Pine Cones in Plantations as Refuge and Substrate of Lichens and Bryophytes in the Tropical Andes
Abstract
1. Introduction
2. Materials and Methods
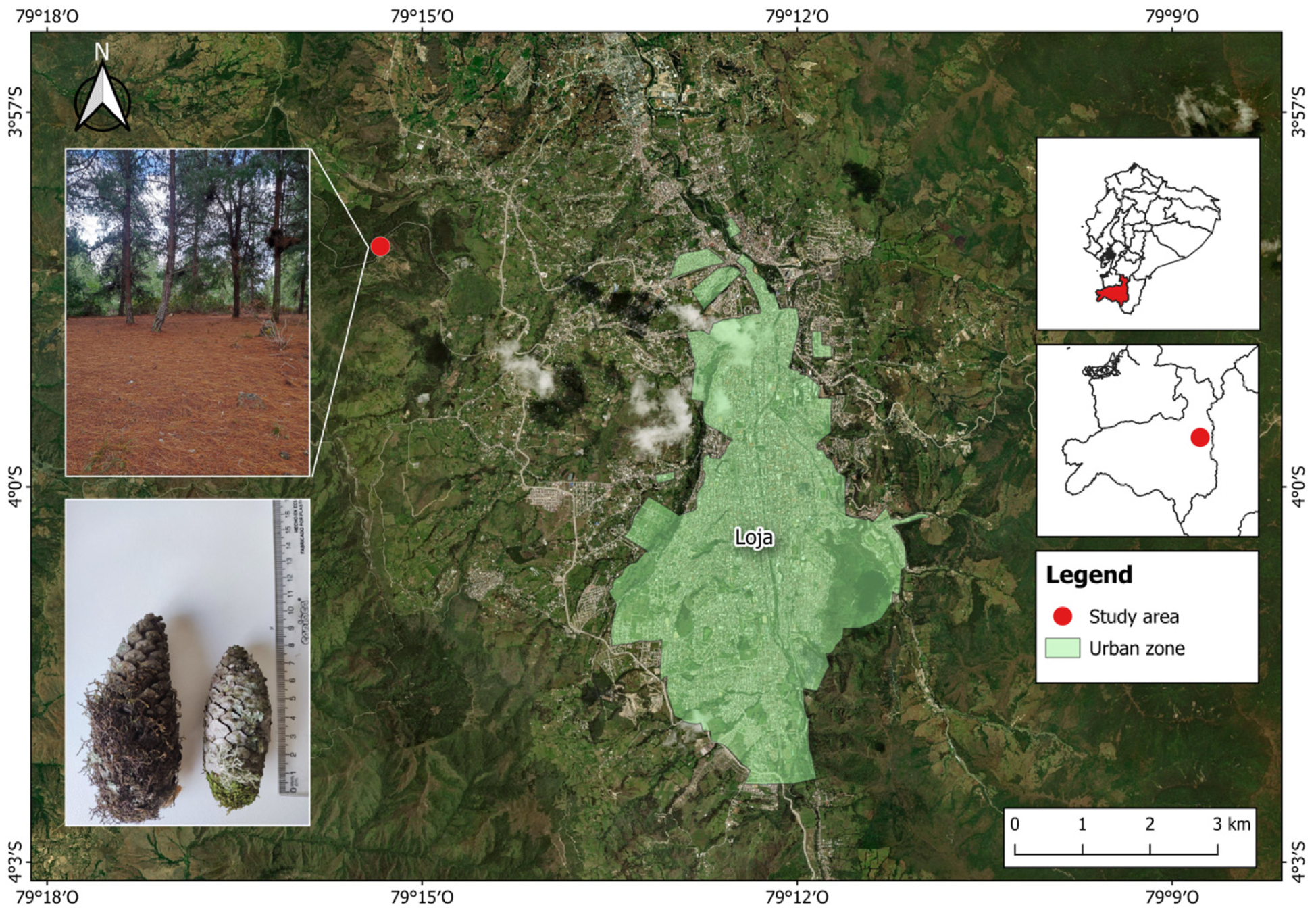
2.1. Study Area
2.2. Sampling Design and Data Collecting
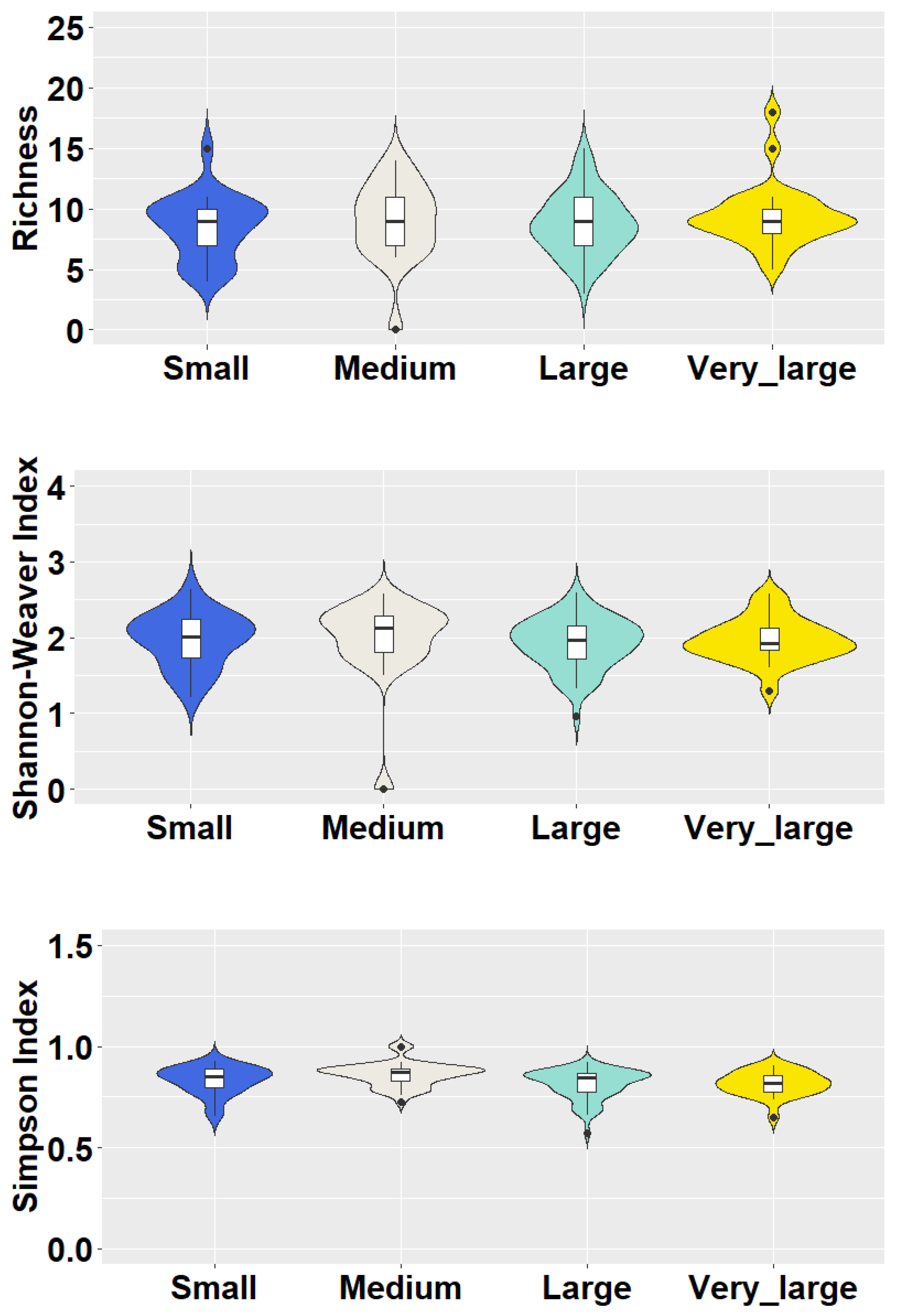
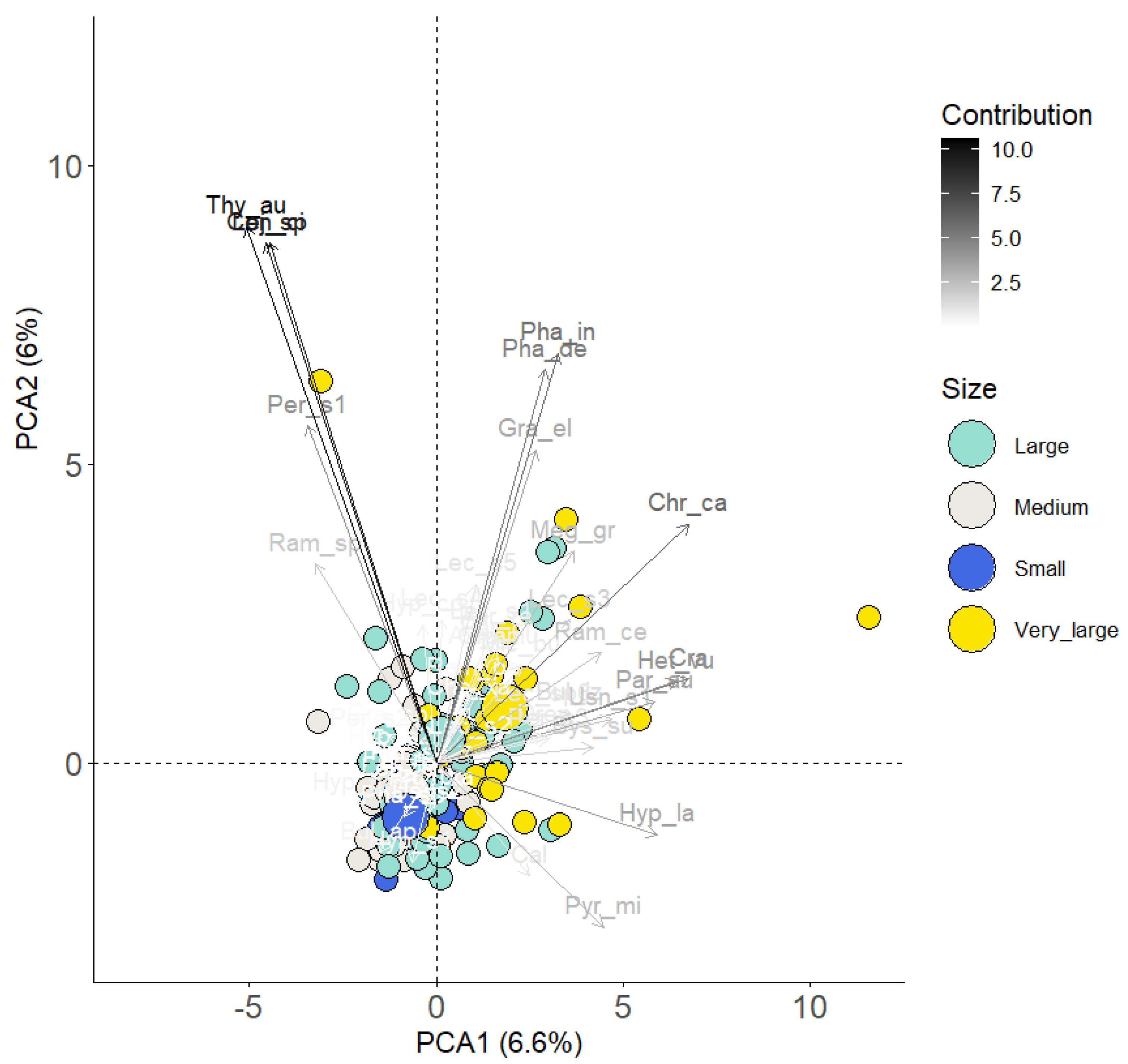
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Species | Small | Medium | Large | Very Large | Total | Growth Form |
|---|---|---|---|---|---|---|---|
| Lichens | |||||||
| Arthoniaceae | Arthonia sp. | 0 | 2 | 2 | 2 | 6 | Crustose |
| Arthoniaceae | Coniocarpon cinnabarinum DC. | 0 | 0 | 1 | 0 | 1 | Crustose |
| Caliciaceae | Cratiria sp. | 13 | 28 | 25 | 17 | 83 | Crustose |
| Chrysotrichaceae | Chrysothrix candelaris (L.) J. R. Laundon | 21 | 42 | 53 | 25 | 141 | Crustose |
| Graphidaceae | Allographa nuda (H. Magn.) Lücking & Kalb | 6 | 23 | 22 | 11 | 62 | Crustose |
| Graphidaceae | Graphis elegans (Borrer ex Sm.) Ach. | 11 | 28 | 27 | 12 | 78 | Crustose |
| Graphidaceae | Graphis sp. | 0 | 6 | 2 | 0 | 8 | Crustose |
| Graphidaceae | Phaeographis dendritica (Ach.) Müll.Arg | 14 | 26 | 28 | 18 | 86 | Crustose |
| Graphidaceae | Phaeographis inconspicua (Fée) Müll.Arg | 13 | 28 | 33 | 18 | 92 | Crustose |
| Graphidaceae | Phaeographis sp. | 0 | 1 | 0 | 0 | 1 | Crustose |
| Lecanoraceae | Lecanora sp1 | 0 | 1 | 3 | 1 | 5 | Crustose |
| Lecanoraceae | Lecanora sp2 | 0 | 0 | 2 | 0 | 2 | Crustose |
| Lecanoraceae | Lecanora sp3 | 14 | 31 | 31 | 19 | 95 | Crustose |
| Lecanoraceae | Lecanora sp4 | 0 | 8 | 5 | 1 | 14 | Crustose |
| Lecanoraceae | Lecanora sp5 | 9 | 24 | 20 | 9 | 62 | Crustose |
| Lecanoraceae | Lecanora sp6 | 7 | 7 | 4 | 6 | 24 | Crustose |
| Parmeliaceae | Bulbothrix apophysata (Hale & Kurok.) Hale | 8 | 29 | 22 | 7 | 66 | Foliose |
