Abstract
The Chiapas Highlands of Mexico represent a critical center of herpetofaunal diversity and endemism within Mesoamerica. This study provides the first comprehensive inventory of amphibians and reptiles in the Mexican part of this biogeographic province, documenting 354 species, 112 amphibians, and 242 reptiles. Amphibian richness is highest in the families Hylidae, Plethodontidae, and Craugastoridae, whereas reptile richness is highest in the families Dipsadidae and Colubridae. Ninety-eight species are endemic to the Chiapas Highlands, with forty restricted to its Mexican portion. The herpetofauna of the Chiapas Highlands shows a high level of endemism. A higher proportion of reptile species are shared with neighboring provinces than amphibians. Conservation assessments reveal that 64 species are listed as Vulnerable, Endangered, or Critically Endangered on the International Union for Conservation of Nature’s (IUCN) Red List, 39 species are classified as Threatened (A) or Endangered (P) under Mexican environmental legislation, and 98 species are considered high-risk according to the Environmental Vulnerability Score (EVS), with habitat loss and chytrid fungi identified as principal threats. Our summary and synthesis of its amphibian and reptile species highlights the Chiapas Highlands as a biogeographic and ecological hotspot deserving urgent conservation attention.
1. Introduction
The Mexican Transition Zone comprises five biogeographic provinces, four of which are exclusive to Mexico: the Sierra Madre del Sur, Transvolcanic Belt, Sierra Madre Oriental, and Sierra Madre Occidental [1]. The fifth province, the Chiapas Highlands, is shared with Guatemala, Honduras, El Salvador, and Nicaragua and correspond to the Central American Nucleus [2]. In Mexico, this province includes the Sierra Madre de Chiapas, extending along the Pacific coast of Chiapas to the Isthmus of Tehuantepec, and the Central Massif of Chiapas, which also runs parallel to the Pacific coast but farther inland [2]. These two mountain systems are separated by the Central Depression of Chiapas, a wide valley formed by the Grijalva River, which is part of the Veracruzan biogeographic province [3].
The Chiapas Highlands represents a biogeographic province characterized by high mountain systems, rugged topography, and exceptional biodiversity. As part of the Mexican Transition Zone, the Chiapas Highlands lie at the intersection of the Nearctic and Neotropical domains, where independently evolved biotas come into contact, overlap, and mix to varying degrees [4,5]. The resulting high number of endemic species reflects speciation processes triggered by this confluence of biogeographic regions [6,7]. Importantly, the long-term stability of paleoclimatic conditions in the highlands has supported the persistence of these endemic lineages [8]. Pleistocene climate oscillations, particularly glacial–interglacial cycles, shifted the distributions of montane forest species, alternately fragmenting populations into refugia during drier periods and allowing the re-expansion of populations during wetter periods, thereby promoting lineage diversification and persistence [9]. The Chiapas Highlands likely served as a Pleistocene refugium, offering a stable, humid habitat that buffered local taxa from the more extreme climatic fluctuations experienced elsewhere in Mesoamerica. Thus, both mixing of biota and persistence of species within paleoclimatic refugia underlie the region’s remarkable herpetofaunal endemism [8]. The Chiapas Highlands are thus a biodiversity hotspot for amphibians and reptiles [10,11].
Unfortunately, the Chiapas Highlands has undergone rapid environmental change, resulting in urgent conservation needs. Deforestation and forest fragmentation, driven by traditional agriculture, timber extraction, and urban expansion, have significantly reshaped its landscape [12,13,14,15], with alarming rates of forest loss of up to 4.8% annually, and a dramatic increase in the number and isolation of forest fragments [15]. These reductions in forests have also been accompanied by an increase in urbanization in the region [16]. These changes have led to degraded forest structure, reduced core habitat areas, and a decline in ecological integrity [15]. Fragmentation patterns are closely linked to land tenure, poor soil conditions, and limited economic alternatives for local communities [13,14].
Despite increasing efforts to document the herpetofauna of the Chiapas Highlands at various scales, from statewide assessments [17,18,19,20,21,22] to regional inventories and studies in protected areas [13,14,23,24,25], there remains a critical gap in our understanding of the amphibians and reptiles inhabiting the Chiapas Highlands as a distinct biogeographic province. The herpetofaunal diversity of the province and its conservation status and biogeographic affinities has not been summarized or synthesized. We address this gap by presenting the first comprehensive species list of amphibians and reptiles for the Chiapas Highlands biogeographic province of Mexico. We also assess the conservation status of its amphibian and reptile species using three key references: the International Union for Conservation of Nature’s (IUCN) Red List [26], Mexico’s official list of species at risk [27], and the Environmental Vulnerability Score [28,29]. Additionally, we identify major threats to these species and compare the herpetofaunal composition of the Chiapas Highlands with that of adjacent biogeographic provinces. By establishing a baseline for the region’s herpetofauna, this work aims to support ongoing conservation planning, inform future ecological and evolutionary research, and highlight the urgent need for targeted protection measures in one of Mexico’s most biologically significant montane regions.
2. Methods
2.1. Physiographic Characteristics
The Chiapas Highlands is a biogeographic province located in southern Mexico, spanning latitudes from 15.0838° to 17.3796° and longitudes from −91.2535° to −94.6642°. It features an altitudinal range from 200 to 3800 m above sea level (masl), though most of the region lies between 500 and 2000 masl (modified from [30,31,32]). Covering an area of 27,880 km2, it is the second smallest of Mexico’s 14 biogeographic provinces, comprising approximately 1.43% of the country’s total land area [32]. The province has a total perimeter of 2748 km, including borders of 2136 km with the Veracruzan Province, 490 km with the Pacific Lowlands, and 122 km with Guatemala. It encompasses most of central Chiapas and a small portion of eastern Oaxaca. The region is divided into two main mountainous areas: the Sierra Madre de Chiapas in the south, bordered by the Pacific Lowlands biogeographic province to the south, the Veracruzan Province to the west and north, and Guatemala to the east, and the Central Massif of Chiapas, which is similarly bordered by the Veracruzan province to the south, west, and north, and Guatemala to the east (modified from [3]; Figure 1).
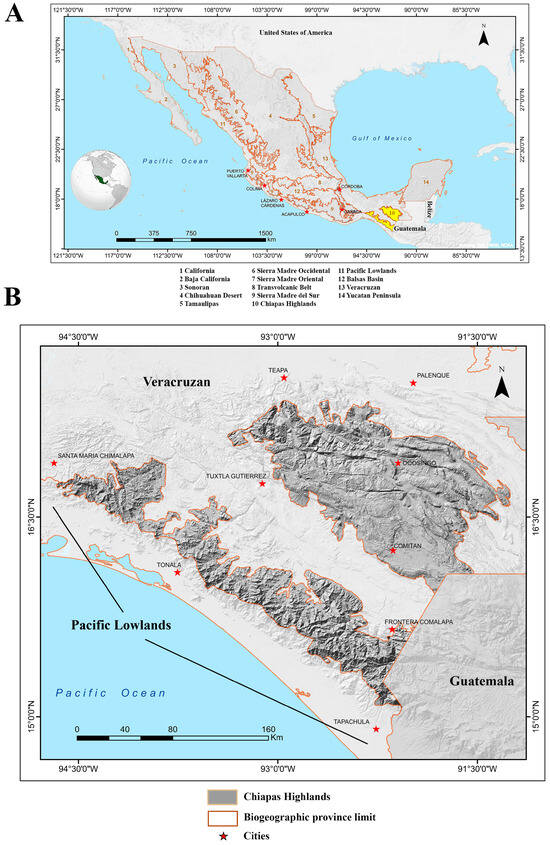
Figure 1.
Topographic map of the Chiapas Highlands of Mexico. (A) Overview of Mexico’s fourteen biogeographic provinces, with the Chiapas Highlands highlighted in bright yellow. The map also shows portions of the southern United States and northern Central America (Guatemala and Belize) for geographic context. (B) Detailed view of the Chiapas Highlands of Mexico and surrounding provinces, illustrating regional topography and adjacent areas [33].
Due to the substantial altitudinal range and rugged topography of the Chiapas Highlands, this region contains a wide variety of climates [34] (Figure 2). Semi-warm climates predominate at mid-altitudes in both the Sierra Madre and the Chiapas Central Massif, while warm climates are found along the slopes of the mountain ranges. Temperate climates cover extensive areas in the higher elevations, and small patches of semi-cold climate occur in the highest zones, such as the area surrounding San Cristóbal de las Casas. Additionally, two small patches of semi-arid climate are present, one in the lower northern extremes of the Sierra Madre de Chiapas and another in the central part of this mountain range.
