Abstract
Crops of cocoa, avocado, cassava, yam, and maize are of utmost importance to the economy of the Colombian Caribbean, as they serve as the primary source of income for many families. However, establishing these crops requires the replacement of natural ecosystems, with limited understanding of how these areas contribute to biodiversity conservation. This study analyzed the diversity of dung beetles in both transitory and permanent crops within a landscape in San Jacinto, Bolívar, to assess their contribution to the conservation of diversity within this insect group. Dung beetle communities were sampled in permanent crops of avocado and cocoa, transitory crops (cassava, yam, and maize), and a forest fragment. The forest fragment exhibited high levels of species richness, abundance, and diversity regardless of the sampling period; these values were only matched by those of the permanent cocoa crop, and only during the rainy season. Our findings highlight the necessity of preserving forest fragments for biodiversity conservation, while also indicating that certain permanent crops may contribute to this effort.
1. Introduction
Uncontrolled deforestation of forest areas for agricultural and livestock expansion has been one of the main drivers of tropical ecosystem transformation, altering landscape structure, causing the loss of plant and animal species, accelerating the decline of certain species populations, and generating changes in the composition of biotic communities [1,2]. Despite these impacts, agriculture and biodiversity are clearly intertwined, which makes it possible to find alternatives that balance agricultural production with biological conservation, such as agroecological practices [3,4,5].
Part of this harmony between agriculture and conservation has been demonstrated through certain permanent crops, such as cocoa, which offer hope for biodiversity conservation. Due to their high vegetative cover and complex structure, they provide significant benefits to biological diversity [6,7,8]. These benefits range from stable microclimates to corridors for wildlife in fragmented landscapes [8,9,10]. The advantages offered by permanent crops contrast with those of intensive, non-permanent agricultural systems such as maize, wheat, rice, sorghum, cassava, and yam, which directly impact biodiversity, as their production requires the total removal of vegetative cover, thereby reducing the number of native animal and plant species in the planting area [1,11,12,13].
According to data from the FAO (Food and Agriculture Organization of the United Nations) (FAOSTAT data on food and agriculture), approximately 26,287,932 hectares in Colombia were dedicated to agricultural production between 2017 and 2022. In this area, focusing solely on the most common and extensive crops, it is observed that 40% of the crops are transitory (rice 14.2%, sugarcane 10.2%, maize 8.9%, potato 3.0%, plantain 2.2%, and cassava 2.2%), while 35% corresponds to permanent crops (coffee 19.6%, oil palm 11.0%, avocado 1.7%, and cocoa 3.4%) [14]. This indicates similarities in the land area occupied by each crop type; however, the distribution of these areas across Colombian territory depends on the regional climate. For instance, in the Colombian Caribbean, where dry conditions prevail for much of the year, agriculture relies primarily on transitory crops (maize, yam, cassava, sorghum, banana, and chili) and, to a lesser extent, on permanent crops (cocoa and avocado) [15,16,17,18]. This scenario entails greater vegetation clearance for establishing transitory crops.
Given the above, it is necessary to address research questions that provide insight into the roles of transitory and permanent crops in the conservation of biological diversity. This is particularly relevant in areas of the Colombian Caribbean, such as the Montes de María, one of the ecoregions where little is known about Scarabaeinae diversity and where the expansion of agriculture is replacing natural ecosystems like tropical dry forest [19,20,21].
To address these types of questions, it is essential to use biological indicators such as dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). This group of insects constitutes a well-defined guild with distinct morphological, functional, ecological, and behavioral traits that identify them as indicators of environmental disturbances. Additionally, species within this group play a significant role in providing ecosystem services to the soil, such as nutrient recycling, soil aeration, fertilization, and the control of larvae and eggs of parasitic organisms [22,23,24,25,26,27,28].
An approach to studying beetles in permanent and temporary crops in Colombia has shown that plantations of borojo (Borojoa patinoi Cuatrecasas) and coffee (Coffea arabica L.) can host a beetle diversity comparable to that of forests [29,30]. Moreover, these plantations act as biological corridors, connecting different landscape elements and facilitating beetle movement between them [31]. By contrast, low diversity values have been recorded in areas with temporary crops such as maize (Zea mays L.), yam (Dioscorea esculenta (Lour.) Burkill), cassava (Manihot esculenta Crantz), and chili pepper (Capsicum annuum L.) in the Colombian Caribbean, a situation exacerbated during the dry season [11,12].
These studies have demonstrated how both transitory and permanent crops can independently contribute to the conservation of beetles within a landscape. However, none have conducted a simultaneous analysis to determine the combined contribution of both crop types to the conservation of this insect group’s diversity.
Considering the above, this document analyzed the diversity of dung beetles (Scarabaeidae: Scarabaeinae) in permanent and transitory crops in the La Flecha reserve, San Jacinto-Bolívar, to contribute to understanding the role of agricultural systems in the maintenance and conservation of beetle diversity. The hypothesis proposed for this study was that the forest fragment, by providing stable environmental conditions and resources necessary for a wide range of beetle species, would exhibit high diversity values. Similarly, permanent crops, which share environmental characteristics with forest fragments, such as vegetation cover, could sustain a comparable beetle diversity. However, this diversity would be limited to favorable periods, such as the rainy season.
Finally, this study aims to provide key information for decision making in ecosystem management, specifically regarding the landscape elements that can contribute to the conservation of biological diversity and its ecosystem functions.
2. Materials and Methods
2.1. Study Area
This study was conducted in the Montes de María ecoregion, which is characterized by extensive areas of well-preserved tropical dry forest, some of which are included within the national park protection system [32,33]. A constant threat to this ecoregion is the reduction in forest areas due to the expansion of agricultural and livestock frontiers in the region. These activities primarily affect areas not included within protected systems.
The fieldwork was conducted in the villages of La Flecha and El Rastro, within the jurisdiction of the municipality of San Jacinto-Bolívar. The village of La Flecha is located at coordinates 09°50′40″ N and 75°10′53″ W, at an altitude between 300 and 500 m, while the village of El Rastro is situated at coordinates 9°52′15.1″ N and 75°09′58.1″ W, at an altitude between 310 and 450 m (Figure A1). This area is part of the climatic subunit D2, according to the classification proposed by Rangel-Ch and Carvajal-Cogollo [34], with an average precipitation of 1972 mm and a bimodal, four-season rainfall pattern characterized by an intense dry season from December to March and a shorter dry spell between June and July. The average annual temperatures are 35 °C during the dry season and 24 °C during the rainy season, with the relative humidity ranging from 42% in the dry season to 93% during the rainy season. In the dry season, there is a deficit of available water in the region, and many water sources, such as streams, dry up, leaving small water pools in the deep areas of streams that act as reservoirs [35,36,37,38,39].
The area corresponds to a dry forest landscape, where forest fragments are interspersed within low-intensity livestock and agricultural matrices aimed toward economic production on a small scale. Agricultural areas are composed of traditional permanent crops of avocado (Persea americana Mill.), bitter palm (Sabal mauritiiformis (H. Karst.) Griseb. & H. Wendl.), and cocoa (Theobroma cacao L.). Transitory crops consist of polycultures of maize (Zea mays L.), yam (Dioscorea esculenta (Lour.) Burkill), cassava (Manihot esculenta Crantz), and chili pepper (Capsicum annuum L.) established in deforested areas for this purpose and without any associated vegetation to provide shade or canopy cover [35,39]. Neither the transitory nor the permanent crops are managed for biological conservation, and all are located on small plots (two to three hectares) owned by farmers whose economic livelihood depends on these harvests.
The forest area corresponds to a fragment of tropical dry forest with an area of 149 hectares, located in the village of La Flecha, where the predominant plant species are Aspidosperma polyneuron Müll. Arg, Lecythis sp., Bursera simaruba (L.) Sarg., Hura crepitans L., Terminalia amazónica (J. F. Gmel.) Exell, Quadrella odoratissima (Jacq.) Hutchinson, Pseudobombax septenatum (Jacq.) Dugand, Uribea tamarindoides Dugand & Romero, Enterolobium cyclocarpum (Jacq.) Griseb and Spondias mombin Jacq.
