New Records of Wild Bees from Calabria and Basilicata Highlight the Hidden Diversity of Anthophila in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxonomic Framework
2.2. DNA Barcoding
3. Results
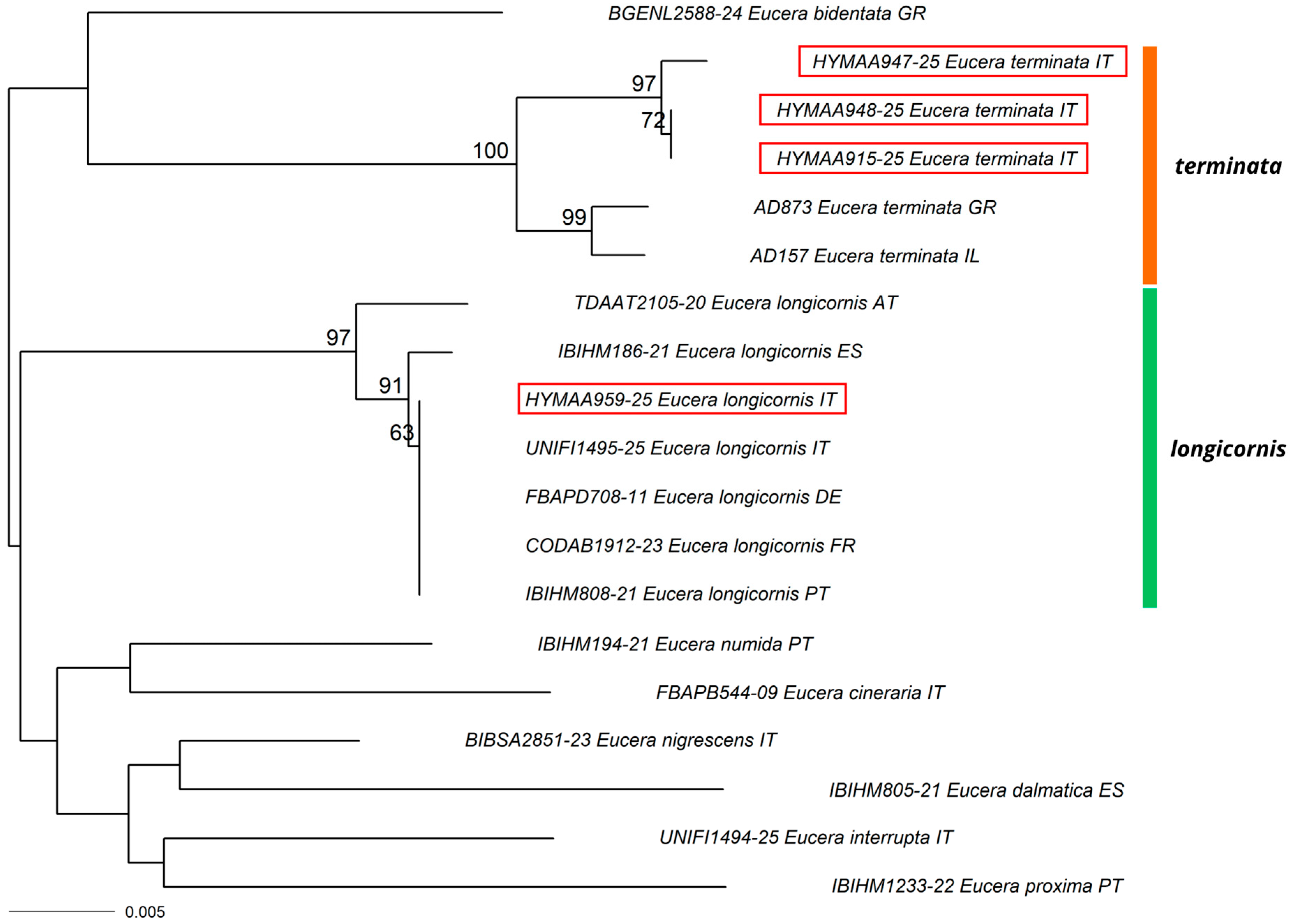
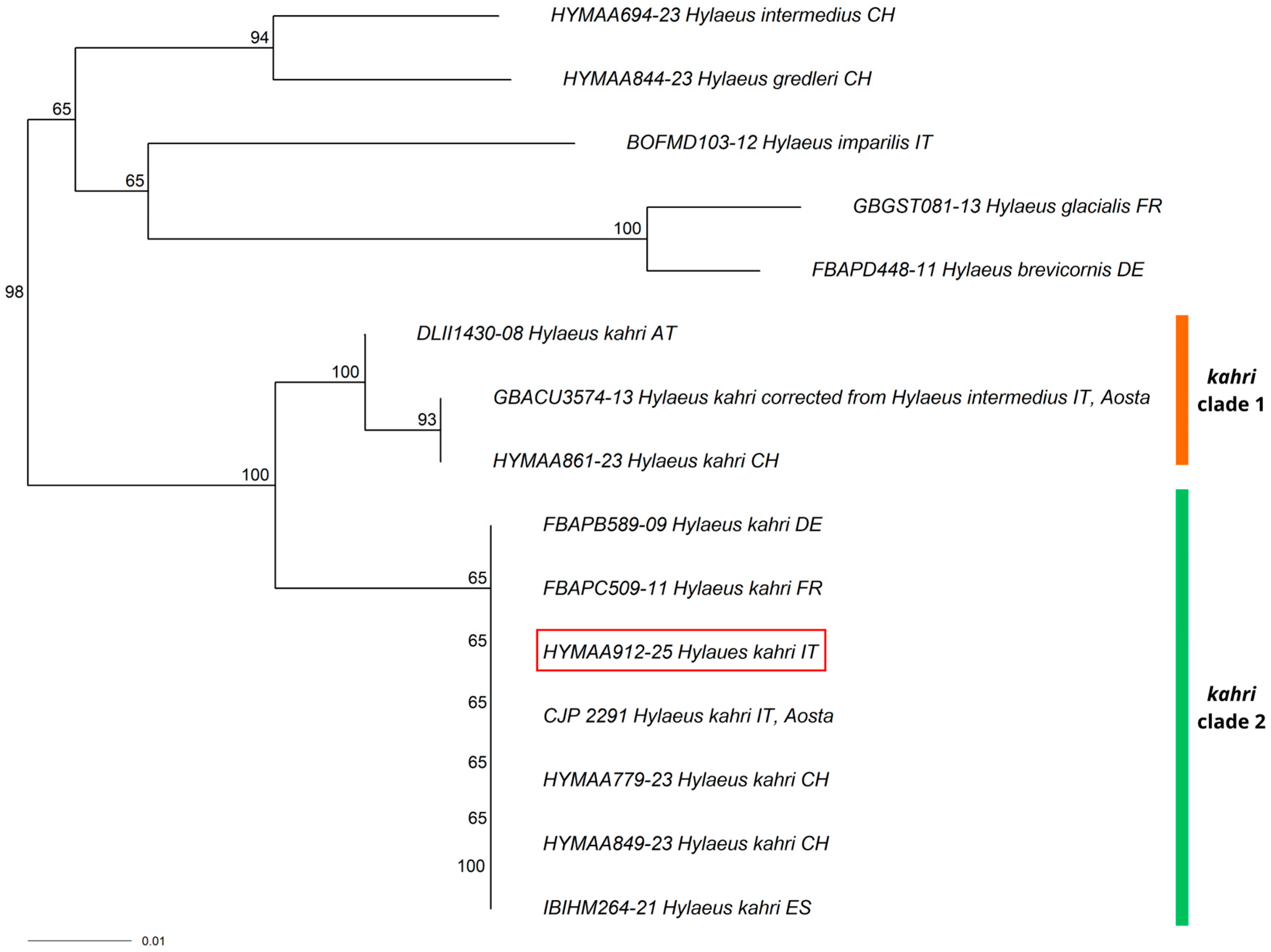
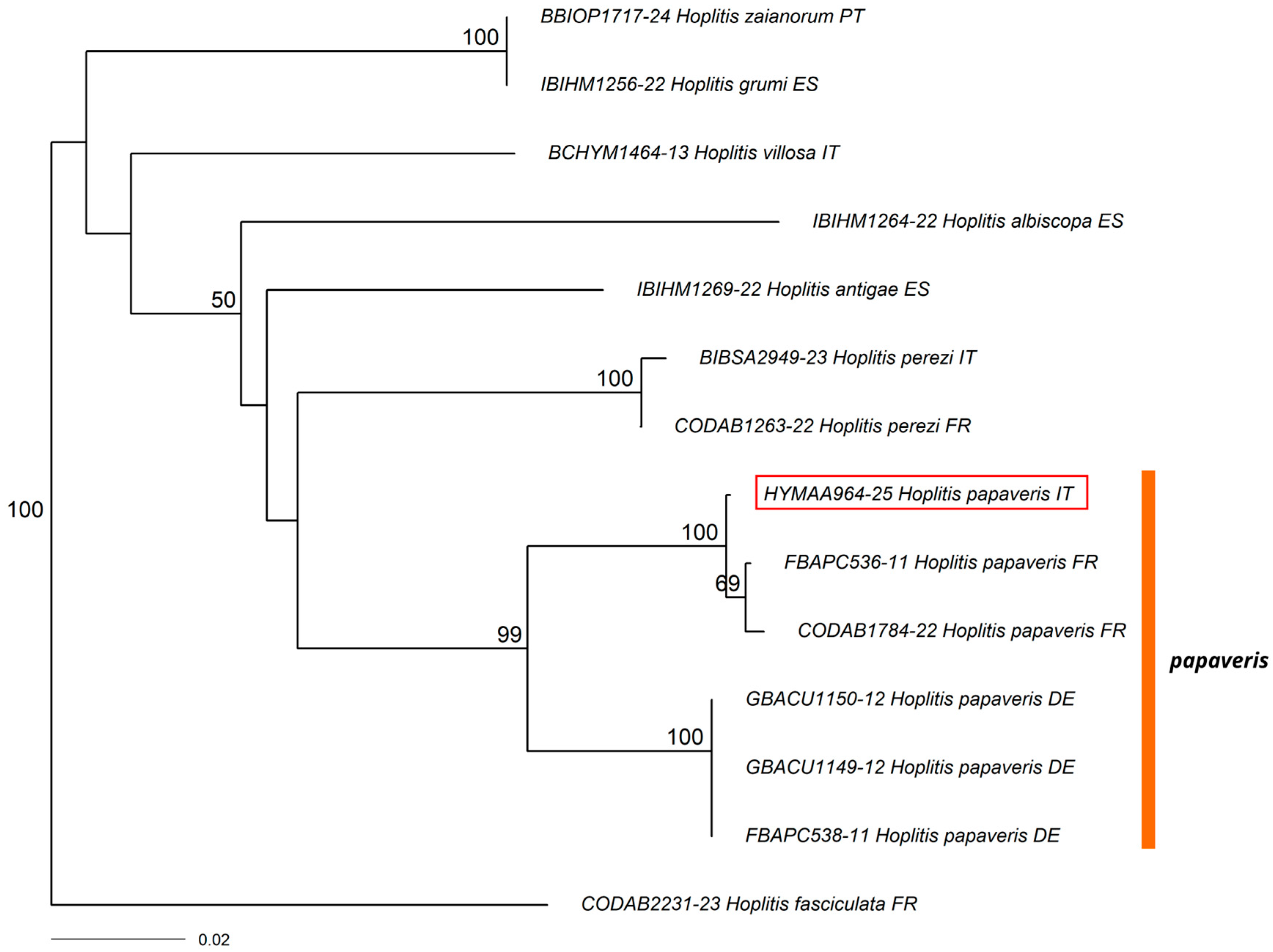
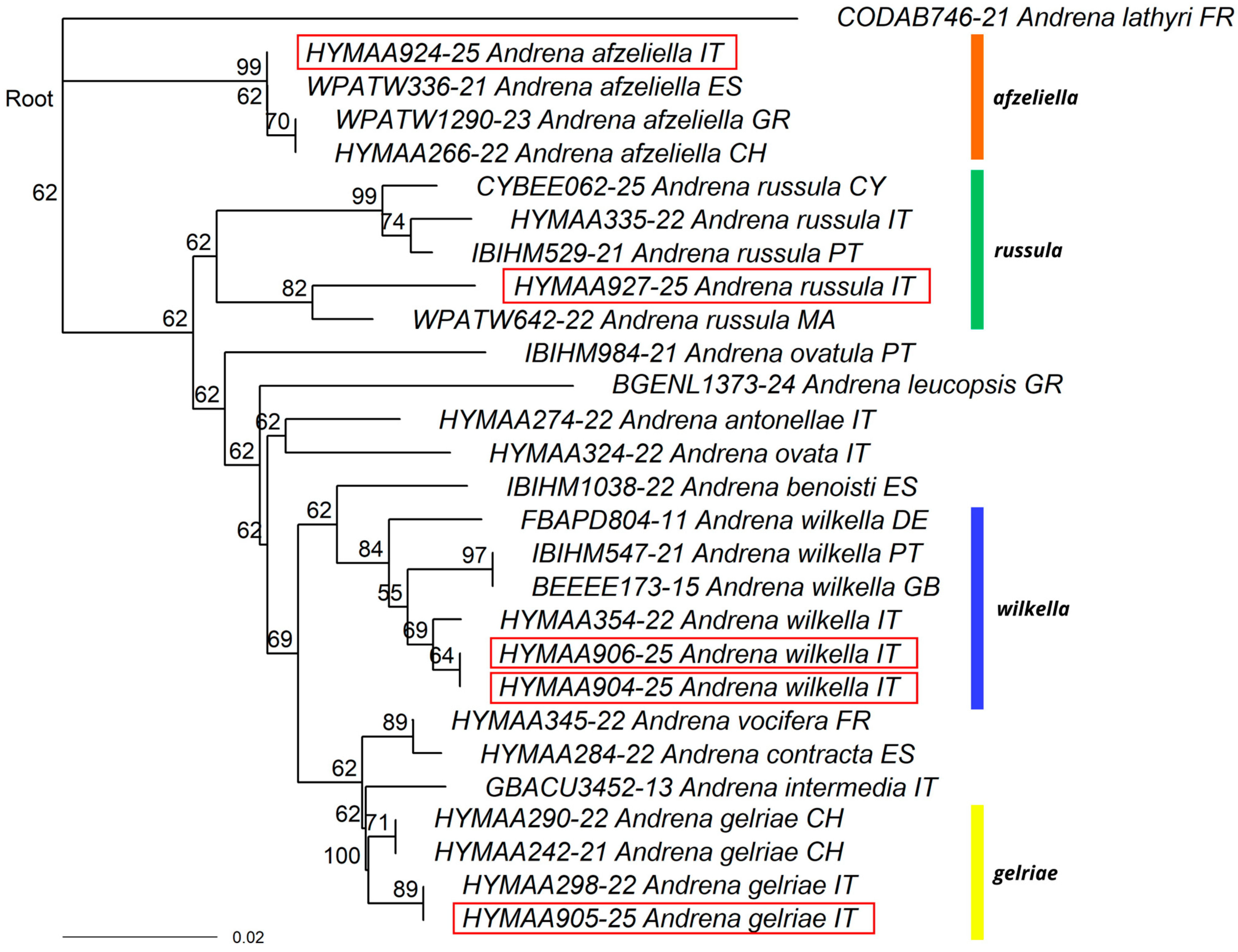
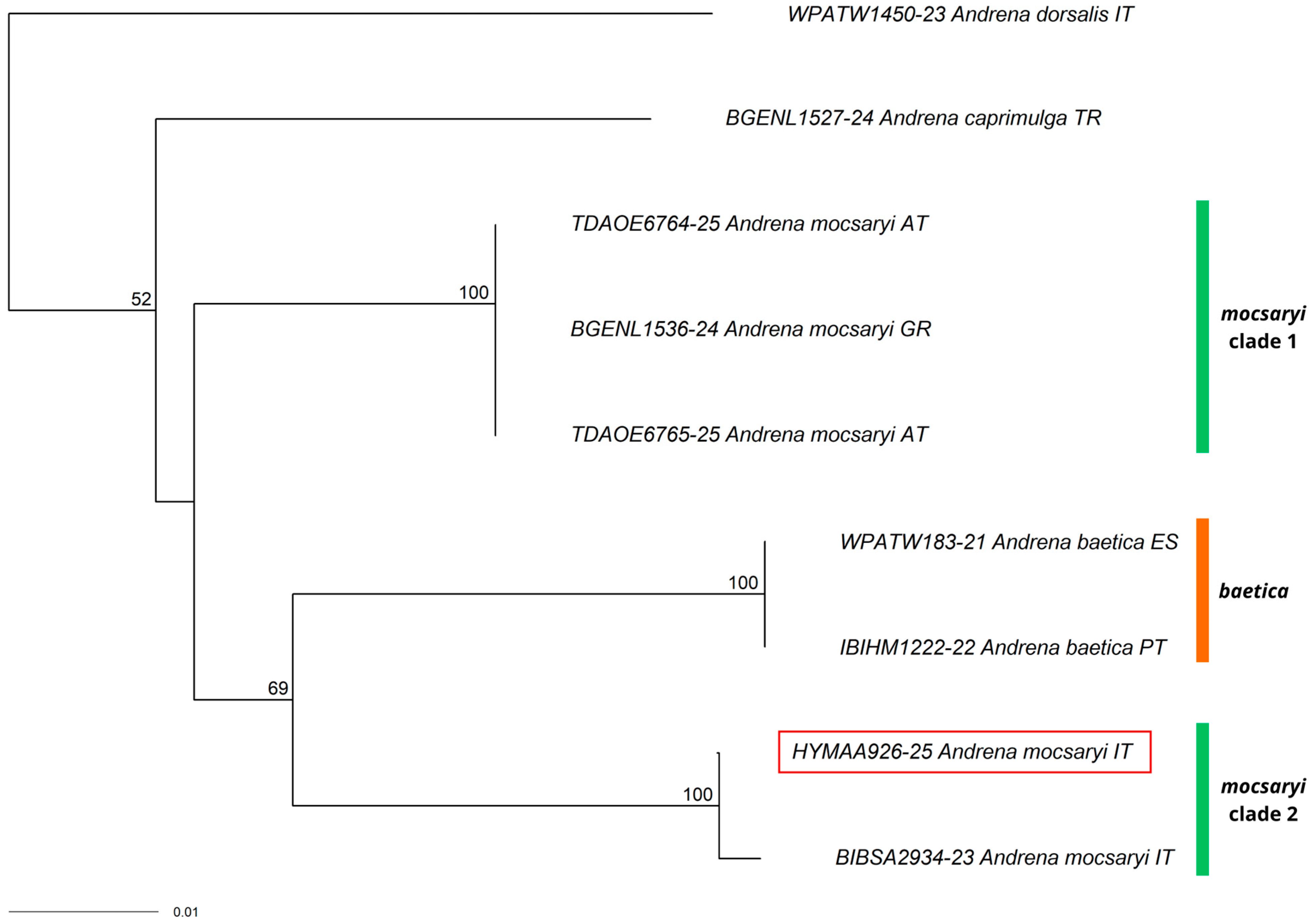
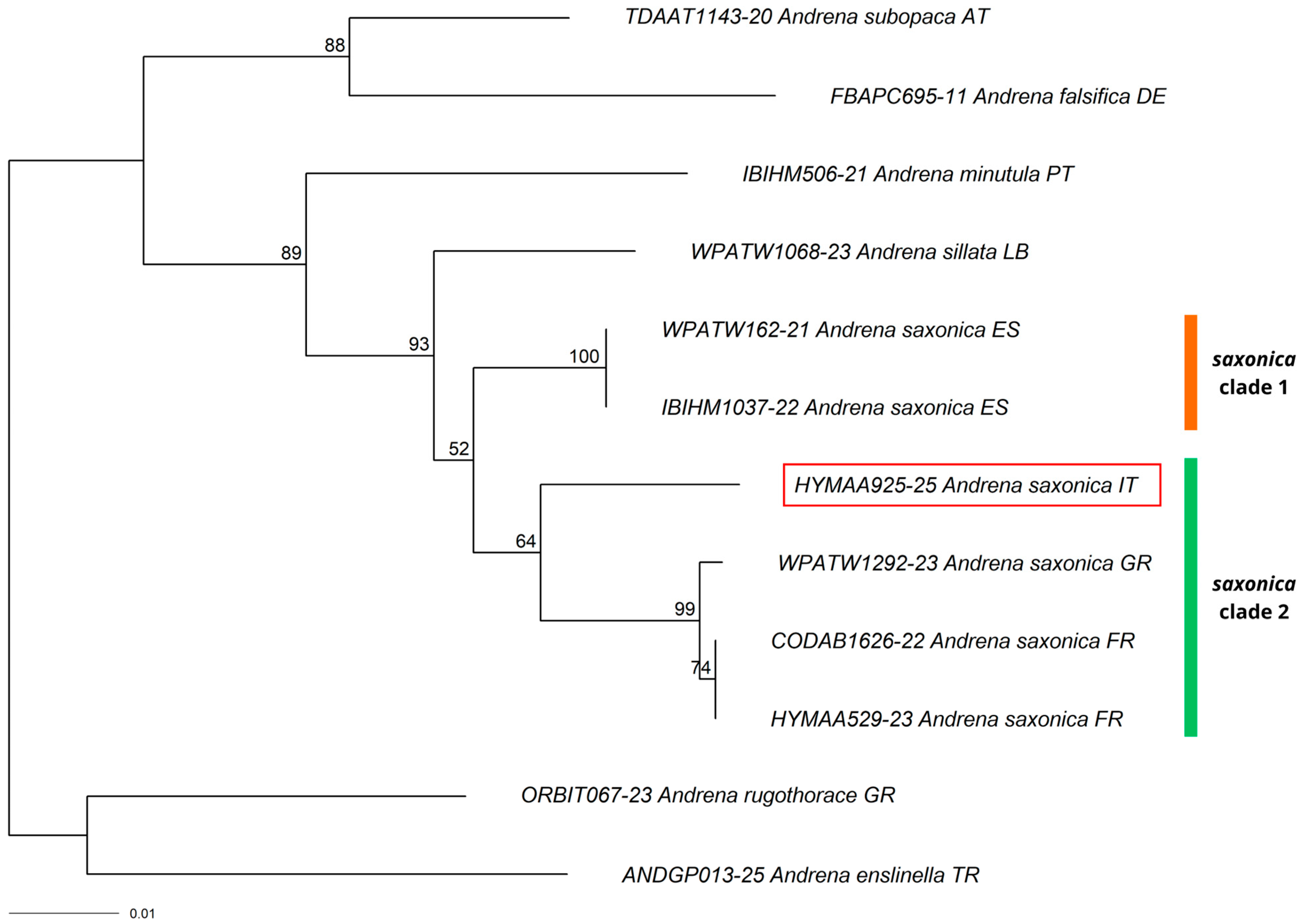
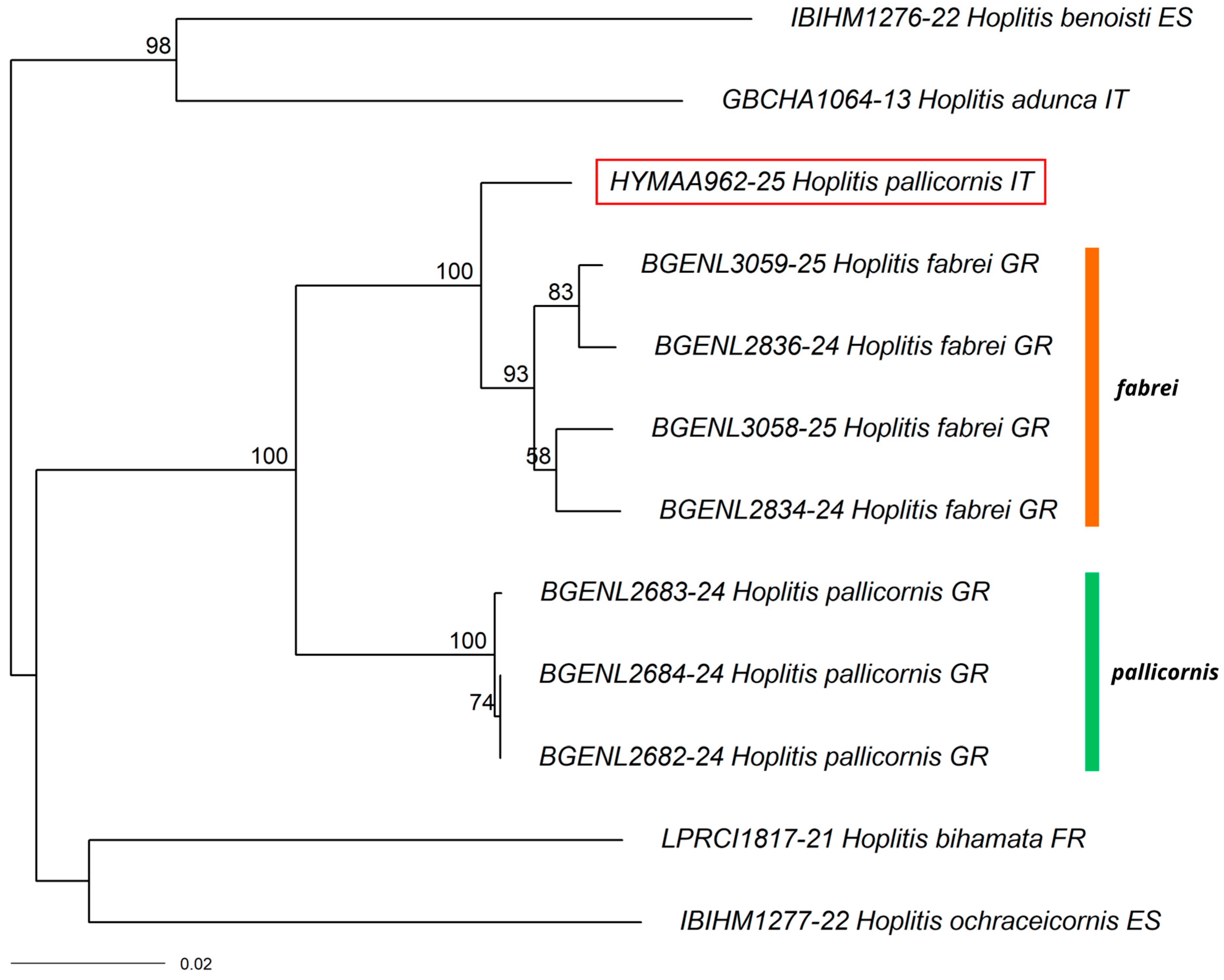
3.1. New Record for Italy
3.2. New Records for the Italian Mainland
3.3. New Records for Southern Italy
3.4. Other Interesting Records
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBC | Teresa Bonacci Collection, University of Calabria, Rende, Italy. |
| CPCN | Christophe Praz Collection, University of Neuchâtel, Neuchâtel, Switzerland. |
| ADCM | Achik Dorchin research Collection, University of Mons, Mons, Belgium. |
References
- Primack, R.B. Conservazione Della Natura; Zanichelli: Bologna, Italy, 2007; 514p. [Google Scholar]
- Ghisbain, G.; Rosa, P.; Bogusch, P.; Flaminio, S.; Le Divelec, R.; Dorchin, A.; Kasparek, M.; Kuhlmann, M.; Litman, J.; Mignot, M.; et al. The new annotated checklist of the wild bees of Europe (Hymenoptera: Anthophila). Zootaxa 2023, 5327, 1–147. [Google Scholar] [CrossRef]
- Reverté, S.; Milicic, M.; Acanski, L.; Andric, A.; Aracil, A.; Aubert, M.; Balzan, M.V.; Bartomeus, I.; Bogusch, P.; Bosch, J.; et al. National records of 3000 European bee and hoverfly species: A contribution to pollinator conservation. Insect Conserv. Divers. 2023, 16, 758–775. [Google Scholar] [CrossRef]
