New Insights into the Telomere Structure in Hemiptera (Insecta) Inferred from Chromosome-Level and Scaffold-Level Genome Assemblies
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
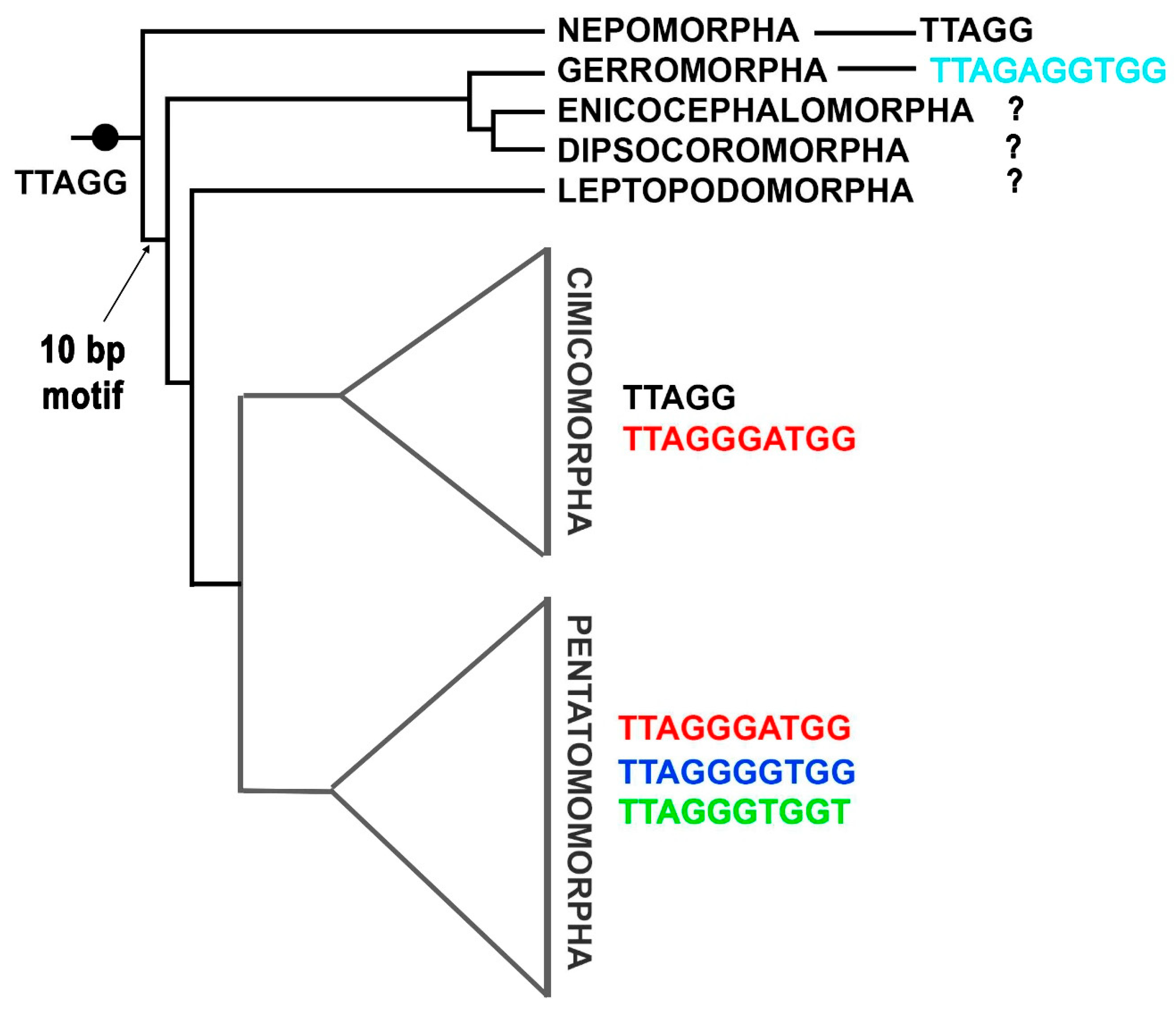
3.1. Variability of Telomeric Motifs in Chromosomes of Analyzed Hemiptera Species
| Family | Species | Meioformula | Repeats at Chromosomes’ Ends; Non-LTR Retrotransposons | GenBank |
|---|---|---|---|---|
| Auchenorrhyncha | ||||
| Aphrophoridae | Aphrophora alni (Fallén, 1805) | n = 14 + X | (TGAC)n: Chr. 1 left end (94% matches), Chr. 4 left end, Chr. 11 left end, Chr. 14 right end; no TRAS or SART. | GCA_963513935.1 |
| Cicadellidae | Allygus modestus Scott, 1876 | n = 6 + X | (TTAGG)n: Chr. 2 and 3 at both ends, Chr. 4 left end, Chr. 5 right end; no TRAS or SART. | GCA_963675035.1 |
| Cicadellidae | Arthaldeus pascuellus (Fallén, 1826) | n = 8 + X | (TTAGG)n: Chr. 1 right end, Chr. 2 left end Chr. X left end; Chr. 3 and Chr. 4 right end, Chr. 5 left end. Chr. 7 both ends; no TRAS or SART. | GCA_964204695.1 |
| Cicadellidae | Cicadella viridis (Linnaeus, 1758) * | n = 10 + X | (TTAGG)n: Chr, 1 left end; TTTGCGTGAG, TTTGCGTTAG, TTTGCTTGAG, TTTGCGTGTG, TTTGCGAGAG, TTTGCGTTAG, and many other 10 bp motifs: Chr. 1 right end, Chr. 2 left end, Chr. 5 left end, Chr. 6 right end; Chr. 7 and Chr. 8 both ends, Chr. 9 and Chr. 10 left end; no TRAS or SART. | GCA_964205205.1 |
| Cicadellidae | Empoasca decipiens Paoli, 1930 | n = 10 + X | (TTAGG)n: Chr. 3 right end, Chr. 7 right end; no TRAS or SART. | GCA_964267455.1 |
| Cicadellidae | Ribautiana ulmi (Linnaeus, 1758) | n = 8 + X | (TTAGG)n: Chr. 1 left end, Chr. 2 left end, Chr. 7 right end; no TRAS or SART. | GCA_964468525.1 |
| Sternorrhyncha | ||||
| Aphididae | Metopolophium dirhodum (Walker, 1849) | n = 9 | (TTAGG)n: Chr. 3 left end, Chr. 4 left end, Chr. 7 right end, 8 right end; SART: on the chromosomes where the (TTAGG)n was present (after the 380 position counting from the ends). | GCA_019925205.1 |
| Aphididae | Rhopalosiphum padi (Linnaeus, 1758) * | n = 4 | (TTAGG)n: Chr. 1 right end; SART: same chr. end (after the 3431 position). | GCA_020882245.1 |
| Aphididae | Schizaphis graminum (Rondani, 1852) * | n = 6 | (TTAGG)n: Chr. 2 left end; SART: same chr. end (after the 633 position). | GCA_020882235.1 |
| Aphididae | Tuberolachnus salignus (Gmelin, 1790) | n = 10 | (TTAGG)n: Chr. 1 and Chr. 10 both ends, Chr. 4 right end, Chr. 7 right end; SART: Chr. 7 right end (after the 1357 position counting from the end). | GCA_956483605.1 |
| Eriococcidae | Acanthococcus lagerstroemiae Borchsenius, 1960 | n = 9 | (TTAGG)n: Chr. 1 both ends, Chr. 2 right end, Chr. 9 right end; SART: Chr. 1 both ends (after the 4800 position), Chr. 2 right end (after the 4800 position), Chr. 9 right end (after the 665 position). | GCA_031841125.1 |
| Pseudococcidae | Trionymus diminutus (Leonardi, 1918) | n = 5 | (TTAGG)n Chr. 1, Chr. 3, Chr. 4 at both ends, Chr. 2 right end, Chr. 5 at both ends; TRAS: Chr. 2 left end (after the 3491 position), Chr. 3 left end (after the 7352 position). | GCA_959613365.1 |
| Pseudococcidae | Planococcus citri (Risso, 1813) | n = 5 | (TTAGG)n: Chr. 2 right end; Chr. 5 right. end, Chr. 3 right end, Chr. 4 right end, Chr. 1 both ends; TRAS and SART: same chromosome ends after the 5000 position. | GCA_950023065.1 |
