Responses of Cryptofaunal Species Richness and Trophic Potential to Coral Reef Habitat Degradation
Abstract
:1. Introduction
2. Evidence from Eastern Pacific Pocilloporid Reefs
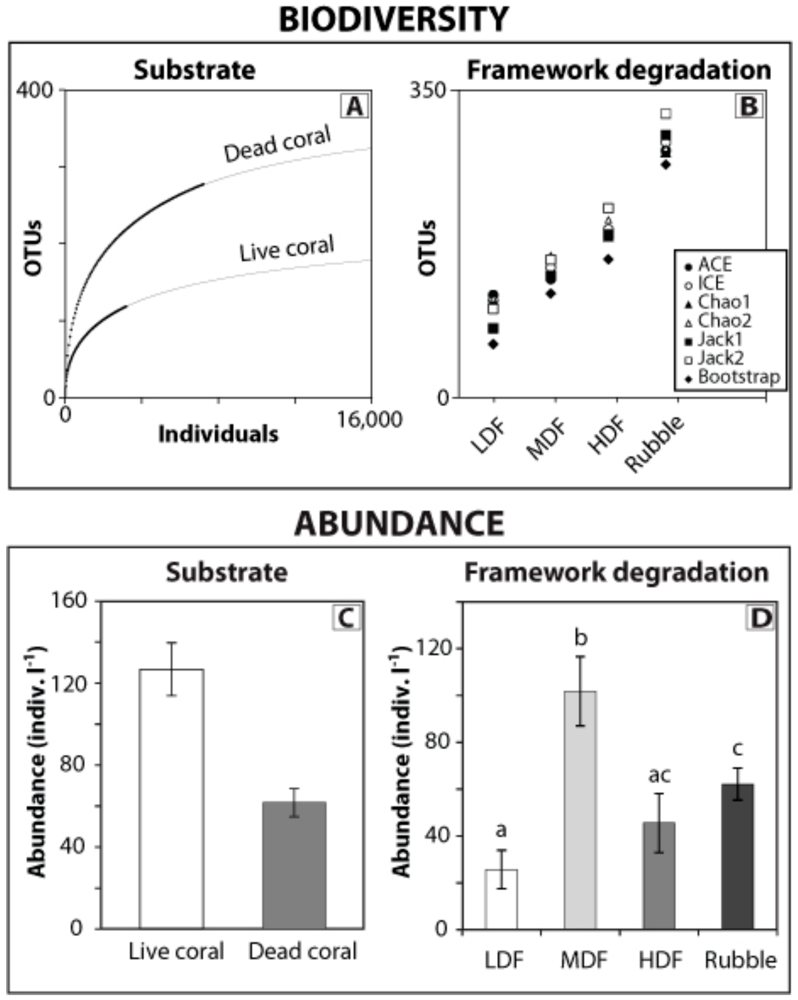
3. Predicting Ecosystem Responses to Climate Change
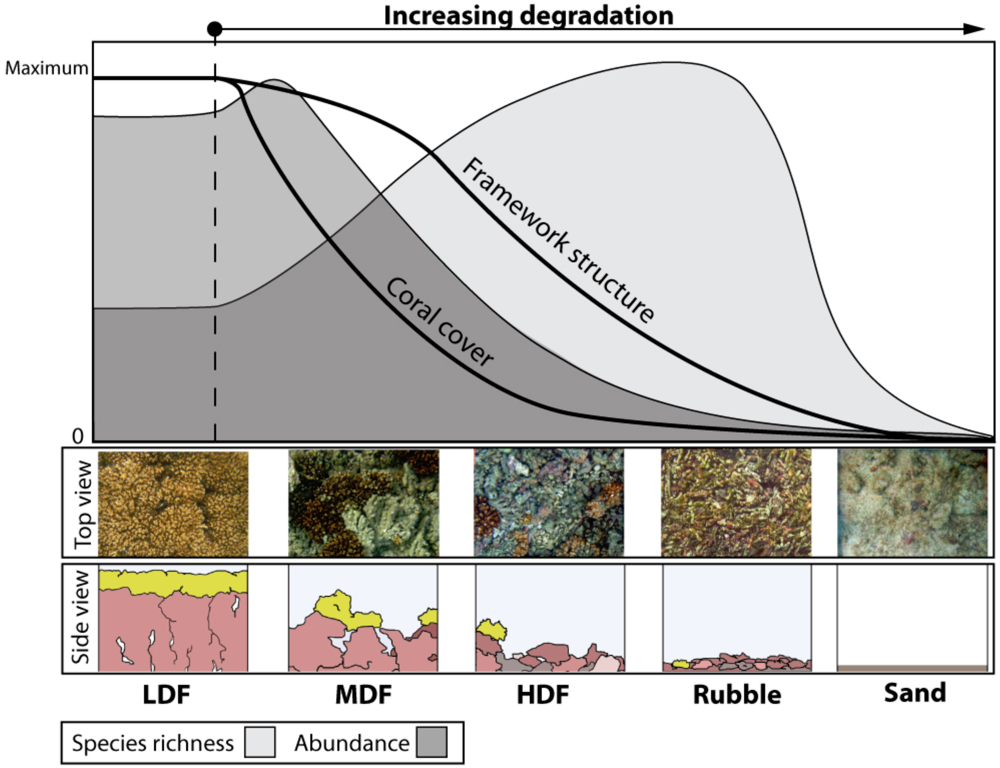
4. Parallels with Forest Ecosystems
5. Conclusions
Acknowledgments
References
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–638. [Google Scholar]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.A.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar]
- Reaka-Kudla, M.L. The global biodiversity of coral reefs. In Biodiversity II: Understanding and Protecting Our Biological Resources; Reaka-Kudla, M.L., Wilson, D.E., Wilson, E.O., Eds.; Joseph Henry Press: Washington, DC, USA, 1997; pp. 83–108. [Google Scholar]
- Adrianov, A.V. Current problems in marine biodiversity studies. Russ. J. Mar. Biol. 2004, 30, S1–S16. [Google Scholar] [CrossRef]
- Small, A.; Adey, W.; Spoon, D. Are current estimates of coral reef biodiversity too low? The view through the window of a microcosm. Atoll Res. Bull. 1998, 458, 1–20. [Google Scholar] [CrossRef]
- Reaka, M.L.; Rodgers, P.J.; Kudla, A.U. Patterns of biodiversity and endemism on Indo-West Pacific coral reefs. Proc. Natl. Acad. Sci. USA 2008, 105, 11474–11581. [Google Scholar]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.N.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar]
- Munday, P.L. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Change Biol. 2004, 10, 1642–1647. [Google Scholar] [CrossRef]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Chiriboga, A.; Cortés, J.; Delbeek, J.C.; DeVantier, L.; et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 2008, 321, 560–563. [Google Scholar]
