One Genome, Multiple Phenotypes: Would Rhodnius milesi Carcavallo, Rocha, Galvão & Jurberg, 2001 (Hemiptera, Triatominae) Be a Valid Species or a Phenotypic Polymorphism of R. neglectus Lent, 1954? †
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Analyses
2.1.1. DNA Extraction
2.1.2. Cytochrome B Amplification
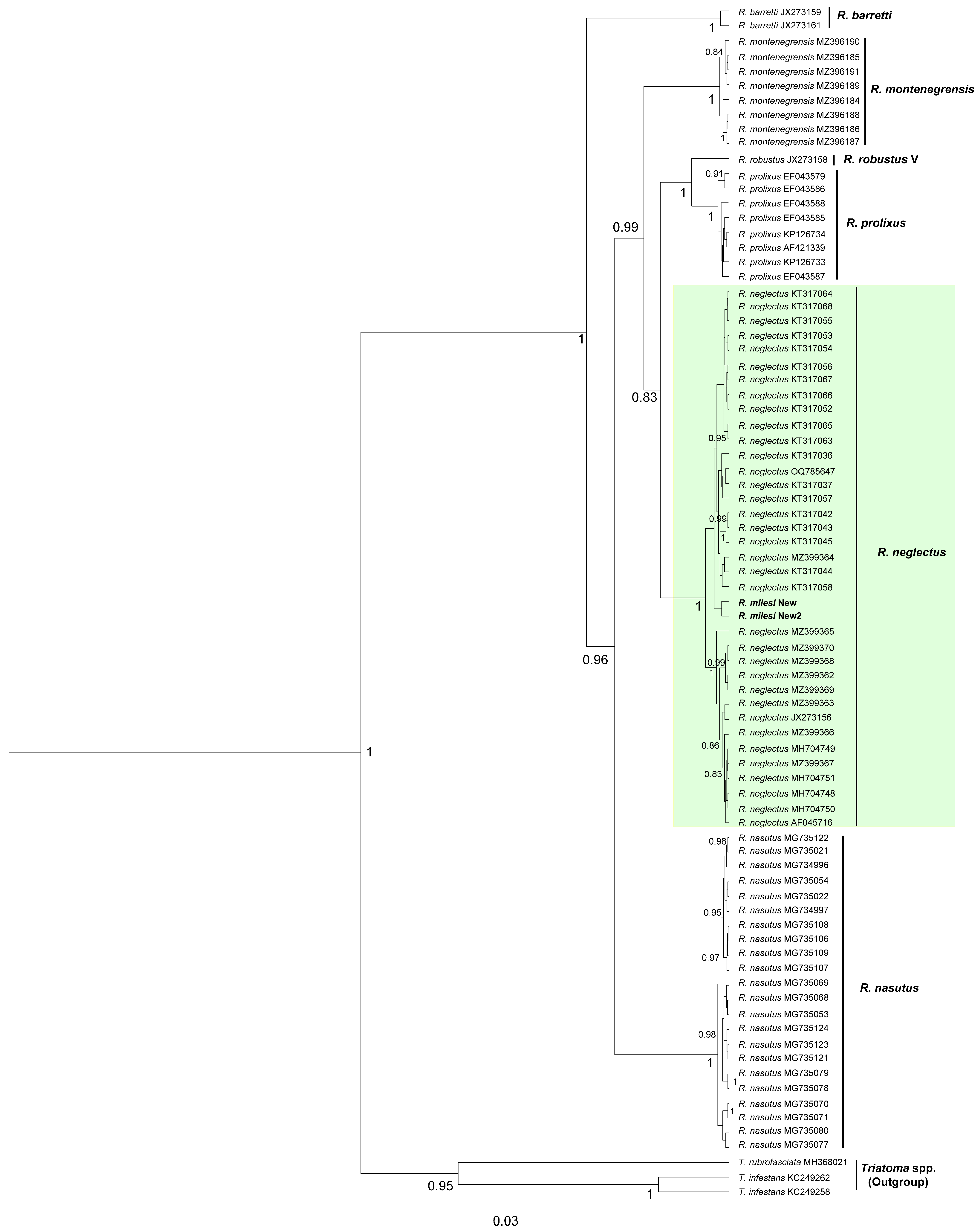
2.1.3. Phylogenetic and Genetic Distance Analyses
2.2. Experimental Crosses
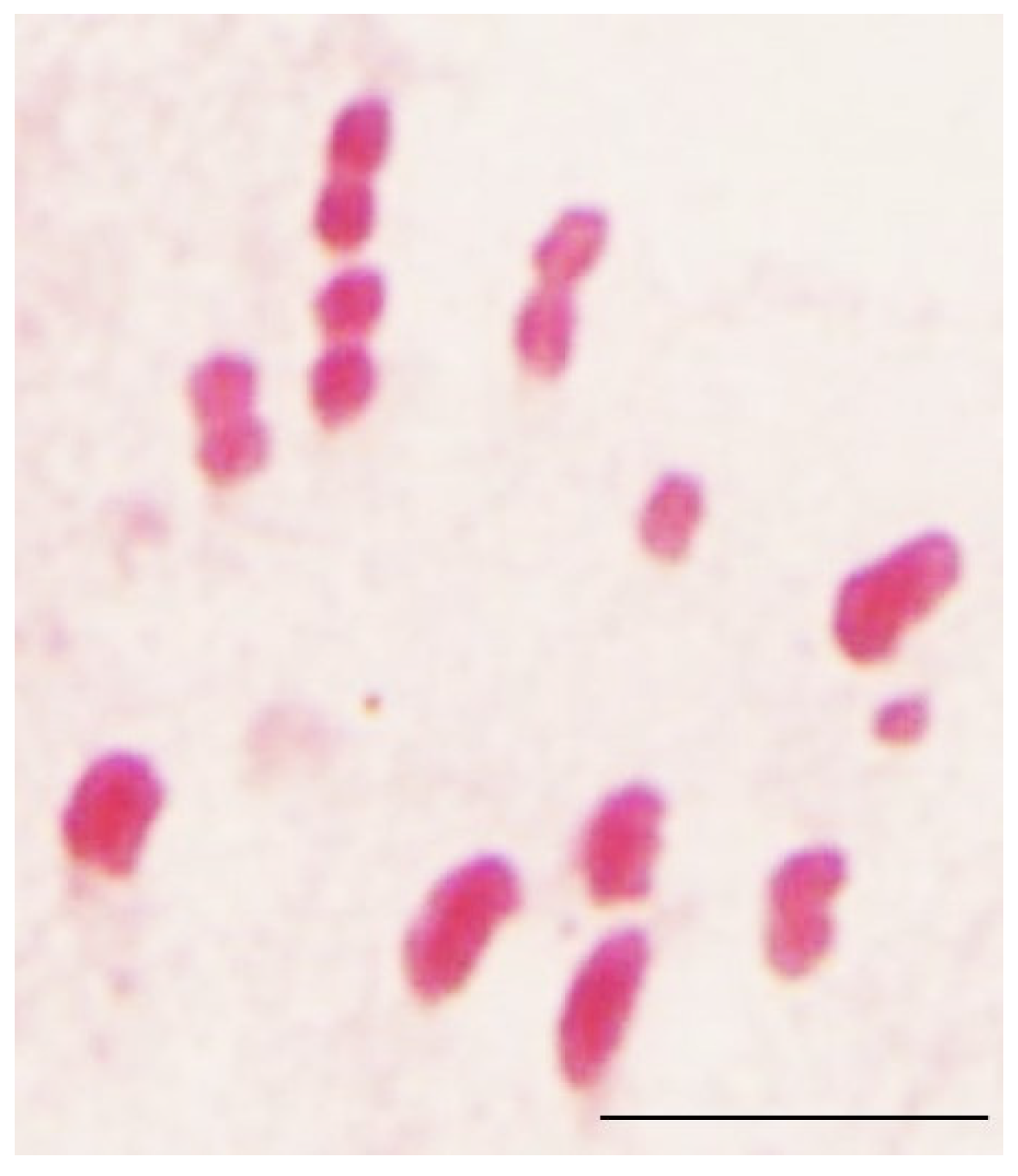
2.3. Cytogenetic Analysis
3. Results and Discussion
4. Conclusions
- Taxonomy
- Kingdom Animalia Linnaeus, 1758
- Phylum Arthropoda von Siebold, 1848
- Class Insecta Linnaeus, 1758
- Order Hemiptera Linnaeus, 1758
- Suborder Heteroptera Latreille, 1810
- Family Reduviidae Latreille, 1807
- Subfamily Triatominae Jeannel, 1919
- Tribe Rhodniini Pinto, 1926
- Genus Rhodnius Stål, 1859
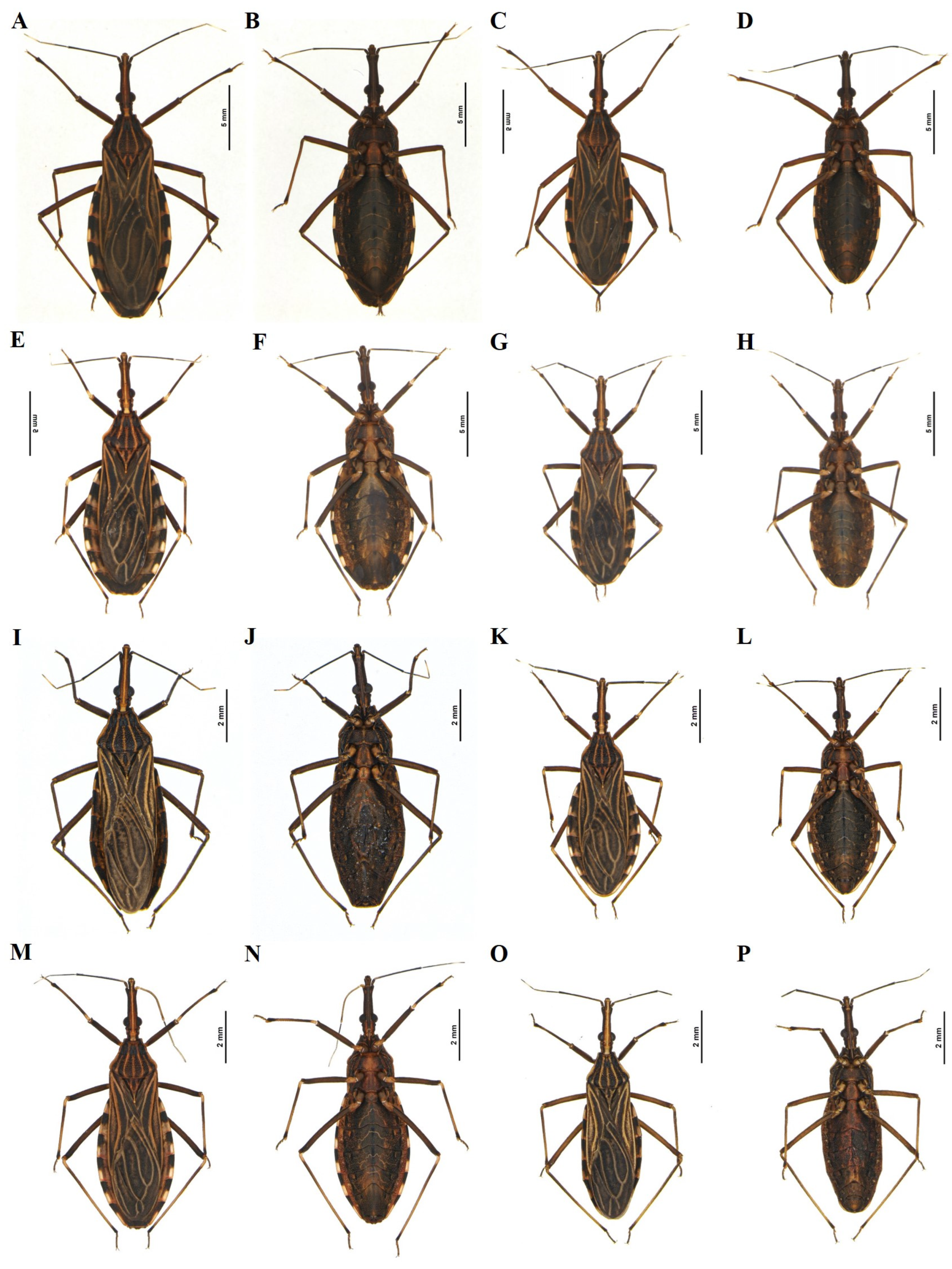
- Rhodnius neglectus Lent, 1954 (Figure 5D–F)
- Rhodnius milesi Carcavallo, Rocha, Galvão & Jurberg, 2001 (in: Valente et al. 2001), syn. nov. (Figure 5A–C)
- urn:lsid:zoobank.org:pub:DC76CE99-5F14-4D6C-886A-4AD0DD20073E
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 24 April 2024).
- Alevi, K.C.C.; Oliveira, J.; Rocha, D.S.; Galvão, C. Trends in Taxonomy of Chagas Disease Vectors (Hemiptera, Reduviidae, Triatominae): From Linnaean to Integrative Taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef]
- Oliveira Correia, J.P.S.O.; Gil-Santana, H.R.; Dale, C.; Galvão, C. Triatoma guazu Lent and Wygodzinsky Is a Junior Synonym of Triatoma williami Galvão, Souza and Lima. Insects 2022, 13, 591. [Google Scholar] [CrossRef]
- Gil-Santana, H.R.; Chavez, T.; Pita, S.; Panzera, F.; Galvão, C. Panstrongylus noireaui, a remarkable new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys 2022, 1104, 203–225. [Google Scholar] [CrossRef]
- Téllez-Rendón, J.; Esteban, L.; Rengifo-Correa, L.; Díaz-Albiter, H.; Huerta, H.; Dale, C. Triatoma yelapensis sp. nov. (Hemiptera: Reduviidae) from Mexico, with a Key of Triatoma Species Recorded in Mexico. Insects 2023, 14, 331. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, M.; Li, H.; Cai, W. Review of Kissing Bugs (Hemiptera: Reduviidae: Triatominae) from China with Descriptions of Two New Species. Insects 2023, 14, 450. [Google Scholar] [CrossRef]
- Oliveira-Correia, J.P.S.; de Oliveira, J.; Gil-Santana, H.R.; da Silva Rocha, D.; Galvão, C. Taxonomic reassessment of Rhodnius zeledoni Jurberg, Rocha & Galvão: A morphological and morphometric analysis comparing its taxonomic relationship with Rhodnius domesticus Neiva & Pinto. BMC Zool. 2024, 9, 6. [Google Scholar]
- Justi, S.; Galvão, C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. 2017, 33, 42–52. [Google Scholar] [CrossRef]