| Heteroptera | ||||
| Aradidae | Aradus depressus (Fabricius, 1794) * | n = 12 + XY | (TTAGGGATGG)n: Chr. 1 left end. SART: same chromosome end (after the 7906 position). | GCA_963662175.1 |
| Aradidae | Aradus truncatus Fieber, 1860 | n = 13 + XY | (TTAGGGATGG)n: Chr. Y left end, Chr. 12 left end. SART: same chromosome ends (after the 1443 position). | GCA_965153375.1 |
| Gerridae | Gerris lacustris (Linnaeus, 1758), | n = 10 + X | (TTAGAGGTGG)n: Chr. 2, 3, 4, 8, 10 both ends, Chr. 7 left end, Chr. X right end; no TRAS or SART. | GCA_951217055.1 |
3.2. Occurrence of SART and TRAS Retrotransposons in Hemiptera
3.3. Diversity of Telomeric Repeats in Hemiptera
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller, H.J. The remaking of chromosomes. Collect. Net. 1938, 13, 181–198. [Google Scholar]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- Zakian, V.A. Telomeres: Beginning to understand the end. Science 1995, 270, 1601–1607. [Google Scholar] [CrossRef]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W. Telomere length regulation. Annu. Rev. Biochem. 1996, 65, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Fajkus, J.; Sýkorová, E.; Leitch, A.R. Telomeres in evolution and evolution of telomeres. Chromosome Res. 2005, 13, 469–479. [Google Scholar] [CrossRef]
- Traut, W.; Szczepanowski, M.; Vítková, M.; Opitz, C.; Marec, F.; Zrzavý, J. The telomere repeat motif of basal Metazoa. Chromosome Res. 2007, 15, 371–382. [Google Scholar] [CrossRef]
- Lukhtanov, V.A.; Kuznetsova, V.G. What genes and chromosomes say about the origin and evolution of insects and other arthropods. Russ. J. Genet. 2010, 46, 1115–1121. [Google Scholar] [CrossRef]
- Anokhin, B.A.; Kuznetsova, V.G. FISH-based karyotyping of Pelmatohydra oligactis, Hydra oxycnida, and H. magnipapillata (Cnidaria, Hydrozoa). Comp. Cytogenet. 2018, 12, 539–548. [Google Scholar] [CrossRef]
- Čapková Frydrychová, R. Telomerase as a possible key to bypass reproductive cost. Mol. Ecol. 2023, 32, 2134–2143. [Google Scholar] [CrossRef]
- Peška, V.; García, S. Origin, diversity, and evolution of telomere sequences in plants. Front. Plant Sci. 2020, 11, 117. [Google Scholar] [CrossRef]
- Frydrychová, R.; Grossmann, P.; Trubač, P.; Vítková, M.; Marec, F. Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome 2004, 47, 163–178. [Google Scholar] [CrossRef]
- Vítková, M.; Král, J.; Traut, W.; Zrzavý, J.; Marec, F. The evolutionary origin of insect telomeric repeats, (TTAGG)n. Chromosome Res. 2005, 13, 145–156. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Grozeva, S.; Gokhman, V. Telomere structure in insects: A review. J. Zool. Syst. Evol. Res. 2020, 58, 127–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Xiong, X.; Appel, A.G.; Zhang, C.; Wang, X. Profiles of telomeric repeats in Insecta reveal diverse forms of telomeric motifs in Hymenopterans. Life Sci. Alliance 2022, 5, e202101163. [Google Scholar] [CrossRef]
- Prušáková, D.; Peška, V.; Pekár, S.; Bubeník, M.; Čížek, L.; Bezděk, A.; Čapková Frydrychová, R. Telomeric DNA sequences in beetle taxa vary with species richness. Sci. Rep. 2021, 11, 13319. [Google Scholar] [CrossRef]