| Parmeliaceae | Bulbothrix isidiza (Nyl.) Hale | 0 | 3 | 19 | 7 | 29 | Foliose |
| Parmeliaceae | Hypotrachyna costaricensis (Nyl.) Hale | 0 | 1 | 4 | 4 | 9 | Foliose |
| Parmeliaceae | Hypotrachyna horrescens (Taylor) Krog & Swinsc. | 4 | 4 | 4 | 1 | 13 | Foliose |
| Parmeliaceae | Hypotrachyna laevigata (Sm.) Hale | 19 | 30 | 42 | 20 | 111 | Foliose |
| Parmeliaceae | Hypotrachyna sp1 | 1 | 3 | 2 | 1 | 7 | Foliose |
| Parmeliaceae | Hypotrachyna sp2 | 0 | 3 | 0 | 0 | 3 | Foliose |
| Parmeliaceae | Parmotrema austrosinense (Zahlbr.) Hale | 10 | 18 | 20 | 9 | 57 | Foliose |
| Parmeliaceae | Parmotrema cristiferum (Taylor) Hale | 0 | 1 | 2 | 0 | 3 | Foliose |
| Parmeliaceae | Parmotrema reticulatum (Taylor) M. Choisy | 0 | 4 | 5 | 4 | 14 | Foliose |
| Parmeliaceae | Parmotrema subisidiosum (Müll. Arg.) Hale & Fletcher | 0 | 1 | 0 | 0 | 1 | Foliose |
| Parmeliaceae | Usnea sp1 | 9 | 7 | 21 | 10 | 47 | Fruticose |
| Parmeliaceae | Usnea sp2 | 4 | 11 | 6 | 4 | 25 | Fruticose |
| Pertusariaceae | Pertusaria cf. amara (Ach.) Nyl. | 0 | 3 | 15 | 1 | 19 | Crustose |
| Pertusariaceae | Pertusaria sp1 | 0 | 1 | 0 | 0 | 1 | Crustose |
| Pertusariaceae | Pertusaria sp2 | 0 | 0 | 0 | 1 | 1 | Crustose |
| Physciaceae | Heterodermia vulgaris (Vain.) Follman & Redón | 0 | 0 | 0 | 1 | 1 | Foliose |
| Physciaceae | Leucodermia leucomelos (L.) Kalb | 1 | 1 | 0 | 0 | 2 | Foliose |
| Pilocarpaceae | Byssoloma subdiscordans (Nyl.) P. James. | 1 | 3 | 0 | 1 | 5 | Crustose |
| Pyrenulaceae | Pyrenula aff. microcarpa Müll.Arg. | 12 | 17 | 27 | 8 | 64 | Crustose |
| Ramalinaceae | Megalaria aff. grossa (Pers. ex Nyl.) Hafellner. | 1 | 4 | 8 | 4 | 17 | Crustose |
| Ramalinaceae | Ramalina peruviana Ach. | 0 | 0 | 1 | 2 | 3 | Fruticose |