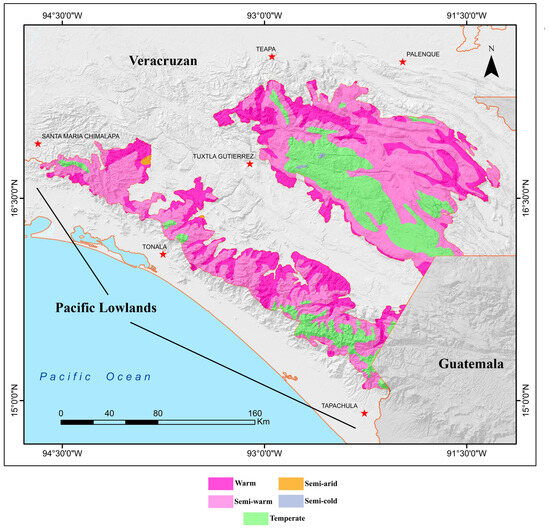
Figure 2.
Map showing the climatic regions found in the Chiapas Highlands biogeographic province of Mexico [34].
The vegetation of the Chiapas Highlands is remarkably diverse ([2,35,36]; Figure 3). At higher elevations, the landscape is dominated by extensive pine and pine–oak forests, the most widespread vegetation type in the region. Along the lower-elevation margins and slopes of the highlands, these temperate forests often transition to tropical montane forests, including montane cloud forests. At the highest elevations, montane scrublands, characterized by thorny or shrubby vegetation adapted to cooler and drier conditions, prevail. In some upland areas, natural grasslands also occur, often interspersed with patches of forest or scrub.
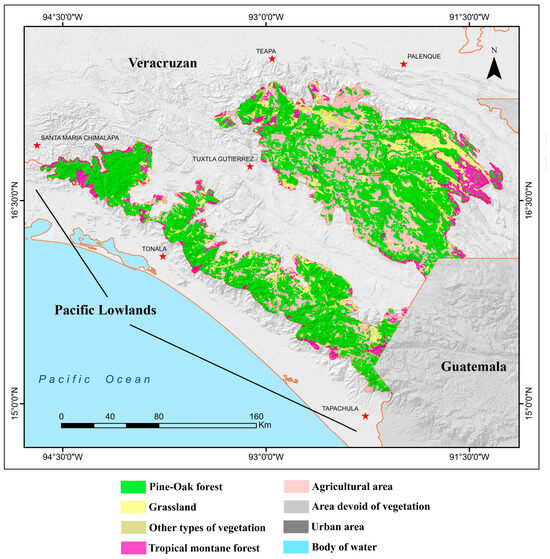
Figure 3.
Map showing the vegetation and land cover types in the Chiapas Highlands biogeographic province of Mexico, based on the classification provided by Instituto Nacional de Estadística y Geografía (INEGI) [35]. Categories represent generalized land cover types and are intended to provide broad landscape context for the province: Pine–Oak Forest includes temperate forests dominated by Pinus and Quercus species; Grassland refers to natural or semi-natural open herbaceous areas; Other types of vegetation encompasses heterogeneous or transitional areas not assigned to a single dominant category; Tropical montane forest includes humid, closed-canopy forests at lower elevations, such as cloud forest; Agricultural area includes croplands and pasture; Area devoid of vegetation includes bare soil or rocky surfaces; Urban area corresponds to developed land; and Body of water includes both marine and freshwater habitats.
2.2. Methodology
We compiled a list of the species of amphibians and reptiles of the Mexican Chiapas Highlands. We used the species lists for the states of Chiapas and Oaxaca found in [22] and updated with [37]. We verified species records using VertNet, GBIF, and additional sources, and found no inconsistencies with the published state lists provided by [22,37], which were compiled by regional experts and peer-reviewed and incorporate records compiled up to 2023 and 2024, respectively. These lists incorporate data from VertNet, GBIF, museum collections, literature reviews, and fieldwork, further supporting their reliability. We used the definition of the Mexican Chiapas Highlands of [2,3,32]. Using this definition, we refined the species lists to derive our lists for the herpetofaunas of the Mexican Chiapas Highlands. We also compiled species for the amphibians and reptiles of the neighboring provinces, Pacific Lowlands, and Veracruzan, provided by [37]. We used these lists to determine how many species of amphibians and reptiles were shared between the Chiapas Highlands and its neighboring provinces. For amphibian names, we used [38] and [39], and [40] for reptile names. For each species in our list that has been evaluated by the International Union for Conservation of Nature’s (IUCN) Red List, we determined its conservation status and population trend [26]. We also recorded its listing by the Mexican government [27] and its Environmental Vulnerability Score (EVS) [28,29].
3. Results and Discussion
3.1. Species Richness, Endemism, and Distribution
To date, the Chiapas Highlands in Mexico are known to support a total of 354 herpetofaunal species, including 112 amphibians and 242 reptiles (Supplementary Table S1 and Table 1). The high diversity of the Chiapas Highlands is not surprising since the two states that include portions of the Chiapas Highlands in Mexico, Chiapas and Oaxaca, are the two most diverse states for amphibians [41] and reptiles [42] (see also [20,43]. The amphibian species belong to three orders: Anura (85 species), Caudata (25 species), and Gymnophiona (two species), spanning 11 families, 9 anurans, 1 salamander, and 1 gymnophionan, and 36 genera (27 anurans, 8 salamanders, and 1 gymnophionan). The most diverse amphibian families are Hylidae (34 species), Plethodontidae (25 species), and Craugastoridae (22 species). Of the 112 amphibian species, 31 are endemic to Mexico, with 14 of these restricted to the Chiapas Highlands (Supplementary Table S1). Given the region’s topographic complexity and climatic variability, it is likely that additional species remain undocumented, especially cryptic or fossorial species.

Table 1.
Summary of native species present in the Chiapas Highlands biogeographic province of Mexico by family, order or suborder, and class. Status summary indicates the number of species found in each IUCN conservation status in the order Data-Deficient (DD), Least Concern (LC), Near-Threatened (NT), Vulnerable (VU), Endangered (EN), and Critically Endangered (CR) [26]. In some cases, species have not been assigned a status by the IUCN, and therefore, these may not add up to the total number of species in a taxon). Mean EVS is the mean environmental vulnerability score; scores ≥ 14 are considered high vulnerability [28,29] and conservation status in Mexico according to Secretaría de Medio Ambiente y Recursos Naturales [27] in the order not listed (NL), subject to special protection (Pr), threatened (A), and in danger of extinction (P).
For reptiles, the Chiapas Highlands in Mexico currently harbors 242 species, representing three orders: Crocodylia (2 species), Squamata (103 lizards and 123 snakes), and Testudines (12 species). There are 30 reptilian families: 1 crocodilian, 15 lizards, 9 snakes, and 5 turtles, comprising 93 genera, 1 crocodile, 26 lizards, 59 snakes, and 7 turtles (Supplementary Table S1 and Table 1). The most species-rich reptile families are Dipsadidae (52 species) and Colubridae (40 species). Of the reptile species recorded, 66 are endemic to Mexico, with 26 found exclusively in the Chiapas Highlands. Additionally, one introduced species, the Common House Gecko (Hemidactylus frenatus), occurs in the region (Supplementary Table S1).
According to [37], Mexico supports a total of 1399 native herpetofaunal species, including 435 amphibians and 964 reptiles. These species represent 55 families (16 amphibian, 39 reptile) and 210 genera (55 amphibian, 155 reptile) (see also [43]). The Chiapas Highlands houses 74.5% of these families (68.8% for amphibians and 76.9% for reptiles), 61.4% of the genera (65.5% for amphibians and 60.0% for reptiles), and 25.3% of the country’s species (25.7% of amphibians and 25.1% of reptiles). As field surveys continue and understudied habitats are explored, these numbers will likely increase, further underscoring the region’s biological significance.
The distributions of amphibian species inhabiting the Chiapas Highlands can be categorized into three main groups based on their geographic range. The first group includes 31 species that are endemic to Mexico, of which 14 are restricted to the Chiapas Highlands (Figure 4). The second and largest group comprises 77 species with broader distributions across Mexico and into Central or even South America. Within this group, 30 species are endemic to the Chiapas Highlands but not specifically to the Mexican portion. Of these, only 2, Hypopachus barberi and Plectrohyla hartwegi, extend their range as far south as Honduras. The remaining 28 reach their southernmost distribution limit in Guatemala. The third group includes 4 widely distributed species, occurring from the southern United States through Central America (Smilisca baudinii and Hypopachus variolosus) or extending into South America (Rhinella horribilis and Leptodactylus fragilis).

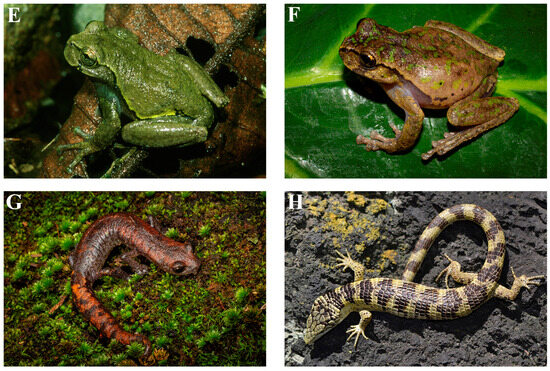
Figure 4.