- Justi, S.A.; Russo, C.A.M.; dos Santos Mallet, J.R.; Obara, M.T.; Galvão, C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasites Vectors 2014, 7, 149. [Google Scholar] [CrossRef]
- Justi, S.A.; Galvão, C.; Schrago, C.G. Geological changes of the Americas and their influence on the diversification of the Neotropical kissing bugs (Hemiptera: Reduviidae: Triatominae). PLoS Negl. Trop. Dis. 2016, 10, e0004527. [Google Scholar] [CrossRef]
- Monteiro, F.A.; Weirauch, C.; Felix, M.; Lazoski, C.; Abad-Franch, F. Evolution, Systematics, and Biogeography of the Triatominae, Vectors of Chagas Disease. Adv. Parasitol. 2018, 99, 265–344. [Google Scholar]
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera: Reduviidae) and their significance as vector of Chagas’s disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 123–520. [Google Scholar]
- Hernández, C.; da Rosa, J.A.; Vallejo, G.A.; Guhl, F.; Ramírez, J.D. Taxonomy, Evolution, and Biogeography of the Rhodniini Tribe (Hemiptera: Reduviidae). Diversity 2020, 12, 97. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Pavan, M.G.; Jaramillo, N.; Palomeque, F.S.; Dale, C.; Chaverra, D.; Monteiro, F.A. Rhodnius barretti, a new species of Triatominae (Hemiptera: Reduviidae) from western Amazonia. Mem. Inst. Oswaldo Cruz 2013, 108, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.B.S.; Jaramillo, N.; Diotaiuti, L. Description and characterization of the melanic morphotype of Rhodnius nasutus Stål, 1859 (Hemiptera: Reduviidae: Triatominae). Rev. Soc. Bras. Med. Trop. 2014, 47, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Mougel, F.; Jacquin-Joly, E.; Almeida, C.E.; Blanchet, D.; Bérenger, J.M.; da Rosa, J.A.; Harry, M. Chemosensory Gene Expression for Two Closely Relative Species Rhodnius robustus and R. prolixus (Hemiptera, Reduviidade, Triatominae) Vectors of Chagas Disease. Front. Ecol. Evol. 2021, 9, 725504. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Monteiro, F.A.; Pavan, M.G.; Patterson, J.S.; Bargues, M.B.; Zuriaga, M.A.; Aguilar, M.; Beard, C.B.; Mas-Coma, S.; Miles, M.A. Under pressure: Phenotypic divergence and convergence associated with microhabitat adaptations in Triatominae. Parasites Vectors 2021, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Catalá, S.; Carbajal de la Fuente, L.; Junqueira, A.C.V. Phenotypic variability oh teh Amazon species Rhodnius brethesi (Hemiptera: Reduviidae). J. Med. Entomol. 2017, 54, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.D.; Rosa, J.A.; Salgado-Roa, F.C.; Hernández, C.; Pardo-Diaz, C.; Alevi, K.C.C.; Ravazi, A.; Oliveira, J.; Azeredo-Oliveira, M.T.V.; Salazar, C.; et al. Taxonomical over splitting in the Rhodnius prolixus (Insecta: Hemiptera: Reduviidae) clade: Are R. taquarussuensis (da Rosa et al., 2017) and R. neglectus (Lent, 1954) the same species? PLoS ONE 2019, 14, e0211285. [Google Scholar] [CrossRef] [PubMed]