| Ramalinaceae | Ramalina celastri (Sprengel) Krog & Swinscow | 1 | 2 | 2 | 4 | 9 | Fruticose |
| Ramalinaceae | Ramalina sp. | 5 | 12 | 9 | 0 | 26 | Fruticose |
| Teloschistaceae | Caloplaca sp. | 1 | 5 | 7 | 3 | 16 | Crustose |
| Bryophytes | |||||||
| Frullaniaceae | Frullania brasiliensis Raddi | 0 | 0 | 0 | 1 | 1 | Foliose |
| Frullaniaceae | Frullania riojaneirensis (Raddi) Spruce | 2 | 5 | 1 | 2 | 10 | Foliose |
| Lejeuneaceae | Cheilolejeunea xanthocarpa (Lehm. & Lindenb.) Malombe | 0 | 3 | 4 | 0 | 7 | Foliose |
| Lejeuneaceae | Drepanolejeunea sp. | 0 | 0 | 2 | 2 | 4 | Foliose |
| Lejeuneaceae | Lejeunea sp. | 0 | 0 | 1 | 1 | 2 | Foliose |
| Lejeuneaceae | Microlejeunea bullata (Taylor) Steph. | 0 | 2 | 1 | 2 | 5 | Foliose |
| Lejeuneaceae | Thysananthus auriculatus (Wilson & Hook.) Sukkharak & Gradst. | 0 | 0 | 3 | 1 | 4 | Foliose |
Appendix B



References
- Günter, S.; Gonzalez, P.; Alvarez, G.; Aguirre, N.; Palomeque, X.; Haubrich, F.; Weber, M. Determinants for successful reforestation of abandoned pastures in the Andes: Soil conditions and vegetation cover. For. Ecol. Manag. 2009, 258, 81–91. [Google Scholar] [CrossRef]
- Wassenaar, T.; Gerber, P.; Verburg, P.H.; Rosales, M.; Ibrahim, M.; Steinfeld, H. Projecting land use changes in the neotropics: The geography of pasture expansion into forest. Glob. Environ. Change 2007, 17, 86–104. [Google Scholar] [CrossRef]
- Weber, E.; Sun, S.G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef]
- Carrión-Paladines, V.; Benítez, Á.; García-Ruíz, R. Conversion of Andean montane forest to exotic forest plantation modifies soil physicochemical properties in the buffer zone of Ecuador’s Podocarpus National Park. For. Ecosyst. 2022, 9, 100076. [Google Scholar] [CrossRef]