- Glynn, P.W. In tandem reef coral and cryptic metazoan declines and extinctions. Bull. Mar. Sci. 2011, 87, 1–28. [Google Scholar] [CrossRef]
- Wilson, E.O. The Diversity of Life; W. W. Norton and Company: New York, NY, USA, 1992. [Google Scholar]
- Carlton, J.T.; Geller, J.B.; Reaka-Kudla, M.L.; Norse, E.A. Historical extinctions in the sea. Ann. Rev. Ecol. Syst. 1999, 30, 515–538. [Google Scholar] [CrossRef]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast Shelf Sci. 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Wilkinson, C. Status of Coral Reefs of the World: 2000; Australian Institute of Marine Science: Townsville, Australia, 2000. [Google Scholar]
- Maynard, J.A.; Baird, A.H.; Pratchett, M.S. Revisiting the Cassandra syndrome; the changing climate of coral reef research. Coral Reefs 2008, 27, 745–749. [Google Scholar] [CrossRef]
- Enochs, I.C.; Toth, L.T.; Brandtneris, V.W.; Afflerbach, J.C.; Manzello, D.P. Environmental determinants of motile cryptofauna on an eastern Pacific coral reef. Mar. Ecol. Prog. Ser. 2011, 438, 105–118. [Google Scholar] [CrossRef]
- Ginsburg, R.N. Geological and biological roles of cavities in coral reefs. In Perspectives on Coral Reefs; Barnes, D.J., Ed.; Australian Institute of Marine Science: Townsville, Australia, 1983; pp. 148–153. [Google Scholar]
- Richter, C.; Wunsch, M.; Rasheed, M.; Kötter, I.; Badran, M.I. Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature 2001, 413, 726–730. [Google Scholar]
- Caley, M.J.; Buckley, K.A.; Jones, G.P. Separating ecological effects of habitat fragmentation, degradation, and loss on coral commensals. Ecology 2001, 82, 3435–3448. [Google Scholar]
- Enochs, I.C.; Hockensmith, G. Effects of coral mortality on the community composition of cryptic metazoans associated with Pocillopora damicornis. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 1368–1372.
