Resilience of Florida Keys Coral Communities Following Large-Scale Disturbances
Abstract
: The decline of coral reefs in the Caribbean over the last 40 years has been attributed to multiple chronic stressors and episodic large-scale disturbances. This study assessed the resilience of coral communities in two different regions of the Florida Keys reef system between 1998 and 2002 following hurricane impacts and coral bleaching in 1998. Resilience was assessed from changes in coral abundance, diversity, disease, and bleaching prevalence in reefs near the remote off-shore islands of the Dry Tortugas compared to reefs near Key West, a center of high population density and anthropogenic influences. During the first assessment in spring 1998, Key West and Dry Tortugas coral communities had similar abundance, species diversity, and disease prevalence. Bleaching and disease significantly increased in all reef areas during the summer 1998 El Niño event, with Key West reefs exhibiting higher bleaching and disease prevalence and severity compared to Dry Tortugas. Acroporids and total coral abundance significantly declined in both regions during 1998 following mass-coral bleaching and hurricane impact, but remained reduced only on Key West reefs during the 5-year assessment. These results provide additional evidence that coral reef systems distant from anthropogenic influences may have greater resilience to large-scale disturbances.1. Introduction
The Florida Keys reef tract has had substantial declines in coral cover and condition, and although multiple factors appear to contribute, the specific causes remain uncertain [1-4]. Fossilized reefs of Florida coral communities show general stability for the last several thousand years prior to the rapid changes observed in the last four decades [5]. Analysis of growth rates in the massive star coral Montastraea faveolata also indicates relative stability in past decades [6]. Recent reductions in the condition of Florida Keys coral reefs have included a 45% decline in live coral coverage between 1996 and 2004, to an average of only 6.6% [3]. Increased coral disease prevalence of 28% has also been reported in the Lower Keys [7,8]. Perhaps of greatest concern are the acroporids which have declined to historically low densities and were federally listed as threatened in 2008 [9]. Overall, the Florida Keys reef track represents a degraded coral ecosystem that continues to experience periodic large-scale disturbances.
The resilience of an ecosystem has been defined as its capacity to absorb recurrent disturbances without switching to an alternate stable state [10,11]. Coral reef resilience can be measured as the time taken to return to its former state after disturbance or the resistance of the system to perturbation [12]. Resilient coral ecosystems will maintain their critical functions and biodiversity after major perturbations, such as a hurricane or massive bleaching event. Change from a coral to an algal dominated reef is a common phase shift observed on less resilient reefs. The causes of phase shifts appear to be diverse but are believed to be closely aligned with continued human influences [13].
Recent research from coral ecosystems across the globe has shown that local stressors and regional scale disturbances can have differential effects on species composition and diversity, coral cover, growth rates, and disease prevalence [3,14-18]. Studies of hurricane impacts have shown loss of coral cover and impaired recruitment from both physical damage and subsequent increased mortality, with impacts appearing to increase with increasing storm frequency and intensity [19,20]. There is conflicting evidence on the degree that local stressors such as degraded water quality can influence coral resilience when exposed to large-scale disturbances [2,14,21,22]. Recent studies suggest that multiple factors may affect the impact of coral bleaching, mortality and recovery [10].
This study assessed the resilience of coral communities near Key West and the more remote Dry Tortugas following large-scale disturbances of massive coral bleaching and Hurricane Georges in 1998. The Florida Keys, including Key West, are influenced by the Gulf Stream, the more turbid waters of Florida Bay, and from land-based stressors [23,24]. In contrast, the Dry Tortugas are a remote island system located at the western edge of the Florida reef tract and have water quality more comparable to oceanic conditions [25]. The reefs in these two regions had similar exposure to hurricane and El Niño bleaching conditions, but differed in water flow patterns and quality, and the intensity of human usage [26,27]. Resilience was assessed over a five-year period by quantifying coral abundance, species diversity, disease prevalence, and bleaching prevalence.
2. Materials and Methods
2.1. Survey Design
A stratified random sampling design was used for selecting survey stations and has been detailed previously [8,28]. Briefly, hard coral bottoms were demarcated as reefs within each geographic region using a prototype of the Florida Marine Research Institute benthic habitat maps of the Florida Keys [29]. Potential stations were identified by generating 20 random points within each reef. Surveyors visited each site in the order it was generated and accepted the site if it contained 5% or greater coral cover. Most potential stations were rejected because of an absence of live coral. If a site was suitable for sampling, a permanent station was installed and surveyed. The random stations were matched by approximate depths between regions to allow comparison of condition (Figure 1). SCUBA-based surveys were conducted between 1998 and 2002 at twelve reef stations in each region, with four survey locations in each depth zone: shallow, mid-depth and deep. Surveys were conducted during May–June 1998 (prior to bleaching), September 1998 (during bleaching, prior to hurricane impact), and in June 1999, August 2000, August 2001, and August–September 2002 following large-scale bleaching and hurricane disturbances.
