Assessing Ecological Restoration of Père David’s Deer Habitat Using Soil Quality Index and Bacterial Community Structure
Abstract
1. Introduction
2. Materials and Methods
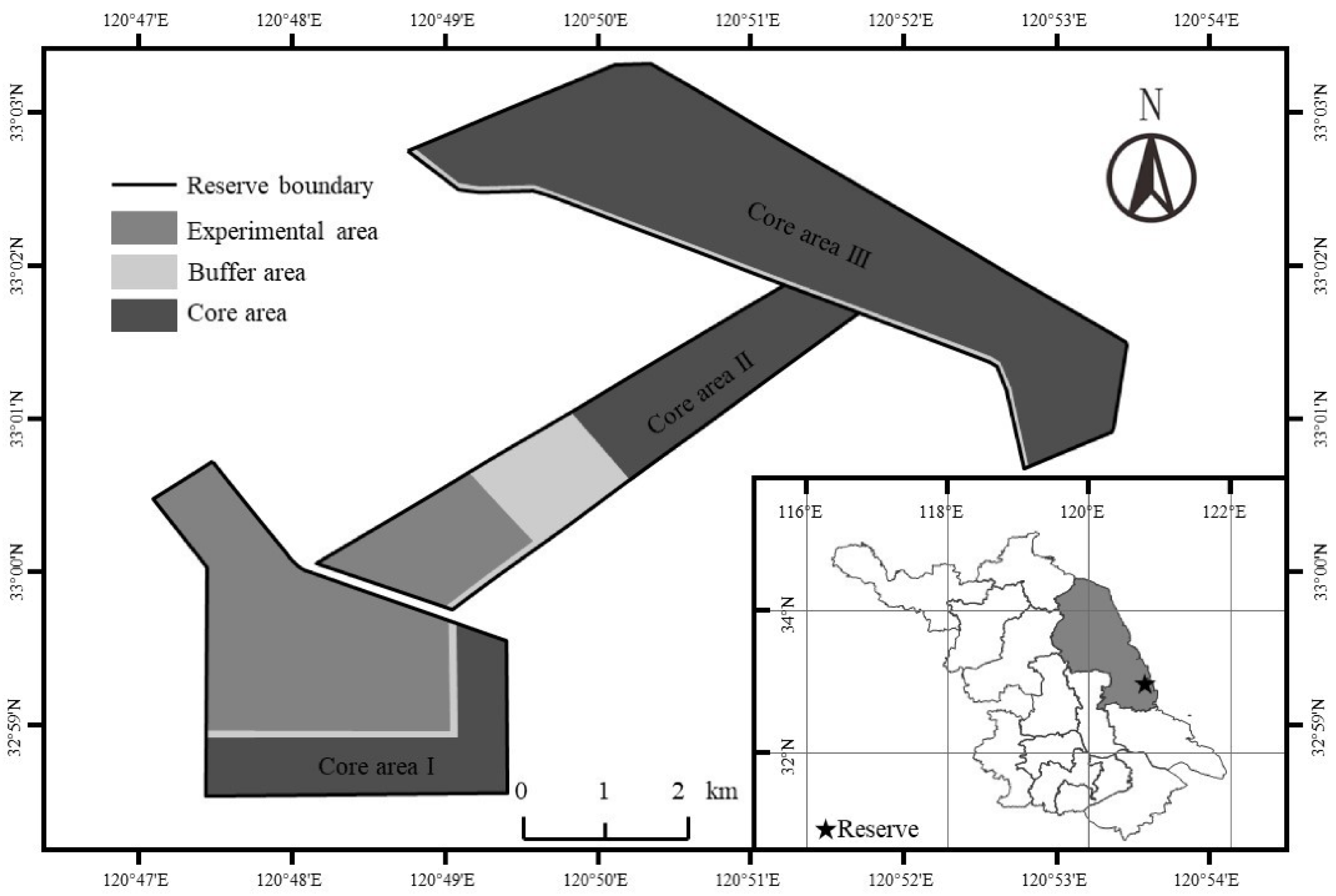
2.1. Study Area
2.2. Experimental Design
2.3. Determination of Soil Factors
2.4. Soil Bacterial Community DNA Extraction, Amplification, and Sequencing
2.5. Data Processing and Analysis
2.5.1. Significance Analysis
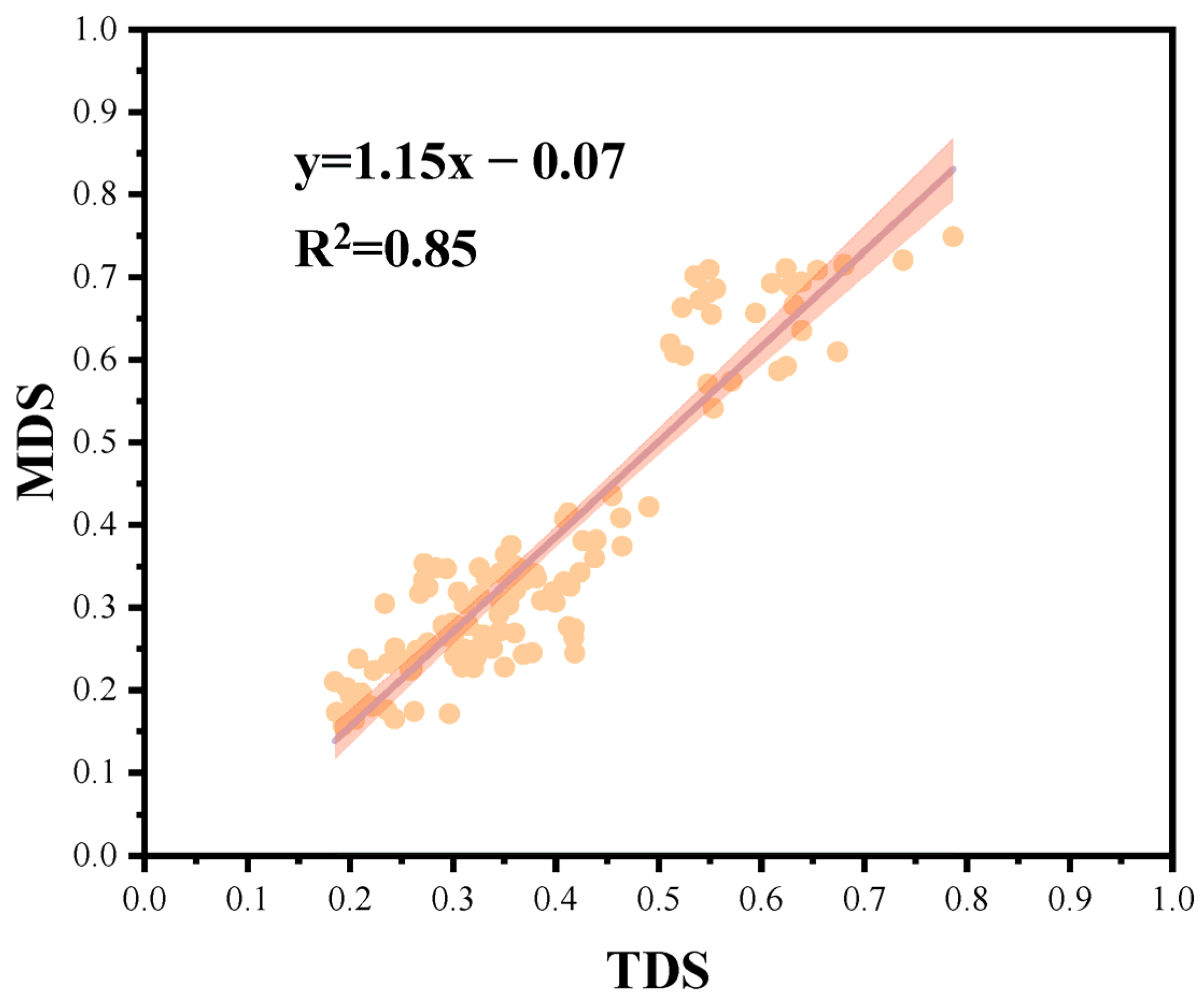
2.5.2. Minimum Data Set Filtering
2.5.3. Dimensionless Data
2.5.4. Indicator Weights
2.5.5. Calculation of the Soil Quality Index
2.6. Analysis of Bacterial Community Data
3. Results
3.1. Soil Properties
3.2. Soil Quality Index
3.2.1. Minimum Data Set and Indicator Weight Construction
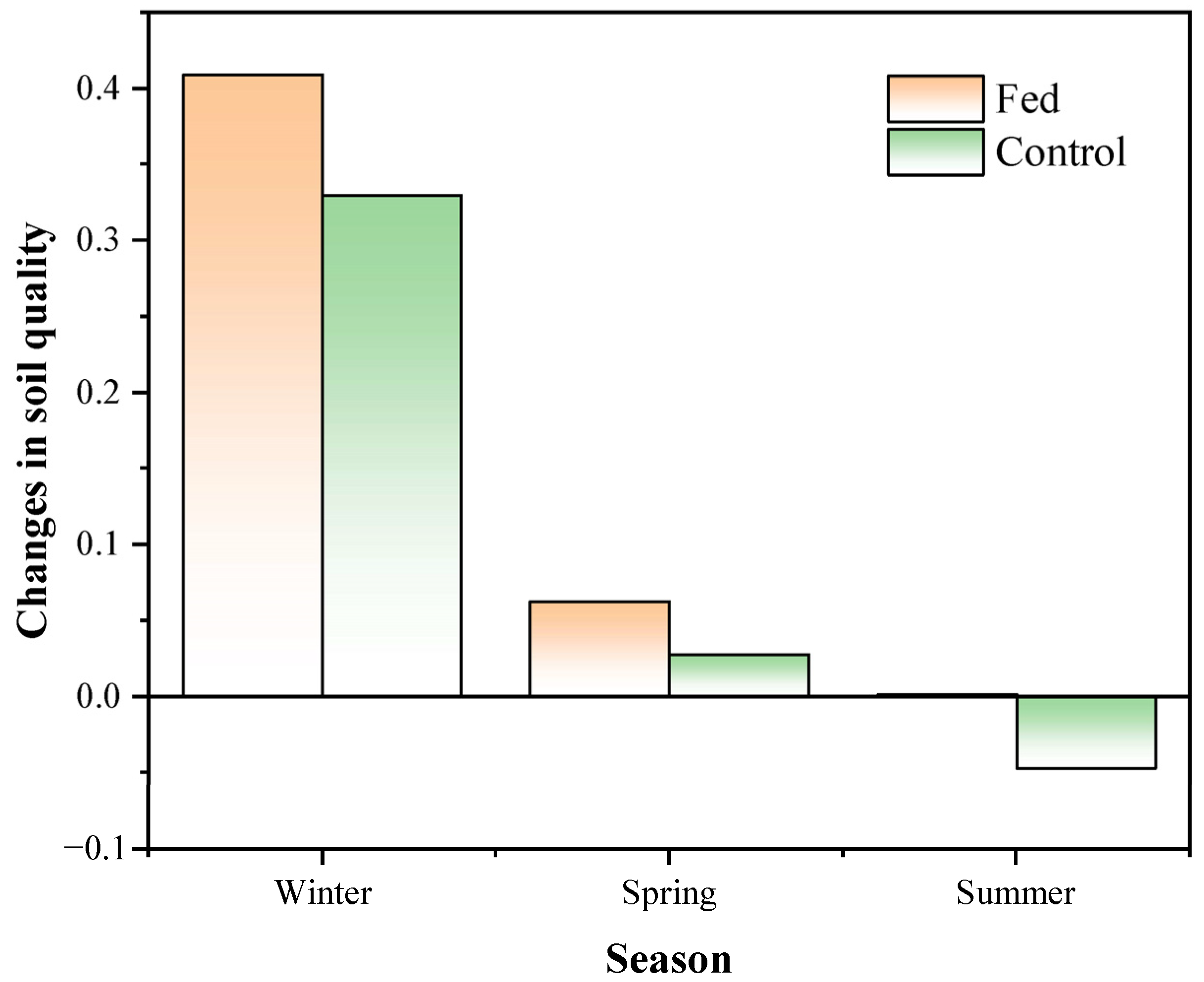
3.2.2. Soil Quality Index in Different Seasons
3.3. Soil Bacterial Community
3.3.1. Bacterial Community Diversity
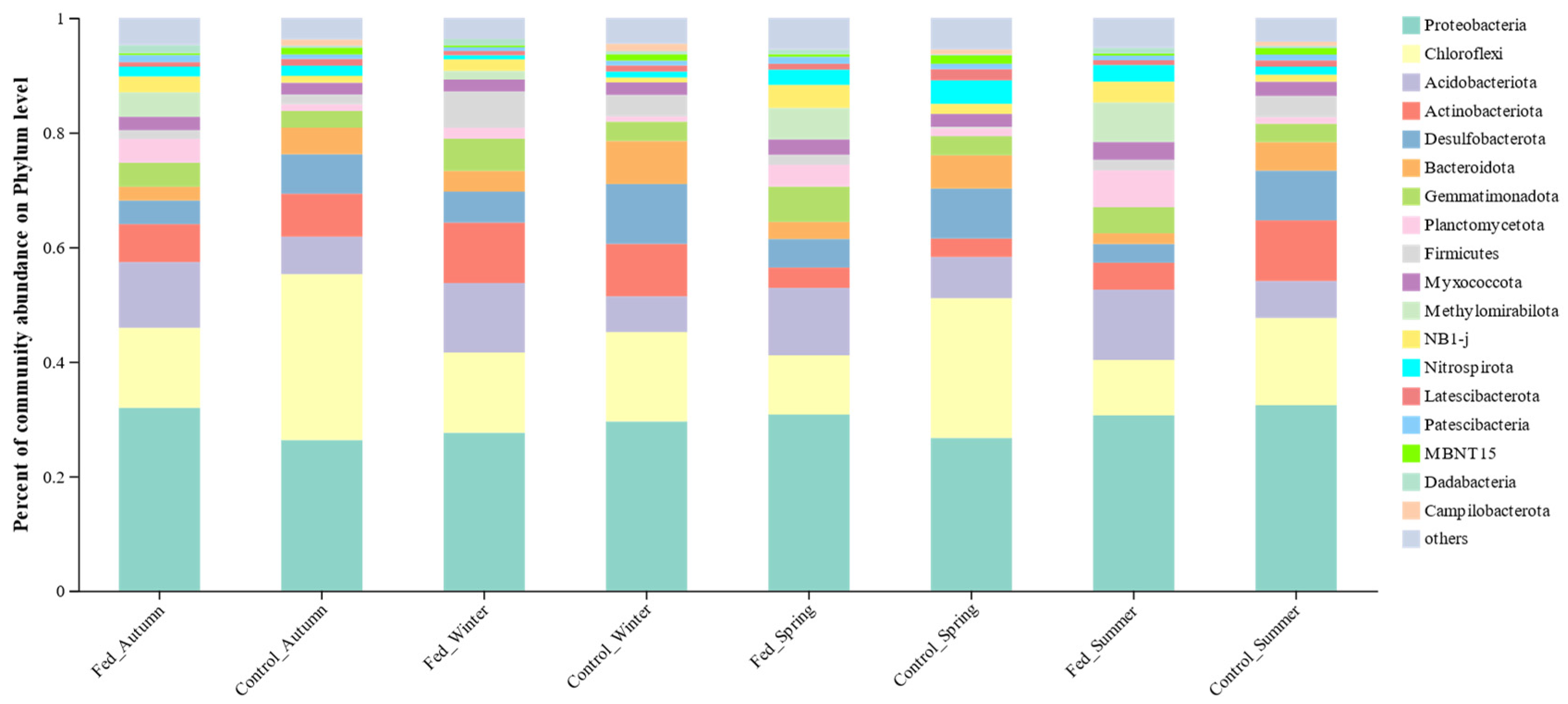
3.3.2. Bacterial Community Structure
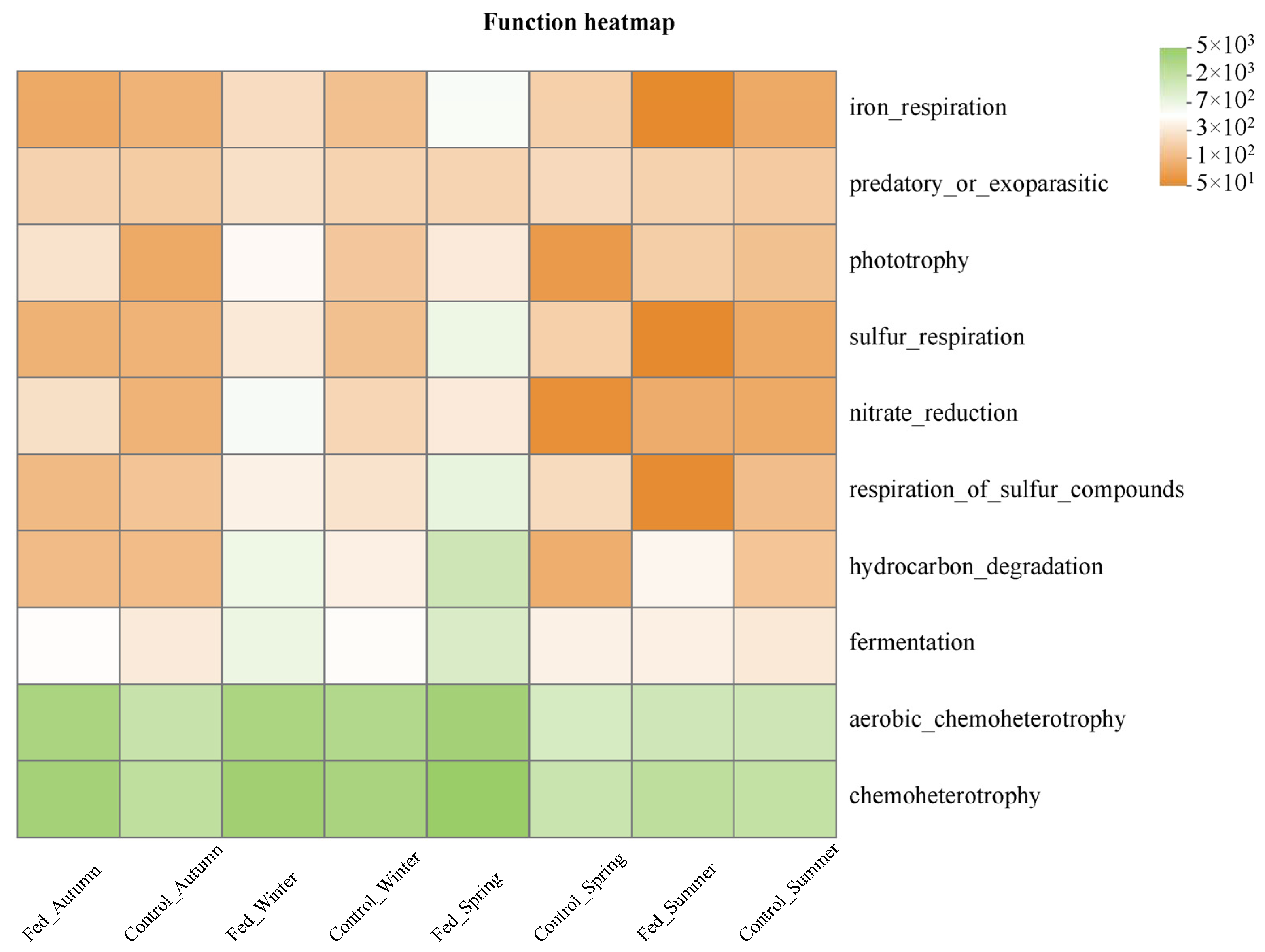
3.3.3. Bacterial Community Function
3.3.4. Bacterial Community Co-Occurring Network
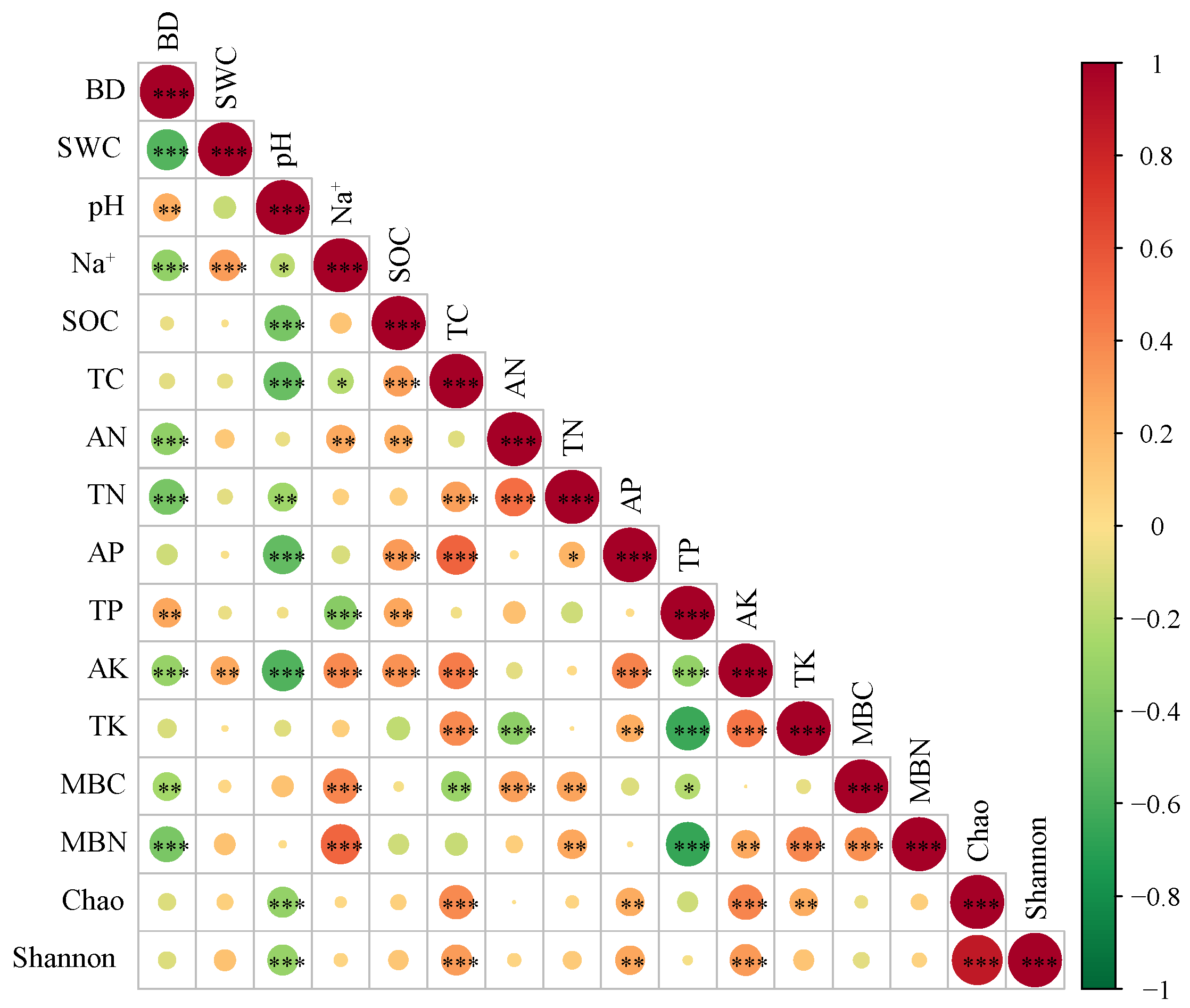
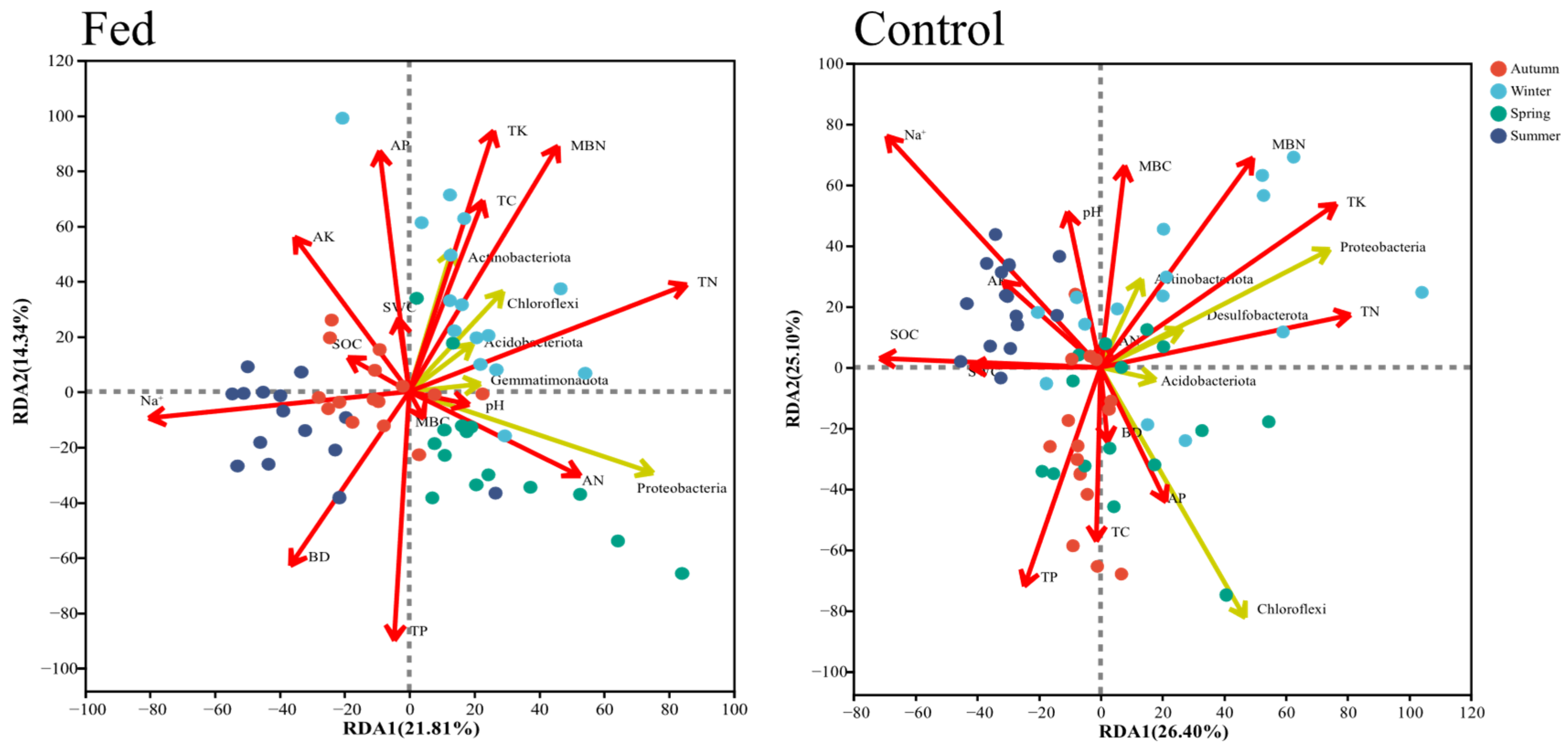
3.4. Soil Property and Bacterial Community
4. Discussion
4.1. The Cluster of Père David’s Deer at Feeding Site Exerts Negative Effects on Soil Properties
4.2. Microbial and Soil Nutrient Indicators as Key Factors in Soil Quality Assessment
4.3. Bacterial Community Analysis Indicates Ongoing Carbon Loss
4.4. Regional Recovery Requires Human Intervention
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.Y.; Li, D.L.; Chen, Y.N.; Zhao, Z.Z. Spatial-Temporal Evolution Monitoring and Ecological Risk Assessment of Coastal Wetlands on Hainan Island, China. Remote Sens. 2023, 15, 1035. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Q.M.; Huang, F.M.; Xue, X.Z.; Zhang, Y. Identifying ecological risk and cost-benefit value for supporting habitat restoration: A case study from Sansha Bay, southeast China. Ecol. Process 2023, 12, 20. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, L.H.; Yang, P.; Tong, C.; Lin, Y.X.; Lai, D.Y.F.; Yang, H.; Tian, Y.L.; Zhu, W.Y.; Tang, K.W. Responses of coastal sediment organic and inorganic carbon to habitat modification across a wide latitudinal range in southeastern China. Catena 2023, 225, 107034. [Google Scholar] [CrossRef]
- Zhang, T.; Song, B.; Han, G.X.; Zhao, H.L.; Hu, Q.L.; Zhao, Y.; Liu, H.J. Effects of coastal wetland reclamation on soil organic carbon, total nitrogen, and total phosphorus in China: A meta-analysis. Land Degrad. Dev. 2023, 34, 3340–3349. [Google Scholar] [CrossRef]
- Margriter, S.C.; Bruland, G.L.; Kudray, G.M.; Lepczyk, C.A. Using indicators of land-use development intensity to assess the condition of coastal wetlands in Hawai’i. Landscape Ecol. 2014, 29, 517–528. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B.; Mondal, P.; Mitra, S.; Sarkar, S.K. Assessment of trace metal contamination level and toxicity in sediments from coastal regions of West Bengal, eastern part of India. Mar. Pollut. Bull. 2015, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Grillo-Avila, D.; Antón-Pardo, M.; Armengol, J.; Puche, E.; Carballeira, R.; Moratalla-López, J.; Palacios-Abella, J.F.; López, I.; Minaglia, M.C.C.; Amador, P.; et al. Effects of the herbicide bentazone on the structure of plankton and benthic communities representative of Mediterranean coastal wetlands: A mesocosm experiment. Hydrobiologia 2025, 852, 2709–2728. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, G.M.; Ye, S.Y.; Li, G.X.; Yuan, H.M.; He, L.; Su, D.P.; Ding, X.G.; Xie, L.J.; Pei, S.F.; et al. Spatial distribution and sources of heavy metals in the sediment and soils of the Yancheng coastal ecosystem and associated ecological risks. Environ. Sci. Pollut. Res. 2023, 30, 18843–18860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Li, C.; Li, Y.P.; Chen, Q.; Hu, D.F.; Cheng, Z.B.; Wang, X.; Shan, Y.F.; Bai, J.D.; Liu, G. Genetic Differentiation of Reintroduced Pere David’s Deer (Elaphurus davidianus) Based on Population Genomics Analysis. Front. Genet. 2021, 12, 705337. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Y.B.; An, Y.T. Effects of David Deer Grazing on Soil Bacterial and Fungal Communities in an Eastern Coastal Wetland of China. Diversity 2022, 14, 976. [Google Scholar] [CrossRef]
- An, Y.T.; Liu, B.; Wang, L.B.; Xie, S.B.; Xue, D.D.; Wu, Y.B. Effects of Père David deer (Elaphurus davidianus) grazing on soil physicochemical properties. Acta Ecol. Sin. 2020, 40, 3571–3578. [Google Scholar]
- Bogunovic, I.; Kljak, K.; Dugan, I.; Grbesa, D.; Telak, L.J.; Duvnjak, M.; Kisic, I.; Solomun, M.K.; Pereira, P. Grassland Management Impact on Soil Degradation and Herbage Nutritional Value in a Temperate Humid Environment. Agriculture 2022, 12, 7–921. [Google Scholar] [CrossRef]
- Liu, L.K.; Zhao, G.J.; An, Z.F.; Mu, X.M.; Jiao, J.Y.; An, S.S.; Tian, P. Effect of grazing intensity on alpine meadow soil quality in the eastern Qinghai-Tibet Plateau, China. Ecol. Indic. 2022, 141, 109111. [Google Scholar] [CrossRef]
- Valani, G.P.; Martini, A.F.; Pezzopane, J.R.M.; Bernardi, A.C.D.C.; Cooper, M. Soil physical quality in the topsoil of integrated and non-integrated grazing systems in a Brazilian Ferralsol. Soil. Tillage Res. 2022, 220, 105357. [Google Scholar] [CrossRef]
- Rezaei, S.A.; Gilkes, R.J.; Andrews, S.S.; Arzani, H. Soil quality assessment in semiarid rangeland in Iran. Soil. Use Manag. 2005, 21, 402–409. [Google Scholar] [CrossRef]
- Samaei, F.; Emami, H.; Lakzian, A. Assessing soil quality of pasture and agriculture land uses in Shandiz county, northwestern Iran. Ecol. Indic. 2022, 139, 108974. [Google Scholar] [CrossRef]
- Fierer, N.; Wood, S.A.; de Mesquita, C.P.B. How Microbes Can, and Cannot, Be Used to Assess Soil Health. Soil. Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Singh, B.K.; Nunan, N.; Millard, P. Response of fungal, bacterial and ureolytic communities to synthetic sheep urine deposition in a grassland soil. FEMS Microbiol. Ecol. 2009, 70, 109–117. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zhang, Q.T.; Zheng, M.M.; Gao, Y.; Yuan, T.; Hale, L.; Van Nostrand, J.D.; Zhou, J.Z.; Wan, S.Q.; Yang, Y.F. Microbial functional traits are sensitive indicators of mild disturbance by lamb grazing. ISME J. 2019, 13, 1370–1373. [Google Scholar] [CrossRef]
- Mahajan, G.; Das, B.; Morajkar, S.; Desai, A.; Murgaokar, D.; Kulkarni, R.; Sale, R.; Patel, K. Soil quality assessment of coastal salt-affected acid soils of India. Environ. Sci. Pollut. Res. 2020, 27, 26221–26238. [Google Scholar] [CrossRef]