2.2. Sampling Methods and Capture Techniques
This study was conducted between January and July 2018, covering both the dry and rainy periods for the area. Data collection was carried out in four land cover types: permanent avocado crop, cocoa crop, transitory crops, and a forest fragment as a reference habitat. The avocado and transitory crops, as well as the forest fragment, were located in the village of La Flecha, while the cocoa crop was situated in the village of El Rastro. Each land cover type had two replicates, and in each replicate, two samplings were conducted—one during the dry season and one during the rainy season. Within each cover type, a 200 m linear transect was established, with five pitfall traps spaced 50 m apart [40]. The pitfall trap consisted of a 32-ounce plastic container buried at ground level, containing a solution of detergent and alcohol. Around each trap, a thin 15 cm iron rod was placed, with its upper end bent into a hook shape to hold the bait. The bait consisted of 60 g of a mixture of human and pig feces wrapped in gauze [41,42]. To protect the traps from rain, an inverted disposable plate (30 cm in diameter) was suspended over each trap using three stakes, approximately 30 cm above ground level. The traps were removed 24 h after installation [11,42].
The collected material was washed, preserved in 70% alcohol, and transported to the Ecology and Entomology Laboratory at the Universidad del Atlántico for identification and biomass estimation. The beetles were identified using available taxonomic keys for genera [43,44] and species [45,46,47,48,49,50,51,52,53,54,55]. Following identification, biomass estimation was conducted by selecting between 10 and 20 individuals of each species, depending on material availability. These beetles were dried in a muffle furnace at 120 °C for 48 h [56,57]. They were then weighed using an analytical balance (Radwag AS 220, 0.0001 g, Radwag, Cholet, France), and an average biomass was calculated for each species.
In addition to beetle sampling at each point, environmental variables were measured, including ambient temperature and humidity (Extech RHT50 Datalogger, Extech Instruments, Nashua, NH, USA), soil temperature (Extech 39240 Soil Thermometer, Extech Instruments, Nashua, NH, USA), soil moisture (Extech MO750 Soil Hygrometer, Extech Instruments, Nashua, NH, USA), light intensity (Extech LT 300 Lux Meter Extech Instruments, Nashua, NH, USA), soil compaction (Agratronix 08180, Agratronix, Streetsboro, OH, USA), and vegetation cover (Forestry Suppliers Spherical Densitometer, Forestry-Suppliers, Jackson, MS, USA).
2.3. Data Analysis
Sample coverage was calculated using the coverage estimator recommended by Chao and Jost [58], as follows:
where f1 and f2 represent the numbers of species with one and two individuals in the sample, respectively, and n is the total number of individuals. Sampling coverage varies between 1 and 100%, with values of ≈ 100% indicating that the sampling is complete relative to the capture technique. The sampling coverage was estimated for each crop type as well as for the reference habitat in each sampling season.
To observe changes in dung beetle richness and diversity across sampling seasons in different crop types and the forest fragment, interpolation and extrapolation analysis was used [59]. For this analysis, Hill numbers [60] were employed in units of the effective number of species [61]. The following true diversity values were calculated: 0D (species richness), 1D (common species [the exponential of Shannon’s index]), and 2D (very abundant species [the inverse of Simpson’s index]). The calculated diversity was compared using 95% confidence intervals and their overlap was assessed to determine if there were differences in beetle diversity [62].
The abundance and biomass of dung beetles by season across crop types and reference habitats were compared using the Kruskal–Wallis test, followed by paired Mann–Whitney U tests. To observe changes in community structure and the distribution of relative abundances of each beetle species by season for each crop type and forest fragment, rank-abundance curves were constructed. These curves enable the observation of differences in abundance distributions among dominant and rare species, as well as community evenness [63].
Additionally, to examine the spatial distribution and similarity in the composition and structure of Scarabaeinae by season across crop types and the forest fragment, a non-metric multidimensional scaling (nMDS) analysis was performed using the Bray–Curtis dissimilarity index. This index is appropriate for identifying dissimilarity relationships between sites and ecological distances among species [64]. To determine if the groupings observed in the nMDS showed significant differences, a one-way ANOSIM (analysis of similarity) was conducted. This analysis detects differences in species composition both between and within groups and is useful because it considers each species’ contribution to community structure through its abundance [65].
To identify interactions between beetle species and habitats across climatic seasons, a species–habitat interaction analysis was conducted, calculating the following indices: the specialization index H2′, which ranges from 0 to 1 and describes the level of specialization within the network, with higher values of H2′ indicating greater selectivity among species in the network [66]; the d′ index, which indicates a species preference or specialization for a specific habitat type within the network, with values ranging from 0 (no specialization) to 1 (perfect specialist) [66]; and the network module connectivity index, which measures the connectivity between modules (c), with a critical value established at 0.62. Habitats meeting or exceeding this threshold are considered network connectors, as they link different modules and combine high inter-module connectivity [67,68]. Additionally, the resistance index, defined as the sum of species dependencies on a habitat (specialist species), was calculated [69].
Finally, generalized linear models (GLMs) were conducted along with analysis of variance (ANOVA) to determine the effect of categorical predictor variables (season and habitat) and continuous variables (ambient temperature, soil temperature, soil moisture, and soil compaction) on the community attributes of copronecrophagous beetles (richness, abundance, and biomass). The variables ambient humidity, vegetation cover, and light intensity were excluded due to high collinearity with other variables (rs < 0.7). Abundance was fitted to a negative binomial distribution for both categorical and continuous variables, while richness was fitted to a Poisson distribution for categorical variables and a negative binomial distribution for continuous variables. Biomass was fitted to a Gamma distribution for both datasets. A logarithmic link function was used in all models, and the models with the lowest AIC values were selected [70]. All analyses were performed using R software version 4.4.1 [71].
3. Results
A total of 1566 individuals were collected, representing 15 genera and 36 species. Overall, without discriminating by sampling season, 345 individuals and 20 species were captured in the transitory crops, 157 individuals and 21 species in the avocado crop, 534 individuals and 24 species in the cacao crop, and 530 individuals and 28 species in the forest fragment (Table A1).
The species Canthon septemmaculatus Latreille (1811), with 63 individuals, and Onthophagus marginicollis Harold (1880), with 50 individuals, were the most abundant in the transitory crops. Onthophagus lebasi Boucomont (1932), with 25 individuals, was the most abundant species in the avocado crop. In the cacao crop, Onthophagus lebasi with 94 individuals, Onthophagus marginicollis with 84 individuals, and Sylvicanthon aequinoctialis Harold (1868) with 81 individuals were the most numerous species. Meanwhile, O. lebasi with 94 individuals and S. aequinoctialis with 78 individuals were the species with the highest abundance in the forest fragment. The highest values for these species across the crops and the reference habitat were recorded during the rainy season (Table A1).
The interpolation and extrapolation (iNEXT) analysis revealed that sampling coverage, considering seasons and crop types, ranged between 89% and 99% (Table A1). A seasonal pattern in beetle diversity was also observed, with lower values during the dry season that increased significantly during the rainy season for all diversity orders and analyzed habitats (Figure 1). At the habitat level, the analysis showed that during the dry season, the diversity of zero-order (0D, species richness), first-order (1D, common species), and second-order (2D, very abundant species) was significantly higher in the forest fragment compared to that in the transitory and permanent crops. The avocado crop followed, showing differences in 0D and 1D diversity compared to the cacao crop (Figure 1). During the rainy season, high values of zero-order (0D) and first-order (1D) diversity were observed in the cacao crop and the forest fragment, which did not differ significantly from each other but did differ from the avocado and transitory crops (Figure 1A,B). Regarding second-order diversity (2D), while high values were observed in the forest fragment and cacao crop, these did not differ significantly from those in the transitory and avocado crops (Figure 1C).
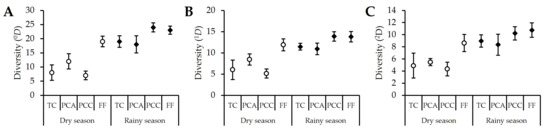
Figure 1.
Diversity analysis for 0D (A), 1D (B), and 2D (C), conducted for different crop types and the reference forest fragment across seasons. Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), and forest fragment (FF).