All stations were assessed using a radial belt transect survey by SCUBA that has been used in coral reef surveys in US and Australia and has been detailed in other reports [7,8,28]. Transects were conducted by positioning a 2 m long rod into a permanent stainless steel pipe in the center of the survey station. A 10 m survey line was extended from the center and positioned horizontally to encompass the 8–10 m swath around the rod. The 8 and 10 m points were marked on the line with plumb lines; all coral colonies which fell within the 8 to 10 m radius of the line were included in the arc survey, for a total area of 113 m2. An arbitrary start/end point was marked with a buoy just outside the 10 m mark in the radial belt. Three surveyors swam directly over the survey line recording the number of each coral species, coral diseases on each colony, and the degree of bleaching of each colony within the transect area. A fourth diver moved the line along the arc segment.
2.2. Coral Assessments
Eighteen coral species previously known to be affected by disease were evaluated to characterize coral condition in colonies 10 cm or greater in diameter. The coral species assessed included Acropora cervicornis, Acropora palmata, Colpophyllia natans, Dichocoenia stokesii, Montastraea cavernosa, Solenastrea bournoni, Siderastrea siderea, Diploria strigosa, Diploria labyrinthiformis, Stephanocoenia intersepta, Mycetophyllia ferox, Mycetophyllia lamarkiana, and Mycetophyllia danaae. Montastraea annularis, M. faveolata, and M. franksi were aggregated as the M. annularis complex because of uncertain species identification at early survey times. The sea fans Gorgonia ventalina and G. flabellum were not differentiated in the field and were recorded as Gorgonia spp. They were included in the assessment since several diseases affected them. Each colony in the transect area was assessed for disease and bleaching. Ten coral disease categories were assessed including black-band disease, red-band disease, white-band disease, aspergillosis, white plague, white pox disease/patchy necrosis, dark spots disease, yellow blotch/band disease, skeletal anomalies and unknown. The unknown category was recorded when a disease condition was encountered which had not been previously described [8,28]. Colonies were evaluated as diseased only if they contained active lesions. Specific diseases were not scored if the cause of mortality was uncertain. Distinguishing signs for coral diseases have been detailed elsewhere [7,8,30]. White pox described the lesions found on Acropora palmata colonies that could not be attributed to white-band disease or predation. White plague types 1 and 2 were only identified as white plague. If multiple diseases were found on the same colony, both conditions were recorded as one so disease prevalence was not overestimated. Each coral colony was also assessed for bleaching and assigned one of three categories based on an estimate of the proportion of colony surface affected: no bleaching (less than 10% surface area bleached), partial bleaching (10–50%) and severe bleaching (>50%).
2.3. Data Analyses
The data were partitioned to examine the responses of all surveyed coral species in aggregate, and also three functional groups: the acroporids (A. cervicornis, A. palmata), sea fans (Gorgonia spp.), and massive corals comprised of the largest hemispherical colonies: C. natans, M. cavernosa, and the three species of the M. annularis complex. Coral abundance rather than percent areal coverage was assessed to allow determination of the prevalence of coral disease and bleaching based on presence or absence on each colony. Total and functional group data were examined for differences over time within each region and for differences between the regions at each time period. All metrics were standardized to the number of colonies per annular area of one arc (113 m2).
Nonparametric statistics were used in the analysis because the data failed normality requirements. Data were analyzed using SAS (SAS Institute, Cary, SC) and PRIMER™ [27]. The experimental design was developed after a power analysis of data from a 1997 pilot study supported adopting α = 0.10 to allow a regional comparison through sampling more sites less intensively [8]. The first null hypothesis tested for temporal trends within each region, and the second tested for differences between the regions at each survey time.
To test the null hypotheses for no differences in abundance, a rank-based analysis of variance (ANOVA) was applied for unbalanced data and discriminated statistically different main effects at α = 0.10 using Dunnett's test. A Poisson regression model was fitted to over-dispersed abundance data to test for temporal responses in Key West and Dry Tortugas coral communities. Regression coefficients were estimated by maximum likelihood and statistical significance levels were adjusted to reflect over-dispersion. Four metrics were used to assess changes in coral species composition as species richness, evenness, dominance and diversity, including Shannon's diversity index (H), Simpson's dominance index (λ), Margalef's species richness index (d) and Pielou's evenness index (J). The statistical model included terms for year, region, and interaction of year and region. The model assumed the response could be approximated by an exponent function of time, and recovery rate might vary between Dry Tortugas and Key West regions.