- Pita, S.; Panzera, F.; Ferrandis, I.; Galvao, C.; Gomez-Palacio, A.; Panzera, Y. Chromosomal divergence and evolutionary inferences in Rhodniini based on the chromosomal location of ribosomal genes. Mem. Inst. Oswaldo Cruz 2013, 108, 376–382. [Google Scholar] [CrossRef]
- Kieran, T.J.; Bayona-Vásquez, N.J.; Varian, C.P.; Saldaña, A.; Samudio, F.; Calzada, J.E.; Gottdenker, N.L.; Glenn, T.C. Population genetics of two chromatic morphs of the Chagas disease vector Rhodnius pallescens Barber, 1932 in Panamá. Infec. Genet. Evol. 2020, 84, 104369. [Google Scholar] [CrossRef]
- Galvão, C.; Carcavallo, R.; Rocha, D.S.; Jurberg, J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa 2003, 202, 1–36. [Google Scholar] [CrossRef]
- Filée, J.; Merle, M.; Bastide, H.; Mougel, F.; Bérenger, J.M.; Folly-Ramos, E.; Almeida, C.E.; Harry, M. Phylogenomics for Chagas Disease Vectors of the Rhodnius Genus (Hemiptera, Triatominae): What We Learn from Mito-Nuclear Conflicts and Recommendations. Front. Ecol. Evol. 2022, 9, 750317. [Google Scholar] [CrossRef]
- Valente, V.C.; Valente, S.; Carcavallo, R.U.; Rocha, D.S.; Galvão, C.; Jurberg, J. Considerações sobre uma nova espécie do gênero Rhodnius Stål, do estado do Pará, Brasil (Hemíptera, Reduviidae, Triatominae). Entomol. Vectores 2001, 8, 65–80. [Google Scholar]
- Massaro, D.C.; Rezende, D.S.; Camargo, L.M.A. Study of the triatomine fauna and occurrence of Chagas disease in Monte Negro, Rondonia, Brazil. Rev. Bras. Epidemiol. 2008, 11, 228–240. [Google Scholar] [CrossRef]
- Diotaiuti, L.; de Oliviera, M.A.; dos Santos, J.P.; Barbosa, S.E. Triatomíneos, 1st ed.; CPqRR: Belo Horizonte, Brazil, 2015; p. 296. [Google Scholar]
- da Rosa, J.A.; Mendonça, V.J.; Gardim, S.; Carvalho, D.B.; Oliveira, J.; Nascimento, J.D.; Pinotti, H.; Pinto, M.C.; Cilense, M.; Galvão, C.; et al. Study of the external female genitalia of 14 Rhodnius species (Hemiptera, Reduviidae, Triatominae) using scanning electron microscopy. Parasites Vectors 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Jurberg, J.; Galvão, C.; Rosa, J.A.; Ceretti-Júnior, W.; Barata, J.M.S.; Obara, M.T. Comparative descriptions of eggs from three species of Rhodnius (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 2009, 104, 1012–1018. [Google Scholar] [CrossRef]
- Galvão, C. Vetores da Doença de Chagas no Brasil, 1st ed.; Sociedade Brasileira de Zoologia: Curitiba, Brazil, 2014; p. 289. [Google Scholar]
- Jurberg, J.; Rodrigues, J.M.S.; Moreira, F.F.F.; Dale, C.; Cordeiro, I.R.S.; Lamas, V.D., Jr.; Galvão, C.; Rocham, D.S. Atlas Iconográfico dos Triatomíneos do Brasil (Vetores da Doença de Chagas), 2nd ed.; Laboratório Nacional e Internacional de Referência em Taxonomia de Triatomíneos: Rio de Janeiro, Brazil, 2014; p. 61. [Google Scholar]