Regarding abundance and biomass, during the dry season, the Kruskal–Wallis test detected differences among habitats (Abundance: H = 22.19; p = 4 × 10−5, Biomass: H = 22.99; p = 4 × 10−5), with higher values observed in the forest fragment compared to the transitory and permanent crops (Table 1 and Table 2). In the rainy season, abundance showed differences among habitats (H = 11.8; p = 0.008), with a higher value in the avocado crop than in the other habitats. Biomass, however, did not show significant differences among habitats (H = 5.13; p = 0.163) (Table 1).

Table 1.
Results of the Kruskal–Wallis test for comparisons of abundance (light gray values) and biomass (dark gray values) across different crop types by season. Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), and forest fragment (FF). Asterisk (*) indicates significant differences for comparison.

Table 2.
Results of the generalized linear models. Abbreviations: soil temperature (S.T), soil moisture (S.M), soil compaction (S.C), and ambient temperature (A.T). Variables with significant values are noted in bold.
The rank-abundance curves showed changes in the composition and structure of beetle communities by season across habitats. For instance, during the dry season in the forest fragment, four species contributed more than 10 individuals to the habitat’s abundance, whereas in the avocado and cacao crops, only two species reached this threshold, and in the transitory crops, no species contributed more than 10 individuals. Moreover, the species structuring of the communities in terms of abundance varied between habitats (Figure 2). During the rainy season, there was a notable increase in the number of species contributing more than 10 individuals to the abundance of each habitat. However, the species structuring of the communities in terms of abundance differed for each habitat (Figure 2).
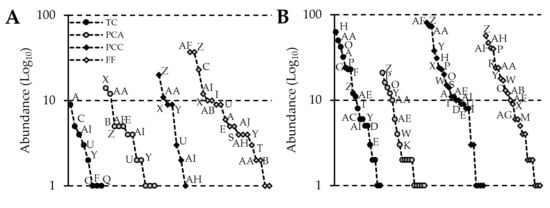
Figure 2.
Rank-abundance curves constructed for each crop type during the dry season (A) and the rainy season (B). Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), and forest fragment (FF).
This was supported by the non-metric multidimensional scaling (nMDS) analysis and the analysis of similarity (ANOSIM), which showed that during both the dry season (ANOSIM: R = 0.482; p = 0.001) and the rainy season (R = 0.388; p = 0.001), each habitat exhibited a distinct beetle composition and structure. However, similarities were observed between transitory and permanent crops during the rainy season (Figure 3).
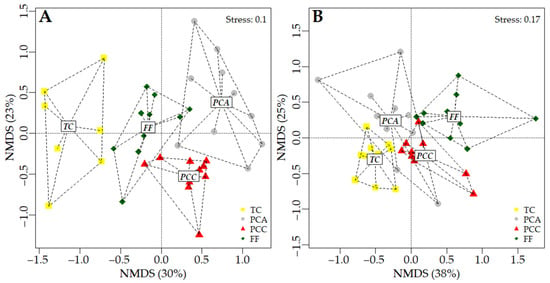
Figure 3.
Non-metric multidimensional scaling (nMDS) analysis based on a Bray–Curtis similarity matrix, performed for the different crop types during the dry season (A) and the rainy season (B). Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), and forest fragment (FF).
The species–habitat interaction network analysis revealed a nested network for both sampling periods. During the dry season, the highest number of interactions occurred in the forest, while the lowest number was observed in the transitory crops. Conversely, during the rainy season, the highest numbers of interactions were recorded in the cacao crop and the forest fragment (Figure 4). According to the specialization index (H2′), the network exhibited a generalist species composition for both periods, with species utilizing different habitats interchangeably (Dry = 0.33, Rainy = 0.27). The habitat specialization index (d′) showed no species with high specialization (d′ > 0.40). However, during the rainy season, species such as C. mutabilis and C. lituratus had the highest values (d′ = 0.34), with their entire abundance concentrated in the transitory crops. In the dry season, Canthidium sp. 1 exhibited the highest value (d′ = 0.36), with its abundance concentrated in two land-use types (transitory crops and forest). Regarding module connectivity (c), during the rainy season, the avocado crop acted as a connecting habitat (c = 0.63), followed by the transitory crops (c = 0.46), cacao (c = 0.45), and forest (c = 0.31). In the dry season, the cacao crop served as the connector habitat (c = 0.67), while other habitats showed lower values (transitory = 0.39, avocado = 0.41, forest = 0.53). In terms of network resistance, during the rainy season, the cacao crop and the forest fragment had the highest values (transitory = 7.4; avocado = 2.5; cacao = 10.8; forest = 10.2). During the dry season, only the forest fragment exhibited the highest resistance value, significantly exceeding the other habitats (transitory = 1.2; avocado = 3.5; cacao = 2.0; forest = 11.2).
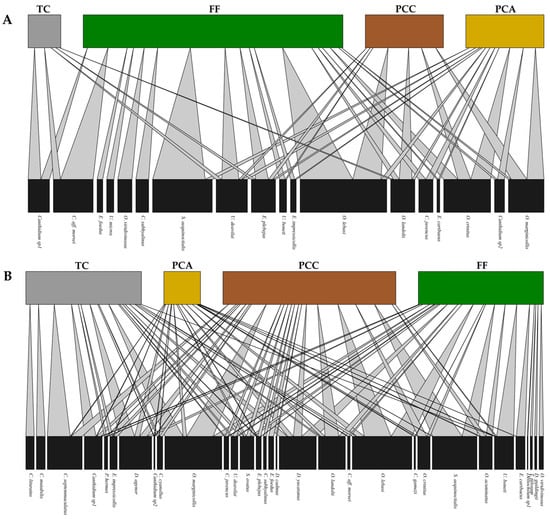
Figure 4.
Species–habitat interaction networks constructed for the dry season (A) and the rainy season (B). The thickness of the bands represents the strength of the interaction between the species and the habitat. Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), and forest fragment (FF).
Finally, generalized linear models (GLMs) determined that the interaction between climatic season and land use had a significant effect (p < 0.05) on the abundance, richness, and biomass of dung beetles. Regarding environmental variables, the interaction of soil temperature, soil moisture, and ambient temperature showed a significant effect (p < 0.05) on the abundance, richness, and biomass of beetles. Additionally, soil moisture, ambient temperature and soil compaction independently also had a significant effect (p < 0.05) on dung beetle abundance, richness, and biomass (Table 2).
4. Discussion
The results of this research highlight two key aspects for the management and conservation of dung beetles in modified landscapes of the Colombian Caribbean: (I) the conservation of forest fragments over any other vegetation cover, and (II) the management of transitory and permanent crops as tools for conservation. Regarding these two aspects, the maintenance of forest areas is crucial, as forests represent the original ecosystems inhabited by species prior to landscape modification processes. These habitats provide stable environmental conditions and resource availability (e.g., primate feces), which are not found in modified areas. Such conditions favor the persistence of many species, especially those with strict habitat and resource requirements [26,72,73,74,75].
Additionally, findings such as those provided by the resistance index in the interaction network analysis underscore the importance of this habitat for sustaining dung beetle communities, highlighting the need for its conservation. Many species rely on this habitat [69,76], including S. aequinoctialis, C. aff. morsei, C. subhyalinus, E. caribaeus, O. viridivinosus, T. pilosum, U. micros, Deltochilum sp. 1, and D. guildingii, which concentrated their abundances in forest areas. Recent studies support this assertion, such as research on Deltochilum guildingii in the dry forest of the Colombian Caribbean. This research found that the species exhibits high sensitivity and limited dispersal in habitats other than forests. Therefore, to maintain its genetic diversity and ensure its preservation, it is essential to conserve and connect dry forest patches while also protecting mammal species like Alouatta seniculus. This is due to the close ecological relationship between these two species, driven by the preference of D. guildingii for the feces of this primate [77].
On the other hand, our results demonstrated that, regardless of seasonality, the forest fragment consistently exhibited higher diversity values compared to the permanent and transitory crops, which only showed diversity levels similar to those of the forest fragment during the rainy season. This indicates the stability of forest habitats and their importance in maintaining beetle communities and other organisms during periods of significant stress, such as the dry season [11]. Some authors suggest that the diversity of certain landscape elements within a sampling window largely depends on forest fragments. Studies have found that greater quantity and better conservation status of these fragments lead to higher diversity across different habitats within the landscape, compared to areas with few forest patches in critical conservation states. This is attributed to the ability of large forest areas to connect with other habitats in the landscape, facilitating the movement of forest-native beetles to these areas in search of resources without establishing permanent populations, thereby increasing overall diversity. In a strict sense, forests may function as source habitats for certain beetle species, which underscores the critical importance of their conservation in sustaining beetle diversity within landscapes [11,30,78,79,80,81,82].