Coral disease and bleaching prevalence were standardized as the percentage of diseased or bleached coral colonies at each station for each survey time. Both untransformed and arcsine-transformed data failed normality assumptions. Data were analyzed using the multivariate nonparametric statistics for analysis of similarities (ANOSIM [31]). Bray Curtis similarity coefficients were used in the ANOSIM test of temporal changes within each region and differences between regions for each survey. The 90% confidence intervals for prevalence were calculated using bootstrapping [32]. Statistically significant relationships were determined by the ANOSIM R statistic [33].
3. Results
3.1. Similarity in Coral Condition Prior to Disturbance
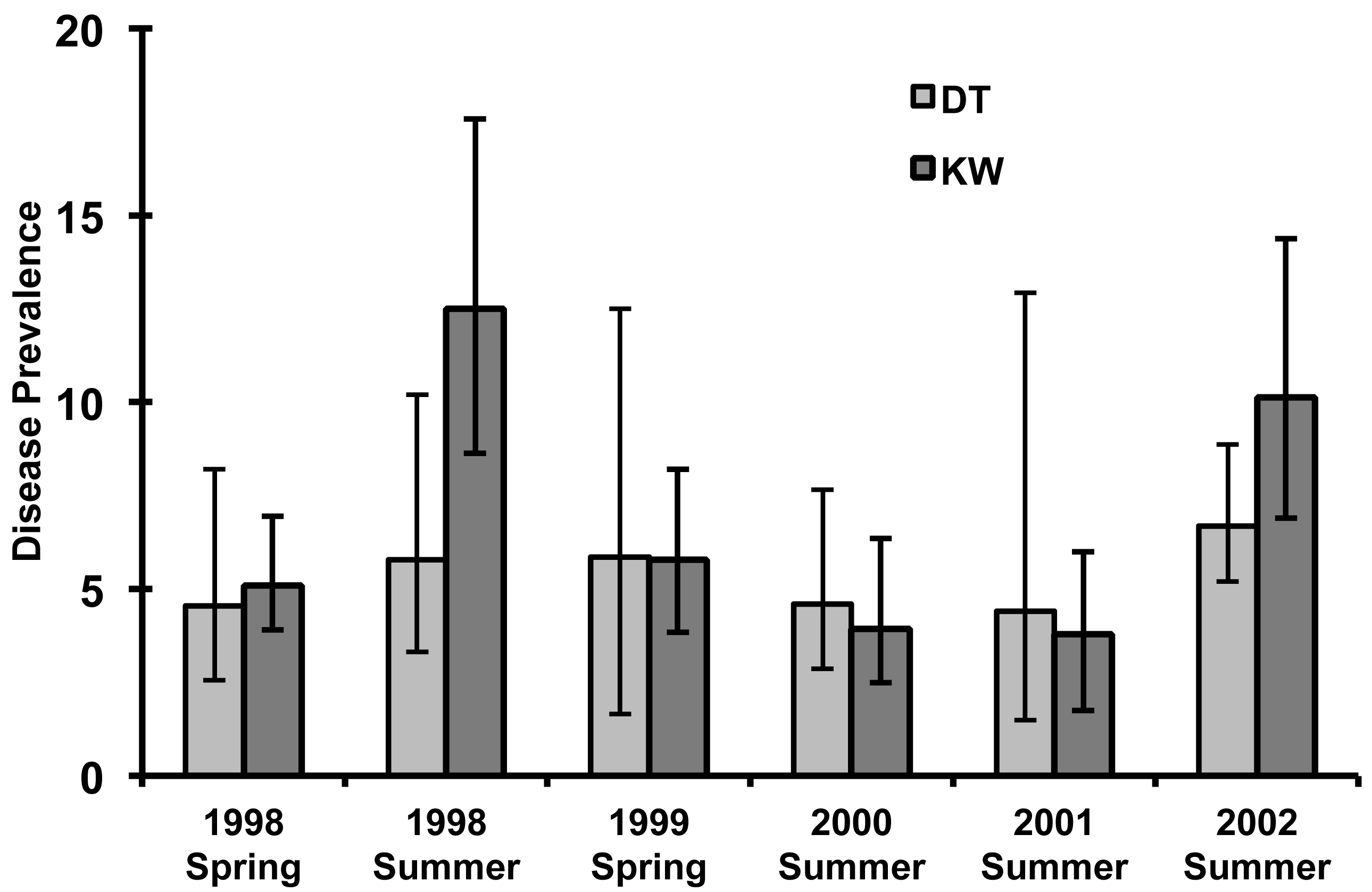
Surveys in spring 1998 conducted prior to the large-scale disturbances of hurricane impact and massive coral bleaching showed that coral condition was similar in reefs near Key West and the more remote Dry Tortugas. There were no significant differences in coral abundance, either as total species in aggregate or for the three functional groups of sea fans, acroporids, and massive species (Figure 2). The four community metrics of species richness, evenness, dominance and diversity all indicated similar community attributes in the two locations (Table 1). There also were no significant differences in disease prevalence in aggregate or for the three functional groups prior to the large-scale disturbances impacting Key West and Dry Tortugas reefs (Figure 3). Bleaching was not quantified in spring 1998, because there was no evidence of coral bleaching in either area based on qualitative observations and bleaching conditions were absent.
