, J.E. Watkins, Jr. and F. Frey at Colgate University.
References and Notes
- Bhagwat, S.A.; Rutte, C. Sacred groves: Potential for biodiversity management. Front. Ecol. Environ. 2006, 4, 519–524. [Google Scholar] [CrossRef]
- Cardelús, C.L.; Lowman, M.D.; Eshete, A.W. Uniting church and science for conservation. Science 2012, 335, 915–917. [Google Scholar] [CrossRef]
- Ormsby, A.; Ormsby, C. Tafi atome monkey sanctuary, ghana: Community-based ecotourism at a sacred site. In Sacred Natural Sites: Conserving Nature and Culture; Routledge: Geneva, Switzerland, 2010. [Google Scholar]
- Dudley, N.; Higgins-Zogib, L.; Mansourian, S. The links between protected areas, faiths, and sacred natural sites. Conserv. Biol. 2009, 23, 568–577. [Google Scholar] [CrossRef]
- Bhagwat, S.A. Ecosystem services and sacred natural sites: Reconciling material and non-material values in nature conservation. Environ. Valu. 2009, 18, 417–427. [Google Scholar] [CrossRef]
- Powledge, F. The millennium assessment. BioScience 2006, 56, 880–886. [Google Scholar] [CrossRef]
- McCann, J.C. The plow and the forest: Narratives of deforestation in ethiopia, 1840–1992. Environmen.Hist. 1997, 2, 138–159. [Google Scholar] [CrossRef]
- Wassie, A.; Sterck, F.J.; Bongers, F. Species and structural diversity of church forests in a fragmented ethiopian highland landscape. J.Veg. Sci. 2010, 21, 938–948. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Sodhi, N.S.; Brook, B.W. Tropical turmoil: A biodiversity tragedy in progress. Front. Ecol. Environ. 2008, 7, 79–87. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K. Global consequences of land use. Science 2005, 309, 570. [Google Scholar] [CrossRef]
- Laurance, W.; Wright, S.J. Special section: New insights into the tropical biodiversity crisis. Conserv. Biol. 2009, 23, 1382–1671. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Malhi, Y.; Roberts, J.T.; Betts, R.A.; Killeen, T.J.; Li, W.; Nobre, C.A. Climate change, deforestation, and the fate of the amazon. Science 2008, 319, 169–172. [Google Scholar] [CrossRef]
- Nascimento, H.E.M.; Laurance, W.F. Biomass dynamics in amazonian forest fragments. Ecol. Appl. 2004, 14, 127–138. [Google Scholar] [CrossRef]
- Boucher, D.; Elias, P.; Lininger, K.; May-Tobin, C.; Roquemore, S.; Saxon, E. The Root of the Problem: What’s Driving Tropical Deforestation Today? Union of Concerned Scientists: Cambridge, MA, USA, 2011. [Google Scholar]
- Franklin, S. Remote Sensing for Sustainable Forest Management; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Bongers, F.; Wassie, A.; Sterck, F.; Bekele, T.; Teketay, D. Ecological restoration and church forests in northern ethiopia. J. Drylands 2006, 1, 35–45. [Google Scholar]
- Jorge, L.A.B.; Garcia, G.J. A study of habitat fragmentation in southeastern brazil using remote sensing and geographic information systems (gis). Forest Ecol. Manag. 1997, 98, 35–47. [Google Scholar] [CrossRef]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.P.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Alvarez, P. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef]
- Lehouck, V.; Spanhove, T.; Vangestel, C.; Cordeiro, N.J.; Lens, L. Does landscape structure affect resource tracking by avian frugivores in a fragmented afrotropical forest? Ecography 2009, 32, 789–799. [Google Scholar] [CrossRef]
- Lawes, M.J.; Joubert, R.; Griffiths, M.E.; Boudreau, S.; Chapman, C.A. The effect of the spatial scale of recruitment on treediversity in afromontane forest fragments. Biol. Conserv. 2007, 139, 447–456. [Google Scholar] [CrossRef]
- Laurance, W.F.; Ferreira, L.V.; Merona, J.M.; Laurance, S.G.; Hutchings, R.W.; Lovejoy, T.E. Effects of forest fragmentation on recruitment patterns in amazonian tree communities. Conserv. Biol. 1998, 12, 460–464. [Google Scholar] [CrossRef]
- Wuyts, K.; De Schrijver, A.; Staelens, J.; Gielis, L.; Vandenbruwane, J.; Verheyen, K. Comparison of forest edge effects on throughfall deposition in different forest types. Environmen. Pollut. 2008, 156, 854–861. [Google Scholar] [CrossRef]