- Lukhtanov, V.A.; Pazhenkova, E.A. Diversity and evolution of telomeric motifs and telomere DNA organization in insects. Biol. J. Linn. Soc. 2023, 140, 536–555. [Google Scholar] [CrossRef]
- Stoianova, D.; Grozeva, S.; Golub, N.V.; Anokhin, B.A.; Kuznetsova, V.G. The first FISH-confirmed non-canonical telomeric motif in Heteroptera: Cimex lectularius and C. hemipterus have a 10 bp motif (TTAGGGATGG)n. Genes 2024, 15, 1026. [Google Scholar] [CrossRef]
- Lukhtanov, V.A. Telomere DNA in the insect order Dermaptera and the first evidence for the non-canonical telomeric motif TTCGG in Arthropoda. Comp. Cytogenet. 2025, 19, 13–18. [Google Scholar] [CrossRef]
- Rico-Porras, J.M.; Mora, P.; Palomeque, T.; Cabral-de-Mello, D.C.; Lorite, P. Comparative cytogenetics of Lachnaia species (Coleoptera: Chrysomelidae) reveals a novel telomeric motif (TTTGG) in insects. Org. Divers. Evol. 2025, 25, 329–342. [Google Scholar] [CrossRef]
- Menezes, R.S.T.; Bardella, V.B.; Cabral-de-Mello, D.C.; Lucena, D.A.A.; Almeida, E.A.B. Are the TTAGG and TTAGGG telomeric repeats phylogenetically conserved in aculeate Hymenoptera? Sci. Nat. 2017, 104, 85. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Golub, N.; Anokhin, B.; Stoianova, D.; Lukhtanov, V. Diversity of telomeric sequences in true bugs (Heteroptera): New data on the infraorders Pentatomomorpha and Cimicomorpha. Cytogenet. Genome Res. 2025, in press. [CrossRef]
- López-Fernández, C.; Pradillo, E.; Zabal-Aguirre, M.; Fernández, J.L.; Garcia de la Vega, C.; Gosálvez, J. Telomeric and interstitial telomeric-like DNA sequence in Orthoptera genomes. Genome 2004, 47, 757–763. [Google Scholar] [CrossRef]
- Jetybayev, I.E.; Bugrov, A.G.; Karamysheva, T.V.; Camacho, J.P.M.; Rubtsov, N.B. Chromosomal localization of ribosomal and telomeric DNA provides new insights on the evolution of Gomphocerinae grasshoppers. Cytogenet. Genome Res. 2012, 138, 36–45. [Google Scholar] [CrossRef]
- Sasaki, T.; Fujiwara, H. Detection and distribution patterns of telomerase activity in insects. Eur. J. Biochem. 2000, 267, 3025–3031. [Google Scholar] [CrossRef]
- Cabral-de-Mello, D.C.; Gasparotto, A.E.; Rico-Porras, J.M.; Ferretti, A.B.S.; Mora-Ruiz, P.; Alves-Gomes, R.T.; Lourejan, V.; Scudeler, E.L.; Lorite, P.; Bardella, V.B. First insights into the satellitomes and new evidence for the absence of canonical insect telomere in the Neuroptera order. Genome, 2025; ahead of print. [Google Scholar] [CrossRef]
- Frydrychová, R.; Marec, F. Repeated losses of TTAGG telomere repeats in evolution of beetles (Coleoptera). Genetica 2002, 115, 179–187. [Google Scholar] [CrossRef]
- Mravinac, B.; Meštrović, N.; Čavrak, V.; Plohl, M. TCAGG, an alternative telomeric sequence in insects. Chromosoma 2011, 120, 367–376. [Google Scholar] [CrossRef]
- Vershinina, A.O.; Anokhin, B.A.; Lukhtanov, V.A. Ribosomal DNA clusters and telomeric (TTAGG)n repeats in blue butterflies (Lepidoptera, Lycaenidae) with low and high chromosome numbers. Comp. Cytogenet. 2015, 9, 161–171. [Google Scholar] [CrossRef]
- Rego, A.; Marec, F. Telomeric and interstitial telomeric sequences in holokinetic chromosomes of Lepidoptera: Telomeric DNA mediates association between postpachytene bivalents in achiasmatic meiosis of females. Chromosome Res. 2003, 11, 681–694. [Google Scholar] [CrossRef]