3.2. Similarity in Large-scale Disturbances
The available information indicated that the reefs near Key West and the Dry Tortugas were exposed to similar temporal and spatial scales of disturbance. Based on remote sensed sea surface temperature data for the Lower Florida Keys and Dry Tortugas, both reef areas experienced elevated levels of thermal stress during the summers of both 1997 and 1998 (Figure 4). In 1998, sea surface temperatures remained above 30 °C in both regions for over six consecutive weeks, and both regions reached their maxima in early July 1998 (DT 31.3 °C, KW 30.6 °C) (Figure 4). Key West and Dry Tortugas reefs were also exposed to similar impacts from the Category 3 Hurricane Georges. The eye of the hurricane moved rapidly over both the Florida Keys and Dry Tortugas reef areas on 25 September, 1998 in a northwestern trajectory [27]. Satellite imagery indicated region-wide impact across the Florida Keys reef tract.
3.3. Changes in Coral Abundance and Diversity Following Disturbance
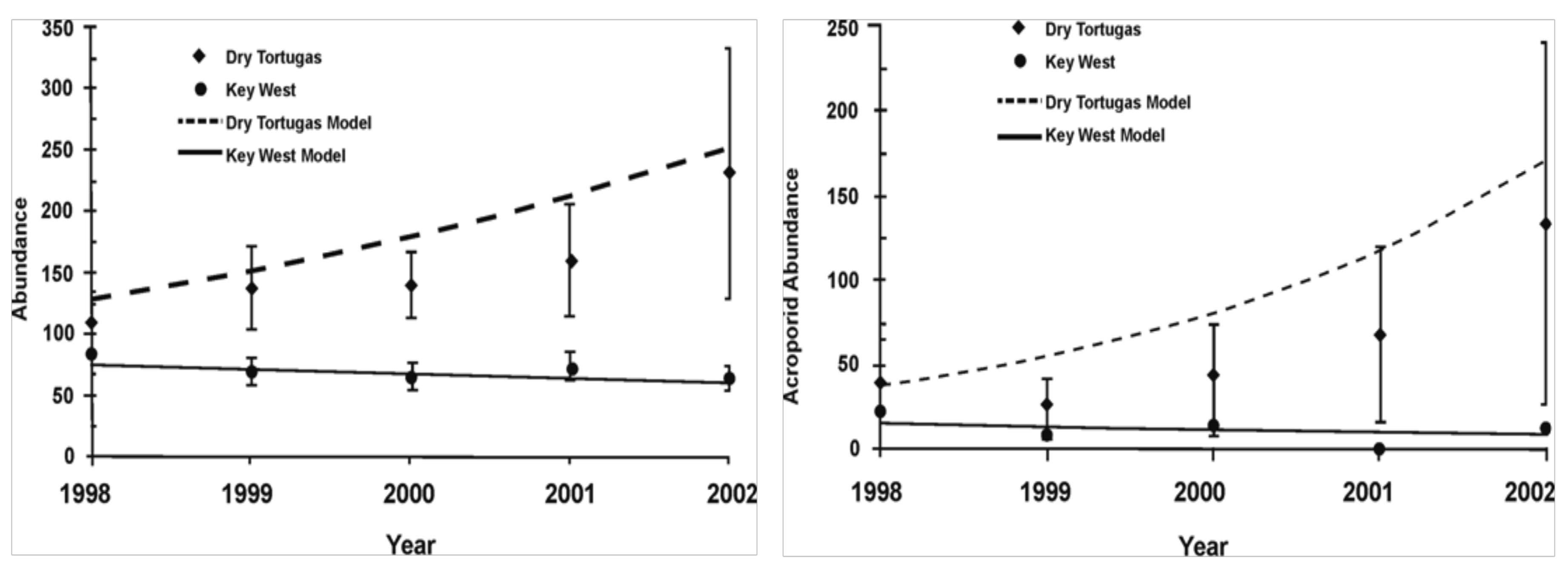
Coral abundance in the Dry Tortugas and Key West differed significantly in their responses following large-scale disturbance, whereas species diversity metrics showed only limited variation. A significant decline in mean total coral abundance and density (F = 3.18, p ≤ 0.013) occurred between spring 1998 (151 ± 36 SE) and summer 2002 on Key West reefs (65 ±10, Figure 2). In contrast, live coral abundance and density on Dry Tortugas reefs were not statistically different over the five year assessment period for the surveyed species in aggregate as well as the abundance of each of the three functional groups (p ≤ 0.660).