- Coker, D.J.; Pratchett, M.S.; Munday, P.L. Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav. Ecol. 2009, 20, 1204–1210. [Google Scholar] [CrossRef]
- Enochs, I.C.; Manzello, D.P. Species richness of motile coral reef cryptofauna across a gradient of framework erosion in Pacific Panamá. Coral Reefs 2012, in press.. [Google Scholar]
- Enochs, I.C. Motile cryptofauna associated with live and dead coral substrates: Implications for coral mortality and framework erosion. Mar. Biol. 2012, in press.. [Google Scholar]
- Lang, J.C.; Chornesky, E.A. Competition between scleractinian reef corals: A review of mechanisms and effects. In Ecosystems of the World: Coral Reefs; Dubinsky, Z., Ed.; Elsevier Press: Amsterdam, The Netherlands, 1990; pp. 209–252. [Google Scholar]
- Moran, D.P.; Reaka, M.L. Bioerosion and availability of shelter for benthic reef organisms. Mar. Ecol. Prog. Ser. 1988, 44, 249–263. [Google Scholar] [CrossRef]
- Jackson, J.B.C. Distribution and ecology of clonal and aclonal benthic invertebrates. In Population Biology and Evolution of Clonal Organisms; Jackson, J.B.C., Buss, L.W., Cook, R.E., Eds.; Yale University Press: New Haven, CT, USA, 1985; pp. 297–355. [Google Scholar]
- Bailey-Brock, J.; Brock, R.; Kam, A.; Fukunaga, A.; Akiyama, H. Anthropogenic disturbances on shallow cryptofaunal communities in a marine life conservation district on Oahu, Hawai’i. Int. Rev. Hydrobiol. 2007, 92, 291–300. [Google Scholar] [CrossRef]
- Patton, W.K. Community structure among the animals inhabiting the coral Pocillopora damicornis at Heron Island, Australia. In Symbiosis in the Sea; Vernberg, W., Ed.; University of South Carolina Press: Columbia, SC, USA, 1974; pp. 219–243. [Google Scholar]
- Stimson, J. Stimulation of fat-body production in the polyps of the coral Pocillopora damicornis by the presence of mutualistic crabs of the genus Trapezia. Mar. Biol. 1990, 106, 211–218. [Google Scholar] [CrossRef]
- Rotjan, R.D.; Lewis, S.M. Impact of coral predators on tropical reefs. Mar. Ecol. Prog. Ser. 2008, 367, 73–91. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. Roy. Soc. Lond. B 2009, 276, 3019–3025. [Google Scholar] [CrossRef]
- Manzello, D.P. Reef development and resilience to acute (El Niño Warming) and chronic (high-CO2) disturbances in the eastern tropical Pacific: A real-world climate change model. In Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, FL, USA, 7–11 July 2008; pp. 1299–1304.