- Rosén, M.; Edström, J. DNA structures common for chironomid telomeres terminating with complex repeats. Insect Mol. Biol. 2000, 9, 341–347. [Google Scholar] [CrossRef]
- Danilevskaya, O.N.; Tan, C.T.; Wong, J.; Alibhai, M.; Pardue, M.L. Unusual features of the Drosophila melanogaster telomere transposable element HeT-A are conserved in Drosophila yakuba telomere elements. Proc. Natl. Acad. Sci. USA 1998, 95, 3770–3775. [Google Scholar] [CrossRef] [PubMed]
- Madalena, C.R.G.; Fernandes, T.; Villasante, A.; Gorab, E. Curiously composite structures of a retrotransposon and a complex repeat associated with chromosome ends of Rhynchosciara americana (Diptera: Sciaridae). Chromosome Res. 2010, 18, 587–598. [Google Scholar] [CrossRef]
- Biessmann, H.; Zurovcova, M.; Yao, J.G.; Lozovskaya, E.; Walter, M.F. A telomeric satellite in Drosophila virilis and its sibling species. Chromosoma 2000, 109, 372–380. [Google Scholar] [CrossRef]
- Shcherbakov, D.E.; Popov, Y.A. Order Hemiptera Linné, 1758. The bugs, cicadas, plantlice, scale insects, etc. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 143–157. [Google Scholar]
- Kieran, T.J.; Gordon, E.R.; Forthman, M.; Hoey-Chamberlain, R.; Kimball, R.T.; Faircloth, B.C.; Weirauch, C.; Glenn, T.C. Insight from an ultraconserved element bait set designed for hemipteran phylogenetics integrated with genomic resources. Mol. Phylogenet. Evol. 2019, 130, 297–303. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; pp. 1–755. [Google Scholar]
- Chen, J.; Szwedo, J.; Wang, B.; Zheng, Y.; Jiang, H.; Jiang, T.; Wang, X.; Zhang, H. A new bizarre cicadomorph family in mid-Cretaceous Burmese amber (Hemiptera, Clypeata). Cretac. Res. 2019, 97, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wu, H.Y.; Rédei, D.; Xie, Q.; Chen, Y.; Chen, P.P.; Dong, Z.E.; Dang, K.; Damgaard, J.; Štys, P. When did the ancestor of true bugs become stinky? Disentangling the phylogenomics of Hemiptera-Heteroptera. Cladistics 2019, 35, 42–66. [Google Scholar] [CrossRef]
- Okazaki, S.; Tsuchida, K.; Maekawa, H.; Ishikawa, H.; Fujiwara, H. Identification of a pentanucleotide telomeric sequence, (TTAGG)n, in the silkworm Bombyx mori and in other insects. Mol. Cell. Biol. 1993, 13, 1424–1432. [Google Scholar] [CrossRef]
- Spence, J.M.; Blackman, R.L.; Testa, J.M.; Ready, P.D. A 169-base-pair tandem repeat DNA marker for subtelomeric heterochromatin and chromosomal rearrangements in aphids of the Myzus persicae group. Chromosome Res. 1998, 6, 167–175. [Google Scholar] [CrossRef]
- Sahara, K.; Marec, F.; Traut, W. TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res. 1999, 7, 449–460. [Google Scholar] [CrossRef]
- Bizzaro, D.; Mandrioli, M.; Zanotti, M.; Giusti, M.; Manicardi, G.C. Chromosome analysis and molecular characterization of highly repeated DNAs in the aphid Acyrthosiphon pisum (Aphididae, Hemiptera). Genetica 2000, 108, 197–202. [Google Scholar] [CrossRef] [PubMed]
- International Aphid Genomics Consortium. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010, 8, e1000313. [Google Scholar] [CrossRef]