Declines in Key West coral abundance were attributed to significant declines in acroporids (F = 3.23, p ≤ 0.012), rather than Gorgonia spp. (F = 0.64, p ≤ 0.669) or massive coral abundance (F = 1.06, p ≤ 0.390) (Figure 2). Regression analysis showed that both total abundance and acroporid abundance declined on Key West reefs, but increased over time in the Dry Tortugas (p < 0.0001; Figure 5). Three of the coral species diversity metrics (Shannon's diversity index, Simpson's dominance index, and Margalef's species richness index) did not vary significantly within or between regions over time (Table 1). Only Pielou's evenness index significantly differed between each region in 1999 (F = 6.59, p ≤ 0.018), 2000 (F = 6.26, p ≤ 0.020), and 2002 (F = 3.73, p ≤ 0.067), with species evenness remaining the same in the Dry Tortugas, and increasing in Key West.
3.4. Change in Coral Disease and Bleaching Prevalence Following Disturbance
The prevalence of coral disease significantly increased from 5.1% to 12.5% of affected colonies on Key West reefs following large-scale disturbance, but remained relatively constant in the Dry Tortugas (4.4–6.7% of colonies) (Figure 3). Disease prevalence was also significantly elevated on Key West reefs in 2002 (10% of colonies; R = 0.055, p ≤ 0.032). Species specific and coral functional group disease information are available in the epizootic report of Santavy et al. [7].
Extensive and significantly elevated coral bleaching occurred during summer 1998 on both Key West (43% of colonies) and Dry Tortugas reefs (14% of colonies), and was the only time bleaching prevalence exceeded 3% (Figure 6). Key West reefs exhibited significantly greater aggregate bleaching prevalence than the Dry Tortugas (R = 0.186, p ≤ 0.008). In the Dry Tortugas, 6 of 15 surveyed species exhibited severe bleaching (>50% of surface tissue) compared to 12 of 15 species severely bleaching in Key West [5,6]. Acroporid bleaching was significantly greater in Key West (52% of colonies) than in Dry Tortugas (8.3%) during summer 1998, and accounted for the majority of differences in aggregate species bleaching. Bleaching prevalence in massive corals was not significantly different between Key West colonies (46%) and Dry Tortugas (37%) whereas gorgonians were never observed to bleach during any survey period. Following the 1998 bleaching event, severely bleached corals were never more than 1% of the coral community, and most bleached colonies exhibited only a low frequency of partial bleaching (Figure 6).
4. Discussion
Ecological impacts of catastrophic stressors on coral reefs in remote versus more anthropogenically affected regions is an area of increasing interest in coral reef, conservation, and global change research. There is conflicting evidence on the degree that local stressors such as degraded water quality and human uses can influence coral resilience to large-scale disturbances [2,15,21,22]. Studies in the Great Barrier and Mesoamerican reef systems have indicated that local stressors can increase susceptibility to mass-coral bleaching events [21,22]. In the upper, middle and lower Florida Keys, proximity to land-based stressors did not appear to influence coral recruitment and population structure of patch reefs surveyed between 2001 and 2003 [2]. In a 10-year study of Virgin Island coral reef community structure, areas of greater coral community resilience to repeated hurricanes was associated with lower human uses impacts [35]. Studies in the Western Indian Ocean show geographically heterogeneous responses of coral to thermal stress, further indicating that a diversity of factors control reef resilience [10].
In the current study, Key West reefs exhibited greater bleaching and disease prevalence and severity compared to the reefs of the more remote Dry Tortugas following similar large-scale hurricane impact and mass-coral bleaching events. Coral abundance significantly declined on Key West reefs and coral density remained lower during the 5-year assessment. These results provide additional evidence that coral reef ecosystems distant from anthropogenic influences may have greater resilience to large-scale disturbances [15,21,22]. Additional research is needed on the determinants of local stressors effects on coral reef resilience, as well as the importance of recent disturbance history, coral community condition, species composition, and other factors [10-12].
Acroporid responses to disturbance were a primary difference in the lower resilience of Key West reefs. Acroporid bleaching was significantly elevated on Key West reefs (52% of colonies) compared to Dry Tortugas acroporids (8% of colonies). The abundance of acroporids on Key West reefs continued to decline following hurricane and bleaching impacts, whereas Dry Tortugas acroporids rebounded. Acroporids were once a dominant reef-building coral in the Caribbean, but have experienced substantial regional declines, resulting in U.S. Federal listing as a protected species [9]. Miller et al. [36] reported that acroporid abundance declined over 90% between 1983 and 2000 in the lower Keys (Looe Key), and that substantial population declines may have occurred prior to 1983. Additionally, Williams et al. [20] reported recruitment failure in A. palmata at upper Keys sites between 2004 and 2007. Hughes et al. [11] and others have noted the increase in phase shifts from once common coral communities, and the need for research directed from monitoring ecosystem declines to understanding the processes controlling degradation.