- Alvarez, A.C.P.C.; Dale, C.; Galvão, C. Geometric morphometry of the Rhodnius prolixus complex (Hemiptera, Triatominae): Patterns of intraspecific and interspecific allometry and their taxonomic implications. ZooKeys 2024, 1202, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.A.; Rocha, C.S.; Gardim, S.; Pinto, M.C.; Mendonça, V.J.; Ferreira-Filho, J.C.R.; Carvalho, E.O.C.; Camargo, L.M.A.; Oliveira, J.; Nascimento, J.D.; et al. Description of Rhodnius montenegrensis n. sp. (Hemiptera, Reduviidae: Triatominae) from the state of Rondônia, Brazil. Zootaxa 2012, 3478, 62–76. [Google Scholar] [CrossRef]
- Souza, E.S.; Atzinger, N.C.B.V.; Furtado, M.B.; Oliveira, J.; Damieli, J.N.; Vendramini, D.P.; Gardim, S.; Rosa, J.A. Description of Rhodnius marabaensis sp. n. (Hemiptera: Reduviidae: Triatominae) from Pará State, Brazil. Zookeys 2016, 621, 45–62. [Google Scholar]
- Cracraft, J. Species Concepts and Speciation Analysis. In Current Ornithology, 1st ed.; Johnston, R.F., Ed.; Springer: New York, NY, USA, 1983; Volume 1, pp. 159–187. [Google Scholar]
- Mayr, E. Animal Species and Evolution; Harvard University Press: Cambridge, MA, USA, 1963; p. 797. [Google Scholar]
- Mayr, E. Populations, Species, and Evolution; Harvard University Press: Cambridge, MA, USA, 1970; p. 453. [Google Scholar]
- Adams, E.R.; Hamilton, P.B.; Malele, I.I.; Gibson, W.C. The identification, diversity and prevalence of trypanosomes in field caught tsetse in Tanzania using ITS-1 primers and fluorescent fragment length barcoding. Infect. Genet. Evol. 2008, 8, 439–444. [Google Scholar] [CrossRef]
- Lyman, D.F.; Monteiro, F.A.; Escalante, A.A.; Cordon-Rosales, C.; Wesson, D.M.; Dujardin, J.P.; Beard, C.B. Mitochondrial DNA sequence variation among triatomine vectors of Chagas disease. Am. J. Trop. Med. Hyg. 1999, 60, 377–386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Meth. 2012, 9, 772. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yule, G.U. A mathematical theory of evolution, based on the conclusions of Dr. J. C. Willis, F.R.S. Philos. Trans. R. Soc. Ser. B 1925, 213, 21–87. [Google Scholar]
- Gernhard, T. The conditioned reconstructed process. J. Theor. Biol. 2008, 253, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree–Tree Figure Drawing Tool; Version v.1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Dobzhansky, T. Genetics of the Evolutionary Process; Columbia University Press: New York, NY, USA, 1970. [Google Scholar]
- Mendonça, V.J.; Alevi, K.C.C.; Medeiros, L.M.; Nascimento, J.D.; Azeredo-Oliveira, M.T.V.; Rosa, J.A. Cytogenetic andmorphologic approaches of hybrids from experimental crosses between Triatoma lenti Sherlock & Serafim, 1967 and T. sherlocki Papa et al., 2002 (Hemiptera: Reduviidae). Infect. Genet. Evol. 2014, 26, 123–131. [Google Scholar] [PubMed]