- Mandrioli, M.; Azzoni, P.; Lombardo, G.; Manicardi, G.C. Composition and epigenetic markers of heterochromatin in the aphid Aphis nerii (Hemiptera: Aphididae). Cytogenet. Genome Res. 2011, 133, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Novotná, J.; Havelka, J.; Starý, P.; Koutecký, P.; Vítková, M. Karyotype analysis of the Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Hemiptera: Aphididae) reveals a large X chromosome with rRNA and histone gene families. Genetica 2011, 139, 281–289. [Google Scholar] [CrossRef]
- Mohan, K.N.; Rani, B.S.; Kulashreshta, P.S.; Kadandale, J.S. Characterization of TTAGG telomeric repeats, their interstitial occurrence and constitutively active telomerase in the mealybug Planococcus lilacinus (Homoptera; Coccoidea). Chromosoma 2011, 120, 165–175. [Google Scholar] [CrossRef]
- Grozeva, S.; Kuznetsova, V.; Anokhin, B. Karyotypes, male meiosis and comparative FISH mapping of 18S ribosomal DNA and telomeric (TTAGG)n repeat in eight species of true bug (Hemiptera, Heteroptera). Comp. Cytogenet. 2011, 5, 355–374. [Google Scholar] [CrossRef]
- Kuznetsova, V.G.; Grozeva, S.M.; Anokhin, B.A. The first finding of (TTAGG)n telomeric repeat in chromosomes of true bugs (Heteroptera, Belostomatidae). Comp. Cytogenet. 2012, 6, 341–346. [Google Scholar] [CrossRef]
- Maryańska-Nadachowska, A.; Kuznetsova, V.G.; Karamysheva, T.V. Chromosomal location of rDNA clusters and TTAGG telomeric repeats in eight species of the spittlebug genus Philaenus (Hemiptera: Auchenorrhyncha: Aphrophoridae). Eur. J. Entomol. 2013, 110, 411–418. [Google Scholar] [CrossRef]
- Monti, V.; Manicardi, G.C.; Mandrioli, M. Cytogenetic and molecular analysis of the holocentric chromosomes of the potato aphid Macrosiphum euphorbiae (Thomas, 1878). Comp. Cytogenet. 2011, 5, 163–172. [Google Scholar] [CrossRef]
- Monti, V.; Giusti, M.; Bizzaro, D.; Manicardi, G.C.; Mandrioli, M. Presence of a functional (TTAGG)n telomere–telomerase system in aphids. Chromosome Res. 2011, 19, 625–633. [Google Scholar] [CrossRef]
- Monti, V.; Serafini, K.; Manicardi, G.C.; Mandrioli, M. Characterization of non-LTR retrotransposable TRAS elements in the aphids Acyrthosiphon pisum and Myzus persicae (Aphididae, Hemiptera). J. Hered. 2013, 104, 547–553. [Google Scholar] [CrossRef]
- Golub, N.V.; Kuznetsova, V.G.; Rakitov, R.A. First karyotype data on the family Myerslopiidae (Hemiptera, Auchenorrhyncha, Cicadomorpha). Comp. Cytogenet. 2014, 8, 293–300. [Google Scholar] [CrossRef]
- Korandová, M.; Krůček, T.; Vrbová, K.; Frydrychová, R. Distribution of TTAGG-specific telomerase activity in insects. Chromosome Res. 2014, 22, 495–503. [Google Scholar] [CrossRef]
- Mandrioli, M.; Zanasi, F.; Manicardi, G.C. Karyotype rearrangements and telomere analysis in Myzus persicae (Hemiptera, Aphididae) strains collected on Lavandula sp. plants. Comp. Cytogenet. 2014, 8, 259–274. [Google Scholar] [CrossRef]
- Kuznetsova, V.G.; Maryańska-Nadachowska, A.; Anokhin, B.; Aguin Pombo, D. Evidence for TTAGG telomere repeats and rRNA gene clusters in leafhoppers of the genus Alebra (Hemiptera: Auchenorrhyncha: Cicadellidae). Eur. J. Entomol. 2015, 112, 207–214. [Google Scholar] [CrossRef]
- Kuznetsova, V.G.; Grozeva, S.M.; Hartung, V.; Anokhin, B.A. First evidence for (TTAGG)n telomeric sequence and sex chromosome post-reduction in Coleorrhyncha (Insecta, Hemiptera). Comp. Cytogenet. 2015, 9, 523–532. [Google Scholar] [CrossRef]