Making inferences on multiple stressor effects on ecological systems from environmental data is uncertain, but is critical to assessing large-scale complex problems. The current study utilized a survey design focused on individual colonies that could be identified to species, and included measures of abundance, diversity, disease and bleaching prevalence. Together, these measures indicated generally similar condition in the Dry Tortugas and Key West prior to the large-scale disturbances of mass-coral bleaching and hurricane impacts during 1998, and continued declines in coral condition on Key West reefs. Other studies have used different metrics of coral condition, and reported similar trends of declining coral reef health and changes in species composition in the Florida Keys [3,5]. The Dry Tortugas have been less studied, but are generally considered to be in better condition as reported in the current study [37]. Florida's reefs can be considered marginal environments where corals are less resilient than many reefs in the Caribbean. For example, an estimated 30,800 km2 of shallow inshore waters in the Florida Reef Tract could potentially support coral reef ecosystems [38], but only 1150 km2 contain live coral reef habitat [29]. The results of the current study and other recent reports show that despite large-scale disturbances, coral reef ecosystems can still exhibit positive population growth if there are minimal additional stressors [14,16,17]. Several authors have proposed frameworks for understanding both the factors that control reef resilience and indicators of sustainable reef ecosystems [10-12]. The continued global decline in coral reef ecosystems creates an urgent need for management strategies that promote reef resilience [10-12].




| Region | Survey | d | H | λ | J |
|---|---|---|---|---|---|
| Dry Tortugas | Spring 1998 | 1.50 ± 0.189 | 1.37 ± 0.170 | 0.376 ± 0.0703 | 0.655 ± 0.0667 |
| Summer 1998 | 1.52 ± 0.188 | 1.37 ± 0.164 | 0.381 ± 0.0661 | 0.668 ± 0.0649 | |
| Spring 1999 | 1.38 ± 0.145 | 1.12 ± 0.130 | 0.487 ± 0.0578 | 0.561 ± 0.0530 | |
| Summer 2000 | 1.42 ± 0.154 | 1.10 ± 0.134 | 0.491 ± 0.0601 | 0.540 ± 0.0543 | |
| Summer 2001 | 1.46 ± 0.178 | 1.23 ± 0.162 | 0.437 ± 0.0702 | 0.593 ± 0.0673 | |
| Summer 2002 | 1.49 ± 0.199 | 1.25 ± 0.165 | 0.434 ± 0.0673 | 0.597 ± 0.0643 | |
| Key West | Spring 1998 | 1.40 ± 0.202 | 1.32 ± 0.166 | 0.393 ± 0.0720 | 0.693± 0.0425 |
| Summer 1998 | 1.48 ± 0.197 | 1.35 ± 0.159 | 0.383 ± 0.0687 | 0.723± 0.0378 | |
| Spring 1999 | 1.36 ± 0.166 | 1.31 ± 0.140 | 0.372 ± 0.0574 | 0.730 ± 0.0393 | |
| Summer 2000 | 1.26 ± 0.184 | 1.15 ± 0.160 | 0.447 ± 0.0693 | 0.715 ± 0.0438 | |
| Summer 2001* | 1.97 ± 0.192 | 1.49 ± 0.128 | 0.345 ± 0.0537 | 0.670 ± 0.0435 | |
| Summer 2002 | 1.40 ± 0.199 | 1.28 ± 0.153 | 0.401 ± 0.0652 | 0.731 ± 0.0267 | |
*Summer 2001: only 5 of 12 Key West stations sampled.
Acknowledgments
This article is dedicated to the memory of Captain Robert Quarles who served on multiple coral monitoring surveys. Support was provided by United States Environmental Protection Agency (US EPA), Office of Research and Development, Gulf Ecology Division (GED), to National Oceanographic and Atmospheric Association through an Interagency Agreement (RW13937452) and by Mote Marine Laboratory. Ship support by US EPA, Office of Water on the OSV/Peter W. Anderson. Small vessel and logistical support was provided by the Florida Keys National Marine Sanctuary and Dry Tortugas National Park. We thank J. Campbell, J. Patrick, M. Parsons, K. Potts, J. Hawkridge, J. Porter, K. Patterson, and D. Marcinek for field support, and J. Kern for statistical advice. Mention of trade names or commercial products does not constitute endorsement by US EPA. Contribution #1296 US EPA, GED, Gulf Breeze, FL.
