Urban Forest Fragmentation Reshapes Soil Microbiome–Carbon Dynamics
Abstract
1. Introduction
2. Materials and Methods
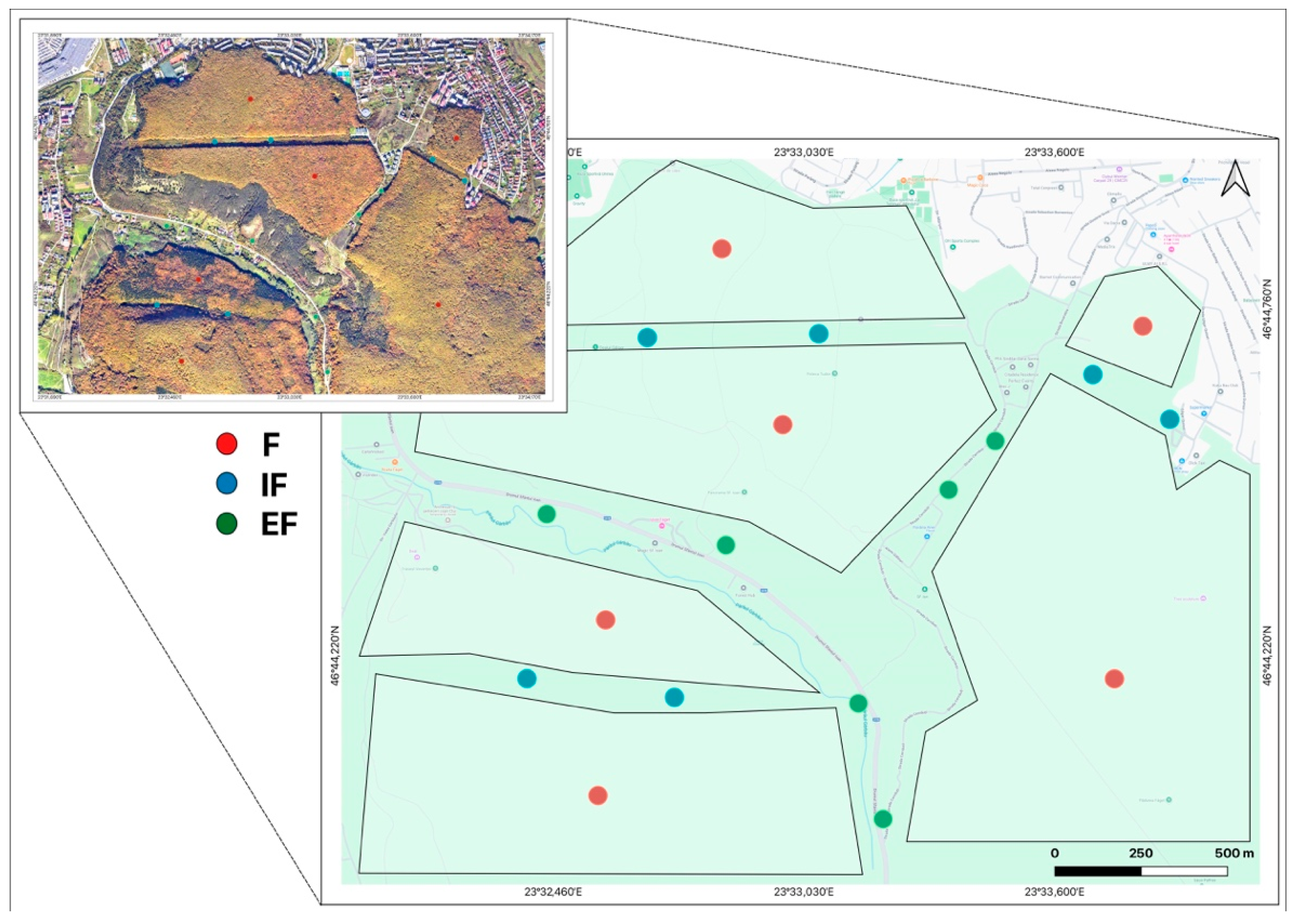
2.1. Sampling Site Description and Sampling Strategy
2.2. Assessment of Soil Physicochemical Properties
2.3. Microbial Diversity and Phenotypic Approach
2.4. Microbial Necromass Assessment
2.5. Statistical Analysis
3. Results
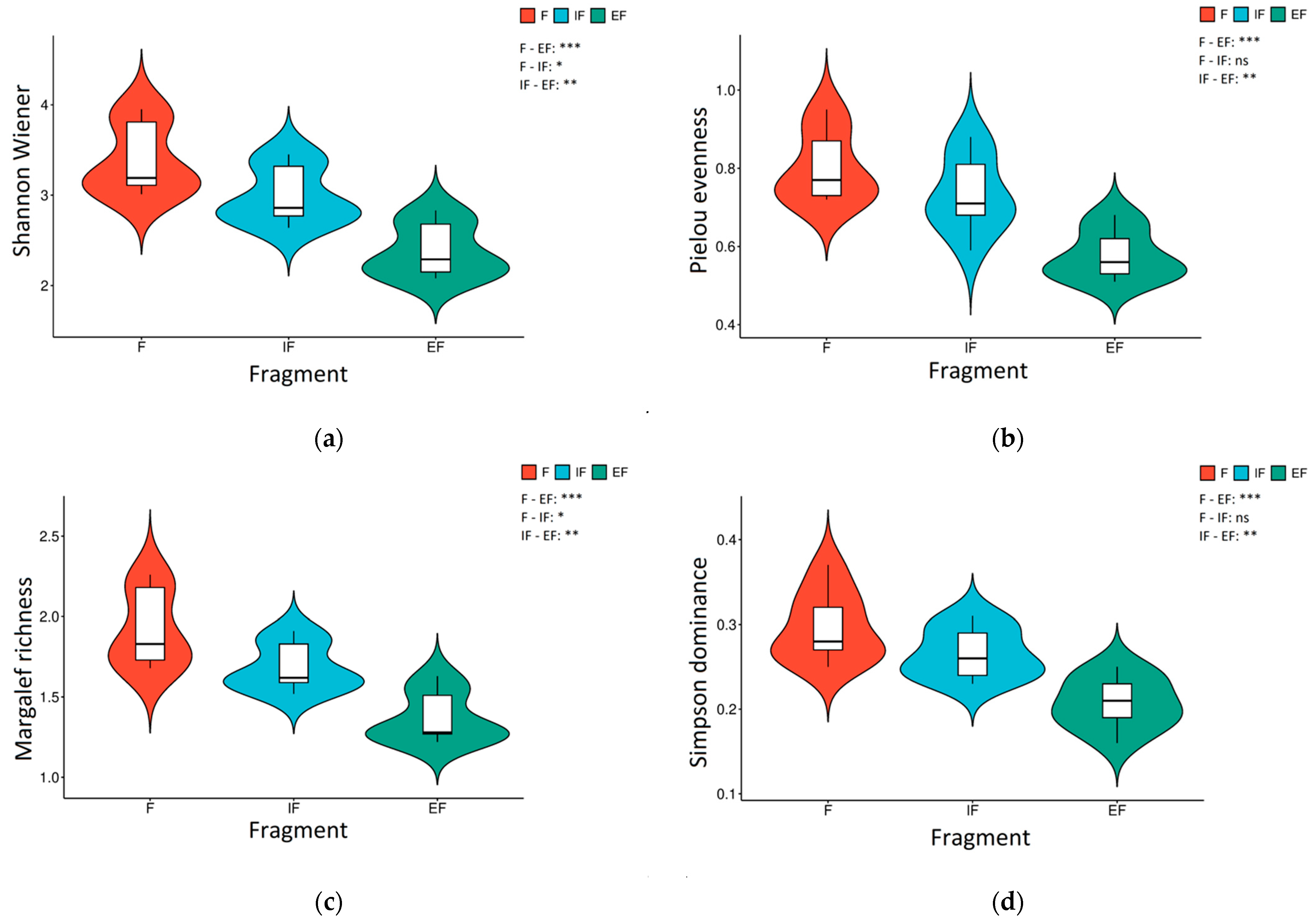
3.1. Microbial PLFA Diversity in Urban Forest Fragment Soil
3.2. Microbial Continuum Characteristics in Urban Forest Fragment Soil
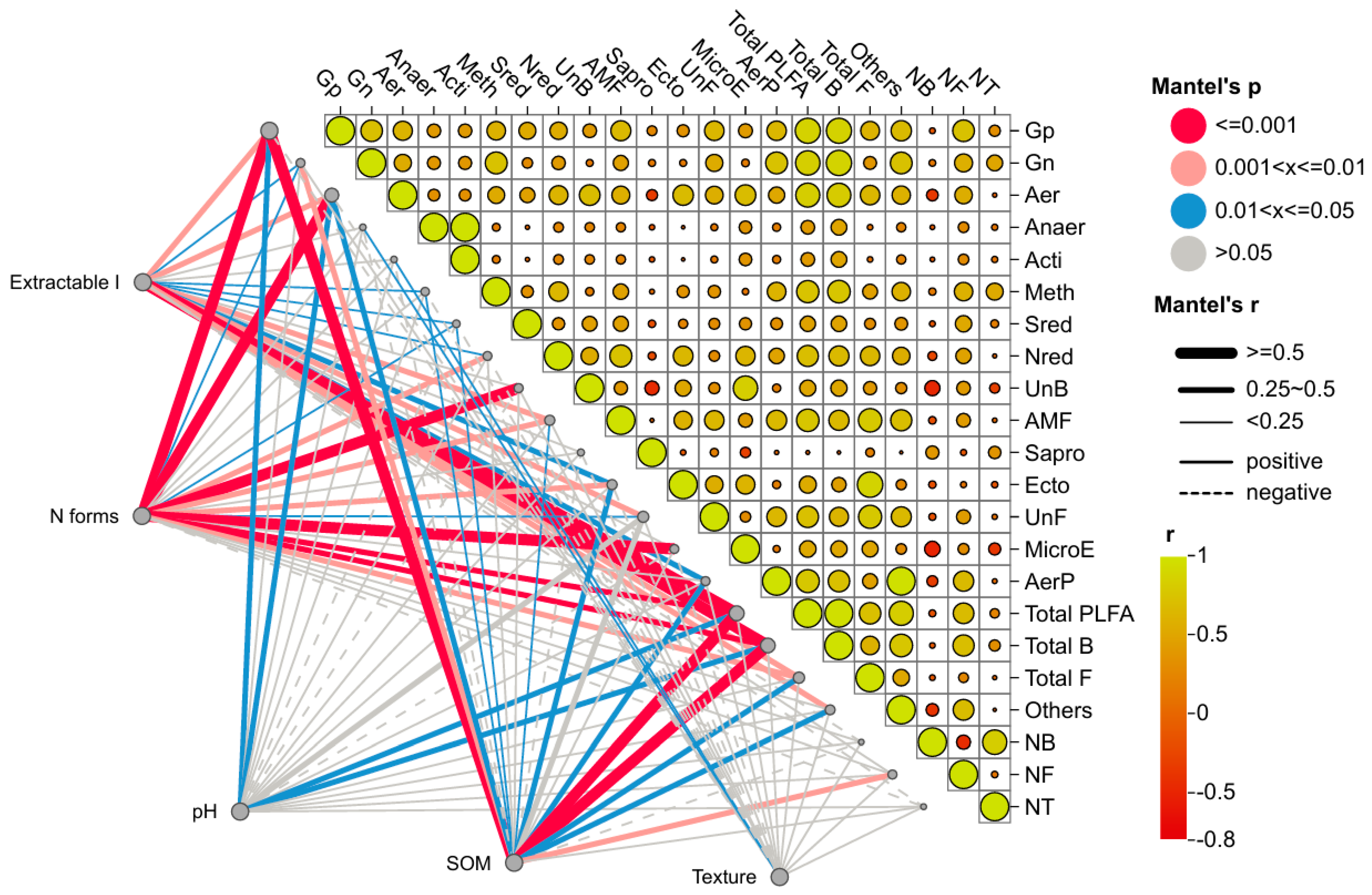
3.3. Abiotic Properties of Forest Fragments Affect the Soil Microbial Continuum
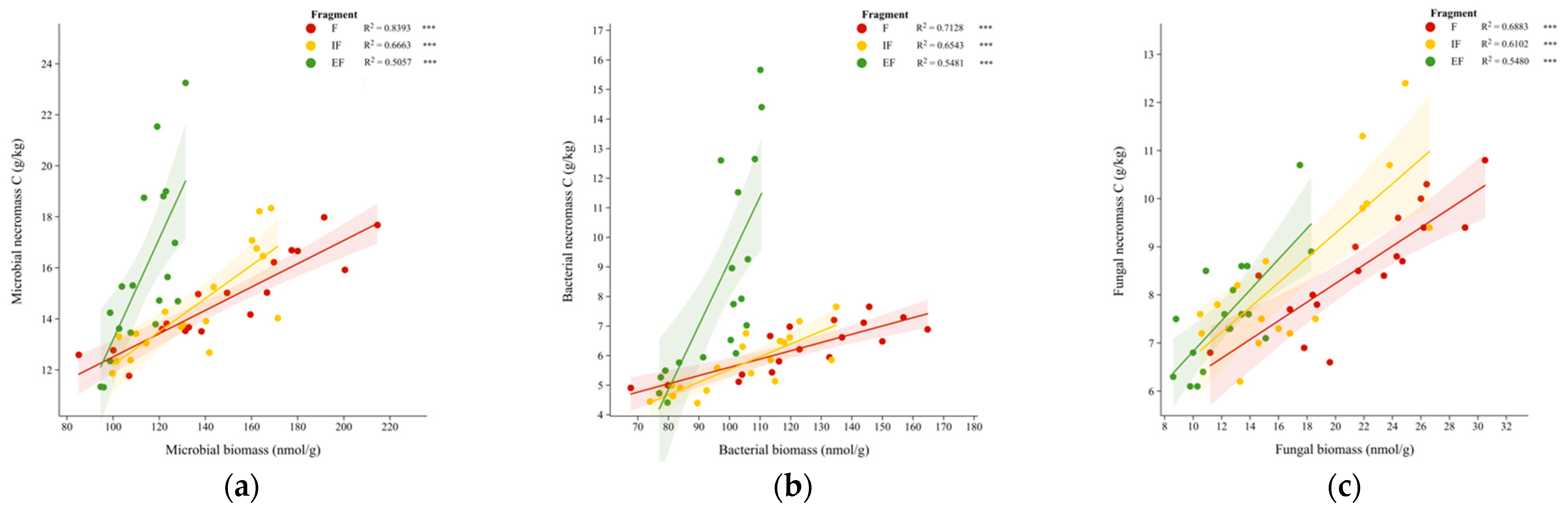
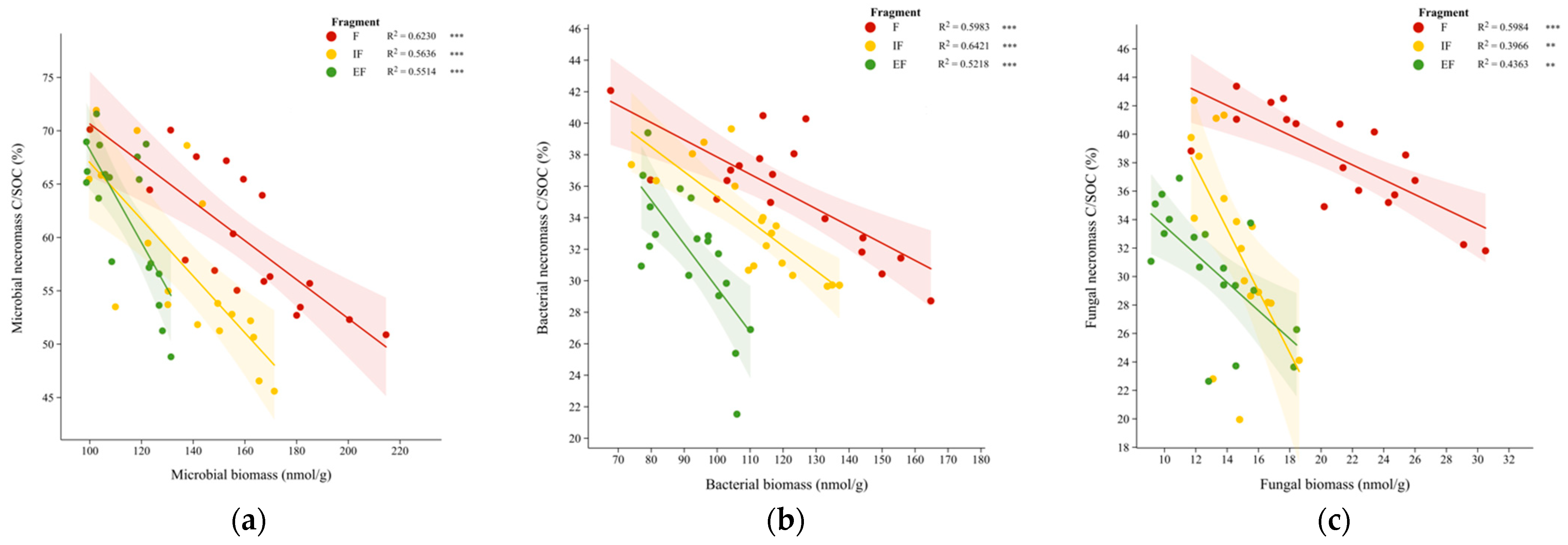
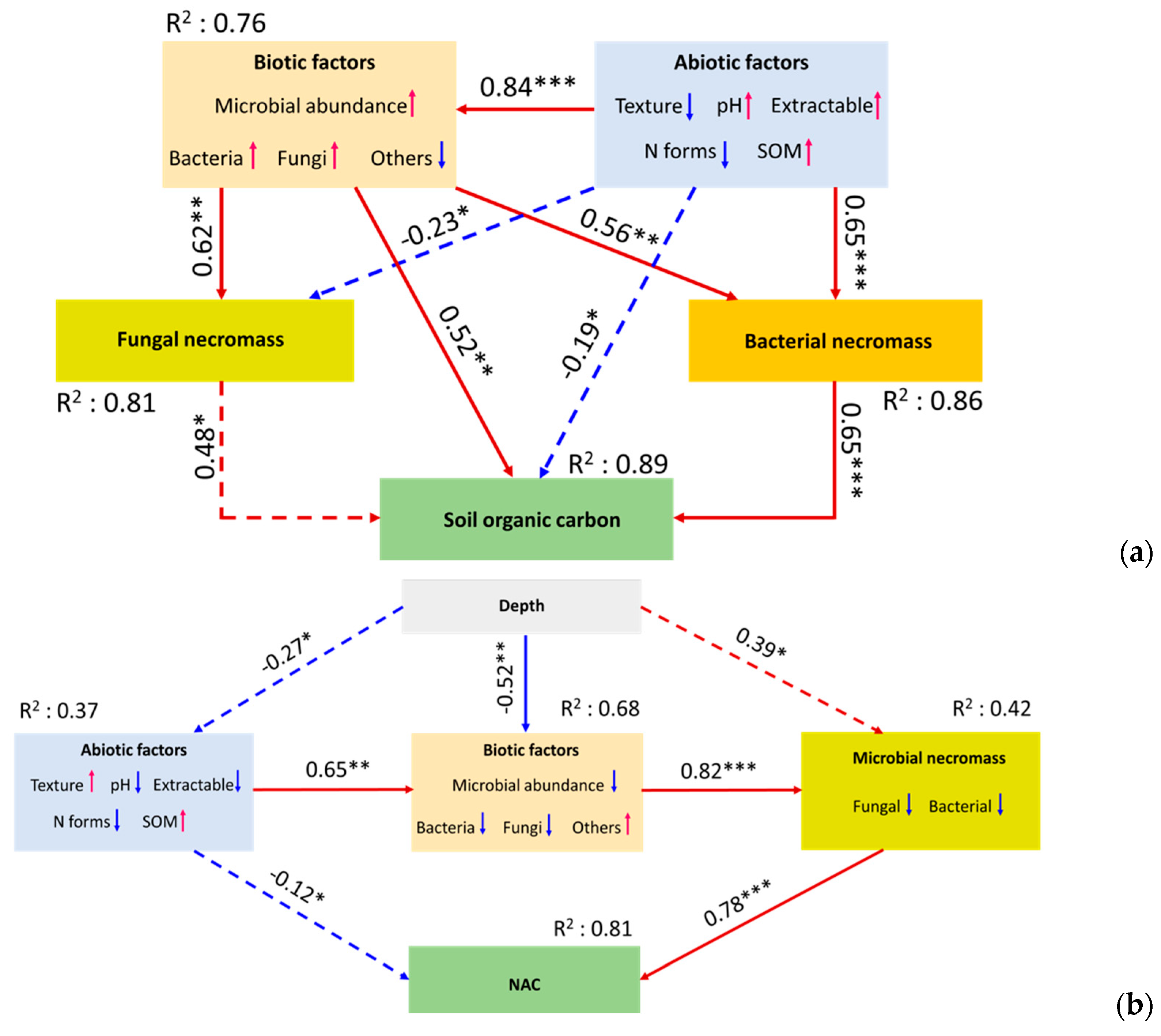
3.4. Microbial Continuum Control over Soil Organic Carbon in Forest Fragments
4. Discussion
4.1. Impact of Forest Fragmentation on the Soil Microbial Continuum
4.2. Biogeochemical Variables Controlling SOC Accumulation in Urban Forest Fragments
4.3. Biodiversity Implications and Environmental Considerations
4.4. Limitations of Current Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joy, M.S.; Jha, P.; Yadav, P.K.; Bansal, T.; Rawat, P.; Begam, S. Forest fragmentation and forest cover dynamics: Mining induced changes in the West Singhbhum District of Jharkhand. Remote Sens. Appl. Soc. Environ. 2024, 36, 101350. [Google Scholar] [CrossRef]
- Dutt, S.; Kumar Batar, A.; Sulik, S.; Kunz, M. Forest ecosystem on the edge: Mapping forest fragmentation susceptibility in Tuchola Forest, Poland. Ecol. Indic. 2024, 161, 111980. [Google Scholar] [CrossRef]
- De Pauw, K.; Depauw, L.; Calders, K.; Caluwaerts, S.; Cousins, S.A.O.; De Lombaerde, E.; Diekmann, M.; Frey, D.; Lenoir, J.; Meeussen, C.; et al. Urban forest microclimates across temperate Europe are shaped by deep edge effects and forest structure. Agric. For. Meteorol. 2023, 341, 109632. [Google Scholar] [CrossRef]
- Wu, L.M.; Quan, R.C.; Wang, B. Forest fragmentation effects on plant-animal interaction do not always show consistent patterns—Evidence from a seed removal experiment of 31 woody species. Bio. Conserv. 2024, 299, 110803. [Google Scholar] [CrossRef]