Photos of some of the amphibian and reptile species endemic to the Chiapas Highlands: (A) Incilius tutelarius, Rodulfo Figueroa, Oaxaca; (B) Exerodonta chimalapa, Rodulfo Figueroa, Oaxaca; (C) Plectrohyla acanthodes, San Cristobal de las Casas, Chiapas; (D) Plectrohyla avia, Volcán Tacaná, Chiapas; (E) Plectrohyla ixil, Rayón Mescalapa, Chiapas; (F) Plectrohyla matudai, Rodulfo Figueroa, Oaxaca; (G) Bolitoglossa stuarti, Las Margaritas Chiapas; (H) Abronia bogerti, Cerro Baúl, Chiapas. Photos (A,B,F,G), by Eric Centenero Alcalá; Photos (C–E,H), by Peter Heimes.
The distributions of reptile species found in the Chiapas Highlands closely mirror those of amphibians and can likewise be categorized into three main groups based on their geographic range. The first group includes 66 species that are endemic to Mexico, with 26 of these restricted to the Mexican portion of the Chiapas Highlands (Figure 4, Figure 5 and Figure 6). The second and largest group comprises 162 species that range from Mexico into Central or South America. Within this group, 28 species are endemic to the Chiapas Highlands but not specifically to the Mexican portion. Of these, 18 reach their southernmost distribution in Guatemala, while the remaining 10 extend farther south into Honduras, El Salvador, or Nicaragua. A third group includes 13 species with broader distributions extending from southern Canada (Coluber constrictor and Storeria dekayi) or the United States into Central or South America. Additionally, a single species, Oxybelis microphthalmus, is found from the southwestern United States to southern Mexico.


Figure 5.
Photos of some of the lizard species endemic to the Chiapas Highlands: (A) Abronia lythrochila, San Cristobal de las Casas, Chiapas; (B) Abronia moreletii, San José Bocomtenelté, San Cristóbal de las Casas, Chiapas; (C) Abronia smithi, Rincón Estrella, Motocintla, Chiapas; (D) Anolis anisolepis, San Cristóbal de las Casas, Chiapas; (E) Anolis crassulus, Volcán Tacaná, Chiapas; (F) Anolis hobartsmithi, Rayón Mescalapa, Chiapas; (G) Anolis parvicirculatus, Berriozabal, Chiapas; (H) Sceloporus smaragdinus, Rincón Estrella, Siltepec, Chiapas. Photos (A–F,H) by Peter Heimes; Photo (G) by Eric Centenero Alcalá.

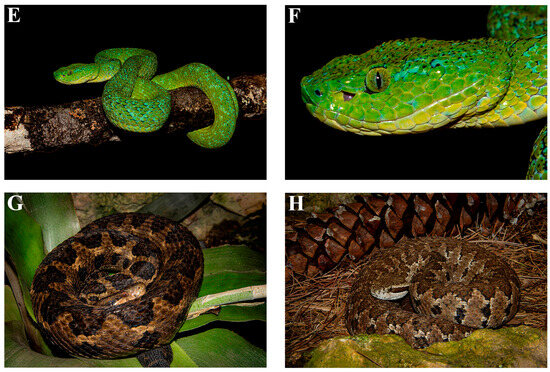
Figure 6.
Photos of some of the lizard and snake species endemic to the Chiapas Highlands: (A) Sceloporus taeniocnemis, Las Margaritas, Chiapas; (B) Adelphicos nigrilatum; (C) Coniophanes alvarezi, San Cristobal de las Casas, Chiapas; (D) Bothriechis aurifer, in captivity; (E,F) Bothriechis bicolor, in captivity; (G) Cerrophidion godmani, in captivity; (H) Cerrophidion tzotzilorum, in captivity. Photos (A,C–H), by Eric Centenero Alcalá; Photo (B) by Peter Heimes.
These findings confirm that the Chiapas Highlands harbor a rich and highly endemic herpetofauna, distinguishing the region as a biodiversity hotspot within Mesoamerica (see also [10,11]). The region supports over a quarter (25.3%) of Mexico’s amphibian and reptile species, despite occupying a relatively small portion of its territory [37,43]. This richness reflects both the region’s ecological complexity and its biogeographic position at the intersection of the Nearctic and Neotropical realms, which facilitates species accumulation and diversification [44,45], as well as species turnover among biogeographic subprovinces [46]. Amphibians, though numerically fewer than reptiles, display especially high levels of endemism, with 14 species restricted solely to this province, highlighting the province’s importance for amphibian conservation (Supplementary Table S1; [26]), especially in the cloud forests [47]. The prominence of families such as Hylidae, Plethodontidae, and Craugastoridae aligns with patterns observed in other humid montane systems of Mesoamerica, where moisture-dependent and habitat-specialist taxa thrive [48,49]. Reptile diversity is even higher in absolute terms, driven by speciose snake families, such as Dipsadidae and Colubridae, that are widespread across diverse Neotropical habitats [50]. While reptiles show broader distributions overall, the presence of 26 reptile species endemic to the Chiapas Highlands still points to strong regional distinctiveness and localized evolutionary processes [28,29]. Collectively, these findings affirm the Chiapas Highlands as a biodiversity hotspot with both taxonomic breadth and a high concentration of unique lineages, reinforcing the province’s priority status for conservation planning and ecological research.
These results highlight the Chiapas Highlands as a key center of herpetofaunal endemism and biogeographic distinctiveness in Mesoamerica. The presence of a substantial proportion of amphibian (39.3%) and reptile (22.3%) species that are endemic to the province, including 14 amphibians and 26 reptiles found exclusively in the Mexican portion, underscores the region’s role as both a national and regional biodiversity hotspot [51,52]. The limited geographic range of many species, particularly those that reach their southernmost distribution in Guatemala or northern Central America, reflects strong biogeographic filtering driven by topographic complexity and historical barriers to dispersal [45,53]. Amphibians in particular show highly localized distributions, which may be attributed to their reduced dispersal ability and sensitivity to environmental gradients [48,54], as well as isolation due to geographic barriers among the highland areas of the region [55]. Reptiles, while exhibiting broader distributions overall, also include a notable group of species with restricted ranges, especially within snake and lizard taxa, reflecting both ecological specialization and regional evolutionary history [50,56]. These patterns are consistent with the high levels of endemism observed in other montane systems across the Neotropics, where elevational and climatic gradients create opportunities for speciation and isolation [57,58]. However, this study does not include a direct comparison of the Chiapas Highlands’ herpetofauna with other important montane systems in Central America, such as the Sierra de los Cuchumatanes in Guatemala or the Cordillera de Talamanca in Costa Rica. Such comparisons would offer valuable insights into the broader patterns of endemism, community composition, and species turnover across the Mesoamerican highlands. In addition, future research should examine potential faunal differences between the distinct biogeographic subprovinces within the Chiapas Highlands, such as the Central Massif of Chiapas and the Sierra Madre de Chiapas, which may harbor unique assemblages due to their ecological and topographic divergence. The exceptional concentration of narrow-range species in the Chiapas Highlands thus affirms its importance as a conservation priority, particularly given that such taxa are disproportionately vulnerable to habitat loss, climate change, and emerging diseases [59,60]. In addition, there may be substantial hidden diversity in the region with cryptic species likely present (e.g., [61]). The presence of such species with narrow ranges makes it important for future studies to evaluate the factors that drive the distribution of these species within the Chiapas Highlands.
3.2. Species Overlap and Faunal Similarity
The amphibian fauna of the Chiapas Highlands is more exclusive: only 45.5% and 29.5% of its amphibian species are shared with the Veracruzan and Pacific Lowlands provinces, respectively (Table 2). Furthermore, 39.3% of amphibian species are endemic to the Chiapas Highlands. In contrast, reptiles exhibit higher levels of interprovincial species overlap, with 62.0% and 39.7% of Chiapas Highlands reptile species shared with the Veracruzan and Pacific Lowlands provinces, respectively, and only 22.3% of reptile species endemic to the Highlands. However, this appears contradictory to the observed biological pattern, where amphibians show higher endemism and lower species overlap with neighboring regions [44,45]. Amphibians often show strong environmental filtering, and similar montane habitats across provinces may support ecologically analogous species despite their taxonomic uniqueness [58]. Reptiles, in contrast, occupy a broader range of habitats and show more geographically structured faunal assemblages, which may lead to greater observed dissimilarity among provinces, despite a higher proportion of shared species [50,56].

Table 2.