References
- Keller, B.D.; Causey, B.D. Linkages between the Florida Keys National Marine Sanctuary and the South Florida ecosystem restoration initiative. Ocean Coast Manag. 2005, 48, 869–900. [Google Scholar]
- Lirman, D.; Fong, P. Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida Reef Tract. Mar. Poll Bull. 2007, 54, 779–791. [Google Scholar]
- Somerfield, P.J.; Jaap, W.C.; Clarke, K.R.; Callahan, M.; Hackett, K.; Porter, J.; Lybolt, M.; Tsokos, C.; Yanev, G. Changes in coral reef communities among the Florida Keys, 1996–2003. Coral Reefs 2008, 27, 951–965. [Google Scholar]
- Yee, S.H.; Santavy, D.L.; Barron, M.G. Assessing the effects of disease and bleaching on Florida Keys corals by fitting population models to data. Ecol. Model. 2011, 222, 1323–1332. [Google Scholar]
- Precht, W.F.; Miller, S.L. Ecological shifts along the Florida Reef tract: The past as a key to the future. In Geological Approaches to Coral Reef Ecology; Aronson, R.B., Ed.; Springer: New York, NY, USA, 2007; pp. 237–312. [Google Scholar]
- Helme, K.P.; Dodge, R.E.; Swart, P.K.; Gledhill, D.K.; Eakin, C.M. Growth rates of Florida corals from 1937 to 1996 and their response to climate change. Nat. Com. 2011, 2011, 1–6. [Google Scholar]
- Santavy, D.L.; Campbell, J.; Quarles, R.L.; Patrick, J.M.; Hawkridge, J. The Epizootiology of Coral Diseases in South Florida; EPA/600/R-05/146U; SEPA: Gulf Breeze, FL, USA; GPO: Washington, DC, USA, 2006. [Google Scholar]
- Santavy, D.L.; Mueller, E.; Peters, E.C.; MacLaughlin, L.; Porter, J.W.; Patterson, K.L.; Campbell, J. Quantitative assessment of coral diseases in the Florida Keys: Strategy and methodology. Hydrobiologia 2001, 460, 39–52. [Google Scholar]
- USDOC. Endangered and threatened species; Critical habitat for threatened Elkhorn and Staghorn Corals. Final rule: 50 CFR Parts 223 and 226. Fed. Regist. 2008, 73, 72210–72240. [Google Scholar]
- Obura, D.O. Resilience, coral bleaching and MPA design. Estuar. Coast. Shelf Sci. 2005, 603, 353–372. [Google Scholar]
- Hughes, T.P.; Graham, A.J.; Jackson, J.B.C.; Mumby, P.J.; Stenick, R.S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 2010, 25, 619–680. [Google Scholar]
- Nystrom, M.; Graham, N.A.J.; Lokrantz, J.; Norstrom, A.V. Capturing the cornerstones of coral reef resilience: Linking theory to practice. Coral Reefs 2008, 27, 795–809. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar]
- Smith, L.D.; Gilmour, J.P.; Heyward, A.J. Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching. Coral Reefs 2008, 27, 197–205. [Google Scholar]
- Yee, S.H.; Santavy, D.L.; Barron, M.G. Comparing environmental influences on coral bleaching across and within species using clustered binomial regression. Ecol. Model. 2008, 218, 162–174. [Google Scholar]
- Mallela, J.; Crabbe, M.J.C. Hurricanes and coral bleaching linked to changes in coral recruitment in Tobago. Mar. Environ. Res. 2009, 68, 158–162. [Google Scholar]
- Trapon, M.L.; Pratchett, M.S.; Penin, L. Comparative effects of different disturbances in coral reef habitats in Moorea, French Polynesia. J. Mar. Biol. 2011, 2011, 1–11. [Google Scholar]
- Alvarez-Filip, L.; Cote, I.M.; Gill, J.A.; Watkinson, A.R.; Dulvy, N.K. Region-wide temporal and spatial variation in Caribbean reef architecture: Is coral cover the whole story. Glob. Change Biol. 2010, 17, 2470–2477. [Google Scholar]
- Gardner, T.A.; Cote, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Hurricanes and Caribbean coral reefs: Impacts, recovery patterns, and role in long-term decline. Ecology 2005, 86, 174–184. [Google Scholar]
- Williams, D.E.; Miller, M.W.; Kramer, K.L. Recruitment failure in Florida Keys Acropora palmata, a threatened Caribbean coral. Coral Reefs 2008, 27, 687–705. [Google Scholar]
- Wooldridge, S.A.; Done, T.J. Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 2009, 19, 1492–1499. [Google Scholar]
- Carilli, J.E.; Norris, R.D.; Black, B.; Walsh, S.M.; McField, M. Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob. Change Biol. 2010, 16, 1247–1257. [Google Scholar]
- Lee, T.N.; Leaman, K.; Williams, E.; Berger, T.; Atkinson, L. Florida current meanders and gyre formation in the southern Straits of Florida. J. Geophys. Res. 1995, 100, 8607–8620. [Google Scholar]
- Porter, J.W.; Lewis, S.K.; Porter, K.G. The effect of multiple stressors on the Florida Keys coral reef ecosystem: A landscape hypothesis and a physiological test. Limnol. Oceanogr. 2007, 44, 941–949. [Google Scholar]
- Boyer, J.N.; Jones, R.D. A View from the bridge: External and internal forces affecting the ambient water quality of the Florida Keys National Marine Sanctuary (FKNMS). In The Everglades, Florida Bay, and Coral Reefs of the Florida Keys An Ecosystem Sourcebook; Porter, J.W., Porter, K.G., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Volume 27, pp. 609–628. [Google Scholar]
- Manzello, D.P.; Berkelmans, R.; Hendee, J.C. Coral bleaching indices and thresholds for the Florida reef tract, Bahamas, and St. Croix, US Virgin Islands. Mar. Poll. Bull. 2007, 54, 1923–1931. [Google Scholar]
- Guiney, J.L. Report from National Hurricane Center. Hurricane Georges, 15 September–1 October 1998. Available online: http://www.nhc.noaa.gov/1998georges.html (accessed on 5 January 1999).