- Yasuda, K.; Kato, D.; Naoe, S.; Amano, T.; Yoshikawa, T.; Tochigi, K.; Koike, S. Does forest loss and fragmentation reduce woodpecker-associated ecosystem functions? Glob. Ecol. Conserv. 2025, 57, e03366. [Google Scholar] [CrossRef]
- Gómez, A.I.P.; Núñez-Rodríguez, L.X.; Mora-Motta, D.A.; Suárez, J.C.; Silva-Olaya, A.M.; de Araujo Pereira, A.P.; Ortiz-Morea, F.A. Unveiling soil bacterial diversity in the Andes-Amazon transition zone: Impacts of forest conversion to pasture. Appl. Soil Ecol. 2024, 201, 105486. [Google Scholar] [CrossRef]
- Wan, M.; Han, Y.; Song, Y.; Hashimoto, S. Estimating and projecting the effects of urbanization on the forest habitat quality in a highly urbanized area. Urban For Urban Green 2024, 94, 128270. [Google Scholar] [CrossRef]
- Koukianaki, E.A.; Lilli, M.A.; Efstathiou, D.; Matthews, B.; Knaebel, K.; Pröll, G.; Kobler, J.; Dirnböck, T.; Bäck, J.; Mirtl, M.; et al. Modelling soil functions of forested ecosystems. J. Environ. Manag. 2025, 386, 125636. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, F.; Liu, S.; Qi, W.; Zhengchen, R.; Wang, D. Synergistic effects of urban forest on urban heat island-air pollution-carbon stock in mega-urban agglomeration. Urban For. Urban Green 2025, 103, 128590. [Google Scholar] [CrossRef]
- Kiesewetter, K.N.; Afkhami, M.E. Microbiome-mediated effects of habitat fragmentation on native plant performance. New Phytol. 2021, 232, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Du, J.; Zhang, X.; Feng, J.; Feng, J. Tree species influence microbiome-mediated nutrient sequestration in soil aggregates of subtropical plantations in China. Appl. Soil Ecol. 2025, 209, 106034. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Rhizosphere engineering for soil carbon sequestration. Trends Plant Sci. 2024, 29, 447–468. [Google Scholar] [CrossRef]
- Anthony, M.A.; Tedersoo, L.; De Vos, B.; Croisé, L.; Meesenburg, H.; Wagner, M.; Andreae, H.; Jacob, F.; Lech, P.; Kowalska, A.; et al. Fungal community composition predicts forest carbon storage at a continental scale. Nat. Commun. 2024, 15, 2385. [Google Scholar] [CrossRef]