- Ravazi, A.; Oliveira, J.; Campos, F.F.; Madeira, F.F.; Reis, Y.V.; Oliveira, A.B.B.; Azeredo-Oliveira, M.T.V.; Rosa, J.A.; Galvão, C.; Alevi, K.C.C. Trends in evolution of the Rhodniini tribe (Hemiptera, Triatominae): Experimental crosses between Psammolestes tertius Lent & Jurberg, 1965 and P. coreodes Bergroth, 1911 and analysis of the reproductive isolating mechanisms. Parasites Vectors 2021, 14, 350. [Google Scholar]
- Olaia, N.; Alevi, K.C.C.; Oliveira, J.; Cacini, G.L.; Souza, E.D.S.; Pinotti, H.; Silva, L.A.; Rosa, J.A. Biology of Chagas disease vectors: Biological cycle and emergence rates of Rhodnius marabaensis Souza et al., 2016 (Hemiptera, Reduviidae, Triatominae) under laboratory conditions. Parasitol. Res. 2021, 120, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922, 12, 101–109. [Google Scholar] [CrossRef]
- Turelli, M.; Orr, H.A. The dominance theory of Haldane’s Rule. Genetics 1995, 140, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Alevi, K.C.C.; Mendonca, P.P.; Pereira, N.P.; Rosa, J.A.; Azeredo-Oliveira, M.T.V. Karyotype of Triatoma melanocephala Neiva & Pinto (1923). Does this species fit in the Brasiliensis subcomplex? Infect. Genet. Evol. 2012, 12, 1652–1653. [Google Scholar]
- De Vaio, E.S.; Grucci, B.; Castagnino, A.M.; Franca, M.E.; Martinez, M.E. Meiotic differences between three triatomine species (Hemiptera: Reduviidae). Genetica 1985, 67, 185–191. [Google Scholar] [CrossRef]
- Panzera, Y.; Pita, S.; Ferreiro, M.J.; Ferrandis, I.; Lages, C.; Pérez, R.; Silva, A.E.; Guerra, M.; Panzera, F. High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera). Cytogenet. Genome Res. 2012, 138, 56–67. [Google Scholar] [CrossRef]
- Pita, S.; Lorite, P.; Cuadrado, A.; Panzera, Y.; De Oliveira, J.; Alevi, K.C.C.; Rosa, J.A.; Freitas, S.P.C.; Gómez-Palacio, A.; Solari, A.; et al. High chromosomal mobility of rDNA clusters in holocentric chromosomes of Triatominae, vectors of Chagas disease (Hemiptera-Reduviidae). Med. Vet. Entomol. 2022, 36, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Alevi, K.C.C.; Oliveira, J.; Garcia, A.C.C.; Cristal, D.C.; Delgado, L.M.G.; Bittinelli, I.F.; Reis, Y.V.; Ravazi, A.; Oliveira, A.B.B.; Galvão, C.; et al. Triatoma rosai sp. nov. (Hemiptera, Triatominae): A New Species of Argentinian Chagas Disease Vector Described Based on Integrative Taxonomy. Insects 2020, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, V.J.; Alevi, K.C.C.; Pinotti, H.; Gurgel-Gonçalves, R.; Pita, S.; Guerra, A.L.; Panzera, F.; Araújo, R.F.; Azeredo-Oliveira, M.T.V.; Rosa, J.A. Revalidation of Triatoma bahiensis Sherlock & Serafim, 1967 (Hemiptera: Reduviidae) and phylogeny of the T. Brasiliensis species complex. Zootaxa 2016, 4107, 239–254. [Google Scholar] [PubMed]