Number of native amphibian and reptile species shared between the Chiapas Highlands and the adjacent biogeographic provinces of Veracruzan and Pacific Lowlands. The regional pool refers to the total number of unique species recorded across all three provinces. Values in parentheses represent the percentage of species in each family that are shared between the Chiapas Highlands and the adjacent provinces. A dash “—” indicates that no species from that family are shared between provinces, either because the family is absent in the Chiapas Highlands or because there is no species overlap with the others. For example, the family Scaphiopodidae is present in the Veracruzan and Pacific Lowlands (with two species in total), but since it is absent from the Chiapas Highlands, there are no shared species between provinces, even though the family contributes to the Regional Pool. Families without any shared species are thus only represented in the Regional Pool column.
Amphibians in the Chiapas Highlands show moderate overlap with neighboring provinces, sharing 45.5% of species (51/112) with the Veracruzan province and 29.5% (33/112) with the Pacific Lowlands. However, 39.3% of amphibian species are endemic to the Chiapas Highlands, indicating a high level of regional uniqueness. This endemism is especially pronounced among salamanders (Order Caudata), where only 28% of species are shared with the Veracruzan province and a mere 8% with the Pacific Lowlands. Anuran (frog) families such as Craugastoridae and Eleutherodactylidae also display limited overlap, further reinforcing the distinctiveness of the highland amphibian fauna. Lemos-Espinal and Smith [37] found that the Chiapas Highland amphibian fauna clustered with the Transvolcanic Belt, Sierra Madre Oriental, and Veracruzan provinces.
In contrast, reptiles show broader interprovincial distributions. Of the 242 reptile species recorded in the Chiapas Highlands, 62.0% (150 species) are shared with the Veracruzan province and 39.7% (96 species) with the Pacific Lowlands. Only 22.3% of reptile species are endemic to the Chiapas Highlands, suggesting greater faunal connectivity across provinces. This is particularly evident in families like the Colubridae and Dipsadidae (snakes), Iguanidae and Teiidae (lizards), and most turtle families, where interprovincial overlap exceeds 50%. The high proportion of shared reptile species is consistent with their generally broader ecological tolerances and dispersal capacities compared with amphibians. Lemos-Espinal and Smith [37] found that the Chiapas Highland reptile fauna clustered with these provinces as well as the Sierra Madre del Sur and the Balsas Basin.
These data underscore a fundamental difference in the biogeographic structure of amphibian and reptile communities in the Chiapas Highlands. Amphibians appear more geographically and ecologically restricted, consistent with their lower dispersal ability, higher habitat specificity, and sensitivity to environmental barriers. Reptiles, on the other hand, are more widely distributed and exhibit a stronger pattern of regional overlap. Although multivariate analyses such as cluster analysis can aid in visualizing these patterns, in this case, the descriptive metrics of species overlap offer more robust and interpretable insights into the biogeographic relationships of the region’s herpetofauna.
3.3. Conservation Status
Johnson et al. [20] noted that, within the state of Chiapas, the Northern Highlands and the Sierra Madre de Chiapas are regions with a high priority for conservation action. Our survey of the conservation status of the herpetofauna of the Chiapas Highlands confirms this. Of the 354 species of amphibians and reptiles recorded in the Chiapas Highlands of Mexico, 64 (17.7% of the 325 evaluated species) are listed on the IUCN Red List as Vulnerable (VU), Endangered (EN), or Critically Endangered (CR) [26]. In addition, 39 species (11.0%) are classified as Threatened (A) or Endangered (P) under Mexican environmental legislation [27], and 98 species are considered at high conservation risk according to the Environmental Vulnerability Score (EVS) [28,29] (Table 1). Among amphibians, 47 of the 109 species assessed by [26] (43.1%) are assigned a conservation status of concern (VU, EN, or CR). Only 5 of the 112 amphibian species present in the region (4.5%) are listed as Threatened (A) or Endangered (P) by [27]. However, the EVS identifies 39 of the 109 evaluated amphibian species (35.8%) as high-risk for conservation [25,26] (Table 1; Figure 7). For reptiles, 17 of the 216 species evaluated by the IUCN (7.9%) are listed with some level of conservation concern (VU, EN, or CR) [26]. Meanwhile, 34 of the 242 reptile species found in the Chiapas Highlands (14.0%) are classified as Threatened (A) or Endangered (P) by the Mexican government [27]. The EVS further identifies 59 of 223 reptile species (26.5%) as high conservation priority [28,29] (Table 1; Figure 7).
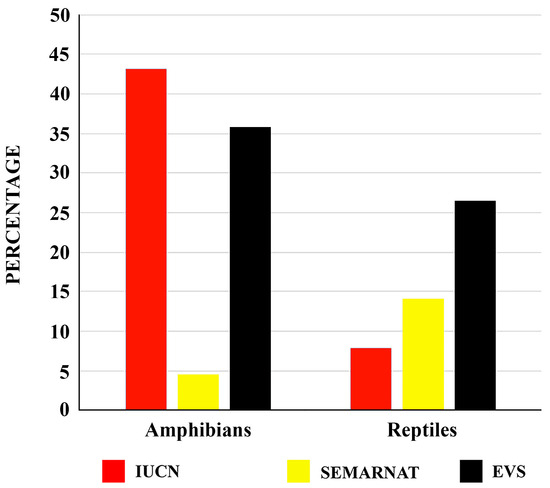
Figure 7.
Percentage of amphibian and reptile species from the Chiapas Highlands biogeographic province of Mexico that are under conservation concern. Species are categorized as Vulnerable, Endangered, or Critically Endangered according to the IUCN Red List [26], as Threatened (A) or in Danger of Extinction (P) by the Mexican government [27], or as high-risk based on the Environmental Vulnerability Score (EVS) [28,29].
According to [26], the primary threat facing most amphibian and reptile species of conservation concern (categorized as Vulnerable, Endangered, or Critically Endangered) is habitat loss (Table 3; see also [62]). This threat is largely driven by habitat fragmentation resulting from excessive logging, agricultural expansion, urbanization, overgrazing, and the widespread use of slash-and-burn practices [23,63,64,65]. In particular, deforestation and ecosystem degradation, especially due to large-scale livestock farming, continue to exert significant pressure on these species [26,65]. Among the most critical drivers of biodiversity loss in the region are clear-cut logging and the conversion of forests into agricultural or residential land [26,62]. Such disruption of native habitats is likely responsible for population declines in amphibians and reptiles in the Chiapas Highlands (e.g., [66]). However, the use of certification programs, such as for coffee plantations (e.g., [67]), may benefit biodiversity while allowing for economic benefits for local residents in the Chiapas Highlands. The effects of climate change on vegetation in the montane regions of southern Mexico could be substantial [68], which has the potential to affect the amphibians and reptiles in the Chiapas Highlands.

Table 3.
Amphibian and reptile species recorded in the Chiapas Highlands biogeographic province of Mexico that are listed as species of conservation concern on the IUCN Red List [26]. The table includes each species’ common name, IUCN conservation status, and reported population trend. Conservation status categories include: Vulnerable (VU), Endangered (EN), and Critically Endangered (CR). Population trends are represented as follows: Decreasing (↓), Increasing (↑), Stable (=), and Unknown (?). Distribution: 0 = Endemic to the Mexican portion of the Chiapas Highlands; 1 = Endemic to the Chiapas Highlands, but not exclusive to Mexico, also found in Guatemala, El Salvador, Honduras, and/or Nicaragua; 2 = Endemic to Mexico; 3 = Found in Mexico and Central America; 4 = Widely distributed from southern Florida (USA) to northern South America. Threat codes: 1 = Habitat loss; 2 = Potential threat from Batrachochytrium dendrobatidis; 2* = Confirmed threat from Batrachochytrium dendrobatidis; 3 = Climate change; 4 = Water pollution; 5 = Potential threat from Batrachochytrium salamandrivorans; 6 = Illegal hunting; 7 = Human persecution; 8 = Human consumption; 9 = International pet trade; 9 * = Potential threat from international pet trade.
Importantly, conservation efforts in the region are embedded within a complex socio-ecological and political context. Since the signing of the San Andrés Accords in February 1996, aimed at recognizing the autonomy, land rights, and natural resource stewardship of indigenous communities in Chiapas, many local populations have assumed a more active role in land and resource management. Although these accords were not fully implemented at the federal level, they served as a clear mandate for the community-led governance of natural areas [69]. Indeed, social conservation initiatives, including private and community protected areas with formal certification, now protect hundreds of thousands of hectares across Mexico, demonstrating the potential of localized stewardship for biodiversity conservation [70]. Moreover, recent studies focusing on indigenous community conservation zones, such as those in Oaxaca, underscore how local governance models can support the conservation of amphibians and reptiles via culturally-informed and ecologically sustainable practices [71]. Conservation of amphibians is especially pressing given their high sensitivity to environmental change and their role as indicators of ecosystem health [72]. Recognizing and supporting community-based conservation strategies, particularly those that align with traditional ecological knowledge and local autonomy, could be essential for the long-term protection of herpetofauna in the Chiapas Highlands.