- Quaranta, M.; Cornalba, M.; Biella, P.; Comba, M.; Battistoni, A.; Rondinini, C.; Teofili, C. Lista Rossa IUCN Delle Api Italiane Minacciate; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare; Stamperia: Roma, Italy, 2018; 68p.
- IUCN Red List. Available online: https://www.iucnredlist.org (accessed on 15 January 2026).
- Kevan, P.G. Pollinator as bioindicator of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Katumo, D.M.; Liang, H.; Ochola, A.C.; Lv, M.; Wang, Q.; Yang, C. Pollinator diversity benefits natural and agricultural ecosystems, environmental health, and human welfare. Plant Divers. 2022, 44, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Maggi, T.; Pardo, L.; Chreil, R. Pollinator Diversity: A key to Ecosystem Resilience and Food Security. Pollinators 2023, 6, 33–48. [Google Scholar]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of Apoidea as Main Pollinators. Ecological and Economic Impact and Implications for Human Nutrion. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Rhodes, J.C. Are insect species imperilled? Critical factors and prevailing evidence for a potential global loss of the entomofauna: A current commentary. Sci. Prog. 2019, 102, 181–196. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Further evidence for a global decline of the entomofauna. Aust. Entomol. 2021, 60, 9–26. [Google Scholar]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J.; et al. Scientists’ warning on climate change and insects. Ecol. Monogr. 2022, 93, e1553. [Google Scholar] [CrossRef]