- Azevedo, L.M.S.; Cesaretto, N.R.; de Oliveira, J.; Ravazi, A.; Dos Reis, Y.V.; Tadini, S.C.A.F.; da Silva Masarin, I.; Borsatto, K.C.; Galvão, C.; da Rosa, J.A.; et al. First evidence of gonadal hybrid dysgenesis in Chagas disease vectors (Hemiptera, Triatominae): Gonad atrophy prevents events of interspecific gene flow and introgression. Parasites Vectors 2023, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Alevi, K.C.C.; Pinotti, H.; Araújo, R.F.; Azeredo-Oliveira, M.T.V.; Rosa, J.A.; Mendonça, V.J. Hybrid colapse confirm the specific status of Triatoma bahiensis Sherlock and Serafim, 1967 (Hemiptera, Triatominae). Am. J. Trop. Med. Hyg. 2018, 98, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Campos-Soto, R.; Panzera, F.; Pita, S.; Lages, C.; Solari, A.; Botto-Mahan, C. Experimental crosses between Mepraia gajardoi and M. spinolai and hybrid chromosome analyses reveal the occurrence of several isolation mechanisms. Infect. Genet. Evol. 2016, 45, 205–212. [Google Scholar] [CrossRef]
- Pérez, R.; Hérnandez, M.; Quintero, O.; Scvortzoff, E.; Canale, D.; Méndez, L.; Cohanoff, C.; Martino, M.; Panzera, F. Cytogenetic analysis of experimental hybrids in species of Triatominae (Hemiptera-Reduviidae). Genetica 2005, 125, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Villacís, A.G.; Dujardin, J.P.; Panzera, F.; Yumiseva, C.A.; Pita, S.; Santillán-Guayasamín, S.; Orozco, M.I.; Mosquera, K.D.; Grijalva, M.J. Chagas vectors Panstrongylus chinai (Del Ponte, 1929) and Panstrongylus howardi (Neiva, 1911): Chromatic forms or true species? Parasites Vectors 2020, 13, 226. [Google Scholar] [CrossRef]
- Perlowagora-Szumlewics, A.; Correia, M.V. Induction of male sterility manipulation of genetic mechanisms present in vector species of Chagas disease (remarks on integrating sterile-male release with insecticidal control measures against vectors of Chagas disease). Rev. Inst. Med. Trop. 1972, 14, 360–371. [Google Scholar]


| Specie | Acession Number | Country | State—City |
|---|---|---|---|
| R. milesi | PQ094218 * | Brazil | Pará—Bragança |
| PQ094219 * | Brazil | Pará—Bragança | |
| R. neglectus | AF045716 | Brazil | - |
| MZ399364 | Brazil | Bahia—Ibotirama | |
| MZ399370 | Brazil | Bahia—São Desiderio | |
| KT317037 | Brazil | Bahia—Xique-Xique | |
| KT317036 | Brazil | Bahia—Xique-Xique | |
| MH704748 | Brazil | Goiás—Formoso | |
| MH704749 | Brazil | Goiás—Formoso | |
| MH704751 | Brazil | Goiás—Formoso | |
| MH704750 | Brazil | Goiás—Formoso | |
| MZ399362 | Brazil | Goiás—Mambai | |
| MZ399366 | Brazil | Maranhão—Vargem Grande | |
| MZ399365 | Brazil | Maranhão—Loreto | |
| MZ399367 | Brazil | Minas Gerais—Buritizeiro | |
| MZ399368 | Brazil | Minas Gerais—Januaria | |