- Armenise, E.; Redmile-Gordon, M.A.; Stellacci, A.M.; Ciccarese, A.; Rubino, P. Developing a soil quality index to compare soil fitness for agricultural use under different managements in the Mediterranean environment. Soil. Tillage Res. 2013, 130, 91–98. [Google Scholar] [CrossRef]
- Teng, L.D.; Jiang, G.H.; Ding, Z.L.; Wang, Y.; Liang, T.B.; Zhang, J.Z.; Dai, H.X.; Cao, F.B. Evaluation of tobacco-planting soil quality using multiple distinct scoring methods and soil quality indices. J. Clean. Prod. 2024, 441, 140883. [Google Scholar] [CrossRef]
- Yu, P.J.; Han, D.L.; Liu, S.W.; Wen, X.; Huang, Y.X.; Jia, H.T. Soil quality assessment under different land uses in an alpine grassland. Catena 2018, 171, 280–287. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Nimkar, A.M.; Anantwar, S.G. Soil Quality Index (SQI) as a Tool to Evaluate Crop Productivity in Semi-arid Deccan Plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, F.W.; Chen, L.; Cheng, Y.P. Comprehensive assessment on quality of soil and water resources of Heilonggang area by entropy weights. Bull. Soil. Water Conserv. 2012, 6, 268–272. [Google Scholar]
- Geng, S.B.; Shi, P.L.; Zong, N.; Zhu, W.R. Using Soil Survey Database to Assess Soil Quality in the Heterogeneous Taihang Mountains, North China. Sustainability 2018, 10, 3443. [Google Scholar] [CrossRef]
- Yuan, B.D.; Xie, S.B.; Liu, B.; Xue, D.D.; Sun, D.M. Differential movement pattern of Pere David’s deer associated with the temporal rhythm using GPS collar fix. Glob. Ecol. Conserv. 2019, 18, e00641. [Google Scholar] [CrossRef]
- Zha, J.J.; Wu, Y.B.; An, Y.T. Diet analysis of Père David’s deer (Elaphurus davidianus) based on stable isotope analysis. Wildlife Biol. 2024, 2024, e01136. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, S.Y.; Fu, Y.H.; Li, Y.; Nawaz, S.; Chen, J.; Yang, G.X.; Li, J.K.; Shi, D.L. Isolation of a Virulent Clostridium perfringens Strain from Elaphurus davidianus and Characterization by Whole-Genome Sequence Analysis. Curr. Issues Mol. Biol. 2024, 46, 7169–7186. [Google Scholar] [CrossRef]
- Mo, Q.Y.; Yao, H.Y.; Wu, H.; Zhao, D.P. Impact of Environmental Food Intake on the Gut Microbiota of Endangered Père David’s Deer: Primary Evidence for Population Reintroduction. Animals 2024, 14, 728. [Google Scholar] [CrossRef]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Vaieretti, M.V.; Cingolani, A.M.; Harguindeguy, N.P.; Cabido, M. Effects of differential grazing on decomposition rate and nitrogen availability in a productive mountain grassland. Plant Soil. 2013, 371, 675–691. [Google Scholar] [CrossRef]
- Yu, P.J.; Liu, S.W.; Zhang, L.; Li, Q.; Zhou, D.W. Selecting the minimum data set and quantitative soil quality indexing of alkaline soils under different land uses in northeastern China. Sci. Total Environ. 2018, 616–617, 564–571. [Google Scholar] [CrossRef]
- Ekosse, G.I.E.; Fouche, P.S. Multivariate analysis and spatial distribution of selected cations in soils within the proximity of an abandoned manganese mine, Kgwakgwe, Botswana. Fresen Environ. Bull. 2007, 16, 261–271. [Google Scholar]
- Zhang, M.; Song, R.Q.; Zhang, R.X.; An, X.L.; Chu, G.X. The pattern of soil microbe metabolic limitation was altered by the increased sheep grazing intensity in two contrasting grasslands: Implications for grassland management in semiarid regions. Land Degrad. Dev. 2023, 34, 72–83. [Google Scholar] [CrossRef]