- Golub, N.V.; Golub, V.B.; Kuznetsova, V.G. Variability of 18 rDNA loci in four lace bug species (Hemiptera, Tingidae) with the same chromosome number. Comp. Cytogenet. 2015, 9, 513–522. [Google Scholar] [CrossRef]
- Anjos, A.; Rocha, G.C.; Paladini, A.; Mariguela, T.C.; Cabral-de-Mello, D.C. Karyotypes and repetitive DNA evolution in six species of the genus Mahanarva (Auchenorrhyncha: Cercopidae). Cytogenet. Genome Res. 2016, 149, 321–327. [Google Scholar] [CrossRef]
- Maryańska-Nadachowska, A.; Anokhin, B.A.; Gnezdilov, V.M.; Kuznetsova, V.G. Karyotype stability in the family Issidae (Hemiptera, Auchenorrhyncha) revealed by chromosome techniques and FISH with telomeric (TTAGG)n and 18S rDNA probes. Comp. Cytogenet. 2016, 10, 347–369. [Google Scholar] [CrossRef]
- Pita, S.; Panzera, F.; Mora, P.; Vela, J.; Palomeque, T.; Lorite, P. The presence of the ancestral insect telomeric motif in kissing bugs (Triatominae) rules out the hypothesis of its loss in evolutionarily advanced Heteroptera (Cimicomorpha). Comp. Cytogenet. 2016, 10, 427–437. [Google Scholar] [CrossRef]
- Chirino, M.G.; Dalíková, M.; Marec, F.; Bressa, M.J. Chromosomal distribution of interstitial telomeric sequences as signs of evolution through chromosome fusion in six species of the giant water bugs (Belostoma, Hemiptera). Ecol. Evol. 2017, 7, 5227–5235. [Google Scholar] [CrossRef]
- Angus, R.B.; Jeangirard, C.; Stoianova, D.; Grozeva, S.; Kuznetsova, V.G. Chromosomal analysis of Nepa cinerea Linnaeus, 1758 and Ranatra linearis (Linnaeus, 1758) (Heteroptera, Nepidae). Comp. Cytogenet. 2017, 11, 641–657. [Google Scholar] [CrossRef]
- Golub, N.V.; Golub, V.B.; Kuznetsova, V.G. Distribution of the major rDNA loci among four hemipteran species of the family Tingidae (Heteroptera, Cimicomorpha). Folia Biol. 2017, 65, 155–158. [Google Scholar] [CrossRef]
- Maryańska-Nadachowska, A.; Kuznetsova, V.G.; Golub, N.V.; Anokhin, B.A. Detection of telomeric sequences and ribosomal RNA genes in holokinetic chromosomes of five jumping plant-lice species: First data on the superfamily Psylloidea (Hemiptera: Sternorrhyncha). Eur. J. Entomol. 2018, 115, 632–640. [Google Scholar] [CrossRef]
- Luan, J.; Sun, X.; Fei, Z.; Douglas, A.E. Maternal inheritance of a single somatic animal cell displayed by the bacteriocyte in whitefly Bemisia tabaci. Curr. Biol. 2018, 28, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Golub, N.V.; Golub, V.B.; Kuznetsova, V.G. New data on karyotypes of lace bugs (Tingidae, Cimicomorpha, Hemiptera) with analysis of the 18S rDNA clusters distribution. Comp. Cytogenet. 2018, 12, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Grozeva, S.; Anokhin, B.A.; Simov, N.; Kuznetsova, V.G. New evidence for the presence of the telomere motif (TTAGG)n in the family Reduviidae and its absence in the families Nabidae and Miridae (Hemiptera, Cimicomorpha). Comp. Cytogenet. 2019, 13, 283. [Google Scholar] [CrossRef]
- Karagyan, G.; Golub, N.; Sota, T. Cytogenetic characterization of periodical cicadas (Hemiptera: Cicadidae: Magicicada). Eur. J. Entomol. 2020, 117, 474–480. [Google Scholar] [CrossRef]