The results reported for the forest fragment in this study contrasted sharply with those of the transitory crops, which consistently exhibited the lowest diversity values regardless of the season. In terms of beetle composition and structure, the transitory crops formed a distinct group, separate from the other habitats analyzed. These types of crops, characterized by polycultures, undergo constant soil modification due to continuous harvesting throughout the year and soil preparation for each crop cycle. This process includes soil cover removal, elimination of vegetation cover, and the use of fertilizers, herbicides, and insecticides. The first two practices limit beetle presence in these habitats by creating adverse environmental conditions, such as elevated soil and ambient temperatures, high soil compaction, and intense light exposure. These conditions pose physiological barriers for many beetle species, preventing their colonization of such habitats [11,79]. Meanwhile, the use of herbicides and insecticides directly causes the death of the few beetles that occupy these areas [11,12,13,83,84]. Despite the intensive agricultural practices observed in the studied crop fields, some species, such as C. lituratus, C. mutabilis, and C. septemmaculatus, were exclusive to this habitat. Others, such as D. agenor, D. yucatanus, and O. marginicollis, were found in moderate abundances. This suggests that changes in current agricultural practices could promote the arrival of beetles, thereby enhancing the ecosystem services they provide, particularly those related to pathogen control that could benefit crop health [85].
On the other hand, the presence of C. lituratus and C. mutabilis in this type of habitat during the rainy season has been reported by other authors, such as Rangel-Acosta and Martínez-Hernández [11] and Rangel-Acosta et al. [12], who associated the occurrence of these species in such areas with their tolerance to the environmental conditions of open spaces, as well as their dietary flexibility. Specimens of these species have been observed using uncommon resources, such as the feces of domestic dogs (Canis familiaris) and other introduced mammals, such as cattle dung (Bos sp.). These traits have possibly emerged as adaptations to avoid competition for resources and space, which frequently occur in habitats like forest fragments, thereby enabling the persistence of these species.
Unlike the transitory crops, the permanent crops retained a portion of the beetle diversity recorded in the forest fragment. This supports the findings from other studies suggesting that agricultural systems, such as agroforestry systems, serve as refuges, feeding areas, and recolonization zones for insects [86,87,88]. These systems are significant landscape elements for biodiversity conservation, as they host a greater number of beetle species compared to systems with more open canopies, such as transitory crops and pastures [82,89,90].
This contribution to maintaining diversity by permanent crops has been found to be associated with the similarity in canopy cover between forests and these crops, as this factor determines the composition of beetle communities [25,91]. The canopy cover in permanent plantations creates favorable environmental conditions for beetles, resembling those found in natural areas (e.g., air temperature, humidity, light intensity), in contrast to the adverse conditions recorded in open areas [86]. Additionally, by preserving vegetation cover, these crops help to maintain dung quality for longer periods by retaining its moisture, which prolongs its usability for beetles [11,92]. Similar results were reported by Horgan [93] in coffee permanent plantations, where the diversity of dung beetles was found to be comparable to that of forest fragments and higher than that observed in transitory crops, such as banana, cassava, and maize. This study also reported a positive and significant relationship between canopy cover and beetle diversity, highlighting the importance of canopy conservation in protecting dung beetles. Canopy cover maintains stable environmental conditions, unlike deforested areas, where increased light intensity and higher air and soil temperatures, resulting from the lack of canopy cover, prevent the establishment of these insects in open areas [94,95].
Although our results indicated that the permanent crops could harbor a portion of the beetle diversity found in the forest fragment, particularly during the rainy season, it is important to note that diversity is not the only parameter to consider when taking conservation and management decisions. Other community attributes, such as abundance, biomass, and species size, must also be evaluated to avoid erroneous decisions caused by potential biases in diversity indices. From this perspective, while the permanent crops (e.g., cacao) exhibited diversity values similar to those of the forest fragment, only 12% of the 24 species reported in this habitat had abundances exceeding 10 individuals, and 25% were represented by just one to five individuals. This suggests that species may use these areas merely to access resources or as corridors to reach more stable environments, such as forest fragments, while avoiding open areas, without necessarily establishing populations in these habitats. Data recorded in this beetle marking and recapture project supported this interpretation, since species such as Coprophanaeus gamezi and Phanaeus hermes were marked in avocado crops and subsequently recaptured within 48 h in the forest interior and edge.
Some studies have highlighted that the environmental conditions of agroforestry systems and permanent crops are favorable for temporarily hosting certain beetle species during specific periods. However, owing to their low complexity, these systems lack the capacity to sustain abundance levels comparable to those observed in natural areas during extended periods [29,86,90,96,97]. This trend was evident in our study, where the permanent crops showed high diversity and abundance values during the rainy season, but during adverse periods, such as the dry season, these community attributes were similar to those observed in the transitory crops and significantly lower than those recorded in the forest fragment.
Considering the above, the adoption of permanent crops is a viable option for biodiversity planning, management, and conservation, but it should not be regarded as a strategy for replacing natural ecosystems such as forest fragments [29]. Replacing forests with these types of crops still produces negative effects on community attributes (species richness, abundance, and dung beetle biomass) and ecological functions (dung removal, soil excavation, and secondary seed dispersal) [98]. Furthermore, these habitat changes often have a pronounced impact on larger dung beetle species, which are more susceptible to declines in abundance. Consequently, this leads to the loss of the ecosystem functions they provide [98,99,100,101,102].
The results presented in this research demonstrate how permanent crops can contribute to the conservation of dung beetles. However, this study had some limitations that could be addressed in future research. For instance, it would be important to analyze how plantation age and management practices might affect beetle diversity. Additionally, future studies should increase the number of replicates per crop type, as this study had a limited number. Finally, further research in this area should focus on analyzing the ecosystem functions performed by dung beetles, such as dung removal and nutrient recycling into the soil, with particular emphasis on agricultural areas.
5. Conclusions
Our results provide clear evidence of the need to protect and conserve natural ecosystems for the preservation of biological diversity. They also demonstrate that replacing these ecosystems with productive systems, such as permanent or transitory crops, leads to changes in the composition and structure of beetle communities. While these new habitats can conserve part of the forest’s diversity, they fail to support a large number of species with strict habitat and resource requirements that only forests can provide. From this perspective, the use of permanent crops could be a strategy integrated into management plans to maintain beetle diversity at the landscape level. However, this should not imply the replacement of forests with these land-use types.
As a recommendation for planning landscape management and contributing to the conservation of beetles, we suggest, based on our results, maintaining large forest areas connected to different landscape elements through biological corridors. This would facilitate movement not only for beetles but also for mammals that provide the essential resources upon which these insects depend.
In crop management, we recommend reducing or eliminating the use of insecticides and herbicides that may directly harm beetles. Punctually, for transitory crops, adopting elements that provide shade could improve the environmental conditions in these areas and extend the availability of the limited resources deposited by native and introduced mammals that visit them. This could increase the number of beetle species in these areas, thereby enhancing their diversity.
Author Contributions
Conceptualization, all authors; methodology, all authors; software, J.L.R.-A. and C.A.D.-G.; validation, all authors; investigation, all authors; resources, all authors; data curation, J.L.R.-A.; writing—original draft preparation, N.J.M.-H., A.S.-G. and J.L.R.-A.; writing—review and editing, all authors; visualization, J.L.R.-A. and C.A.D.-G.; supervision, N.J.M.-H.; project administration, N.J.M.-H.; funding acquisition, N.J.M.-H. All authors have read and agreed to the published version of the manuscript.
Funding
Partial financial support was provided by COLCIENCIAS 2015 through doctoral grant no. 727 and by the Universidad del Atlántico for the development of degree research projects 2018 to N.J.M.-H.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study will be available upon request from all authors.