- Mason-Jones, K.; Breidenbach, A.; Dyckmans, J.; Banfield, C.C.; Dippold, M.A. Intracellular carbon storage by microorganisms is an overlooked pathway of biomass growth. Nat. Commun. 2023, 14, 2240. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- He, X.; Abs, E.; Allison, S.D.; Tao, F.; Huang, Y.; Manzoni, S.; Abramoff, R.; Bruni, E.; Bowring, S.P.K.; Chakrawal, A.; et al. Emerging multiscale insights on microbial carbon use efficiency in the land carbon cycle. Nat. Commun. 2024, 15, 8010. [Google Scholar] [CrossRef]
- Pagel, H.; Kriesche, B.; Uksa, M.; Poll, C.; Kandeler, E.; Schmidt, V.; Streck, T. Spatial Control of Carbon Dynamics in Soil by Microbial Decomposer Communities. Front. Environ. Sci. 2020, 8, 2. [Google Scholar] [CrossRef]
- Ferreira, V.; Elosegi, A.; Tiegs, S.D.; von Schiller, D.; Young, R. Organic matter decomposition and ecosystem metabolism as tools to assess the functional integrity of streams and rivers—A systematic review. Water 2020, 12, 3523. [Google Scholar] [CrossRef]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The role of microbial communities in the formation and decomposition of soil organic matter. In Soil Microbiology and Sustainable Crop Production; Dixon, G., Tilson, E., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Zaman, W.; Ayaz, A.; Puppe, D. Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions. Biology 2025, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Takele, L.; Yang, S.; Chen, Z.; Yan, J.; Ding, W. Contribution of microbial necromass to soil organic carbon in profile depths exhibited opposite patterns across ecosystems: A global meta-analysis. Soil Biol. Biochem. 2025, 207, 109842. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Rusu, T.; Kovacs, M.H. Sustainable soil volatilome: Discrimination of land uses through GC-MS-identified volatile organic compounds. Separations 2025, 12, 92. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Liu, M.; Xu, Z.; Wei, H. Coupled changes in soil organic carbon fractions and microbial community composition in urban and suburban forests. Sci. Rep. 2020, 10, 15933. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.D.; Kovacs, M.H.; Barcelo, D.; Pereira, P. Nonsteroidal anti-inflammatory drugs impact the microbial community in three different soil types—A laboratory experiment. Case Stud. Chem. Environ. Eng. 2024, 10, 100833. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Silaghi-Dumitrescu, L.; Roman, C.; Tian, D. Structural and metabolic profiling of Lycopersicon esculentum rhizosphere microbiota artificially exposed at commonly used non-steroidal anti-inflammatory drugs. Microorganisms 2022, 10, 254. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, S.; Zhang, C.; Liu, Z.; Zhou, L.; Fu, S. Contributions of understorey and/or overstory vegetations to soil microbial PLFA and nematode diversities in Eucalyptus monocultures. PLoS ONE 2014, 9, e85513. [Google Scholar] [CrossRef]
- Liang, C.; Balser, T.C. Warming and nitrogen deposition lessen microbial residue contribution to soil carbon pool. Nat. Commun. 2012, 3, 1222. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Nie, Y.; Tan, X.; Hu, A.; Li, Z.; Dai, S.; Ye, Q.; Zhang, G.; Shen, W. Latitudinal patterns and drivers of plant lignin and microbial necromass accumulation in forest soils: Disentangling microbial and abiotic controls. Soil Biol. Biochem. 2024, 194, 109438. [Google Scholar] [CrossRef]
- Ding, L.J.; Ren, X.Y.; Zhou, Z.Z.; Zhu, D.; Zhu, Y.G. Forest-to-cropland conversion reshapes microbial hierarchical interactions and degrades ecosystem multifunctionality at a national scale. Environ. Sci. Technol. 2024, 58, 11027–11040. [Google Scholar] [CrossRef]
- Xiao, H.; Sheng, H.; Zhang, L.; Zhang, L.; Pan, B.; Zhou, P. How does land-use change alter soil microbial diversity, composition, and network in subtropical China? Catena 2023, 231, 107335. [Google Scholar] [CrossRef]
- Benalcazar, P.; Seuradge, B.; Diochon, A.C.; Kolka, R.K.; Phillips, L.A. Conversion of boreal forests to agricultural systems: Soil microbial responses along a land-conversion chronosequence. Environ. Microbiome 2024, 19, 32. [Google Scholar] [CrossRef]
- Hellenbrand, J.P.; Kelly-Voicu, P.; Bowers, J.T.; Reinmann, A.B. Edge and the city: Evaluating the role of edge effects on urban forest structure and tree species composition. Urban For Urban Green 2025, 107, 128745. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, X.; Wei, H.; Zhang, J.; Saleem, M.; Li, D.; Zhang, Y.; Ma, R.; He, Y.; Zhong, J.; et al. Idiosyncratic responses of microbial communities and carbon utilization to acid rain frequency in the agricultural and forest soils. Glob. Ecol. Conserv. 2021, 26, e01429. [Google Scholar] [CrossRef]