- Reaka-Kudla, M.L.; Feingold, J.S.; Glynn, P.W. Experimental studies of rapid bioerosion of coral reefs in the Galápagos Islands. Coral Reefs 1996, 15, 101–107. [Google Scholar]
- Wilson, S.K.; Dolman, A.M.; Cheal, A.J.; Emslie, M.J.; Pratchett, M.S.; Sweatman, H.P.A. Maintenance of fish diversity on disturbed coral reefs. Coral Reefs 2009, 28, 3–14. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Robinson, J.; Bijoux, J.P.; Daw, T.M. Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 2007, 21, 1291–1300. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Pratchett, M.S.; Polunin, N.V.C.; Spalding, M.D. Coral mortality versus structural collapse as drivers of corallivorous butterflyfish decline. Biodiver. Conserv. 2009, 18, 3325–3336. [Google Scholar] [CrossRef]
- Peyrot-Clausade, M. Motile cryptofauna of Tuléar reef flats. Mar. Biol. 1980, 59, 43–47. [Google Scholar] [CrossRef]
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Science: Townsville, Australia, 2000. [Google Scholar]
- Coles, S.L. Species diversity of decapods associated with living and dead reef coral Pocillopora meandrina. Mar. Ecol. Prog. Ser. 1980, 2, 281–291. [Google Scholar] [CrossRef]
- Shirayama, Y.; Horikoshi, M. A new method of classifying the growth form of corals and its application to a field survey of coral-associated animals in Kabira Cove, Ishigaki Island. J. Oceanogr. Soc. Jpn. 1982, 28, 193–207. [Google Scholar] [CrossRef]
- Kirsteuer, E. Quantitative and qualitative aspects of the Nemertean Fauna in tropical coral reefs. In Proceedings of the 1st International Symposium Coral Reefs, Mandapam Camp, India, 12–16 January 1969; pp. 363–371.
- Preston, N.P.; Doherty, P.J. Cross-shelf patterns in the community structure of coral-dwelling Crustacea in the central region of the Great Barrier Reef. I. Agile shrimps. Mar. Ecol. Prog. Ser. 1990, 66, 47–61. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Grigg, R.W.; Maragos, J.E. Recolonization of hermatypic corals on submerged lava flows in Hawaii. Ecology 1974, 55, 387–395. [Google Scholar] [CrossRef]
- Siitonen, J. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Schiegg, K. Saproxylic insect diversity of beech: Limbs are richer than trunks. For. Ecol. Manag. 2001, 149, 295–304. [Google Scholar] [CrossRef]
- Nordén, B.; Ryberg, M.; Götberg, F.; Olausson, B. Relative importance of coarse and fine woody debris for the diversity of wood-inhabiting fungi in temperate broadleaf forests. Biol. Cons. 2004, 117, 1–10. [Google Scholar] [CrossRef]
- Nilsson, S.G.; Hedin, J.; Niklasson, M. Biodiversity and its assessment in boreal and nemoral forests. Scand. J. For. Res.Suppl. 2001, 3, 10–26. [Google Scholar]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar]
- Peyrot-Clausade, M.; Hutchings, P.; Richard, G. Temporal variations of macroborers in massive Porites lobata on Moorea, French Polynesia. Coral Reefs 1992, 11, 161–166. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Enochs, I.C.; Manzello, D.P. Responses of Cryptofaunal Species Richness and Trophic Potential to Coral Reef Habitat Degradation. Diversity 2012, 4, 94-104. https://doi.org/10.3390/d4010094
Enochs IC, Manzello DP. Responses of Cryptofaunal Species Richness and Trophic Potential to Coral Reef Habitat Degradation. Diversity. 2012; 4(1):94-104. https://doi.org/10.3390/d4010094
Chicago/Turabian StyleEnochs, Ian C., and Derek P. Manzello. 2012. "Responses of Cryptofaunal Species Richness and Trophic Potential to Coral Reef Habitat Degradation" Diversity 4, no. 1: 94-104. https://doi.org/10.3390/d4010094
APA StyleEnochs, I. C., & Manzello, D. P. (2012). Responses of Cryptofaunal Species Richness and Trophic Potential to Coral Reef Habitat Degradation. Diversity, 4(1), 94-104. https://doi.org/10.3390/d4010094