- Rumohr, Q.; Baden, C.U.; Bergtold, M.; Marx, M.T.; Oellers, J.; Schade, M.; Toschki, A.; Maus, C. Drivers and pressures behind insect decline in Central and Western Europe based on long-term monitoring data. PLoS ONE 2023, 18, e0289565. [Google Scholar] [CrossRef]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Rosseels Printing: Leuven, Belgium, 2014; 98p. [Google Scholar]
- Cornalba, M.; Quaranta, M.; Selis, M.; Flaminio, S.; Gamba, S.; Mei, M.; Bonifacino, M.; Cappellari, A.; Catania, R.; Niolu, P.; et al. Exploring the hidden riches: Recent remarkable faunistic records and range extensions in the bee fauna of Italy (Hymenoptera, Apoidea, Anthophila). Biodivers. Data J. 2024, 12, e116014. [Google Scholar] [CrossRef] [PubMed]
- Marziliano, P.A.; Lomabardi, F.; Menguzzato, G.; Scuderi, A.; Altieri, V.; Coletta, V.; Marcianò, C. Biodiversity conservation in Calabria region (southern Italy): Perspectives of management in the sites of the “Natura 2000” network. In Proceedings of the I International Conference on Research for Sustainable Development in Mountain Regions, Braganca, Portugal, 3–7 October 2016. [Google Scholar]
- Gibbs, J. Integrative taxonomy identifies new (and old) species in the Lasioglossum (Dialictus) tegulare (Robertson) species group (Hymenoptera, Halictidae). Zootaxa 2009, 2032, 1–38. [Google Scholar] [CrossRef]