- Choma, M.; Šamonil, P.; Kaštovská, E.; Bárta, J.; Tahovská, K.; Valtera, M.; Šantrůčková, H. Soil microbiome composition along the natural norway spruce forest life cycle. Forests 2021, 12, 410. [Google Scholar] [CrossRef]
- Lasa, A.V.; López-Hinojosa, M.; Villadas, P.J.; Fernández-González, A.J.; Cervera, M.T.; Fernández-López, M. Unravelling the shifts in the belowground microbiota and metabolome of Pinus pinaster trees affected by forest decline. Sci. Total Environ. 2025, 963, 178486. [Google Scholar] [CrossRef] [PubMed]
- Ruijten, P.; Huinink, H.P.; Adan, O.C.G. Hyphal growth of Penicillium rubens in changing relative humidity. Appl. Microb. Cell Physiol. 2021, 105, 5159–5171. [Google Scholar] [CrossRef] [PubMed]
- Mogîldea, D.; Biță-Nicolae, C. Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability. J. Urban Sci. 2024, 8, 159. [Google Scholar] [CrossRef]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil Bio. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Xuan, M.; Ai, L.; Wu, F.; Zhang, X.; Ni, X. Biomarkers evidence shows a preferential occlusion of microbial necromass in mineral-associated and not particle organic matter. Geoderma 2024, 450, 117030. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Bio. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Mattila, T.J.; Vihanto, N. Agricultural limitations to soil carbon sequestration: Plant growth, microbial activity, and carbon stabilization. Agric. Ecosyst. Environ. 2024, 367, 108986. [Google Scholar] [CrossRef]
- Shang, Q.; Li, C.; Liu, Y. The Negative Effects of Tourist Trampling on the Soil Physical Properties and Microbial Community Composition in a Natural Oak Forest. Forests 2024, 15, 1419. [Google Scholar] [CrossRef]
- Min, K.; Lynch, L.; Zheng, T.; Chen, F.; Liang, C. Factors driving microbial biomass and necromass relationships display ecosystem-dependent responses. Eur. J. Soil Sci. 2024, 75, e13555. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Luo, H.; Zhang, R. Labile carbon-induced soil organic matter turnover in a subtropical forest under different redox conditions. J. Environ. Manag. 