- Santavy, D.L.; Summers, J.K.; Engle, V.D.; Harwell, L.C. The condition of coral reefs in south Florida (2000) using coral disease and bleaching as an indicators. Environ. Monitor. Assess. 2005, 100, 129–152. [Google Scholar]
- Benthic habitats of the Florida Keys. In Florida Marine Research Institute Technical Report TR-4; Florida Marine Research Institute: Petersburg, FL, USA, 1998; Volume 53.
- Sutherland, K.P.; Porter, J.W.; Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 2004, 266, 273–302. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v5: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Application; Cambridge University Press: Cambridge, MA, USA, 1997; Chapter 5. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Casey, K.S.; Brandon, T.B.; Cornillon, P.; Evans, R. The Past, present and future of the AVHRR Pathfinder SST Program. Oceanography from Space: Revisited; Barale, V., Gower, J.F.R., Alberotanza, L., Eds.; Springer: New York, USA, 2010. Available online: http://www.nodc.noaa.gov/sog/pathfinder4km/ (date accessed 1 September 2011). [Google Scholar]
- Bythell, J.C.; Hillis-Starr, Z.M.; Rogers, C.S. Local variability but landscape stability in coral reef communities following repeated hurricane impacts. Mar. Ecol. Prog. Ser. 2000, 204, 93–100. [Google Scholar]
- Miller, M.W.; Bourque, A.S.; Bohnsack, J.A. An analysis of the loss of acroporid corals at Looe Key, Florida, USA: 1983–2000. Coral Reefs 2002, 21, 179–182. [Google Scholar]
- Keller, B.D.; Donahue, S. 2002–03 Florida Keys National Marine Sanctuary Science Report: An Ecosystem Report Card after Five Years of Marine Zoning; NMSP-06-12; US Department Commerce, NOAA, NMSP: Silver Spring, MD, USA, 2006. [Google Scholar]
- Rohmann, S.O.; Hayes, J.J.; Newhall, R.C.; Monaco, M.E.; Grigg, R.W. The area of potential shallow-water tropical and subtropical coral ecosystems in the United States. Coral Reefs 2005, 4, 370–383. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Santavy, D.L.; Mueller, E.M.; MacLaughlin, L.; Peters, E.C.; Quarles, R.L.; Barron, M.G. Resilience of Florida Keys Coral Communities Following Large-Scale Disturbances. Diversity 2011, 3, 628-640. https://doi.org/10.3390/d3040628
Santavy DL, Mueller EM, MacLaughlin L, Peters EC, Quarles RL, Barron MG. Resilience of Florida Keys Coral Communities Following Large-Scale Disturbances. Diversity. 2011; 3(4):628-640. https://doi.org/10.3390/d3040628
Chicago/Turabian StyleSantavy, Deborah L., Erich M. Mueller, Lauri MacLaughlin, Esther C. Peters, Robert L. Quarles, and Mace G. Barron. 2011. "Resilience of Florida Keys Coral Communities Following Large-Scale Disturbances" Diversity 3, no. 4: 628-640. https://doi.org/10.3390/d3040628
APA StyleSantavy, D. L., Mueller, E. M., MacLaughlin, L., Peters, E. C., Quarles, R. L., & Barron, M. G. (2011). Resilience of Florida Keys Coral Communities Following Large-Scale Disturbances. Diversity, 3(4), 628-640. https://doi.org/10.3390/d3040628