- Ruzek, L.; Ruzková, M.; Vorísek, K.; Kubát, J.; Friedlová, M.; Mikanová, O. Chemical and microbiological characterization of Cambisols, Luvisols and Stagnosols. Plant Soil. Environ. 2009, 55, 231–237. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Fu, G.; Shen, Z.X.; Zhang, X.Z.; Zhou, Y.T. Response of soil microbial biomass to short-term experimental warming in alpine meadow on the Tibetan Plateau. Appl. Soil. Ecol. 2012, 61, 158–160. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil. Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Karkaj, E.S.; Sepehry, A.; Barani, H.; Motamedi, J.; Shahbazi, F. Establishing a Suitable Soil Quality Index for Semi-arid Rangeland Ecosystems in Northwest of Iran. J. Soil. Sci. Plant Nutr. 2019, 19, 648–658. [Google Scholar] [CrossRef]
- Zhao, Y.; Peth, S.; Krümmelbein, J.; Horn, R.; Wang, Z.Y.; Steffens, M.; Hoffmann, C.; Peng, X.H. Spatial variability of soil properties affected by grazing intensity in Inner Mongolia grassland. Ecol. Model. 2007, 205, 241–254. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Han, X.G.; Wang, S.P. The effects of grazing on grassland soils. Acta Ecol. Sin. 2004, 24, 790–797. [Google Scholar]
- Yu, O.T.; Chmura, G.L. Soil carbon may be maintained under grazing in a St Lawrence Estuary tidal marsh. Environ. Conserv. 2009, 36, 312–320. [Google Scholar] [CrossRef]
- Li, J.W.; Wang, K.B.; Shangguan, Z.P.; Deng, L. Coupling and decoupling of soil carbon, nitrogen and phosphorus stocks following grazing exclusion in temperate grasslands. Catena 2022, 211, 106003. [Google Scholar] [CrossRef]
- Lu, Q.; Ma, H.B.; Zhou, Y.; Liu, J.D.; Shen, Y. Restoration of soil quality of degraded grassland can be accelerated by reseeding in an arid area of Northwest China. Front. Plant Sci. 2023, 14, 1101295. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Hei, J.; Wang, W.Q.; Sardans, J.; Zhang, Z.H.; Zeng, F.J.; Ge, M.Q.; Liao, Y.Y.; Fang, Y.Y.; et al. Impacts of Spartina alterniflora invasion on soil carbon components of particulate and mineral-associated organic matter and soil organic matter mineralization in estuarine wetlands. Appl. Soil. Ecol. 2025, 206, 105857. [Google Scholar] [CrossRef]
- Qin, W.F.; Zhao, X.C.; Yang, F.; Chen, J.H.; Mo, Q.S.; Cui, S.; Chen, C.; He, S.J.; Li, Z. Impact of fertilization and grazing on soil N and enzyme activities in a karst pasture ecosystem. Geoderma 2023, 437, 116578. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V. Assessing the role of interseeding alfalfa into grass on improving pasture soil health in semi-arid Texas High Plains. Appl. Soil. Ecol. 2020, 147, 103399. [Google Scholar] [CrossRef]
- Oduor, C.O.; Karanja, N.K.; Onwonga, R.N.; Mureithi, S.M.; Pelster, D.; Nyberg, G. Enhancing soil organic carbon, particulate organic carbon and microbial biomass in semi-arid rangeland using pasture enclosures. BMC Ecol. 2018, 18, 45. [Google Scholar] [CrossRef]
- Mao, L.; Tang, L.L.; Ye, S.M.; Wang, S.Q. Soil organic C and total N as well as microbial biomass C and N affect aggregate stability in a chronosequence of Chinese fir plantations. Eur. J. Soil. Biol. 2021, 106, 103347. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.S.; Xin, Y. Changes in and evaluation of surface soil quality in Populus × xiaohei shelterbelts in midwestern Heilongjiang province, China. J. For. Res. 2021, 32, 1221–1233. [Google Scholar] [CrossRef]