Species with restricted distributions are particularly vulnerable to these threats [73]. Many amphibians and reptiles in the Chiapas Highlands are confined to small, isolated habitats, making them especially susceptible to extinction from even minor environmental disturbances (e.g., [74]). This vulnerability is exacerbated by ongoing land-use change and the escalating impacts of climate change, including altered rainfall patterns, extended droughts, and rising temperatures, particularly for montane species [67,75,76]. Together, these factors amplify the threat of habitat loss and pose a severe risk to the region’s highly localized and ecologically sensitive herpetofauna.
High-elevation endemic species in the Chiapas Highlands are especially vulnerable to the impacts of climate change due to their limited elevational ranges, physiological specialization, and dependence on stable, humid montane habitats such as cloud forests. As global temperatures rise, suitable climatic conditions for these species are expected to shift upslope, potentially leading to severe range contractions or even mountaintop extirpations for species already occupying the highest elevations [77,78]. Amphibians are particularly susceptible to climate-related stressors due to their permeable skin, low thermal tolerance, and reliance on microclimatic stability for reproduction and survival [79]. Likewise, reptiles restricted to montane zones, including several endemic lizard and snake species, may face reductions in suitable habitat area and altered community dynamics as temperatures rise and precipitation patterns shift [80]. The fragmentation of highland habitats further compounds these risks, limiting the capacity of species to track shifting climate envelopes across the landscape. These threats are especially concerning for taxa already under pressure from habitat degradation and disease, as synergistic effects may accelerate population declines. Given these factors, future research and conservation planning must integrate climate projections to identify potential refugia, prioritize elevational corridors, and assess the adaptive capacity of montane herpetofauna across the region.
Reflecting this vulnerability, of the 64 amphibian and reptile species in the Chiapas Highlands listed as being of conservation concern on the IUCN Red List, a significant proportion exhibit extremely limited distributions [73] (Table 1). Seventeen species are endemic to the Mexican portion of the Highlands, while another 28 are endemic to the broader Chiapas Highlands, but also occur in nearby countries such as Guatemala, El Salvador, Honduras, and Nicaragua (Table 1). Ten additional species are endemic to Mexico, but are found outside the Highlands as well. Eight species with restricted ranges are shared between the Chiapas Highlands and adjacent regions such as the Pacific Lowlands (e.g., Bolitoglossa flaviventris, Heloderma alvarezi, and Ungaliophis continentalis) or Veracruzan province (e.g., Craugastor amniscola, Craugastor palenque, and Ptychohyla macrotympanum) (Table 1). Two species have slightly wider distributions, including Chelydra rossignonii, which ranges from the Chiapas Highlands to Honduras, and Dermatemys mawii, found from southern Mexico to Belize and Guatemala. The only species on the list with a relatively broad geographic range is Crocodylus acutus, which occurs from southern Sonora to northern South America, including populations in Florida and the Caribbean.
The predominance of species with highly restricted ranges underscores the critical role of the Chiapas Highlands as a conservation hotspot. This region serves as a refuge for a diverse and unique assemblage of amphibians and reptiles, many of which are found nowhere else or in very limited areas beyond its borders. Protecting these habitats is not only essential for preventing further species decline, but also for preserving the biological integrity of one of Mesoamerica’s most ecologically important regions. In particular, it is important to ensure the persistence of connectivity among these isolated habitats, especially since critical connectivity corridors fall outside of established protected areas [73]. To achieve the conservation of the herpetofauna of this region will require expansion of existing protected areas, especially in the highland and montane areas [81].
Another significant threat facing amphibians in the Chiapas Highlands is the emergence of infectious diseases, particularly those caused by chytrid fungi (Batrachochytrium dendrobatidis [Bd] and Batrachochytrium salamandrivorans [Bsal]) (Table 3). Populations of at least seven anuran species (Plectrohyla avia, Plectrohyla euthysanota, Plectrohyla hartwegi, Plectrohyla lacertosa, Plectrohyla matudae, Plectrohyla sagorum, and Charadrahyla chaneque) have tested positive for the presence of Batrachochytrium dendrobatidis, and at least 14 additional species are potentially at risk of infection [18,26,82]. It is possible the Bd led to the significant decline in or extirpation of Incilius tacanensis [83]. Although Batrachochytrium salamandrivorans has not yet been detected in the Americas [84,85], it continues to spread across Europe and poses a serious threat of introduction to other regions [86]. If Bsal were to arrive in Mexico, the impact on native salamander populations could be severe [87]. Notably, species found in the Chiapas Highlands are considered to inhabit areas that are highly suitable for Bsal colonization [26,88]. Currently, 15 of the 18 salamander species from the region that are listed as Vulnerable (VU), Endangered (EN), or Critically Endangered (CR) on the IUCN Red List [26] are identified as being at potential risk from the possible arrival of Bsal in the area [26].
In addition to fungal pathogens, emerging viral threats such as Ranavirus also warrant concern. While no cases of Ranavirus infection have yet been reported in wild amphibian populations in Mexico, a notable outbreak was documented in a captive population of American bullfrogs (Rana catesbeiana) in northern Mexico [89], raising alarms about potential spillover to native species. The risk is amplified by activities such as amphibian aquaculture, translocation, and the maintenance of animals for research or educational purposes, all of which have been implicated in the spread of Ranavirus globally [90,91]. Given the presence of amphibian farming and informal trade in parts of Mexico, including Chiapas, the potential introduction of Ranavirus into wild populations, particularly the diverse and highly endemic anurans of the Chiapas Highlands, represents a serious conservation concern, especially for species already under stress from habitat loss and climate change. Proactive surveillance and biosecurity measures should be prioritized to mitigate this emerging risk.
While habitat loss and disease remain the predominant threats to amphibians and reptiles in the Chiapas Highlands, a smaller but significant number of species are also directly impacted by specific human activities (Table 3). These threats include human persecution, consumption, illegal trade, chemical pollution, and poaching [26]; see also [62]. Several species are exploited for human consumption, such as Ctenosaura oaxacana, Chelydra rossignonii, and Dermatemys mawii, the latter also being affected by chemical contamination of aquatic habitats. Heloderma alvarezi, the endangered Chiapan beaded lizard, is often killed due to fear or cultural beliefs, despite its protected status. The illegal international pet trade poses an additional threat to species like Ungaliophis continentalis, Bothriechis aurifer, and potentially Bothriechis rowleyi. Meanwhile, water pollution from agricultural runoff and chemical use (e.g., [92]) threatens aquatic and semi-aquatic species, including Craugastor pelorus and again Dermatemys mawii. Finally, Crocodylus acutus is subject to illegal hunting for its skin, despite existing regulations. Although these threats affect a relatively limited number of species, they represent direct human impacts that can cause rapid population declines, especially when combined with other pressures such as habitat fragmentation and climate change. The harvesting of bromeliads has led to the mortality of Abronia lythrochila [24].
The conservation status of 1 amphibian and 20 reptile species from the Chiapas Highlands is unknown and they are classified on the IUCN Red List as Data-Deficient (DD) due to a lack of information on their taxonomy, population status, or the impact of threats [26]; (Supplementary Table S1). A further 29 species, 3 amphibians and 26 reptiles, have not been evaluated (Supplementary Table S1). These species likely include some of conservation concern, but their uncertain status means that they are often overlooked in conservation planning and funding. This poses a significant problem, as multiple studies indicate that DD species are not necessarily at low risk; in fact, many may be just as or more threatened than data-sufficient species. Data-deficient species are often narrowly distributed, poorly studied, and frequently found in biodiversity hotspots, where habitat loss and other threats are severe [93,94]. With evidence suggesting that over half of DD reptiles and up to 85% of DD amphibians may be threatened [94], and with strategic methods now available to prioritize their reassessment [95], it is imperative that these neglected species in the Chiapas Highlands receive attention. For example, [96,97] suggested the newly described Abronia morenica and Abronia cunemica from the Chiapas Highlands should be considered endangered under the IUCN Red List criteria. Otherwise, critical components of regional biodiversity may silently slip toward extinction. The existing protected areas in Chiapas appear to contain more threatened and endemic species of reptiles than unprotected areas [11]; however, the opposite is true for amphibians [10]. Conservation of the herpetofauna of the Chiapas Highlands may be affected by the perceptions, positive and negative, of local inhabitants [98], suggesting any conservation efforts will have to engage with local residents to be successful. Whitfield et al. [63] suggest that persecution of Abronia lythrochila due to the mistaken belief that they are venomous contributes to a possible population decline.