| KT317058 | Brazil | Paraíba—Olivedos | |
| KT317053 | Brazil | Piauí—Canto do Buriti | |
| KT317056 | Brazil | Piauí—Canto do Buriti | |
| KT317052 | Brazil | Piauí—Canto do Buriti | |
| KT317054 | Brazil | Piauí—Canto do Buriti | |
| KT317055 | Brazil | Piauí—Canto do Buriti | |
| KT317063 | Brazil | Piauí—Colônia do Gurgueia | |
| KT317065 | Brazil | Piauí—Colônia do Gurgueia | |
| KT317068 | Brazil | Piauí—Colônia do Gurgueia | |
| KT317064 | Brazil | Piauí—Colônia do Gurgueia | |
| KT317067 | Brazil | Piauí—Colônia do Gurgueia | |
| KT317066 | Brazil | Piauí—Colônia do Gurgueia | |
| KT317045 | Brazil | Piauí—Jaicos | |
| KT317042 | Brazil | Piauí—Jaicos | |
| KT317043 | Brazil | Piauí—Jaicos | |
| KT317044 | Brazil | Piauí—Jaicos | |
| MZ399363 | Brazil | Piauí—Monte Alegre do Piaui | |
| KT317057 | Brazil | Piauí—Oeiras | |
| OQ785647 | Brazil | Piauí—Sao Raimundo Nonato | |
| JX273156 | Brazil | Tocantins—Palmeirantes | |
| MZ399369 | Brazil | Tocantins—Taguatinga | |
| R. montenegrensis | MZ396184 | Bolivia | - |
| MZ396185 | Bolivia | - | |
| MZ396186 | Bolivia | - | |
| MZ396187 | Bolivia | - | |
| MZ396188 | Bolivia | - | |
| MZ396189 | Bolivia | - | |
| MZ396190 | Bolivia | - | |
| MZ396191 | Bolivia | - | |
| R. robustus | JX273158 | Brazil | - |
| R. prolixus | AF421339 | Honduras | - |
| EF043579 | Venezuela | - | |
| EF043585 | Venezuela | - | |
| EF043586 | Venezuela | - | |
| EF043587 | Venezuela | - | |
| EF043588 | Venezuela | - | |
| KP126733 | Colombia | - | |
| KP126734 | Colombia | - | |
| R. nasutus | MG735124 | - | Pernambuco—Serra Talhada |
| MG735123 | - | Pernambuco—Serra Talhada | |
| MG735122 | - | Pernambuco—Serra Talhada | |
| MG735121 | - | Pernambuco—Serra Talhada | |
| MG735109 | - | Paraíba—Sousa | |
| MG735108 | - | Paraíba—Sousa | |
| MG735107 | - | Paraíba—Sousa | |
| MG735106 | - | Paraíba—Sousa | |
| MG735080 | - | Piauí—Piracuruca | |
| MG735079 | - | Piauí—Piracuruca | |
| MG735078 | - | Piauí—Piracuruca | |
| MG735077 | - | Piauí—Piracuruca | |
| MG735071 | - | Piauí—Parnaiba | |
| MG735070 | - | Piauí—Parnaiba | |
| MG735069 | - | Piauí—Parnaiba | |
| MG735068 | - | Piauí—Parnaiba | |
| MG735054 | - | Ceará—Jaguaruana | |
| MG735053 | - | Ceará—Jaguaruana | |
| MG735022 | - | Rio Grande do Norte—Carnauba dos Dantas | |
| MG735021 | - | Rio Grande do Norte—Carnauba dos Dantas | |
| MG734997 | - | Piauí—Campo Maior | |
| MG734996 | - | Piauí—Campo Maior | |
| Outgroup T. infestans | KC249262 | Uruguai | - |
| T. infestans | KC249258 | - | - |
| T. rubrofasciata | HQ333233 | - | - |
| Crosses Eggs Hatching Rate | Eggs | Hatching Rate |
|---|---|---|
| Experimental crosses (to obtain F1) | ||
| R. milesi ♀ x R. neglectus ♂ | 357 | 82% |
| R. neglectus ♀ x R. milesi ♂ | 366 | 89% |