Acknowledgments
The authors express their gratitude to the Ministry of Science, Technology, and Innovation of Colombia and the University of Atlántico for funding the project. We also extend our thanks to the farming families from the villages of La Flecha and El Rastro, especially Oscar Caro García and Adalberto García, as well as the Arrieta and Carval families, for granting access to their properties and providing support during the fieldwork. The authors also extend their sincere thanks to Yelena Sofía Pájaro Esquivia for her contribution in translating this article into English, ensuring the scientific precision and clarity of the manuscript. Finally, we thank all members of the NEOPTERA Arthropod Research Group for their assistance during the fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
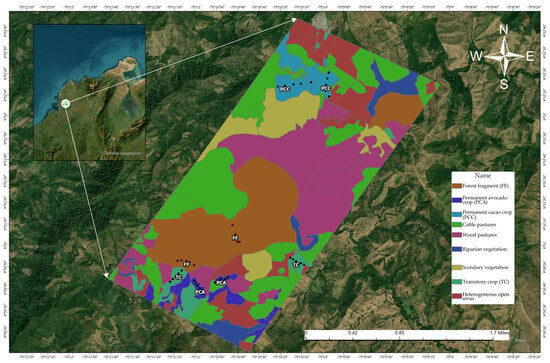
Figure A1.
Geographical location of the study area.
Appendix B

Table A1.
Richness, abundance, biomass, and sampling coverage of dung beetles by climatic season and crop type. Abbreviations: dry season (DS) and rainy season (RS). Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), forest fragment (FF), and species identification in graphs (SIG).
Table A1.
Richness, abundance, biomass, and sampling coverage of dung beetles by climatic season and crop type. Abbreviations: dry season (DS) and rainy season (RS). Abbreviations: transitory crops (TC), permanent avocado crop (PCA), permanent cacao crop (PCC), forest fragment (FF), and species identification in graphs (SIG).
| SIG | Species | TC | PCA | PCC | FF | Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DS | RS | DS | RS | DS | RS | DS | RS | |||
| A | Canthidium sp. 1 | 9 | 32 | 0 | 2 | 0 | 11 | 6 | 4 | 64 |
| B | Canthidium sp. 2 | 0 | 1 | 5 | 1 | 0 | 1 | 2 | 0 | 10 |
| C | Canthon aff. morsei Howden, 1966 | 5 | 2 | 0 | 0 | 0 | 0 | 23 | 5 | 35 |
| D | Canthon cyanellus Leconte, 1859 | 0 | 5 | 0 | 1 | 0 | 9 | 0 | 0 | 15 |
| E | Canthon juvencus Harold, 1868 | 0 | 3 | 5 | 0 | 0 | 8 | 5 | 2 | 23 |
| F | Canthon lituratus (Germar, 1813) | 1 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 24 |
| G | Canthon mutabilis Lucas, 1857 | 1 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| H | Canthon septemmaculatus (Latreille, 1811) | 0 | 63 | 0 | 2 | 0 | 31 | 0 | 0 | 96 |
| I | Canthon subhyalinus Harold, 1867 | 0 | 0 | 0 | 0 | 0 | 8 | 9 | 0 | 17 |
| J | Coprophanaeus corythus (Harold, 1863) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| K | Coprophanaeus gamezi Arnaud, 2002 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 4 |
| L | Deltochilum sp. 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| M | Deltochilum guildingii (Westwood, 1835) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 6 |
| N | Diabroctis cadmus (Harold, 1868) | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| O | Dichotomius agenor (Harold, 1869) | 0 | 42 | 0 | 14 | 0 | 20 | 0 | 13 | 89 |
| P | Dichotomius yucatanus (Bates, 1887) | 0 | 23 | 0 | 16 | 0 | 23 | 0 | 40 | 102 |
| Q | Digitonthophagus gazella (Fabricius, 1787) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| R | Eurysternus caribaeus (Herbst 1789) | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 24 | 27 |
| S | Eurysternus foedus Guérin-Méneville, 1844 | 0 | 0 | 0 | 0 | 0 | 14 | 4 | 0 | 18 |
| T | Eurysternus impressicollis Castelnau, 1840 | 0 | 8 | 1 | 2 | 0 | 10 | 3 | 0 | 24 |
| U | Eurysternus plebejus Harold, 1880 | 3 | 2 | 2 | 2 | 3 | 9 | 9 | 1 | 31 |
| V | Malagoniella astyanax Olivier, 1789 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| W | Onthophagus acuminatus Harold, 1880 | 0 | 0 | 0 | 4 | 0 | 15 | 0 | 17 | 36 |
| X | Onthophagus crinitus Harold, 1869 | 0 | 0 | 14 | 1 | 9 | 24 | 10 | 9 | 67 |
| Y | Onthophagus landolti Harold, 1880 | 2 | 6 | 2 | 12 | 9 | 38 | 4 | 18 | 91 |
| Z | Onthophagus lebasi Boucomont, 1932 | 0 | 12 | 4 | 21 | 20 | 74 | 37 | 57 | 225 |
| AA | Onthophagus marginicollis Harold, 1880 | 0 | 50 | 12 | 10 | 11 | 73 | 2 | 24 | 182 |
| AB | Onthophagus viridivinosus Kohlmann & Solís, 2001 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 12 | 22 |
| AC | Phanaeus hermes Harold, 1868 | 0 | 6 | 0 | 0 | 0 | 1 | 1 | 2 | 10 |
| AD | Pseudocanthon perplexus (LeConte, 1847) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| AE | Scatimus ovatus Harold, 1862 | 0 | 11 | 0 | 6 | 0 | 11 | 0 | 10 | 38 |
| AF | Sylvicanthon aequinoctialis (Harold, 1868) | 0 | 0 | 5 | 1 | 0 | 81 | 37 | 41 | 165 |
| AG | Trichillidium pilosum (Robinson, 1948) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 6 |
| AH | Uroxys boneti Pereira & Halffter, 1961 | 0 | 1 | 0 | 2 | 1 | 3 | 4 | 47 | 58 |
| AI | Uroxys deavilai Delgado & Kolhmann, 2007 | 4 | 5 | 4 | 0 | 2 | 10 | 12 | 4 | 41 |
| AJ | Uroxys micros Bates, 1887 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 6 |
| Number of species | 8 | 19 | 12 | 18 | 7 | 24 | 19 | 23 | 36 | |
| Number of individuals | 26 | 319 | 56 | 101 | 55 | 479 | 184 | 346 | 1566 | |
| Biomass (g) | 0.24 | 18.82 | 0.78 | 8.76 | 0.40 | 20.25 | 3.89 | 21.19 | 74.33 | |
| Sampling coverage (%) | 89 | 99 | 94 | 95 | 98 | 99 | 98 | 99 | - | |
References
- Sánchez, Á.R.; Ulloa, K.H.; Marques, R.A. El Impacto de La Producción de Café Sobre La Biodiversidad, La Transformación Del Paisaje y Las Especies Exóticas Invasoras. Ambiente Desarro. 2012, 16, 93–104. [Google Scholar]
- Harvey, C.A.; Sáenz, J.C. Evaluación y Conservación de Biodiversidad en Paisajes Fragmentados de Mesoamérica; INBio: Heredia, Costa Rica, 2008. [Google Scholar]
- Bos, M.M.; Steffan-Dewenter, I.; Tscharntke, T. The Contribution of Cacao Agroforests to the Conservation of Lower Canopy Ant and Beetle Diversity in Indonesia. Biodivers. Conserv. 2007, 16, 2429–2444. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecología: Principios y Estrategias Para Diseñar Una Agricultura Que Conserva Recursos Naturales y Asegura La Soberanía Alimentaria; Universidad de California: Berkeley, CA, USA, 1995. [Google Scholar]
- Altieri, M.; Nicholls, C. Biodiversity and Pest Management in Agroecosystems, 2nd ed.; CRC press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Klein, A.-M.; Steffan-Dewenter, I.; Buchori, D.; Tscharntke, T. Effects of Land-Use Intensity in Tropical Agroforestry Systems on Coffee Flower-Visiting and Trap-Nesting Bees and Wasps. Conserv. Biol. 2002, 16, 1003–1014. [Google Scholar] [CrossRef]
- Somarriba, E. ¿Cómo evaluar y mejorar el dosel de sombra en cacaotales? Agroforestería Américas 2004, 42, 120–128. [Google Scholar]
- Suatunce, P.; Somarriba, E.; Harvey, C.A.; Finegan, B. Composición florística y estructura de bosques y cacaotales en los territorios indígenas de Talamanca, Costa Rica. Agroforestería Américas 2003, 10, 37–38. [Google Scholar]
- Estrada, A.; Halffter, G.; Coates-Estrada, R.; Meritt, D.A., Jr. Dung Beetles Attracted to Mammalian Herbivore (Alouatta palliata) and Omnivore (Nasua narica) Dung in the Tropical Rain Forest of Los Tuxtlas, Mexico. J. Trop. Ecol. 1993, 9, 45–54. [Google Scholar] [CrossRef]
- Rice, R.A.; Greenberg, R. Cacao Cultivation and the Conservation of Biological Diversity. AMBIO 2000, 29, 167–173. [Google Scholar] [CrossRef]
- Rangel-Acosta, J.L.; Martínez-Hernández, N.J. Comparación de Los Ensamblajes de Escarabajos Copro-Necrófagos (Scarabaeidae: Scarabaeinae) Entre Fragmentos de Bosque Seco Tropical y La Matriz Adyacente En El Departamento Del Atlántico-Colombia. Rev. Mex. Biodivers. 2017, 88, 389–401. [Google Scholar] [CrossRef]
- Rangel-Acosta, J.; Blanco-Rodríguez, O.; Martínez-Hernández, N. Escarabajos Copro-Necrófagos (Scarabaeidae: Scarabaeinae) En Diferentes Usos Del Suelo En La Reserva Campesina La Montaña (RCM) En El Departamento Del Atlántico, Colombia. Boletín Científico Cent. Mus. Mus. Hist. Nat. 2016, 20, 78–97. [Google Scholar] [CrossRef]
- Campos, R.C.; Hernández, M.I.M. The Importance of Maize Management on Dung Beetle Communities in Atlantic Forest Fragments. PLoS ONE 2015, 10, e0145000. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Datos Sobre Alimentación y Agricultura. Available online: https://www.fao.org/faostat/es/#home (accessed on 1 November 2024).