- Praz, C.; Genoud, D.; Vaucher, K.; Bénon, D.; Monks, J.; Wood, T.J. Unexpected levels of cryptic diversity in European bees of the genus Andrena subgenus Taeniandrena (Hymenoptera, Andrenidae): Implications for conservation. J. Hymenopt. Res. 2022, 91, 375–428. [Google Scholar] [CrossRef]
- Wood, T.J. Revisions to the Andrena fauna of north-western Africa with a focus on Morocco (Hymenoptera: Andrenidae). Eur. J. Taxon. 2023, 916, 1–85. [Google Scholar] [CrossRef]
- Schmidt, S.; Schmid-Egger, C.; Morinière, J.; Haszprunar, G.; Hebert, P.D.N. DNA barcoding largely supports 250 years of classical taxonomy: Identifications for Central European bees (Hymenoptera, Apoidea partim). Mol. Ecol. Resour. 2015, 15, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Fritz, U.; Delfino, M.; Ulrich, W.; Habel, J.C. Biogeography of Italy revisited: Genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Front. Zool. 2021, 18, 34. [Google Scholar] [CrossRef]
- Annessi, M.; Riccieri, A.; Marconi, M.; Rossi, S.; Di Giulio, A. Integrating taxonomic, genetic and ecological data to explore the species richness of wild bees (Hymenoptera, Apoidea, Anthophila) of the Culuccia Peninsula (NE Sardinia, Italy). J. Hymenopt. Res. 2025, 98, 117–145. [Google Scholar] [CrossRef]
- Hymenoptera: Apoidea: Anthophila of Italy. Available online: https://digilander.libero.it/mario.comba/ (accessed on 15 November 2025).