- Hofstede, R.G.; Groenendijk, J.P.; Coppus, R.; Fehse, J.C.; Sevink, J. Impact of pine plantations on soils and vegetation in the Ecuadorian high Andes. Mt. Res. Dev. 2002, 22, 159–167. [Google Scholar] [CrossRef]
- Farley, K.A. Grasslands to tree plantations: Forest transition in the Andes of Ecuador. Ann. Am. Assoc. Geogr. 2007, 97, 755–771. [Google Scholar] [CrossRef]
- Ministerio del Ambiente de Ecuador. Plan Nacional de Forestación y Reforestación; Ministerio del Ambiente de Ecuador: Quito, Ecuador, 2006.
- Knoke, T.; Bendix, J.; Pohle, P.; Hamer, U.; Hildebrandt, P.; Roos, K.; Beck, E. Afforestation or intense pasturing improve the ecological and economic value of abandoned tropical farmlands. Nat. Commun. 2014, 5, 5612. [Google Scholar] [CrossRef] [PubMed]
- Farley, K.A.; Kelly, E.F. Effects of afforestation of a páramo grassland on soil nutrient status. For. Ecol. Manag. 2004, 195, 281–290. [Google Scholar] [CrossRef]
- Chacón, G.; Gagnon, D.; Paré, D. Comparison of soil properties of native forests, Pinus patula plantations and adjacent pastures in the Andean highlands of southern Ecuador: Land use history or recent vegetation effects? Soil Sci. 2009, 25, 427–433. [Google Scholar] [CrossRef]
- Quichimbo, P.; Jiménez, L.; Veintimilla, D.; Tischer, A.; Günter, S.; Mosandl, R.; Hamer, U. Forest Site Classification in the Southern Andean Region of Ecuador: A Case Study of Pine Plantations to Collect a Base of Soil Attributes. Forests 2017, 8, 473. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L.; Motta, R.; Nimis, P.L. Lichen diversity of coarse woody habitats in a Pinus-Larix stand in the Italian Alps. Lichenologist 2008, 40, 153–163. [Google Scholar] [CrossRef]
- Pharo, E.J.; Lindenmayer, D.B. Biological legacies soften pine plantation effects for bryophytes. Biodivers. Conserv. 2009, 18, 1751–1764. [Google Scholar] [CrossRef]