- Xie, M.Z.; Zhao, L.; Wu, X.D.; Tian, L.M.; Yue, G.Y.; Zhou, H.Y.; Wu, Z.M. Seasonal variations of nitrogen in permafrost-affected soils of the Qinghai-Tibetan Plateau. Catena 2020, 195, 104793. [Google Scholar] [CrossRef]
- Li, H.T.; Wang, L.; Peng, Y.; Zhang, S.W.; Lv, S.Q.; Li, J.; Abdo, A.I.; Zhou, C.J.; Wang, L.Q. Film mulching, residue retention and N fertilization affect ammonia volatilization through soil labile N and C pools. Agr. Ecosyst. Environ. 2021, 308, 107272. [Google Scholar] [CrossRef]
- Yin, S.; Wang, C.K.; Abalos, D.; Guo, Y.; Pang, X.S.; Tan, C.Q.; Zhou, Z.H. Seasonal response of soil microbial community structure and life history strategies to winter snow cover change in a temperate forest. Sci. Total Environ. 2024, 949, 175066. [Google Scholar] [CrossRef] [PubMed]
- Isabel, P.C.M.; Francisco, G.S.; Consolación, W.B.; Antonio, G.M.F.; Ramón, L.S.F.; Eva, R.; Luis, M.O.J.; Manuela, A.A. Application of Soil Multiparametric Indices to Assess Impacts of Grazing in Mediterranean Forests. Land 2024, 13, 411. [Google Scholar] [CrossRef]
- Yoshitake, S.; Soutome, H.; Koizumi, H. Deposition and decomposition of cattle dung and its impact on soil properties and plant growth in a cool temperate pasture. Ecol. Res. 2014, 29, 673–684. [Google Scholar] [CrossRef]
- Mozdzer, T.J.; Kirwan, M.; McGlathery, K.J.; Zieman, J.C. Nitrogen uptake by the shoots of smooth cordgrass Spartina alterniflora. Mar. Ecol-Prog. Ser. 2011, 433, 42–52. [Google Scholar] [CrossRef]
- Lin, X.; Yang, Y.L.; Yang, P.; Hong, Y.; Zhang, L.H.; Tong, C.; Lai, D.Y.F.; Lin, Y.X.; Tan, L.S.; Tian, Y.L.; et al. Soil organic nitrogen content and composition in different wetland habitat types along the south-east coast of China. Catena 2023, 232, 107457. [Google Scholar] [CrossRef]
- Fischer, M.; Wipf, S. Effect of low-intensity grazing on the species-rich vegetation of traditionally mown subalpine meadows. Biol. Conserv. 2002, 104, 1–11. [Google Scholar] [CrossRef]
- Jordan, S.E.; Palmquist, K.A.; Burke, I.C.; Lauenroth, W.K. Small effects of livestock grazing intensification on diversity, abundance, and composition in a dryland plant community. Ecol. Appl. 2022, 32, e2693. [Google Scholar] [CrossRef]
- Cui, H.W.; Liu, Z.Y.; Chen, J.W.; Wang, J.J.; Song, H.X.; Gao, H.N.; Chen, S.Y.; Wang, Y.J.; Liu, K.; Xiao, S.; et al. Grazing induces positive direct effect of shrubs on nematode diversity but suppresses indirect effects through microbial pathways. Plant Soil. 2024, 500, 681–695. [Google Scholar] [CrossRef]
- Olivera, N.L.; Prieto, L.; Bertiller, M.B.; Ferrero, M.A. Sheep grazing and soil bacterial diversity in shrublands of the Patagonian Monte, Argentina. J. Arid. Environ. 2016, 125, 16–20. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Z.; Lin, C.; Guo, B.L.; Yu, J.H.; Ding, H.; Peng, S.Y.; Sveen, T.R.; Zhang, Y.S. Effects of re-vegetation restoration on soil bacterial community structure in degraded land in subtropical China. Eur. J. Soil. Biol. 2020, 98, 103184. [Google Scholar] [CrossRef]