- Atlas Hymenoptera. Available online: www.atlashymenoptera.net (accessed on 13 November 2025).
- GBIF.org. Data Manually Accessed for Distribution Analysis of 223 Bee Species. Available online: https://www.gbif.org (accessed on 15 November 2025).
- Friese, H. Die Bienen Europa’s (Apidae Europaeae) Nach ihren Gattungen, Arten und Varietaten auf Vergleichend Morphologisch-Biologischer Grundlage. Theil I. Schmarotzerbienen; R. Friedlander & Sohn: Berlin, Germany, 1895; 218p. [Google Scholar]
- Friese, H. Die Bienen Europa’s (Apidae Europaeae) Nach Ihren Gattungen, Arten und Varietaten auf Vergleichend Morphologisch-Biologischer Grundlage. Theil II. Solitare Apiden. Genus Eucera; R. Friedlander & Sohn: Berlin, Germany, 1896; 216p. [Google Scholar]
- Friese, H. Die Bienen Europa’s (Apidae Europaeae) Nach Ihren Gattungen, Arten und Varietaten auf Vergleichend Morphologisch-Biologischer Grundlage. Theil III. Solitare Apiden. Genus Podalirius; R. Friedlander & Sohn: Berlin, Germany, 1897; 316p. [Google Scholar]
- Friese, H. Die Bienen Europa’s (Apidae Europaeae) Nach Ihren Gattungen, Arten und Varietaten auf Vergleichend Morphologisch-Biologischer Grundlage. Theil IV. Solitare Apiden: Genus Eriades, Genus Trachusa, Genus Anthidium; R. Druck u. Verlag von C. Lampe: Innsbruck, Austria, 1898; 304p. [Google Scholar]
- Friese, H. Die Bienen Europa’s (Apidae Europaeae) Nach Ihren Gattungen, Arten und Varietaten auf vergleichend Morphologisch-Biologischer Grundlage. Theil V. Solitare Apiden: Genus Lithurgus, Genus Megachile (Chalicodoma); Druck u. Verlag von C. Lampe: Innsbruck, Austria, 1899; 228p. [Google Scholar]
- Friese, H. Die Bienen Europa’s (Apidae Europaeae) Nach Ihren Gattungen, Arten und Varietaten auf Vergleichend Morphologisch-Biologischer Grundlage. Theil VI. Solitare Apiden: Subfam. Panurginae, Melittinae, Xylocopinae; Duck von C. Lampe: Innsbruck, Austria, 1901; 284p. [Google Scholar]
- Ebmer, A.W. Neue westpaläarktische Halictidae (Halictinae, Apoidea), Teil III. Linz. Biol. Beitr. 1975, 7, 41–118. [Google Scholar]
- Dathe, H. Die Arten der Gattung Hylaeus F. in Europa (Hymenoptera: Apoidea, Colletidae). Mitt. Zool. Mus. Berl. 1980, 56, 207–294. [Google Scholar]
- Schmid-Egger, C.; Scheuchl, E. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs unter Berücksichtigung der Arten der Schweiz; Band III: Andrenidae; Eigenverlag: Velden, Germany, 1997; 180p. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica 6. In Apidae 3. Halictus, Lasioglossum; CSCF & SEG: Neuchâtel, Switzerland, 2001; 208p. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica 9. In Apidae 4. Anthidium, Chelostoma, Coelioxys, Dioxys, Heriades, Lithurgus, Megachile, Osmia, Stelis; CSCF & SEG: Neuchâtel, Switzerland, 2004; 274p. [Google Scholar]
- Michez, D.; Terzo, M.; Rasmont, P. Revision des especes ouest-palearctiques du genre Dasypoda Latreille 1802 (Hymenoptera, Apoidea, Melittidae). Linz. Biol. Beitr. 2004, 36, 847–900. [Google Scholar]
- Scheuchl, E. Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs, für Osmia s.l. unter Berücksichtigung der Arten der Schweiz, Ungarns, Sloweniens und der Slowakei; Band II: Megachilidae, Melittidae; Apollo Books: Stenstrup, Denmark, 2006; 192p. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica 20. In Apidae 5. Ammobates, Ammobatoides, Anthophora, Biastes, Ceratina, Dasypoda, Epeoloides, Epeolus, Eucera, Macropis, Melecta, Melitta, Nomada, Pasites, Tetralonia, Thyreus, Xylocopa; CSCF & SEG: Neuchâtel, Switzerland, 2007; 356p. [Google Scholar]
- Amiet, F.; Herrmann, M.; Müller, A.; Neumeyer, R. Fauna Helvetica 26. In Apidae 6. Andrena, Melitturga, Panurginus, Panurgus; CSCF & SEG: Neuchâtel, Switzerland, 2010; 316p. [Google Scholar]
- Amiet, F.; Muller, A.; Neumeyer, R. Fauna Helvetica 4. In Apidae 2. Colletes, Dufourea, Hylaeus, Nomia, Nomioides, Rhophitoides, Rophites, Sphecodes, Systropha; CSCF & SEG: Neuchâtel, Switzerland, 2014; 219p. [Google Scholar]
- Dathe, H.H.; Scheuchl, E.; Ockermüller, E. Illustrierte Bestimmungstabelle für die Arten der Gattung Hylaeus F. (Maskenbienen) in Deutschland, Österreich und der Schweiz; Entomologica Austriaca; Österreichische Entomologische Gesellschaft: Vienna, Austria, 2016; 51p. [Google Scholar]
- Aubert, M. Proposition de Clé D’identification des Eucerini (Hymenoptera: Anthophila) de France Continentale; Version provisoire; Observatoire des Abeilles: Carcassonne, France, 2020; 45p. [Google Scholar]
- Wood, T.J. The Genus Andrena Fabricius, 1775 in the Iberian Peninsula (Hymenoptera, Andrenidae). J. Hymenopt. Res. 2023, 96, 241–484. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Lunt, D.H.; Zhang, D.X.; Szymura, J.M.; Hewitt, G.M. The insect cytochrome oxidase I gene: Evolutionary patterns and conserved primers for phylogenetic studies. Insect Mol. Biol. 1996, 5, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Praz, C.; Müller, A.; Genoud, D. Hidden diversity in European bees: Andrena amieti sp. n., a new Alpine bee species related to Andrena bicolor (Fabricius, 1775) (Hymenoptera, Apoidea, Andrenidae). Alp. Entomol. 2019, 3, 11–38. [Google Scholar] [CrossRef]
- BOLD System. Available online: https://id.boldsystems.org (accessed on 15 November 2025).