- Lommi, S.; Berglund, H.; Kuusinen, M.; Kuuluvainen, T. Epiphytic lichen diversity in late-successional Pinus sylvestris forests along local and regional forest utilization gradients in eastern boreal Fennoscandia. For. Ecol. Manag. 2010, 259, 883–892. [Google Scholar] [CrossRef]
- Hämäläinen, A.; Kouki, J.; Lõhmus, P. The value of retained Scots pines and their dead wood legacies for lichen diversity in clear-cut forests: The effects of retention level and prescribed burning. For. Ecol. Manag. 2014, 324, 89–100. [Google Scholar] [CrossRef]
- Stefańska-Krzaczek, E.; Fałtynowicz, W.; Szypuła, B.; Kącki, Z. Diversity loss of lichen pine forests in Poland. Eur. J. For. Res. 2018, 137, 419–431. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; de Silanes, M.E.L.; Rubido-Bará, M.; Uribarri, J. The potential role of tree plantations in providing habitat for lichen epiphytes. For. Ecol. Manag. 2013, 291, 386–395. [Google Scholar] [CrossRef]
- Wagner, C.; Schram, L.J.; McMullin, R.T.; Anand, M. Lichen communities in two old-growth pine (Pinus) forests. Lichenologist 2014, 46, 697–709. [Google Scholar] [CrossRef]
- Bäcklund, S.; Jönsson, M.; Strengbom, J.; Frisch, A.; Thor, G. A pine is a pine and a spruce is a spruce–the effect of tree species and stand age on epiphytic lichen communities. PLoS ONE 2016, 11, e0147004. [Google Scholar] [CrossRef]
- Sevgi, E.; Yılmaz, O.Y.; Çobanoğlu Özyiğitoğlu, G.; Tecimen, H.B.; Sevgi, O. Factors influencing epiphytic lichen species distribution in a managed Mediterranean Pinus nigra Arnold Forest. Diversity 2019, 11, 59. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Neumann, M.; de Silanés, M.E.L. Contrasting patterns of lichen abundance and diversity in Eucalyptus globulus and Pinus pinaster plantations with tree age. For. Ecol. Manag. 2020, 462, 117994. [Google Scholar] [CrossRef]