| Intercrosses (F1 x F1) (to obtain F2) | ||
| Hybrid F1 x Hybrid F1 * | 284 | 79% |
| Hybrid F1 x Hybrid F1 ** | 233 | 81% |
| Intercrosses (F2 x F2) (to obtain F3) | ||
| Hybrid F2 x Hybrid F2 *** | 546 | 50% |
| Hybrid F2 x Hybrid F2 **** | 703 | 83% |
| Control group | ||
| R. milesi ♀ x R. milesi ♂ | 386 | 81% |
| R. neglectus ♀ x R. neglectus ♂ | 901 | 89% |
| Tribes | Genera | Species (n) |
|---|---|---|
| Alberproseniini | Alberprosenia | 2 |
| Bolboderini | Belminus | 9 |
| Bolbodera | 1 | |
| Microtriatoma | 2 | |
| Parabelminus | 2 | |
| Cavernicolini | Cavernicola | 2 |
| Rhodniini | Psammolestes | 3 |
| Rhodnius | 19 | |
| Triatomini | Dipetalogaster | 1 |
| Eratyrus | 2 | |
| Hermanlentia | 1 | |
| Linshcosteus | 6 | |
| Mepraia | 3 | |
| Nesotriatoma | 3 | |
| Panstrongylus | 18 | |
| Paratriatoma | 2 | |
| Triatoma | 81 | |
| Paleotriatoma | 1 | |
| Total | 158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, F.F.; de Oliveira, J.; Santos Santana, J.K.; Ravazi, A.; dos Reis, Y.V.; Marson Marquioli, L.; Galvão, C.; de Azeredo-Oliveira, M.T.V.; Aristeu da Rosa, J.; Alevi, K.C.C. One Genome, Multiple Phenotypes: Would Rhodnius milesi Carcavallo, Rocha, Galvão & Jurberg, 2001 (Hemiptera, Triatominae) Be a Valid Species or a Phenotypic Polymorphism of R. neglectus Lent, 1954? Diversity 2024, 16, 472. https://doi.org/10.3390/d16080472
Campos FF, de Oliveira J, Santos Santana JK, Ravazi A, dos Reis YV, Marson Marquioli L, Galvão C, de Azeredo-Oliveira MTV, Aristeu da Rosa J, Alevi KCC. One Genome, Multiple Phenotypes: Would Rhodnius milesi Carcavallo, Rocha, Galvão & Jurberg, 2001 (Hemiptera, Triatominae) Be a Valid Species or a Phenotypic Polymorphism of R. neglectus Lent, 1954? Diversity. 2024; 16(8):472. https://doi.org/10.3390/d16080472
Chicago/Turabian StyleCampos, Fabricio Ferreira, Jader de Oliveira, Jociel Klleyton Santos Santana, Amanda Ravazi, Yago Visinho dos Reis, Laura Marson Marquioli, Cleber Galvão, Maria Tercília Vilela de Azeredo-Oliveira, João Aristeu da Rosa, and Kaio Cesar Chaboli Alevi. 2024. "One Genome, Multiple Phenotypes: Would Rhodnius milesi Carcavallo, Rocha, Galvão & Jurberg, 2001 (Hemiptera, Triatominae) Be a Valid Species or a Phenotypic Polymorphism of R. neglectus Lent, 1954?" Diversity 16, no. 8: 472. https://doi.org/10.3390/d16080472
APA StyleCampos, F. F., de Oliveira, J., Santos Santana, J. K., Ravazi, A., dos Reis, Y. V., Marson Marquioli, L., Galvão, C., de Azeredo-Oliveira, M. T. V., Aristeu da Rosa, J., & Alevi, K. C. C. (2024). One Genome, Multiple Phenotypes: Would Rhodnius milesi Carcavallo, Rocha, Galvão & Jurberg, 2001 (Hemiptera, Triatominae) Be a Valid Species or a Phenotypic Polymorphism of R. neglectus Lent, 1954? Diversity, 16(8), 472. https://doi.org/10.3390/d16080472