- Li, T.F.; Wang, Y.Y.; Kamran, M.; Chen, X.Y.; Tan, H.; Long, M.X. Effects of Grass Inter-Planting on Soil Nutrients, Enzyme Activity, and Bacterial Community Diversity in an Apple Orchard. Front. Plant Sci. 2022, 13, 901143. [Google Scholar] [CrossRef]
- Muench, A.; Elsey-Quirk, T. Competitive reversal between plant species is driven by species-specific tolerance to flooding stress and nutrient acquisition during early marsh succession. J. Appl. Ecol. 2019, 56, 2115–2247. [Google Scholar] [CrossRef]
- Dong, H.Y.; Tian, Y.L.; Lu, J.J.; Zhao, J.; Tong, Y.B.; Niu, J.F. Bioaugmented biological contact oxidation reactor for treating simulated textile dyeing wastewater. Bioresour. Technol. 2024, 404, 130916. [Google Scholar] [CrossRef]
- Li, J.Q.; Jiang, M.K.; Pei, J.M.; Fang, C.M.; Li, B.; Nie, M. Convergence of carbon sink magnitude and water table depth in global wetlands. Ecol. Lett. 2023, 26, 797–804. [Google Scholar] [CrossRef]
- Shang, Z.H.; Cao, J.J.; Guo, R.Y.; Henkin, Z.; Ding, L.M.; Long, R.J.; Deng, B. Effect of Enclosure on Soil Carbon, Nitrogen and Phosphorus of Alpine Desert Rangeland. Land Degrad. Dev. 2017, 28, 1166–1177. [Google Scholar] [CrossRef]
- Li, Y.R.; Hu, S.J.; Lang, S.X.; Pu, Y.L.; Zhang, S.R.; Li, T.; Xu, X.X.; Jia, Y.X.; Wang, G.Y.; Yuan, D.G.; et al. Soil quality and ecological benefits assessment of alpine desertified grassland following different ecological restoration measures. Front. Plant Sci. 2023, 14, 1283457. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.C.; Kang, Y.K.; Zhang, Z.M.; Bao, D.H.; Sun, X.M.; Su, J.H. Assessing the win-win situation of forage production and soil organic carbon through a short-term active restoration strategy in alpine grasslands. Front. Plant Sci. 2024, 14, 1290808. [Google Scholar] [CrossRef]
- Hu, Y.G.; Liu, W.J.; Chang, J.C.; Fan, Y.X.; Hou, S.P.; Zhang, Z.H.; Su, X.; Bahram, M.; Wang, S.P. Grazing exclusion-induced alterations of soil microbial biogeographic pattern and co-occurrence network across a Tibetan elevation gradient. Agr. Ecosyst. Environ. 2024, 376, 109231. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Song, Z.L.; Wang, J.; Guo, L. Interactions of soil bacteria and fungi with plants during long-term grazing exclusion in semiarid grasslands. Soil. Biol. Biochem. 2018, 124, 47–58. [Google Scholar] [CrossRef]
- Li, Y.; Kang, E.Z.; Song, B.; Wang, J.S.; Zhang, X.D.; Wang, J.Z.; Li, M.; Yan, L.; Yan, Z.Q.; Zhang, K.R.; et al. Soil salinity and nutrients availability drive patterns in bacterial community and diversity along succession gradient in the Yellow River Delta. Estuar. Coast. Shelf Sci. 2021, 262, 107621. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, E.; Yang, X.D.; Yang, J.J. Shifts in Microbial Community Structure and Co-occurrence Network along a Wide Soil Salinity Gradient. Microorganisms 2024, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Rath, K.M.; Murphy, D.N.; Rousk, J. The microbial community size, structure, and process rates along natural gradients of soil salinity. Soil. Biol. Biochem. 2019, 138, 107607. [Google Scholar] [CrossRef]


| Sampling Sites | Hoofprint Density/ pcs/m2 | Fecal Legacy/ Pellets/m2 | Vegetation Cover Rate/% | Dominant Plant |
|---|---|---|---|---|
| Fed | 67~89 | 99~109 | 0 | -- |
| Control | 3~10 | 0 | 80~100 | Spartina alterniflora |
| Autumn | Winter | Spring | Summer | |||||
|---|---|---|---|---|---|---|---|---|