2023, 348, 119387. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; He, Y.; Du, Z.; Zhou, G.; Zhou, L.; Wang, X.; Li, N.; Yan, E.; Feng, X.; Liang, C.; et al. Root Production and Microbe-Derived Carbon Inputs Jointly Drive Rapid Soil Carbon Accumulation at the Early Stages of Forest Succession. Forests 2022, 13, 2130. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Haddix, M.L.; Kroeger, M.E.; Steward, C.E. The role of plant input physical-chemical properties, and microbial and soil chemical diversity on the formation of particulate and mineral-associated organic matter. Soil Biol. Biochem. 2022, 168, 108648. [Google Scholar] [CrossRef]
- Dashora, K.; Gattupalli, M.; Tripathi, G.D.; Javed, Z.; Singh, S.; Tuohy, M.; Sarangi, P.K.; Diwan, D.; Singh, H.B.; Gupta, V.K. Fungal Assisted Valorization of Polymeric Lignin: Mechanism, Enzymes and Perspectives. Catalysts 2023, 13, 149. [Google Scholar] [CrossRef]
- Rosso, M.N.; Berrin, J.G.; Lomascolo, A. Plant wastes and sustainable refineries: What can we learn from fungi? Curr. Opin. Green Sustain. Chem. 2022, 34, 100602. [Google Scholar] [CrossRef]
- Zheng, T.; Miltner, A.; Liang, C.; Nowak, K.M.; Kastner, M. Turnover of bacterial biomass to soil organic matter via fungal biomass and its metabolic implications. Soil Biol. Biochem. 2023, 180, 108995. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Ma, J.; Zhang, Y.; Li, X.; Pang, D. Aggregate-associated carbon contributes to soil organic carbon accumulation along the elevation gradient of Helan Mountains. Soil Biol. Biochem. 2023, 178, 108926. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, X.; Liu, Y.; Huang, S.; Chen, J.; Guo, T.; Liu, Y.; Guo, S.; Qi, G. The microbial-driven C dynamics within soil aggregates in walnut orchards of different ages based on microbial biomarkers analysis. Catena 2022, 211, 105999. [Google Scholar] [CrossRef]
- Yesilonis, I.; Giorgio, V.; Hu, Y.; Pouyat, R.; Szlavecz, K. Changes in Soil Chemistry After 17 Years in Urban and Rural Forest Patches. Front. Ecol. Evol. 2022, 10, 786809. [Google Scholar] [CrossRef]