- Aguilera Díaz, M.; Reina Aranza, Y.; Orozco Gallo, A.; Yabrudy Vega, J.; Barcos Robles, R. Composición de la economía de la región Caribe de Colombia. In Ensayos Sobre Economía Regional; Banco de la República de Colombia: Bogota, Colombia, 2013; pp. 1–66. [Google Scholar]
- Sánchez, D.B.; Luna, L.L.; Díaz, A.T.; Pérez, J.V.; Cadena, J.; Sánchez López, D.B.; Luna Castellanos, L.L.; Díaz Cabadiaz, A.T.; Pérez Pazos, J.V.; Cadena Torres, J. Identificación de Hongos Asociados a La Pudrición Seca Del Ñame Bajo Condiciones de Almacenamiento. Rev. Investig. Altoandinas 2020, 22, 199–214. [Google Scholar] [CrossRef]
- Sánchez, D.B.S.; Luna-Castellanos, L.L.; Espinosa-Carvajal, M.R.; Pérez-Polo, D.J.; Cadena-Torres, J. Capacidad de infección de hongos asociados a la pudrición seca de los Tubérculos de Ñame: Fungal infection. Rev. Investig. Altoandinas—J. High Andean Res. 2021, 23, 149–158. [Google Scholar] [CrossRef]
- Meza, M.C.M.; Armenteras, D. Uso del suelo y estructura de la vegetación en paisajes fragmentados en la Amazonia, Colombia. Colomb. For. 2018, 21, 205–223. [Google Scholar] [CrossRef]
- Noriega, J.A.; Solis, C.; Garcia, H.; Murillo-Ramos, L.; Renginfo, J.; Olarte, J.L. Sinopsis de Los Escarabajos Coprófagos (Coleoptera: Scarabaeinae) Del Caribe Colombiano. Caldasia 2013, 35, 465–477. [Google Scholar]
- Noriega, J.A.; Camero, E.; Arias-Buriticá, J.; Locarno, L.C.P.; Montes, J.M.; Acevedo, A.; Esparza, A.; Murcia, B.; Garcia, H.; Solís, C. Grado de Cobertura Del Muestreo de Escarabajos Coprófagos (Coleoptera: Scarabaeidae: Scarabaeinae) En Colombia. Int. J. Trop. Biol. Conserv. 2015, 63, 97–125. [Google Scholar] [CrossRef]
- Amell-Caez, Y.; Decastro-Arrazola, I.; García, H.; Monroy-G, J.D.; Noriega, J.A. Spatial Diversity of Dung Beetle Assemblages (Coleoptera: Scarabaeidae: Scarabaeinae) in Five Ecoregions from Sucre, Colombian Caribbean Coast. Rev. Colomb. Entomol. 2019, 45, e7963. [Google Scholar] [CrossRef]
- Spector, S. Scarabaeine Dung Beetles (Coleoptera: Scarabaeidae: Scarabaeinae): An Invertebrate Focal Taxon for Biodiversity Research and Conservation. Coleopt. Bull. 2006, 60, 71–83. [Google Scholar] [CrossRef]
- Favila, M.E.; Halffter, G. The Use of Indicator Groups for Measuring Biodiversity as Related to Community Structure and Function. Acta Zoológica Mex. 1997, 72, 1–25. [Google Scholar] [CrossRef]
- Halffter, G.; Favila, M.E. The Scarabaeinae (Insecta: Coleoptera) an Animal Group for Analyzing, Inventorying and Monitoring Biodiversity in Tropical Rainforest and Modified Landscapes. Biol. Int. 1993, 27, 15–21. [Google Scholar]
- Halffter, G.; Matthews, E.G. The Natural History of Dung Beetles of the Subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomológica Mex. 1966, 12, 3–312. [Google Scholar]
- Nichols, E.; Larsen, T.; Spector, S.; Davis, A.L.; Escobar, F.; Favila, M.; Vulinec, K. Global Dung Beetle Response to Tropical Forest Modification and Fragmentation: A Quantitative Literature Review and Meta-Analysis. Biol. Conserv. 2007, 137, 1–19. [Google Scholar] [CrossRef]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological Functions and Ecosystem Services Provided by Scarabaeinae Dung Beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Nichols, E.S.; Gardner, T.A. Dung Beetles as a Candidate Study Taxon in Applied Biodiversity Conservation Research. In Ecology and Evolution of Dung Beetles; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 267–291. [Google Scholar] [CrossRef]
- Neita, J.C.; Escobar, F. The Potential Value of Agroforestry to Dung Beetle Diversity in the Wet Tropical Forests of the Pacific Lowlands of Colombia. Agrofor. Syst. 2012, 85, 121–131. [Google Scholar] [CrossRef]
- Cultid-Medina, C.A.; Escobar, F. Assessing the Ecological Response of Dung Beetles in an Agricultural Landscape Using Number of Individuals and Biomass in Diversity Measures. Environ. Entomol. 2016, 45, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Cultid-Medina, C.A.; Martínez-Quintero, B.G.; Escobar, F.; de Ulloa, P.C. Movement and Population Size of Two Dung Beetle Species in an Andean Agricultural Landscape Dominated by Sun-Grown Coffee. J. Insect Conserv. 2015, 19, 617–626. [Google Scholar] [CrossRef]
- García, H.; Corzo, G.; Isaacs, P.; Etter, A. Distribución y estado actual de los remanentes del bioma de bosque seco tropical en Colombia: Insumos para su gestión. In El Bosque Seco Tropical en Colombia; Pizano, C., Garcia, H., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogota, Colombia, 2014; Volume I, pp. 229–251. [Google Scholar]
- Pizano, C. Consideraciones históricas sobre la distribución actual del bosque seco en Colombia. In El Bosque Seco Tropical en Colombia; Pizano, C., Gracia, H., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogota, Colombia, 2014; Volume 1, pp. 24–33. [Google Scholar]
- Rangel-Ch, J.O.; Carvajal-Cogollo, J.E. Clima de La Región Caribe Colombiana. In Colombia Diversidad Biótica XII: La Región Caribe de Colombia; Rangel-Ch, J.O., Ed.; Universidad Nacional de Colombia: Bogota, Colombia, 2012; Volume XII, pp. 67–129. [Google Scholar]
- Madrid-Peralta, B.D.; Martínez-Hernández, N.J. Aspectos poblacionales y desplazamiento de mariposas Hamadryas (Nymphalidae) en distintos usos de suelo en los Montes de María, Colombia. Rev. Mex. Biodivers. 2023, 94, e945148. [Google Scholar] [CrossRef]
- Marquez-P, J.; Martinez-H, N. Estructura poblacional de Morpho helenor peleides kollar, 1850 (lepidoptera: Nymphalidae) en un paisaje de bosque seco tropical, departamento de Bolivar, Colombia. Boletín Científico Cent. Mus. Mus. Hist. Nat. 2020, 24, 169–189. [Google Scholar] [CrossRef]
- Ortega-de la Rosa, R.J.; Moreno-Arias, R.Á.; Martínez-Hernández, N.J. Aproximación a la estructuración ecológica de la comunidad de lagartijas Anolis Daudin, 1802 (Squamata: Dactyloidae) de los bosques secos de los Montes de María en el Caribe colombiano. Boletín Científico. Cent. Museos. Mus. Hist. Nat. 2023, 27, 113–129. [Google Scholar] [CrossRef]
- Villarreal, E.; Martínez, N.; Ortiz, C.R. Diversity of Pseudoscorpiones (Arthropoda: Arachnida) in Two Fragments of Dry Tropical Forest in the Colombian Caribbean Region. Caldasia 2019, 41, 139–151. [Google Scholar] [CrossRef]