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Bodenhofer, U.; Bonatesta, E.; Horejš-Kainrath, C.; Hochreiter, S. Msa: An R package for multiple sequence alignment. Bioinformatics 2015, 31, 3997–3999. [Google Scholar] [CrossRef]
- Paradis, E.; Klaus Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar]
- Sedivy, C.; Dorn, S.; Müller, A. Molecular phylogeny of the bee genus Hoplitis (Megachilidae: Osmiini)—How does nesting biology affect biogeography? Zool. J. Linn. Soc. 2013, 167, 28–42. [Google Scholar]
- Pisanty, G.; Richter, R.; Martin, T.; Dettman, J.; Cardinal, S. Molecular phylogeny, historical biogeography and revised classification of andrenine bees (Hymenoptera: Andrenidae). Mol. Phylogenet. Evol. 2022, 170, 107151. [Google Scholar] [CrossRef]
- Bossert, S.; Hung, K.L.; Neff, J. Evolutionary History and Ecology of Andrena (Foveoandrena) androfovea: A New Nearctic Mining Bee (Hymenoptera, Andrenidae) Species and Subgenus. Ecol. Evol. 2024, 14, e70453. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Ghisbain, G.; Michez, D.; Praz, C.J. Revisions to the faunas of Andrena of the Iberian Peninsula and Morocco with the descriptions of four new species (Hymenoptera: Andrenidae). Eur. J. Taxon. 2021, 758, 147–193. [Google Scholar] [CrossRef]
- Baldock, D.; Wood, T.; Cross, I.; Smit, J. Bees of Portugal (Hymenoptera: Apoidea, Anthophila); Entomofauna; Museum Witt: Munich, Germany, 2018; Volume 22, 164p. [Google Scholar]
- Smith, J. Identification Key to the European Species of the Bee Genus Nomada Scopoli, 1770 (Hymenoptera: Apidae), Including 23 New Species; Entomofauna; Museum Witt: Munich, Germany, 2018; Volume 3, 253p. [Google Scholar]
- Atlas of the Swiss Bees. Info Fauna, Neuchâtel. Available online: https://species.infofauna.ch/groupe/1 (accessed on 31 October 2025).
- Praz, C.; Müller, A.; Bénon, D.; Herrman, M.; Neumeyer, R. Annotated checklist of the Swiss bees (Hymenoptera, Apoidea, Anthophila): Hotspots of diversity in the xeric inner Alpine valleys. Alp. Entomol. 2023, 7, 219–267. [Google Scholar] [CrossRef]
- Magnacca, K.N.; Brown, M.J. Mitochondrial heteroplasmy and DNA barcoding in Hawaiian Hylaeus (Nesoprosopis) bees (Hymenoptera: Colletidae). BMC Evol. Biol. 2010, 10, 174. [Google Scholar] [CrossRef]
- Astafurova, Y.V.; Proshchalykin, M.Y. Review of the bee genus Lasioglossum Curtis, 1833 (Hymenoptera: Halictidae) fauna of the European South of Russia. Russ. Entomol. J. 2024, 33, 230–242. [Google Scholar] [CrossRef]
- Bogusch, P.; Straka, J. Review and identification of the cuckoo bees of central Europe (Hymenoptera: Halictidae: Sphecodes). Zootaxa 2012, 3311, 1–41. [Google Scholar] [CrossRef]
- Güler, Y. The Wild Bee Fauna of Afyonkarahisar Province: Andrenidae, Anthophoridae and Megachilidae (Hymenoptera: Apoidea). Linz. Biol. Beitr. 2011, 43, 731–746. [Google Scholar]
- Van Der Zanden, G. Beitrag zur systematik und nomenklatur der paläarktischen osmiini, mit angaben über ihre verbreitung. Zool. Meded. 1988, 62, 113–133. [Google Scholar]
- Wood, T.; Praz, C.; Selis, M.; Flaminio, S.; Mei, M.; Cornalba, M.; Rosa, P.; Le Divelec, R.; Michez, D. Revisions to the Andrena fauna of Italy, with the description of a new species (Hymenoptera: Andrenidae). Fragm. Entomol. 2023, 55, 271–310. [Google Scholar]
- Vommaro, M.L.; Lento, M.; Michez, D.; Flaminio, S.; Flori, S.; Treccosti, I.; Di Prisco, G.; Goglia, L.; Brandmayr, P.; Giglio, A. Assessing wild bee fauna (Hymenoptera: Apoidea: Anthophila) in Calabria (southern Italy). Eur. Zoo. J. 2025, 92, 769–780. [Google Scholar] [CrossRef]
- Warncke, K. Beitrag zur Klärung paläarktischer Andrena-Arten (Hym. Apidae). Eos 1967, 43, 171–318. [Google Scholar]
- Ungricht, S.; Muller, A.; Dorn, S. A taxonomic catalogue of the Palaearctic bees of the tribe Osmiini (Hymenoptera: Apoidea: Megachilidae). Zootaxa 2008, 1865, 1–253. [Google Scholar] [CrossRef]
- Peters, D.S. Systematik und Zoogeographie der west-paläarktischen Arten von Osmia Panzer 1806 s. str., Monosmia Tkalcu 1974 und Orientosmia n. subgen. (Insecta: Hymenoptera: Megachilidae). Senck. Biol. 1978, 58, 287–346. [Google Scholar]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Canestrelli, D.; Cimmaruta, R.; Costantini, V.; Nascetti, G. Genetic diversity and phylogeography of the Apennine yellow-bellied toad Bombina pachypus, with implications for conservation. Mol. Ecol. 2006, 15, 3741–3754. [Google Scholar] [CrossRef]
- Canestrelli, D.; Cimmaruta, R.; Nascetti, G. Population genetic structure and diversity of the Apennine endemic stream frog, Rana italica–insights on the Pleistocene evolutionary history of the Italian peninsular biota. Mol. Ecol. 2008, 17, 3856–3872. [Google Scholar] [CrossRef]