- Ferguson, K.B.; Visser, S.; Dalíková, M.; Provazníková, I.; Urbaneja, A.; Pérez-Hedo, M.; Marec, F.; Werren, J.H.; Zwaan, B.J.; Pannebakker, B.A.; et al. Jekyll or Hyde? The genome (and more) of Nesidiocoris tenuis, a zoophytophagous predatory bug that is both a biological control agent and a pest. Insect Mol. Biol. 2021, 30, 188–209. [Google Scholar] [CrossRef]
- Gapon, D.A.; Kuznetsova, V.G.; Maryańska-Nadachowska, A. A new species of the genus Rhaphidosoma Amyot et Serville, 1843 (Heteroptera, Reduviidae), with data on its chromosome complement. Comp. Cytogenet. 2021, 15, 467. [Google Scholar] [CrossRef]
- Golub, N.V.; Golub, V.B.; Anokhin, B.A.; Kuznetsova, V.G. Comparative cytogenetics of lace bugs (Tingidae, Heteroptera): New data and a brief overview. Insects 2022, 13, 608. [Google Scholar] [CrossRef]
- Golub, N.V.; Maryańska-Nadachowska, A.; Anokhin, B.A.; Kuznetsova, V.G. Expanding the chromosomal evolution understanding of lygaeioid true bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by classical and molecular cytogenetic analysis. Genes 2023, 14, 725. [Google Scholar] [CrossRef]
- Jing, T.; Yang, J.; Pan, J.; Liu, X.; Yang, X.; Farhan, M.; Su, H.; Ma, X.; Zhang, S. A near-complete genome reveals the population evolution of the cotton-melon aphid Aphis gossypii. Insect Biochem. Mol. Biol. 2025, 176, 104215. [Google Scholar] [CrossRef]
- Palmer Droguett, D.H.; Fletcher, M.; Alston, B.T.; Kocher, S.; Cabral-de-Mello, D.C.; Wright, A.E. Neo-sex chromosome evolution in treehoppers despite long-term X chromosome conservation. Genome Biol. Evol. 2024, 16, evae264. [Google Scholar] [CrossRef]
- Lukhtanov, V.A. Diversity and evolution of telomere and subtelomere DNA sequences in insects. bioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.M.; Trieb, J.; Troestl, M.; Renfrew, K.; Mandáková, T.; Fulneček, J.; Shippen, D.E.; Říha, K. A hypomorphic allele of telomerase uncovers the minimal functional length of telomeres in Arabidopsis. Genetics 2021, 219, iyab126. [Google Scholar] [CrossRef]
- Kubo, Y.; Okazaki, S.; Anzai, T.; Fujiwara, H. Structural and phylogenetic analysis of TRAS, telomeric repeat-specific non-LTR retrotransposon families in lepidopteran insects. Mol. Biol. Evol. 2001, 18, 848–857. [Google Scholar] [CrossRef][Green Version]
- Brown, M.R.; De La Rosa, P.G.; Balxter, M. tidk: A toolkit to rapidly identify telomeric repeats from genomic datasets. Bioinformatics 2025, 42, btaf049. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Osanai, M.; Matsumoto, T.; Kojima, K.K. Telomere-specific non-LTR retrotransposons and telomere maintenance in the silkworm Bombyx mori. Chromosome Res. 2005, 13, 455–467. [Google Scholar] [CrossRef]
- Osanai, M.; Kojima, K.K.; Futahashi, R.; Yaguchi, S.; Fujiwara, H. Identification and characterization of the telomerase reverse transcriptase of Bombyx mori and Tribolium castaneum. Gene 2006, 376, 281–289. [Google Scholar] [CrossRef]
- Kirkness, E.F.; Haas, B.J.; Sun, W.; Braig, H.R.; Perotti, M.A.; Clark, J.M.; Lee, S.H.; Robertson, H.M.; Kennedy, R.C.; Elhaik, E.; et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 12168–12173. [Google Scholar] [CrossRef]
- Robertson, H.M.; Gordon, K.H. Canonical TTAGG-repeat telomeres and telomerase in the honey bee, Apis mellifera. Genome Res. 2006, 16, 1345–1351. [Google Scholar] [CrossRef]