4. Conclusions
The Chiapas Highlands stand out as a region of exceptional herpetofaunal richness, endemism, and ecological significance within Mesoamerica. Hosting over a quarter of Mexico’s amphibian and reptile species and a substantial number of regional endemics, the highlands function as a critical biodiversity reservoir shaped by complex biogeographic processes and ecological gradients. Amphibians and reptiles exhibit distinct distributional patterns, driven by their differing physiological constraints, dispersal capacities, and environmental sensitivities. Amphibians, in particular, show high levels of local endemism and vulnerability, underscoring their value as conservation indicators. The presence of numerous species with restricted ranges and threatened statuses highlights the urgency of protecting this region from ongoing threats such as habitat loss, climate change, and emerging diseases. Furthermore, the significant number of Data-Deficient and unevaluated species emphasizes the need for continued research and reassessment to inform conservation action. Together, these findings not only reinforce the Chiapas Highlands as a national and regional conservation priority, but also underscore the broader importance of montane systems in sustaining global biodiversity. Conservation efforts here will have lasting impacts on the preservation of unique evolutionary lineages and the ecological integrity of Mesoamerica.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17090593/s1, Supplementary Table S1: Amphibians and reptiles of the Chiapas Highlands biogeographic province of Mexico.
Author Contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by J.A.L.-E. and G.R.S. The first draft of the manuscript was written by J.A.L.-E. and G.R.S., and both authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was possible through the generous support provided by the Dirección General de Asuntos del Personal Académico, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (DGAPA-PAPIIT), through the Project IN200225.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All of the data that support the findings of this study are available in the main text or the Supplementary Material.
Acknowledgments
We are grateful to Alejandra Núñez Merchand from the National Commission for the Understanding and Use of Biodiversity (CONABIO) for kindly creating and providing the biogeographic province maps used in this publication. We appreciate the opportunity to feature photographs by Eric Centenero Alcalá and Peter Heimes, which help illustrate some of the endemic amphibian and reptile species of the Chiapas Highlands. We thank the three anonymous reviewers for their very helpful comments that improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Morrone, J.J. Halffter’s Mexican transition zone (1962–2014), cenocrons and evolutionary biogeography. J. Zool. Syst. Evol. Res. 2015, 53, 249–257. [Google Scholar] [CrossRef]
- Morrone, J.J. The Mexican Transition Zone. In A Natural Biogeographic Laboratory to Study Biotic Assembly; Springer Nature Switzerland A.G.: Cham, Switzerland, 2020; pp. 1–191. [Google Scholar]
- Morrone, J.J. Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del Nuevo Mundo. Rev. Mex. Biodivers. 2019, 90, 1–68. [Google Scholar] [CrossRef]
- Zunino, M.; Zullini, A. Biogeografía. La Dimensión Espacial de La Evolución; Fondo de Cultura Económica: Mexico City, Mexico, 2003; p. 359. [Google Scholar]
- Corona-Mendoza, W.; Escalante, T. Aspectos ambientales y culturales de los nodos panbiogeográficos prioritarios para mamíferos terrestres del centro-sur de México. Acta Zool. Mex. 2021, 37, 1–28. [Google Scholar] [CrossRef]
- Escalante, T.; Rodríguez, G.; Morrone, J.J. Las provincias biogeográficas del Componente Mexicano de Montaña desde la perspectiva de los mamíferos continentales. Rev. Mex. Biodivers. 2005, 76, 2. [Google Scholar] [CrossRef]
- Flores-Villela, O.; Martínez-Salazar, E.A. Historical explanation of the origin of the herpetofauna of Mexico. Rev. Mex. Biodivers. 2009, 80, 817–833. [Google Scholar]
- Mastretta-Yanes, A.; Moreno-Letelier, A.; Piñero, D.; Jorgensen, T.H.; Emerson, B.C. Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Trans-Mexican Volcanic Belt. J. Biogeogr. 2015, 42, 1586–1600. [Google Scholar] [CrossRef]
- Rico, Y.; León-Tapia, M.Á.; Zurita-Solís, M.; Rodríguez-Gómez, F.; Vásquez-Morales, S.G. Influence of Pleistocene climatic oscillations on the phylogeography and demographic history of endemic vulnerable trees (section Magnolia) of the Tropical Montane Cloud Forest in Mexico. PeerJ 2021, 9, e12181. [Google Scholar] [CrossRef]
- Cabrera-Hernández, R.; Köhler, G.; Tejeda-Cruz, C.; Peralta-Meixueiro, M.A.; López, S. The distribution of amphibian species richness in protected areas of Chiapas, Mexico. J. Nat. Conserv. 2023, 74, 126444. [Google Scholar] [CrossRef]
- Cabrera-Hernández, R.; Köhler, G.; Tejeda-Cruz, C.; Peralta-Meixueiro, M.A.; López, S. The distribution of reptile species richness in protected areas of Chiapas, Mexico. J. Nat. Conserv. 2024, 80, 126629. [Google Scholar] [CrossRef]
- De Jong, B.H.J.; Cairns, M.A.; Haggerty, P.K.; Ramírez-Marcial, N.; Ochoa-Gaona, S.; Mendoza-Vega, J.; González-Espinosa, M.; March-Mifsut, I. Land-use change and carbon flux between 1970s and 1990s in Central Highlands of Chiapas, Mexico. Environ. Manag. 1999, 23, 373–385. [Google Scholar] [CrossRef]
- Ochoa-Gaona, S.; González-Espinosa, M. Land use and deforestation in the highlands of Chiapas, Mexico. Appl. Geogr. 2000, 20, 17–42. [Google Scholar] [CrossRef]
- Ochoa-Gaona, S. Traditional land-use systems and patterns of forest fragmentation in the highlands of Chiapas, Mexico. Environ. Manag. 2001, 27, 571–586. [Google Scholar] [CrossRef]
- Cayuela, L.; Benayas, J.M.R.; Echeverría, C. Clearance and fragmentation of tropical montane forests in the Highlands of Chiapas, Mexico (1975–2000). For. Ecol. Manag. 2006, 226, 208–218. [Google Scholar] [CrossRef]
- Figueroa-Jáuregui, M.L.; Ibáñez-Castillo, L.A.; Arteaga-Ramírez, R.; Arellano-Monterrosas, J.L.; Vázquez-Peña, M. Cambio de uso de suelo en la cuenca de San Cristóbal de las Casas, México. Agrociencia 2011, 45, 531–544. [Google Scholar]
- Álvarez del Toro, M. Los Reptiles de Chiapas; Instituto de Historia Natural del Estado: Tuxtla Gutiérrez, Chiapas, Mexico, 1982; p. 248. [Google Scholar]
- Muñoz-Alonso, L.A. Riqueza, Diversidad y Estatus de Los Anfibios Amenazados en el Sureste de México; una evaluación para determinar las posibles causas de la declinación de sus poblaciones; El Colegio de la Frontera Sur: San Cristóbal de las Casas, Chiapas, Mexico, 2010; p. 55. [Google Scholar]
- Reynoso, V.H.; Paredes-León, R.; González-Hernández, A. Anfibios y reptiles de Chiapas con comentarios sobre los reportes y estudios de diversidad herpetofaunística en la región, su endemismo y conservación. In Chiapas: Estudios Sobre su Diversidad Biológica; Álvarez, F., Ed.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2011; pp. 459–509. [Google Scholar]
- Johnson, J.D.; Mata-Silva, V.; García-Padilla, E.; Wilson, L.D. The herpetofauna of Chiapas, Mexico: Composition, physiographic distribution, and conservation status. Mesoam. Herpetol. 2015, 2, 272–329. [Google Scholar]
- Smith, G.R.; Lemos-Espinal, J.A. Factors related to species richness, endemism, and conservation status of the herpetofauna (Amphibia and Reptilia) of Mexican states. ZooKeys 2022, 1097, 85. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Smith, G.R. An analysis of the inter-state similarity of the herpetofaunas of Mexican states. Nat. Conserv. 2023, 53, 223–256. [Google Scholar] [CrossRef]
- Aguilar-López, J.L.; Pineda, E.; Luría-Manzano, R.; Canseco-Márquez, L. Species diversity, distribution, and conservation status in a Mesoamerican region: Amphibians of the Uxpanapa-Chimalapas Region, Mexico. Trop. Conserv. Sci. 2016, 9, 1940082916670003. [Google Scholar] [CrossRef]
- Aranda-Coello, J.M.; Ochoa-Ochoa, L.M.; Naranjo-Piñera, E.J. Evaluación de algunos efectos de la extracción tradicional de bromelias sobre la herpetofauna de los bosques de Chanal, Chiapas. Acta Zool. Mex. 2012, 28, 621–624. [Google Scholar] [CrossRef]
- Martínez, A.I.M.; Soto, F.R.; Chankayun, E.C.; Padilla, E.G.; Juárez, I.V.; Silva, V.M.; López-Esquivel, E.A.; Fucsko, L.A.; Lavariega, M.C.; Johnson, J.D.; et al. Los anfibios y reptiles del Noreste de la Selva Lacandona: Nahá y Metzabok, Ocosingo, Chiapas, México; con algunas notas etnoherpetológicas. Biol. Soc. 2023, 6, 48–78. [Google Scholar]
- International Union for Conservation of Nature’s (IUCN). The IUCN Red List of Threatened Pecies, Version 2025-1. Available online: https://www.iucnredlist.org/ (accessed on 15 June 2025).