- Ehleringer, J.; Field, C.; Lin, Z.; Kuo, C. Leaf carbon isotope and mineral composition in subtropical plants along an irradiance cline. Oecologia 1986, 70, 520–526. [Google Scholar]
- Laurance, W.; Laurance, S.; Ferreira, L.; Rankin-de Merona, J.; Gascon, C.; Lovejoy, T. Biomass collapse in amazonian forest fragments. Science 1997, 278, 1117. [Google Scholar] [CrossRef]
- Laurance, W.; Lovejoy, T.; Vasconcelos, H.; Bruna, E.; Didham, R.; Stouffer, P.; Gascon, C.; Bierregaard, R.; Laurance, S.; Sampaio, E. Ecosystem decay of amazonian forest fragments: A 22 year investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Laurance, W.F.; Koster, H.; Grooten, M.; Anderson, A.B.; Zuidema, P.A.; Zwick, S.; Zagt, R.J.; Lynam, A.J.; Linkie, M.; Anten, N.P.R. Making conservation research more relevant for conservation practitioners. Biol. Conserv. 2012, 153, 164–168. [Google Scholar] [CrossRef]
- Aerts, R.; Van Overtveld, K.; Haile, M.; Hermy, M.; Deckers, J.; Muys, B. Species composition and diversity of small afromontane forest fragments in northern ethiopia. Plant Ecol. 2006, 187, 127–142. [Google Scholar] [CrossRef]
- Wassie, A.; Teketay, D.; Powell, N. Church forests in north gonder administrative zone, northern ethiopia. Forests trees livelihoods 2005, 15, 349. [Google Scholar] [CrossRef]
- skinner, A.K.; lunt, I.D.; spooner, P.; Mcintyre, S. The effect of soil compaction on germination and early growth of eucalyptus albens and an exotic annual grass. Austral Ecol. 2009, 34, 698–704. [Google Scholar] [CrossRef]
- Murty, D.; Kirschbaum, M.U.F.; Mcmurtrie, R.E.; Mcgilvray, H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Global Change Biol. 2002, 8, 105–123. [Google Scholar] [CrossRef]
- Neill, C.; Melillo, J.M.; Steudler, P.A.; Cerri, C.C.; deMoraes, J.F.L.; Piccolo, M.C.; Brito, M. Soil carbon and nitrogen stocks following forest clearing for pasture in the southwestern brazilian amazon. Ecol. Appl. 1997, 7, 1216–1225. [Google Scholar] [CrossRef]
- Wassie, A. Ethiopian Church Forests: Opportunities and Challenges for Restoration. Ph.D. Thesis, Wageningen University, The Netherlands, 2007. [Google Scholar]
- Forman, R.T.; Gordon, M. Landscape ecology; John Wiley and Sons: New York, NY, USA, 1986; p. 640. [Google Scholar]
- Turner, M.G. Landscape ecology: The effect of pattern on process. Annu. Rev. Ecol. Syst. 1989, 20, 171–197. [Google Scholar]
- Turner, M.G. Spatial and temporal analysis of landscape patterns. Landscape Ecol. 1990, 4, 21–30. [Google Scholar] [CrossRef]
- Wassie, A.; Sterck, F.; Teketay, D.; Bongers, F. Tree regeneration in church forests of ethiopia: Effects of microsites and management. Biotropica 2009, 41, 110–119. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002; p. 436. [Google Scholar]
- Trouse, A., Jr.; Humbert, R. Some effects of soil compaction on the development of sugar cane roots. Soil Sci. 1961, 91, 208–217. [Google Scholar] [CrossRef]
- McGrath, D.A.; Smith, C.K.; Gholz, H.L.; Oliveira, F.d.A. Effects of land-use change on soil nutrient dynamics in amazonia. Ecosystems 2001, 4, 625–645. [Google Scholar] [CrossRef]
- Ewel, J.J.; Mazzarino, M.J.; Berish, C.W. Tropical soil fertility changes under monocultures and successional communities of different structure. Ecol. Appl. 1991, 1, 289–302. [Google Scholar] [CrossRef]
- Haynes, R.; Williams, P. Changes in soil solution composition and ph in urine-affected areas of pasture. J. soil Sci. 1992, 43, 323–334. [Google Scholar]
- FAO, Global forest resources assessment 2010; Country report: Ethiopia; Forest Department, Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; p. 43.
- West, P.C.; Narisma, G.T.; Barford, C.C.; Kucharik, C.J.; Foley, J.A. An alternative approach for quantifying climate regulation by ecosystems. Front. Ecol.Environ. 2011, 9, 126–133. [Google Scholar] [CrossRef]
- Laurance, W.F.; Williamson, G.B. Positive feedbacks among forest fragmentation, drought, and climate change in the amazon. Conser. Biol. 2001, 15, 1529–1535. [Google Scholar] [CrossRef]
- Shukla, J.; Nobre, C.; Sellers, P. Amazon deforestation and climate change. Science 1990, 247, 1322. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