- Grill, A.; Amori, G.; Aloide, G.; Lisi, I.; Tosi, G.; Wauters, L.A.; Randi, E. Molecular phylogeography of European Sciurus vulgaris: Refuge within refugia? Mol. Ecol. 2009, 18, 2687–2699. [Google Scholar] [CrossRef]
- Vega, R.; Amori, G.; Aloise, G.; Cellini, S.; Loy, A.; Searle, J.B. Genetic and morphological variation in a Mediterranean glacial refugium: Evidence from Italian pygmy shrews, Sorex minutus (Mammalia: Soricomorpha). Biol. J. Linn. Soc. 2010, 100, 774–787. [Google Scholar] [CrossRef]
- Colangelo, P.; Aloise, G.; Annesi, F.; Amori, G. Mitochondrial DNA reveals hidden diversity and an ancestral lineage of the bank vole in the Italian peninsula. J. Zool. 2011, 287, 41–52. [Google Scholar] [CrossRef]
- Mattoccia, M.; Marta, S.; Romano, A.; Sbordoni, V. Phylogeography of an Italian endemic salamander (genus Salamandrina): Glacial refugia, postglacial expansions, and secondary contact. Biol. J. Linn. Soc. 2011, 104, 903–992. [Google Scholar] [CrossRef]
- Chiocchio, A.; Colangelo, P.; Aloise, G.; Amori, G.; Bertolino, S.; Bisconti, R.; Castiglia, R.; Canestrelli, D. Population genetic structure of the bank vole Myodes glareolus within its glacial refugium in peninsular Italy. J. Zool. Syst. Evol. Res. 2019, 4, 959–969. [Google Scholar] [CrossRef]
- Fortini, L.; Ruzzier, E.; Mei, M.; Di Giulio, A. The wild bees (Hymenoptera, Apoidea, Anthophila) of the urban nature reserves of Rome (Italy, Latium): A preliminary survey. Biodivers. Data J. 2024, 2, e139087. [Google Scholar] [CrossRef]
- Goglia, L.; Flaminio, S.; Chianese, F.V.; Quaranta, M.; Conti, P.; Di Prisco, G. Italian wild bees biodiversity and Vesuvius National Park. Riv. Studi. Sulla Sostenibilità 2024, 14, 125–140. [Google Scholar] [CrossRef]
- Chiocchio, A.; Maiorano, L.; Pezzarossa, A.; Bisconti, R.; Canestrelli, D. From the mountains to the sea: Rethinking Mediterranean glacial refugia as dynamic entities. J. Biogeogr. 2024, 51, 956–967. [Google Scholar] [CrossRef]
- Pisanty, G.; Scheuchl, E.; Martin, T.; Cardinal, S.; Wood, T.J. Twenty-five new species of mining bees (Hymenoptera: Andrenidae: Andrena) from Israel and the Levant. Zootaxa 2022, 5185, 1–109. [Google Scholar] [CrossRef]
- Boustani, M.; Rasmont, P.; Dathe, H.H.; Ghisbain, G.; Kasparek, M.; Michez, D.; Muller, A.; Pauly, A.; Risch, S.; Straka, J.; et al. The bees of Lebanon (Hymenoptera: Apoidea: Anthophila). Zootaxa 2021, 4976, 001–146. [Google Scholar] [CrossRef]
- Wood, T.J.; Cross, I.; Baldock, D.W. Updates to the bee fauna of Portugal with the description of three new Iberian Andrena species (Hymenoptera: Apoidea: Anthophila). Zootaxa 2020, 4790, 201–228. [Google Scholar] [CrossRef]
- Pauly, A. Les abeilles sauvages de la lande de Streupas (Hymenoptera: Apoidea). Belg. J. Entomol. 2018, 60, 1–36. [Google Scholar]
- Fiordaliso, W.; Revertè, S.; Wood, T.; Barbier, Y.; Rasmont, P.; Lefèbvre, A.; Loockx, M.; Reese, A.; Ruelle, E.; Michez, D. Inventaire et conservation des abeilles sauvages (Hymenoptera: Anthophila) du sillon industriel hainuyer (Belgique). Belg. J. Entomol. 2022, 132, 1–64. [Google Scholar]
- Schedl, W. Stechimmen II im Botanischen Garten Innsbruck (Tirol, Österreich): Artengarnitur, Blütenbesuch, Phänologie (Insecta: Hymenoptera). Linz. Biol. Beitr. 2015, 47, 939–954. [Google Scholar]
- Fateryga, A.V.; Proshchalykin, M.Y. 150 years after Ferdinand Morawitz: A survey of megachilid bees (Hymenoptera, Megachilidae) of Dagestan, Russia. ZooKeys 2024, 1217, 101–117. [Google Scholar] [CrossRef]
- Proshchalykin, M.Y.; Maharramov, M.M.; Aliyev, K.A. New data on the tribe Osmiini (Hymenoptera: Megachilidae) from Azerbaijan. Far East. Entomol. 2019, 383, 12–20. [Google Scholar]
- Quaranta, M.; Sommaruga, A.; Balzarini, P.; Felicioli, A. A new species for the bee fauna of Italy: Megachile sculpturalis continues its colonization of Europe. Bull. Insectology 2014, 67, 287–293. [Google Scholar]
- Ruzzier, E.; Menchetti, M.; Borlotti, L.; Selis, M.; Monterastelli, E.; Forbicioni, L. Updated distribution of the invasive Megachile sculpturalis (Hymenoptera: Megachilidae) in Italy and its first record on a Mediterranean island. Biodiver. Data J. 2020, 8, e57783. [Google Scholar] [CrossRef] [PubMed]
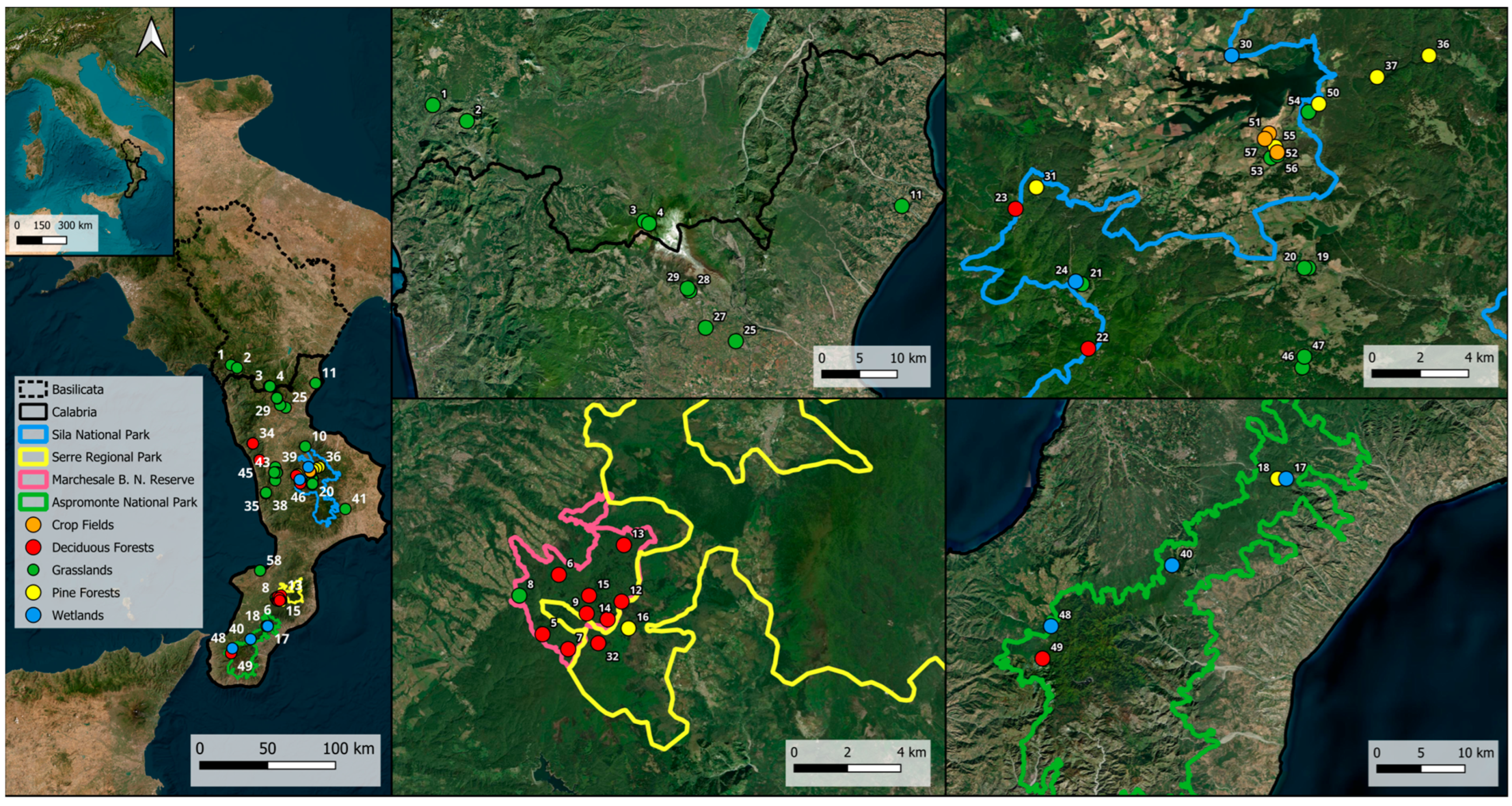
| Code | Region | Province | Municipality | Altitude (in Meters a.s.l.) | Geographical Coordinates |
|---|---|---|---|---|---|
| 1 | Basilicata | PZ | Lauria | 699 | 40.070 N, 15.834 E |
| 2 | Basilicata | PZ | Lauria | 1055 | 40.051 N, 15.887 E |
| 3 | Basilicata | PZ | Viggianello | 1553 | 39.932 N, 16.163 E |
| 4 | Basilicata | PZ | Viggianello | 1450 | 39.929 N, 16.170 E |
| 5 | Calabria | VV | Acquaro | 1096 | 38.505 N, 16.230 E |
| 6 | Calabria | VV | Acquaro | 913 | 38.525 N, 16.237 E |
| 7 | Calabria | VV | Acquaro | 1172 | 38.500 N, 16.241 E |
| 8 | Calabria | VV | Acquaro | 835 | 38.518 N, 16.220 E |