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Modificación al Anexo Normativo III, Lista de Especies en Riesgo de la Norma Oficial Mexicana NOM-059-Ecol-(2010) Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo, Publicado el 30 de diciembre del 2010. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019 (accessed on 15 June 2025).
- Wilson, L.D.; Johnson, J.D.; Mata-Silva, V. A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 97–127. [Google Scholar]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 1–47. [Google Scholar]
- Morrone, J.J. Biogeographic areas and transition zones of Latin America and the Caribbean Islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu. Rev. Entomol. 2006, 51, 467–494. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographical regionalization of the Neotropical region. Zootaxa 2014, 3782, 1–110. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 2017, 4277, 277–279. [Google Scholar] [CrossRef]
- Advanced Spaceborne Thermal Emission and Reflection Radiometer Global Digital Elevation Model Version 2 (ASTER GDEM2)—Modelo Digital de Elevación Global ASTER Versión 2. 1:50,000. 2010. Available online: https://asterweb.jpl.nasa.gov/gdem.asp (accessed on 15 June 2025).
- García, E. Climas (Clasificación de Köppen, Modificado por García). Escala 1:1 000 000; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): Mexico City, Mexico, 1998. [Google Scholar]
- Instituto Nacional de Estadística y Geografía [INEGI]. Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación. Escala 1:250 000. Serie VI (Capa Unión), Escala: 1:250 000, 1st ed.; Instituto Nacional de Estadística y Geografía: Aguascalientes, Mexico, 2016. [Google Scholar]
- Ruan-Soto, F.; Bolom-Ton, F.; Cano-Contreras, E.J.; Domínguez-Torres, L.; Guerrero-Martínez, F.; Mariaca-Méndez, R. Ethnobotany of the Highlands of Chiapas. In Ethnobotany of the Mountain Regions of Mexico; Springer Nature Switzerland A.G.: Cham, Switzerland, 2022; pp. 929–951. [Google Scholar]
- Lemos-Espinal, J.A.; Smith, G.R. The distribution, diversity and conservation of the Mexican herpetofauna among its biogeographic provinces. J. Nat. Conserv. 2024, 82, 126714. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference, version 6.2; American Museum of Natural History: New York, NY, USA, 2025. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 15 June 2025).
- AmphibiaWeb. University of California, Berkeley, CA, USA. Available online: https://amphibiaweb.org (accessed on 15 June 2025).
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Kudera, J.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 3 May 2025).
- Parra-Olea, G.; Flores-Villela, O.; Mendoza-Almeralla, C. Biodiversidad de anfibios en México. Rev. Mex. Biodivers. 2014, 85, S460–S466. [Google Scholar] [CrossRef]
- Flores-Villela, O.; García-Vázquez, U.O. Biodiversidad de reptiles en México. Rev. Mex. Biodivers. 2014, 85, S467–S475. [Google Scholar] [CrossRef]
- Ramírez-Bautista, A.; Torres-Hernández, L.A.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Wilson, L.D.; Johnson, J.D.; Porras, L.W.; Balderas-Valdivia, C.J.; González-Hernández, A.J.; et al. An updated list of the Mexican herpetofauna: With a summary of historical and contemporary studies. ZooKeys 2023, 1166, 287–306. [Google Scholar] [CrossRef]
- Duellman, W.E. Patterns of Distribution of Amphibians: A Global Perspective; Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Wilson, L.D.; Johnson, J.D. Distributional patterns of the herpetofauna of Mesoamerica, a biodiversity hotspot. In Conservation of Mesoamerican Amphibians and Reptiles; Wilson, L.D., Townsend, J.H., Johnson, J.D., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2010; pp. 30–78. [Google Scholar]
- Calderón-Patrón, J.M.; Peña-Joya, K.E.; Téllez-López, J.; Canales-Gómez, E.P. Dissimilarity among species and higher taxa of amphibians in a hotspot of biodiversity and endemism in the Neotropics. Diversity 2024, 16, 224. [Google Scholar] [CrossRef]
- Montiel Canales, G.; Goyenechea Mayer Goyenechea, I. Amphibian areas of endemism: A conservation priority in the threatened Mexican cloud forest. Vert. Zool. 2022, 72, 235–244. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2019. [Google Scholar]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles, 4th ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- Savage, J.M. The Amphibians and Reptiles of Costa Rica: A Herpetofauna Between Two Continents, Between Two Seas; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Navas, C.A. Implications of microhabitat selection and patterns of activity on the thermal ecology of high elevation neotropical anurans. Oecologia 1996, 108, 617–626. [Google Scholar] [CrossRef]
- Johnson, J.D. Biogeographic aspects of the herpetofauna of the Central Depression of Chiapas, México, with comments on surrounding areas. Southwest. Nat. 1990, 35, 268–278. [Google Scholar] [CrossRef]
- Cadle, J.E.; Greene, H.W. Phylogenetic patterns, biogeography, and the ecological structure of neotropical snake assemblages. In Species Diversity in Ecological Communities; Ricklefs, R.E., Schluter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 281–293. [Google Scholar]
- Ghalambor, C.K.; Huey, R.B.; Martin, P.R.; Tewksbury, J.J.; Wang, G. Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integr. Comp. Biol. 2006, 46, 5–17. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H.; Moen, D.S.; Smith, S.A.; Reeder, T.W. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: Treefrog trees unearth the roots of high tropical diversity. Am. Nat. 2006, 168, 579–596. [Google Scholar] [CrossRef]
- Bickford, D.; Howard, S.D.; Ng, D.J.J.; Sheridan, J.A. Impacts of climate change on the amphibians and reptiles of Southeast Asia. Biodivers. Conserv. 2010, 19, 1043–1062. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Castiglia, R.; Flores-Villela, O.A.; Bezerra, A.M.R.; Gornung, E.; Annesi, F.; Muñoz-Alonso, L.A.; Solano, E. Detection of cryptic diversity in lizards (Squamata) from two Biosphere Reserves in Mesoamerica. Comp. Cytogenet. 2020, 14, 613–638. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Vázquez, M.D.R.; Ríos-Rodas, L.; Fucsko, L.A.; Porras, L.W.; Mata-Silva, V.; Rocha, A.; DeSantis, D.L.; García-Padilla, E.; Johnson, J.D.; Wilson, L.D. The herpetofauna of Tabasco, Mexico: Composition, distribution, and conservation status. Amphib. Rept. Conserv. 2022, 16, 1–61. [Google Scholar]
- Whitfield, S.M.; Lips, K.R.; Donnelly, M.A. Amphibian decline and conservation in Central America. Copeia 2016, 104, 351–379. [Google Scholar] [CrossRef]
- Santizo-Nanduca, A.; Rioja-Paradela, T.M.; Carrillo-Reyes, A.; Castañeda-Gaytán, G.; Porras-Murillo, L. Effect of landscape composition and configuration on the diversity of amphibians and reptiles. S. Am. J. Herpetol. 2023, 29, 77–87. [Google Scholar] [CrossRef]
- Soto-Pozos, A.F.; Basanta, M.D.; García-Castillo, M.G.; Parra-Olea, G. The Amphibians of the Mexican Montane Cloud Forest. In Mexican Fauna in the Anthropocene; Springer International Publishing: Cham, Switzerland, 2023; pp. 357–376. [Google Scholar]
- Aranda-Coello, J.M.; Liévano-Oropeza, P. Population size estimate of Abronia lythrochila Smith &Alvarez del Toro, 1963 (Reptilia: Anguidae) in the Estación Biológica San José, Chiapas, Mexico. Acta Zool. Mex. 2023, 39, 1–11. [Google Scholar]
- Philpott, S.M.; Bichier, P.; Rice, R.; Greenberg, R. Field-testing ecological and economic benefits of coffee certification programs. Conserv. Biol. 2007, 21, 975–985. [Google Scholar] [CrossRef]
- Ortega, M.A.; Cayuela, L.; Griffith, D.M.; Camacho, A.; Coronado, I.M.; del Castillo, R.F.; Figueroa-Rangel, B.L.; Fonseca, W.; Garibaldi, C.; Kelly, D.L.; et al. Climate change increases threat to plant diversity in tropical forests of Central America and southern Mexico. PLoS ONE 2024, 19, e0297840. [Google Scholar] [CrossRef]
- Wikipedia. Acuerdos de San Andrés. Available online: https://es.wikipedia.org/wiki/Acuerdos_de_San_Andr%C3%A9s (accessed on 12 August 2025).