- Scholier, T.; Lavrinienko, A.; Brila, I.; Tukalenko, E.; Hindstrom, R.; Vasylenko, A.; Cayol, C.; Ecke, F.; Singh, N.J.; Forsman, J.T.; et al. Urban forest soils harbour distinct and more diverse communities of bacteria and fungi compared to less disturbed forest soils. Mol. Ecol. 2022, 32, 504–517. [Google Scholar] [CrossRef]
- Zech, S.; Schweizer, S.A.; Bucka, F.B.; Ray, N.; Kögel-Knabner, I.; Prechtel, A. Explicit spatial modelling at the pore scale unravels the interplay of soil organic carbon storage and structure dynamics. Glob. Change Biol. 2022, 28, 4589–4604. [Google Scholar] [CrossRef]
- Yi, R.; Liu, Q.; Yang, F.; Dai, X.; Meng, S.; Fu, X.; Li, S.; Kou, L.; Wang, H. Complementary belowground strategies underlie species coexistence in an early successional forest. New Phytol. 2023, 238, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Kudereti, A.; Zhao, S.; Tian, C. Responses of soil fauna community under changing environmental conditions. J. Arid Land 2023, 15, 620–636. [Google Scholar] [CrossRef]
- Koster, G.; Jazwa, M.; Przemieniecki, S.W.; Musielok, L.; Azarbad, H.; Klimek, B. Size matters: Larger fragments of riparian forest in urban areas support functional diversity of soil bacteria more than smaller ones. Front. Microbiol. 2025, 16, 1517545. [Google Scholar] [CrossRef] [PubMed]
| Phenotypic Group | Fragment 1 | Abundance (nmol⋅g−1) | Repeated-Measures ANOVA | ||
|---|---|---|---|---|---|
| 0–15 (cm) | 15–30 (cm) | 30–45 (cm) | |||
| Total PLFA | F | 175.6 (154.5; 195.7) B|a | 147.2 (126.6; 164.4) B|a | 142.1 (120.5; 154.8) A|a | ns |
| IP | 153.5 (133.6; 165.0) AB|a | 132.3 (113.0; 147.7) AB|a | 127.9 (104.3; 140.6) A|a | ns | |
| EF | 125.2 (123.0; 127.7) A|b | 105.5 (102.8; 108.3) A|a | 101.4 (98.7; 105.4) A|a | *** | |
| One-way ANOVA | ** | * | ns | ||
| Total bacteria | F | 140.0 (126.4; 148.9) B|a | 114.8 (104.7; 125.9) B|a | 109.7 (100.7; 122.0) A|a | * |
| IP | 123.5 (107.4; 134.5) AB|a | 106.3 (91.1; 117.6) AB|a | 103.2 (84.3; 114.6) A|a | ns | |
| EF | 103.9 (100.9; 105.9) A|b | 84.3 (80.0; 90.4) A|a | 81.8 (78.1; 87.6) A|a | ** | |
| One-way ANOVA | ** | * | ns | ||
| Total fungi | F | 21.9 (16.9; 27.4) B|a | 19.3 (13.4; 23.3) B|a | 18.7 (12.9; 22.4) A|a | ns |
| IP | 15.7 (14.1; 16.7) A|a | 12.4 (12.4; 14.9) AB|a | 12.6 (11.7; 14.4) A|a | ns | |