| Fed | Control | Fed | Control | Fed | Control | Fed | Control | |
| BD/g∙cm−3 | 1.70 ± 0.10 a | 1.64 ± 0.15 a | 1.37 ± 0.12 a | 1.42 ± 0.27 a | 1.63 ± 0.15 a | 1.49 ± 0.20 b | 1.58 ± 0.09 A | 1.45 ± 0.10 B |
| SWC | 0.14 ± 0.03 a | 0.15 ± 0.05 a | 0.21 ± 0.13 a | 0.13 ± 0.04 b | 0.12 ± 0.03 a | 0.15 ± 0.05 a | 0.18 ± 0.06 a | 0.20 ± 0.01 a |
| pH | 9.29 ± 0.12 A | 8.82 ± 0.11 B | 9.08 ± 0.15 A | 8.82 ± 0.10 B | 9.21 ± 0.17 A | 8.83 ± 0.07 B | 9.00 ± 0.16 a | 8.89 ± 0.09 b |
| Na+/g∙kg−1 | 0.27 ± 0.14 a | 0.29 ± 0.03 a | 0.55 ± 0.13 a | 0.58 ± 0.05 a | 0.32 ± 0.03 a | 0.31 ± 0.15 a | 1.38 ± 0.27 a | 1.40 ± 0.10 a |
| SOC/g∙kg−1 | 2.21 ± 0.76 B | 7.16 ± 2.72 A | 3.23 ± 4.18 a | 5.28 ± 5.31 a | 3.55 ± 0.76 B | 5.68 ± 1.37 A | 4.51 ± 3.63 B | 10.01 ± 3.80 A |
| TC/g∙kg−1 | 14.06 ± 2.89 B | 50.63 ± 18.60 A | 17.20 ± 6.16 a | 20.05 ± 3.68 a | 12.99 ± 0.69 B | 18.85 ± 4.55 A | 12.56 ± 0.69 B | 14.62 ± 1.05 A |
| AN/mg∙kg−1 | 26.27 ± 16.61 B | 43.98 ± 8.74 A | 81.81 ± 35.71 a | 65.38 ± 16.59 a | 114.48 ± 67.38 a | 155.76 ± 84.50 a | 72.46 ± 24.37 b | 93.37 ± 24.03 a |
| TN/g∙kg−1 | 0.21 ± 0.03 B | 0.70 ± 0.26 A | 2.53 ± 0.68 a | 2.72 ± 0.39 a | 2.19 ± 0.11 B | 2.59 ± 0.36 A | 0.81 ± 0.13 B | 1.06 ± 0.18 A |
| AP/mg∙kg−1 | 5.79 ± 1.94 B | 16.38 ± 6.10 A | 13.18 ± 14.03 a | 13.00 ± 4.36 a | 5.23 ± 0.91 B | 10.88 ± 4.94 A | 6.08 ± 2.24 a | 8.96 ± 5.61 a |
| TP/g∙kg−1 | 1.33 ± 0.37 B | 1.85 ± 0.27 A | 0.17 ± 0.14 B | 0.46 ± 0.22 A | 2.17 ± 0.50 a | 2.15 ± 0.65 a | 1.42 ± 0.24 a | 1.52 ± 0.26 a |
| AK/mg∙kg−1 | 284.28 ± 40.65 B | 448.23 ± 52.91 A | 391.87 ± 63.30 a | 455.93 ± 106.77 a | 213.16 ± 21.94 B | 305.65 ± 51.85 A | 362.90 ± 56.00 B | 502.90 ± 137.22 A |
| TK/g∙kg−1 | 7.36 ± 0.61 b | 7.81 ± 0.28 a | 14.55 ± 4.76 a | 16.23 ± 8.05 a | 2.94 ± 0.71 a | 3.10 ± 0.26 a | 4.70 ± 0.46 a | 5.04 ± 0.57 a |
| MBC/mg∙kg−1 | 26.07 ± 10.66 A | 10.16 ± 3.05 B | 39.17 ± 14.32 a | 40.99 ± 12.50 a | 37.75 ± 17.04 a | 36.27 ± 24.62 a | 40.83 ± 19.51 a | 41.47 ± 10.99 a |
| MBN/mg∙kg−1 | 9.20 ± 3.95 A | 1.23 ± 0.45 B | 162.32 ± 79.74 A | 65.64 ± 19.51 B | 6.94 ± 3.90 a | 5.06 ± 4.25 a | 17.12 ± 7.82 a | 16.71 ± 5.42 a |
| Group | Norm | Factors | Weight | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | TDS | MDS | |||
| AK | 1 | 1.53 | 0.724 | 0.281 | 0.062 | −0.294 | 0.28 | 0.118 | 0.02 | 0.04 |
| TP | 1 | 1.51 | −0.571 | 0.545 | 0.283 | 0.315 | 0.027 | −0.165 | 0.08 | 0.13 |
| TK | 1 | 1.46 | 0.602 | −0.347 | −0.488 | −0.048 | −0.082 | 0.23 | 0.08 | |
| BD | 1 | 1.39 | −0.586 | 0.347 | −0.323 | −0.118 | 0.063 | 0.4 | 0.004 | 0.01 |
| pH | 1 | 1.38 | −0.598 | −0.448 | −0.128 | −0.16 | −0.181 | 0.018 | 0.0001 | |
| AP | 1 | 1.24 | 0.534 | 0.195 | −0.256 | 0.335 | 0.261 | −0.103 | 0.08 | |
| MBN | 2 | 1.49 | 0.503 | −0.682 | −0.201 | 0.024 | 0.113 | −0.099 | 0.27 | 0.45 |
| TC | 2 | 1.35 | 0.281 | 0.596 | −0.489 | 0.176 | 0.162 | −0.21 | 0.04 | 0.06 |
| Na+ | 3 | 1.27 | 0.239 | 0.012 | 0.643 | −0.51 | 0.309 | 0.137 | 0.05 | 0.08 |
| MBC | 3 | 1.13 | 0.111 | −0.468 | 0.484 | 0.042 | 0.083 | 0.412 | 0.06 | |
| TN | 4 | 1.36 | 0.378 | −0.417 | 0.048 | 0.717 | −0.052 | 0.085 | 0.10 | 0.16 |
| AN | 4 | 1.16 | −0.051 | −0.043 | 0.541 | 0.664 | −0.114 | 0.008 | 0.08 | |
| Chao | 5 | 1.46 | 0.558 | 0.344 | 0.104 | −0.127 | −0.669 | 0.174 | 0.02 | 0.03 |
| Shannon | 5 | 1.43 | 0.473 | 0.47 | 0.169 | −0.019 | −0.64 | 0.098 | 0.0009 | |