- Vicari, M.R.; Bruschi, D.P.; Cabral-de-Mello, D.C.; Nogaroto, V. Telomere organization and the involvement of interstitial telomeric sites in insect and vertebrate chromosome evolution. Genet. Mol. Biol. 2022, 45, e20220071. [Google Scholar] [CrossRef]
- Nicholson, S.J.; Nickerson, M.L.; Dean, M.; Song, Y.; Hoyt, P.R.; Rhee, H.; Kim, C.; Puterka, G.J. The genome of Diuraphis noxia, a global aphid pest of small grains. BMC Genom. 2015, 16, 429. [Google Scholar] [CrossRef]
- Tang, H. Genome assembly, rearrangement, and repeats. Chem. Rev. 2007, 107, 3391–3406. [Google Scholar] [CrossRef]
- Rhie, A.; McCarthy, S.A.; Fedrigo, O.; Damas, J.; Formenti, G.; Koren, S.; Uliano-Silva, M.; Chow, W.; Fungtammasan, A.; Kim, J.; et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature 2021, 592, 737–746. [Google Scholar] [CrossRef]
- Kim, S.; Chowdhury, T.; Yu, H.J.; Kahng, J.Y.; Lee, C.E.; Kang, H.; Lee, J.H.; Lee, S.-T.; Won, J.-K.; Kim, K.H.; et al. The telomere maintenance mechanism spectrum and its dynamics in gliomas. Genome Med. 2022, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.T.; Mergny, J.-L.; Alberti, P. Stability of telomeric G-quadruplexes. Nucleic Acids Res. 2011, 39, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, C.; Schuh, R.T.; Cassis, G.; Wheeler, W.C. Revisiting habitat and lifestyle transitions in Heteroptera (Insecta: Hemiptera): Insights from a combined morphological and molecular phylogeny. Cladistics 2019, 35, 67–105. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.M. Classification, phylogeny, and zoogeography of the pond skater genus Gerris Fabricius (Hemiptera: Gerridae). Can. J. Zool. 1993, 71, 2473–2508. [Google Scholar] [CrossRef]
- Fajkus, P.; Adámik, M.; Nelson, A.D.L.; Kilar, A.M.; Franek, M.; Bubeník, M.; Frydrychová, R.Č.; Votavová, A.; Sýkorová, E.; Fajkus, J.; et al. Telomerase RNA in Hymenoptera (Insecta) switched to plant/ciliate-like biogenesis. Nucleic Acids Res. 2023, 51, 420–433. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoianova, D.; Grozeva, S.; Todorova, N.; Rangelov, M.; Lukhtanov, V.A.; Kuznetsova, V.G. New Insights into the Telomere Structure in Hemiptera (Insecta) Inferred from Chromosome-Level and Scaffold-Level Genome Assemblies. Diversity 2025, 17, 552. https://doi.org/10.3390/d17080552
Stoianova D, Grozeva S, Todorova N, Rangelov M, Lukhtanov VA, Kuznetsova VG. New Insights into the Telomere Structure in Hemiptera (Insecta) Inferred from Chromosome-Level and Scaffold-Level Genome Assemblies. Diversity. 2025; 17(8):552. https://doi.org/10.3390/d17080552
Chicago/Turabian StyleStoianova, Desislava, Snejana Grozeva, Nadezhda Todorova, Miroslav Rangelov, Vladimir A. Lukhtanov, and Valentina G. Kuznetsova. 2025. "New Insights into the Telomere Structure in Hemiptera (Insecta) Inferred from Chromosome-Level and Scaffold-Level Genome Assemblies" Diversity 17, no. 8: 552. https://doi.org/10.3390/d17080552
APA StyleStoianova, D., Grozeva, S., Todorova, N., Rangelov, M., Lukhtanov, V. A., & Kuznetsova, V. G. (2025). New Insights into the Telomere Structure in Hemiptera (Insecta) Inferred from Chromosome-Level and Scaffold-Level Genome Assemblies. Diversity, 17(8), 552. https://doi.org/10.3390/d17080552