- Ochoa-Ochoa, L.; Urbina-Cardona, J.N.; Vázquez, L.B.; Flores-Villela, O.; Bezaury-Creel, J. The effects of governmental protected areas and social initiatives for land protection on the conservation of Mexican amphibians. PLoS ONE 2009, 4, e6878. [Google Scholar] [CrossRef]
- Simón-Salvador, P.R.; Arreortúa, M.; Flores, C.A.; Santiago-Dionicio, H.; González-Bernal, E. The role of Indigenous and Community Conservation Areas in herpetofauna conservation: A preliminary list for Santa Cruz Tepetotutla, Oaxaca Mexico. ZooKeys 2021, 1029, 185. [Google Scholar] [CrossRef]
- Nori, J.; Lemes, P.; Urbina-Cardona, N.; Baldo, D.; Lescano, J.; Loyola, R. Amphibian conservation, land-use changes and protected areas: A global overview. Biol. Conserv. 2015, 191, 367–374. [Google Scholar] [CrossRef]
- Bolom-Huet, R.; Pacheco, X.P.; Muñoz-Alonso, A.; Sunny, A. Potential distribution and connectivity for two plethodontid salamanders: Conservation areas and landscape corridors for two endemic species of Mexico and Guatemala. Environ. Manag. 2022, 70, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Bolom-Huet, R.; Caloca-Peña, L.; Muñoz-Alonso, A.; Sunny, A. New montane records for the vulnerable Long-nosed Bromeliad Salamander, Dendrotriton megarhinus (Rabb, 1960) (Caudata, Plethodontidae), from Cerro La Bola, Chiapas, México. Check List 2024, 20, 771–777. [Google Scholar] [CrossRef]
- Hernández-Ordóñez, O.; Martínez-Ramos, M.; Arroyo-Rodríguez, V.; González-Hernández, A.; González-Zamora, A.; Zárate, D.A.; Reynoso, V.H. Distribution and conservation status of amphibian and reptile species in the Lacandona rainforest, Mexico: An update after 20 years of research. Trop. Conserv. Sci. 2014, 7, 1–25. [Google Scholar] [CrossRef]
- Ureta, C.; Ramírez-Barrón, M.; Ruán-Soto, F.; Kolb, M.; Martínez-Cruz, A.L.; Gasparello, G.; Sánchez-Cordero, V. Impact of climate change on the distribution of insectivorous bats: Implications for small-scale farming in southern Mexico. PLoS ONE 2024, 19, e0310623. [Google Scholar] [CrossRef]
- Raxworthy, C.J.; Pearson, R.G.; Rabibisoa, N.; Rakotondrazafy, A.M.; Ramanamanjato, J.B.; Raselimanana, A.P.; Wu, S.; Nussbaum, R.A.; Stone, D.A. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: A preliminary appraisal for the highest massif in Madagascar. Glob. Change Biol. 2008, 14, 1703–1720. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Glob. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R.; Sears, M.W. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008, 6, e72. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Urbina-Cardona, J.N.; Flores-Villela, O. Ecological-niche modeling and prioritization of conservation-area networks for Mexican herpetofauna. Conserv. Biol. 2010, 24, 1031–1041. [Google Scholar] [CrossRef]
- Bolom-Huet, R.; Pineda, E.; Andrade-Torres, A.; Díaz-Fleischer, F.; Muñoz, A.L.; Galindo-González, J. Chytrid prevalence and infection intensity in treefrogs from three environments with different degrees of conservation in Mexico. Biotropica 2022, 55, 318–328. [Google Scholar] [CrossRef]
- McCarthy, K.; Shinn, O.; Luna-Reyes, R.; Mendelson, J.R., III. A redescription of the poorly known Central American toad Incilius tacanensis (Anura, Bufonidae), with a summary of its biology and conservation status. ZooKeys 2022, 1102, 149–161. [Google Scholar] [CrossRef]
- Waddle, J.H.; Grear, D.A.; Mosher, B.A.; Campbell Grant, E.H.; Adams, M.J.; Backlin, A.R.; Barichivich, W.J.; Brand, A.B.; Bucciarelli, G.M.; Calhoun, D.L.; et al. Batrachochytrium salamandrivorans (Bsal) not detected in an intensive survey of wild North American amphibians. Sci. Rep. 2020, 10, 13012. [Google Scholar] [CrossRef]
- Basanta, M.D.; Avila-Akerberg, V.; Byrne, A.Q.; Castellanos-Morales, G.; González Martínez, T.M.; Maldonado-López, Y.; Rosenblum, E.B.; Suazo-Ortuño, I.; Parra Olea, G.; Rebollar, E.A. The fungal pathogen Batrachochytrium salamandrivorans is not detected in wild and captive amphibians from Mexico. PeerJ 2022, 10, e14117. [Google Scholar] [CrossRef]
- Yap, T.A.; Nguyen, N.T.; Serr, M.; Shepack, A.; Vredenburg, V.T. Batrachochytrium salamandrivorans and the risk of a second amphibian pandemic. EcoHealth 2017, 14, 851–864. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Basanta, M.D.; García-Castillo, M.G.; Zumbado-Ulate, H.; Neam, K.; Rovito, S.; Searle, C.L.; Parra-Olea, G. Anticipating the potential impacts of Batrachochytrium salamandrivorans on Neotropical salamander diversity. Biotropica 2022, 54, 157–169. [Google Scholar] [CrossRef]
- Basanta, M.D.; Rebollar, E.A.; Parra-Olea, G. Potential risk of Batrachochytrium salamandrivorans in Mexico. PLoS ONE 2019, 14, e0211960. [Google Scholar] [CrossRef]
- Saucedo, B.; Serrano, J.M.; Jacinto-Maldonado, M.; Leuven, R.S.E.W.; Rocha García, A.A.; Méndez Bernal, A.; Gröne, A.; Van Beurden, S.J.; Escobedo-Bonilla, C.M. Pathogen Risk Analysis for Wild Amphibian Populations Following the First Report of a Ranavirus Outbreak in Farmed American Bullfrogs (Lithobates catesbeianus) from Northern Mexico. Viruses 2019, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Ke, F.; Gui, J.-F.; Zhang, Q.-Y. Environmental Factors and Their Threshold Affecting the Survival of Five Aquatic Animal Viruses in Different Animal Cells. Viruses 2022, 14, 2546. [Google Scholar] [CrossRef]
- Lisachova, L.S.; Lisachov, A.P.; Ermakov, O.A.; Svinin, A.O.; Chernigova, P.I.; Lyapkov, S.M.; Zamaletdinov, R.I.; Pavlov, A.V.; Zaks, S.S.; Fayzulin, A.I.; et al. Continent-Wide Distribution of CMTV-Like Ranavirus, from the Urals to the Atlantic Ocean. Ecohealth 2025, 263, e5949. [Google Scholar] [CrossRef]
- Bernardino Hernández, H.U.; Mariaca Méndez, R.; Nazar Beutelspacher, A.; Álvarez Solís, J.D.; Torres Dosal, A.; Herrera Portugal, C. Factores socioeconómicos y tecnológicos en el uso de agroquímicos en tres sistemas agrícolas en los altos de Chiapas, México. Interciencia 2016, 41, 382–392. [Google Scholar]
- Morais, A.R.; Siqueira, M.N.; Lemes, P.; Maciel, N.M.; De Marco, P., Jr.; Brito, D. Unraveling the conservation status of Data Deficient species. Biol. Conserv. 2013, 166, 98–102. [Google Scholar] [CrossRef]
- Borgelt, J.; Dorber, M.; Høiberg, M.A.; Verones, F. More than half of data deficient species predicted to be threatened by extinction. Commun. Biol. 2022, 5, 679. [Google Scholar] [CrossRef]
- Cazalis, V.; Santini, L.; Lucas, P.M.; González-Suárez, M.; Hoffmann, M.; Benítez-López, A.; Pacifici, M.; Schipper, A.M.; Böhm, M.; Zizka, A.; et al. Prioritizing the reassessment of data-deficient species on the IUCN Red List. Conserv. Biol. 2023, 37, e14139. [Google Scholar] [CrossRef] [PubMed]
- Clause, A.G.; Luna-Reyes, R.; Nieto-Montes de Oca, A. A new species of Abronia (Squamata: Anguidae) from a protected area in Chiapas, Mexico. Herpetologica 2020, 76, 330–343. [Google Scholar] [CrossRef]
- Clause, A.G.; Luna-Reyes, R.; Mendoza-Velázquez, O.M.; Nieto-Montes de Oca, A.; Solano-Zavaleta, I. Bridging the gap: A new species of arboreal Abronia (Squamata: Anguidae) from the Northern Highlands of Chiapas, Mexico. PLoS ONE 2024, 19, e0295230. [Google Scholar] [CrossRef]
- Castillo-Huitrón, N.M.; Naranjo, E.J.; Santos-Fita, D.; Peñaherrera-Aguirre, M.; Prokop, P.; Cisneros, R.; Gallegos, S.V.; Jezová, Z. Influence of human emotions on conservation attitudes toward relevant wildlife species in El Triunfo Biosphere Reserve, Mexico. Biodiv. Conserv. 2024, 33, 2423–2439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).