| SOC | 5 | 1.25 | 0.338 | 0.467 | 0.236 | 0.171 | 0.508 | 0.217 | 0.10 | |
| SWC | 6 | 1.18 | 0.316 | −0.137 | 0.331 | −0.26 | −0.05 | −0.75 | 0.03 | 0.04 |
| Characteristic value | 3.496 | 2.648 | 1.935 | 1.7 | 1.466 | 1.164 | ||||
| Variance contribution rate | 21.85 | 16.551 | 12.095 | 10.626 | 9.161 | 7.272 | ||||
| Cumulative variance contribution rate/% | 21.85 | 38.401 | 50.496 | 61.122 | 70.283 | 77.555 | ||||
| Season | Fed | Control |
|---|---|---|
| Autumn | 0.23 ± 0.04 b | 0.32 ± 0.03 a |
| Winter | 0.64 ± 0.15 a | 0.65 ± 0.04 a |
| Spring | 0.29 ± 0.02 b | 0.35 ± 0.05 a |
| Summer | 0.23 ± 0.06 a | 0.27 ± 0.06 a |
| Seasons | Sample | Chao | Shannon | AVD |
|---|---|---|---|---|
| Autumn | Fed | 3888.07 ± 834.01a | 6.21 ± 0.37 b | 0.698 |
| Control | 4465.60 ± 831.85a | 6.49 ± 0.24 a | 0.749 | |
| Winter | Fed | 3672.31 ± 745.04 A | 6.08 ± 0.43 A | 0.720 |
| Control | 5074.16 ± 729.06 B | 6.64 ± 0.20 B | 0.724 | |
| Spring | Fed | 2656.05 ± 905.97 B | 6.04 ± 0.37 B | 0.702 |
| Control | 4297.01 ± 716.76 A | 6.42 ± 0.30 A | 0.720 | |
| Summer | Fed | 3618.52 ± 523.55 B | 6.13 ± 0.30 B | 0.701 |
| Control | 4713.66 ± 651.70 A | 6.63 ± 0.25 A | 0.761 |
| Samples | Seasons | Average Degree | Average Clustering Coefficient | Modularity Index | Average Path Length | Nodes | Edges | Positive Edges/% | Negative Edges/% |
|---|---|---|---|---|---|---|---|---|---|
| Fed | Autumn | 62.133 | 0.509 | 0.238 | 1.912 | 300 | 9320 | 62.3 | 37.7 |
| Winter | 180.221 | 0.813 | 0.104 | 1.414 | 299 | 26,943 | 54.31 | 45.69 | |
| Spring | 58.093 | 0.516 | 0.286 | 1.951 | 300 | 8714 | 64.48 | 35.52 | |
| Summer | 53.34 | 0.485 | 0.238 | 1.986 | 300 | 8001 | 63.09 | 36.91 | |
| Control | Autumn | 64.727 | 0.522 | 0.268 | 1.854 | 300 | 9709 | 59.39 | 40.61 |
| Winter | 56.433 | 0.511 | 0.285 | 1.931 | 300 | 8465 | 75.33 | 24.67 | |
| Spring | 56.613 | 0.491 | 0.298 | 1.907 | 300 | 8492 | 63.05 | 36.95 | |
| Summer | 50.28 | 0.471 | 0.284 | 1.972 | 300 | 7542 | 67.71 | 32.29 |
| Soil Property Indicators | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BD | SWC | pH | Na+ | SOC | TC | AN | TN | AP | TP | AK | TK | MBC | MBN | |
| VIF | 2.55 | 3.27 | 1.617 | 4.057 | 1.82 | 3.18 | 2.20 | 4.31 | 1.56 | 4.537 | 2.06 | 2.06 | 1.97 | 5.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; An, Y.; Wang, L.; Xue, J.; Naka, K.; Wu, Y. Assessing Ecological Restoration of Père David’s Deer Habitat Using Soil Quality Index and Bacterial Community Structure. Diversity 2025, 17, 594. https://doi.org/10.3390/d17090594
Zhu Y, An Y, Wang L, Xue J, Naka K, Wu Y. Assessing Ecological Restoration of Père David’s Deer Habitat Using Soil Quality Index and Bacterial Community Structure. Diversity. 2025; 17(9):594. https://doi.org/10.3390/d17090594
Chicago/Turabian StyleZhu, Yi, Yuting An, Libo Wang, Jianhui Xue, Kozma Naka, and Yongbo Wu. 2025. "Assessing Ecological Restoration of Père David’s Deer Habitat Using Soil Quality Index and Bacterial Community Structure" Diversity 17, no. 9: 594. https://doi.org/10.3390/d17090594
APA StyleZhu, Y., An, Y., Wang, L., Xue, J., Naka, K., & Wu, Y. (2025). Assessing Ecological Restoration of Père David’s Deer Habitat Using Soil Quality Index and Bacterial Community Structure. Diversity, 17(9), 594. https://doi.org/10.3390/d17090594