- Sarmiento-Roa, J.D.; Arenas-Clavijo, A.; Martínez-Hernández, N.J. Variación espacio-temporal de la diversidad de escarabajos Geadephaga (Coleoptera: Carabidae, Cicindelidae) en el bosque seco tropical del Caribe colombiano. Rev. Biol. Trop. 2024, 72, e52855. [Google Scholar] [CrossRef]
- Larsen, T.H.; Forsyth, A. Trap Spacing and Transect Design for Dung Beetle Biodiversity Studies. Biotropica 2005, 37, 322–325. [Google Scholar] [CrossRef]
- Marsh, C.J.; Louzada, J.; Beiroz, W.; Ewers, R.M. Optimising Bait for Pitfall Trapping of Amazonian Dung Beetles (Coleoptera: Scarabaeinae). PLoS ONE 2013, 8, e73147. [Google Scholar] [CrossRef]
- Martínez-Hernández, N.J.; Rangel-Acosta, J.L.; Beltrán-Díaz, H.; Daza-Guerra, C.A. ¡El tamaño sí importa! Incidencia del tamaño del cebo en la captura de escarabajos coprófagos en el bosque seco tropical. Rev. Biol. Trop. 2022, 70, 1–19. [Google Scholar] [CrossRef]
- Medina, C.A.; Lopera, A. Clave Ilustrada Para La Identificación de Géneros de Escarabajos Coprófagos (Coleoptera: Scarabaeinae) de Colombia. Caldasia 2000, 22, 299–315. [Google Scholar]
- Vaz-de-Mello, F.Z.; Edmonds, W.D.; Ocampo, F.C.; Schoolmeesters, P. A Multilingual Key to the Genera and Subgenera of the Subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2011, 2854, 1–73. [Google Scholar] [CrossRef]
- Sarmiento-Garcés, R.; Amat-Garcia, G. Escarabajos del Género Dichotomius Shope 1838 (Scarabaeidae: Scarabaeinae) en Colombia; Fauna de Colombia; Universidad Nacional de Colombia: Bogota, Colombia, 2014. [Google Scholar]
- Delgado, L.; Kohlmann, B. Revisión de Las Especies Del Género Uroxys Westwood de México y Guatemala (Coleoptera: Scarabaeidae: Scarabaeinae). Folia Entomológica Mex. 2007, 46, 1–36. [Google Scholar]
- Kohlmann, B.; Solís-Blanco, A. El Género Dichotomius (Coleoptera: Scarabaeidae) En Costa Rica. G. Ital. Entomologia. 1997, 8, 343–382. [Google Scholar]
- Kohlmann, B.; Solís-Blanco, A. El Género Onthophagus (Coleoptera: Scarabaeidae) En Costa Rica. G. Ital. Entomologia. 2001, 9, 159–261. [Google Scholar]
- Solís-Blanco, A.; Kohlmann, B. El Género Canthidium (Coleoptera: Scarabaeidae) En Costa Rica. G. Ital. Entomologia. 2004, 11, 1–73. [Google Scholar]
- Solís-Blanco, A.; Kohlmann, B. El Género Canthon (Coleoptera: Scarabaeidae) En Costa Rica. G. Ital. Entomologia. 2002, 10, 1–68. [Google Scholar]
- Edmonds, W.; Zidek, J. A Taxonomic Review of the Neotropical Genus Coprophanaeus Olsoufieff, 1924 (Coleoptera: Scarabaeidae, Scarabaeinae). Insecta Mundi 2010, 129, 1–111. [Google Scholar]
- Génier, F. Le Genre Eurysternus Dalman, 1824 (Scarabaeidae: Scarabaeinae: Oniticellini), Revision Taxonomique et Clés de Determination Illustrées; Pensoft Publishers: Sofia, Bulgaria, 2009. [Google Scholar]
- González-Alvarado, A.; Vaz-de-Mello, F.Z. Taxonomic Review of the Subgenus Hybomidium Shipp 1897 (Coleoptera: Scarabaeidae: Scarabaeinae: Deltochilum). Ann. Société Entomol. Fr. 2014, 50, 431–476. [Google Scholar] [CrossRef]
- Vitólo, A. Clave Para La Identificación de Los Géneros y Especies Phanaeinas (Coleoptera: Scarabaeidae: Coprinae: Phanaeini) de Colombia. Rev. Acad. Colomb. Cienc. 2000, 24, 591–601. [Google Scholar]
- González, F.A.; Molano, F.; Medina, C.A. Los Subgéneros Calhyboma Kolbe 1893, Hybomidium Shipp 1897 y Telhyboma Kolbe 1893 de Deltochilum (Coleoptera: Scarabaeidae: Scarabaeinae) En Colombia. Rev. Colomb. Entomol. 2009, 35, 253–274. [Google Scholar]
- Lobo, J.M. Estimation of Dung Beetle Biomass (Coleoptera: Scarabaeoidea). Eur. J. Entomol. 1993, 90, 235–238. [Google Scholar]
- Rangel-Acosta, J.L.; Martínez-Hernández, N.J.; Yonoff-Zapata, R. Respuesta de los escarabajos coprófagos (Scarabaeidae: Scarabaeinae) a la modificación del hábitat causada por un incendio forestal en la Reserva Bijibana, Atlántico-Colombia. Rev. Mex. Biodivers. 2020, 91, e912879. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather than Size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error Bars in Experimental Biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing Company: Oxford, UK, 2004. [Google Scholar]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional Dissimilarity as a Robust Measure of Ecological Distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring Specialization in Species Interaction Networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The Modularity of Pollination Networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Fründ, J.; Schaefer, H.M. Identifying Causes of Patterns in Ecological Networks: Opportunities and Limitations. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 559–584. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Olesen, J.M. Asymmetric Coevolutionary Networks Facilitate Biodiversity Maintenance. Science 2006, 312, 431–433. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- R Development Core Team. R: The R Project for Statistical Computing. 2024. Available online: https://www.r-project.org/ (accessed on 6 November 2024).[Green Version]
- Escobar, F. Diversity and Composition of Dung Beetle (Scarabaeinae) Assemblages in a Heterogeneous Andean Landscape. Trop. Zool. 2004, 17, 123–136. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R. Dung Beetles in Continuous Forest, Forest Fragments and in an Agricultural Mosaic Habitat Island at Los Tuxtlas, Mexico. Biodivers. Conserv. 2002, 11, 1903–1918. [Google Scholar] [CrossRef]
- Larsen, T.H.; Lopera, A.; Forsyth, A. Understanding Trait-Dependent Community Disassembly: Dung Beetles, Density Functions, and Forest Fragmentation. Conserv. Biol. 2008, 22, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Barragán, F.; Moreno, C.E.; Escobar, F.; Bueno-Villegas, J.; Halffter, G. The Impact of Grazing on Dung Beetle Diversity Depends on Both Biogeographical and Ecological Context. J. Biogeogr. 2014, 41, 1991–2002. [Google Scholar] [CrossRef]
- Marini, L.; Bartomeus, I.; Rader, R.; Lami, F. Species–Habitat Networks: A Tool to Improve Landscape Management for Conservation. J. Appl. Ecol. 2019, 56, 923–928. [Google Scholar] [CrossRef]