| EF | 13.0 (11.7; 13.9) A|a | 11.8 (10.5; 12.8) A|a | 11.1 (9.9; 12.4) A|a | ns | |
| One-way ANOVA | ** | * | * | ||
| Other PLFA | F | 13.7 (11.3; 18.1) A|a | 11.0 (9.0; 15.8) A|a | 9.8 (7.2; 12.9) A|a | ns |
| IP | 14.3 (9.4; 16.4) A|a | 12.6 (7.2; 14.8) A|a | 11.6 (6.5; 13.1) A|a | ns | |
| EF | 8.9 (7.7; 9.8) A|a | 7.8 (6.6; 8.6) A|a | 6.8 (5.7; 7.8) A|a | ns | |
| One-way ANOVA | ns | ns | ns | ||
| Microbial Necromass Group | Fragment 1 | Amount (g⋅kg−1) | Repeated-Measures ANOVA | ||
|---|---|---|---|---|---|
| 0–15 (cm) | 15–30 (cm) | 30–45 (cm) | |||
| Total microbial necromass | F | 16.1 (15.2; 17.3) A|b | 15.0 (14.1; 16.3) A|ab | 13.6 (12.8; 13.7) A|a | * |
| IF | 14.6 (13.9; 17.5) A|a | 14.0 (13.5; 16.1) A|a | 12.5 (12.4; 13.0) A|a | ns | |
| EF | 18.0 (16.0; 20.9) A|b | 14.8 (13.8; 17.9) A|ab | 13.1 (11.6; 14.5) A|a | ** | |
| One-way ANOVA | ns | ns | ns | ||
| Total bacterial necromass | F | 6.7 (5.7; 7.1) AB|a | 6.4 (5.6; 6.9) A|a | 5.9 (5.2; 6.5) A|a | ns |
| IF | 6.1 (5.9; 6.6) A|a | 5.8 (5.5; 6.3) A|a | 5.3 (5.0; 5.6) A|a | ns | |
| EF | 9.8 (6.4; 14.0) B|a | 6.8 (5.8; 10.6) A|a | 5.9 (4.9; 7.6) A|a | ns | |
| One-way ANOVA | * | ns | ns | ||
| Total fungal necromass | F | 9.5 (9.1; 10.1) A|a | 8.6 (8.1; 9.3) A|a | 7.3 (6.8; 7.7) A|b | *** |
| IF | 9.0 (7.8; 10.4) A|a | 8.5 (7.6; 9.6) A|a | 7.6 (7.3; 7.7) A|a | ns | |
| EF | 8.1 (7.3; 8.6) A|a | 7.5 (7.3; 8.0) A|a | 6.9 (6.4; 7.2) A|a | ns | |
| One-way ANOVA | ns | ns | ns | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacs, M.H.; Nghia, N.K.; Kovacs, E.D. Urban Forest Fragmentation Reshapes Soil Microbiome–Carbon Dynamics. Diversity 2025, 17, 545. https://doi.org/10.3390/d17080545
Kovacs MH, Nghia NK, Kovacs ED. Urban Forest Fragmentation Reshapes Soil Microbiome–Carbon Dynamics. Diversity. 2025; 17(8):545. https://doi.org/10.3390/d17080545
Chicago/Turabian StyleKovacs, Melinda Haydee, Nguyen Khoi Nghia, and Emoke Dalma Kovacs. 2025. "Urban Forest Fragmentation Reshapes Soil Microbiome–Carbon Dynamics" Diversity 17, no. 8: 545. https://doi.org/10.3390/d17080545
APA StyleKovacs, M. H., Nghia, N. K., & Kovacs, E. D. (2025). Urban Forest Fragmentation Reshapes Soil Microbiome–Carbon Dynamics. Diversity, 17(8), 545. https://doi.org/10.3390/d17080545