| 9 | Calabria | VV | Acquaro | 1165 | 38.512 N, 16.249 E |
| 10 | Calabria | CS | Acri | 673 | 39.531 N, 16.474 E |
| 11 | Calabria | CS | Amendolara | 94 | 39.950 N, 16.563 E |
| 12 | Calabria | VV | Arena | 1147 | 38.516 N, 16.264 E |
| 13 | Calabria | VV | Arena | 1080 | 38.535 N, 16.265 E |
| 14 | Calabria | VV | Arena | 1152 | 38.510 N, 16.258 E |
| 15 | Calabria | VV | Arena | 1153 | 38.518 N, 16.250 E |
| 16 | Calabria | VV | Arena | 1149 | 38.507 N, 16.267 E |
| 17 | Calabria | RC | Canolo | 912 | 38.329 N, 16.151 E |
| 18 | Calabria | RC | Canolo | 979 | 38.329 N, 16.140 E |
| 19 | Calabria | CS | Casali del Manco | 1338 | 39.316 N, 16.538 E |
| 20 | Calabria | CS | Casali del Manco | 1374 | 39.316 N, 16.536 E |
| 21 | Calabria | CS | Casali del Manco | 1699 | 39.310 N, 16.429 E |
| 22 | Calabria | CS | Casali del Manco | 1820 | 39.286 N, 16.432 E |
| 23 | Calabria | CS | Casali del Manco | 1580 | 39.338 N, 16.397 E |
| 24 | Calabria | CS | Casali del Manco | 1720 | 39.311 N, 16.426 E |
| 25 | Calabria | CS | Cassano allo Ionio | 520 | 39.789 N, 16.305 E |
| 26 | Calabria | CS | Castrolibero | 303 | 39.306 N, 16.215 E |
| 27 | Calabria | CS | Castrovillari | 424 | 39.805 N, 16.258 E |
| 28 | Calabria | CS | Castrovillari | 618 | 39.849 N, 16.233 E |
| 29 | Calabria | CS | Castrovillari | 670 | 39.852 N, 16.230 E |
| 30 | Calabria | CS | Celico | 1140 | 39.395 N, 16.501 E |
| 31 | Calabria | CS | Celico | 1425 | 39.346 N, 16.407 E |
| 32 | Calabria | VV | Fabrizia | 1173 | 38.502 N, 16.254 E |
| 33 | Calabria | CS | Fagnano Castello | 1097 | 39.551 N, 16.021 E |
| 34 | Calabria | CS | Fagnano Castello | 1086 | 39.551 N, 16.023 E |
| 35 | Calabria | CS | Longobardi | 1490 | 39.220 N, 16.134 E |
| 36 | Calabria | CS | Longobucco | 1293 | 39.395 N, 16.596 E |
| 37 | Calabria | CS | Longobucco | 1275 | 39.387 N, 16.571 E |
| 38 | Calabria | CS | Mendicino | 1370 | 39.224 N, 16.136 E |
| 39 | Calabria | CS | Montalto Uffugo | 184 | 39.393 N, 16.217 E |
| 40 | Calabria | RC | Oppido Mamertina | 1090 | 38.241 N, 16.003 E |
| 41 | Calabria | KR | Petilia Policastro | 180 | 39.115 N, 16.820 E |
| 42 | Calabria | CS | Rende | 207 | 39.359 N, 16.231 E |
| 43 | Calabria | CS | Rende | 236 | 39.357 N, 16.227 E |
| 44 | Calabria | CS | Rende | 313 | 39.359 N, 16.207 E |
| 45 | Calabria | CS | San Benedetto Ullano | 1370 | 39.440 N, 16.081 E |
| 46 | Calabria | CS | San Giovanni in Fiore | 1555 | 39.279 N, 16.535 E |
| 47 | Calabria | CS | San Giovanni in Fiore | 1561 | 39.283 N, 16.536 E |
| 48 | Calabria | RC | San Roberto | 1322 | 38.179 N, 15.846 E |
| 49 | Calabria | RC | Santo Stefano | 1400 | 38.146 N, 15.835 E |
| 50 | Calabria | CS | Spezzano della Sila | 1162 | 39.377 N, 16.543 E |
| 51 | Calabria | CS | Spezzano della Sila | 1178 | 39.366 N, 16.519 E |
| 52 | Calabria | CS | Spezzano della Sila | 1203 | 39.359 N, 16.523 E |
| 53 | Calabria | CS | Spezzano della Sila | 1240 | 39.357 N, 16.520 E |
| 54 | Calabria | CS | Spezzano della Sila | 1181 | 39.374 N, 16.538 E |
| 55 | Calabria | CS | Spezzano della Sila | 1192 | 39.361 N, 16.522 E |
| 56 | Calabria | CS | Spezzano della Sila | 1218 | 39.358 N, 16.523 E |
| 57 | Calabria | CS | Spezzano della Sila | 1178 | 39.364 N, 16.517 E |
| 58 | Calabria | VV | Vibo Valentia | 38 | 38.703 N, 16.085 E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Mendicino, F.; Carlomagno, F.; Praz, C.J.; Wood, T.J.; Dorchin, A.; Flaminio, S.; Aubert, M.; Le Divelec, R.; Bonelli, D.; Di Biase, E.; et al. New Records of Wild Bees from Calabria and Basilicata Highlight the Hidden Diversity of Anthophila in Italy. Diversity 2026, 18, 74. https://doi.org/10.3390/d18020074
Mendicino F, Carlomagno F, Praz CJ, Wood TJ, Dorchin A, Flaminio S, Aubert M, Le Divelec R, Bonelli D, Di Biase E, et al. New Records of Wild Bees from Calabria and Basilicata Highlight the Hidden Diversity of Anthophila in Italy. Diversity. 2026; 18(2):74. https://doi.org/10.3390/d18020074
Chicago/Turabian StyleMendicino, Federica, Francesco Carlomagno, Christophe J. Praz, Thomas J. Wood, Achik Dorchin, Simone Flaminio, Matthieu Aubert, Romain Le Divelec, Domenico Bonelli, Erica Di Biase, and et al. 2026. "New Records of Wild Bees from Calabria and Basilicata Highlight the Hidden Diversity of Anthophila in Italy" Diversity 18, no. 2: 74. https://doi.org/10.3390/d18020074
APA StyleMendicino, F., Carlomagno, F., Praz, C. J., Wood, T. J., Dorchin, A., Flaminio, S., Aubert, M., Le Divelec, R., Bonelli, D., Di Biase, E., Fumo, F., Pezzi, M., Luzzi, G., Siclari, A., Pellegrino, G., & Bonacci, T. (2026). New Records of Wild Bees from Calabria and Basilicata Highlight the Hidden Diversity of Anthophila in Italy. Diversity, 18(2), 74. https://doi.org/10.3390/d18020074