- González-Molina, M.; Martínez-Hernández, N.; Rico, Y. Genetic Structure and Demographic History of the Dung Beetle Deltochilum Guildingii (Scarabaeinae): Implications for Conservation of the Tropical Dry Forest in the Colombian Caribbean. J. Insect Conserv. 2024, 28, 1211–1221. [Google Scholar] [CrossRef]
- Beiroz, W.; Barlow, J.; Slade, E.M.; Borges, C.; Louzada, J.; Sayer, E.J. Biodiversity in Tropical Plantations Is Influenced by Surrounding Native Vegetation but Not Yield: A Case Study with Dung Beetles in Amazonia. For. Ecol. Manag. 2019, 444, 107–114. [Google Scholar] [CrossRef]
- Rangel-Acosta, J.L.; Hernández, N.J.M.; Gutierrez-Rapalino, B.P.; Gutierrez-Moreno, L.C.; Borja-Acuña, R.A. Efecto del tamaño de la ronda hidráulica sobre las comunidades de escarabajos coprófagos (Scarabaeidae: Scarabaeinae) en la cuenca media y baja del rio Cesar, Colombia. Entomotropica 2016, 31, 109–130. [Google Scholar]
- Waltert, M.; Bobo, K.S.; Kaupa, S.; Montoya, M.L.; Nsanyi, M.S.; Fermon, H. Assessing Conservation Values: Biodiversity and Endemicity in Tropical Land Use Systems. PLoS ONE 2011, 6, e16238. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Louzada, J.; Peres, C.A. Measuring the Conservation Value of Tropical Primary Forests: The Effect of Occasional Species on Estimates of Biodiversity Uniqueness. PLoS ONE 2010, 5, e9609. [Google Scholar] [CrossRef]
- Korasaki, V.; Braga, R.F.; Zanetti, R.; Moreira, F.M.S.; Vaz-de-Mello, F.Z.; Louzada, J. Conservation Value of Alternative Land-Use Systems for Dung Beetles in Amazon: Valuing Traditional Farming Practices. Biodivers. Conserv. 2013, 22, 1485–1499. [Google Scholar] [CrossRef]
- Campos, R.C.; Hernández, M.I.M. Changes in the Dynamics of Functional Groups in Communities of Dung Beetles in Atlantic Forest Fragments Adjacent to Transgenic Maize Crops. Ecol. Indic. 2015, 49, 216–227. [Google Scholar] [CrossRef]
- Alves, V.M.; Hettwer Giehl, E.L.; Lovato, P.E.; Vaz-de-Mello, F.Z.; Agudelo, M.B.; Medina Hernández, M.I. Dung Beetles and the Conservation of Diversity in an Agricultural Landscape with Maize Fields and Atlantic Forest Remnants. Acta Oecologica 2020, 107, 103598. [Google Scholar] [CrossRef]
- Braga, R.F.; Korasaki, V.; Audino, L.D.; Louzada, J. Are Dung Beetles Driving Dung-Fly Abundance in Traditional Agricultural Areas in the Amazon? Ecosystems 2012, 15, 1173–1181. [Google Scholar] [CrossRef]
- Righi, C.A.; Sandoval Rodríguez, C.; Ferreira, E.N.L.; Godoy, W.A.C.; Cognato, A.I. Microclimatic Conditions for Dung Beetle (Coleoptera: Scarabaeidae) Occurrence: Land Use System as a Determining Factor. Environ. Entomol. 2018, 47, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.d.S.P.d.; Louzada, J.N.C. Estrutura da comunidade de Scarabaeinae (Scarabaeidae: Coleoptera) em fitofisionomias do cerrado e sua importância para a conservação. Neotrop. Entomol. 2009, 38, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Briones, R.; Jerez, V. Efecto de La Edad de La Plantación de Pinus Radiata En La Abundancia de Ceroglossus Chilensis (Coleoptera: Carabidae) En La Región Del Biobío, Chile. Bosque 2007, 28, 207–214. [Google Scholar] [CrossRef]
- Reyes, E.; Delfín-González, H.; Morón, M.Á. Copro-Necrophagous Beetle (Coleoptera: Scarabaeidae) Diversity in an Agroecosystem in Yucatan, Mexico. Rev. Biol. Trop. 2007, 55, 83–99. [Google Scholar]
- Shahabuddin; Hidayat, P.; Manuwoto, S.; Noerdjito, W.A.; Tscharntke, T.; Schulze, C.H. Diversity and Body Size of Dung Beetles Attracted to Different Dung Types along a Tropical Land-Use Gradient in Sulawesi, Indonesia. J. Trop. Ecol. 2010, 26, 53–65. [Google Scholar] [CrossRef]
- Santos-Heredia, C.; Andresen, E.; Zárate, D.A.; Escobar, F. Dung Beetles and Their Ecological Functions in Three Agroforestry Systems in the Lacandona Rainforest of Mexico. Biodivers. Conserv. 2018, 27, 2379–2394. [Google Scholar] [CrossRef]
- Rivera, J.D.; Cantarero, K.J. Comunidad de Escarabajos Coprófagos (Coleoptera: Scarabaeidae: Scarabaeinae) en Hábitats bajo Distinta Intensidad de Uso en Yuscarán, Honduras. Ceiba 2011, 52, 212–229. [Google Scholar] [CrossRef]
- Horgan, F.G. Invasion and Retreat: Shifting Assemblages of Dung Beetles amidst Changing Agricultural Landscapes in Central Peru. Biodivers. Conserv. 2009, 18, 3519. [Google Scholar] [CrossRef]
- Scheffler, P.Y. Dung Beetle (Coleoptera: Scarabaeidae) Diversity and Community Structure across Three Disturbance Regimes in Eastern Amazonia. J. Trop. Ecol. 2005, 21, 9–19. [Google Scholar] [CrossRef]
- Breshears, D.D.; Nyhan, J.W.; Heil, C.E.; Wilcox, B.P. Effects of Woody Plants on Microclimate in a Semiarid Woodland: Soil Temperature and Evaporation in Canopy and Intercanopy Patches. Int. J. Plant Sci. 1998, 159, 1010–1017. [Google Scholar] [CrossRef]
- Pineda, E.; Moreno, C.; Escobar, F.; Halffter, G. Biodiversity in Cloud Forest and Shade Coffee: Analysis of Three Indicator Groups. Conserv. Biol. 2005, 19, 400–410. [Google Scholar] [CrossRef]
- Harvey, C.A.; Gonzalez, J.; Somarriba, E. Dung Beetle and Terrestrial Mammal Diversity in Forests, Indigenous Agroforestry Systems and Plantain Monocultures in Talamanca, Costa Rica. Biodivers. Conserv. 2006, 15, 555–585. [Google Scholar] [CrossRef]
- Braga, R.F.; Korasaki, V.; Andresen, E.; Louzada, J. Dung Beetle Community and Functions along a Habitat-Disturbance Gradient in the Amazon: A Rapid Assessment of Ecological Functions Associated to Biodiversity. PLoS ONE 2013, 8, e57786. [Google Scholar] [CrossRef]
- Gardner, T.A.; Barlow, J.; Araujo, I.S.; Ávila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. The Cost-Effectiveness of Biodiversity Surveys in Tropical Forests. Ecol. Lett. 2008, 11, 139–150. [Google Scholar] [CrossRef]
- Slade, E.M.; Mann, D.J.; Villanueva, J.F.; Lewis, O.T. Experimental Evidence for the Effects of Dung Beetle Functional Group Richness and Composition on Ecosystem Function in a Tropical Forest. J. Anim. Ecol. 2007, 76, 1094–1104. [Google Scholar] [CrossRef]
- Larsen, T.H.; Williams, N.M.; Kremen, C. Extinction Order and Altered Community Structure Rapidly Disrupt Ecosystem Functioning. Ecol. Lett. 2005, 8, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Carpio, C.; Woodward, G. Size-Dependent Species Removal Impairs Ecosystem Functioning in a Large-Scale Tropical Field Experiment. Ecology 2012, 93, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).