- Fernández-Prado, N.; Aragón, G.; Prieto, M.; Benítez, Á.; Martínez, I. Differences in epiphytic trunk communities in secondary forests and plantations of southern Ecuador. For. Int. J. For. Res. 2023, 96, 20–36. [Google Scholar] [CrossRef]
- Krannitz, P.G.; Duralia, T.E. Cone and seed production in Pinus ponderosa: A review. Western North American Naturalist. 2004, 64, 208–218. [Google Scholar]
- Nguyen, T.T.; Tai, D.T.; Zhang, P.; Razaq, M.; Shen, H.L. Effect of thinning intensity on tree growth and temporal variation of seed and cone production in a Pinus koraiensis plantation. J. For. Res. 2019, 30, 835–845. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsakaldimi, M.; Thanos, C. Seed and cone diversity and seed germination of Pinus pinea in strofylia site of the natura 2000 network. Biodivers. Conserv. 2008, 17, 2427–2439. [Google Scholar] [CrossRef]
- Calama, R.; Gordo, J.; Madrigal, G.; Mutke, S.; Conde, M.; Montero, G.; Pardos, M. Enhanced tools for predicting annual stone pine (Pinus pinea L.) cone production at tree and forest scale in Inner Spain. For. Syst. 2016, 25, e079. [Google Scholar] [CrossRef]
- Freire, J.A.; Rodrigues, G.C.; Tomé, M. Climate change impacts on Pinus pinea L. Silvicultural system for cone production and ways to contour those impacts: A review complemented with data from permanent plots. Forests 2019, 10, 169. [Google Scholar] [CrossRef]
- Johnson, M.; Vander Wall, S.B.; Borchert, M. A comparative analysis of seed and cone characteristics and seed-dispersal strategies of three pines in the subsection Sabinianae. Plant Ecol. 2003, 168, 69–84. [Google Scholar] [CrossRef]
- Vander Wall, S.B. Seed dispersal in pines (Pinus). Bot. Rev. 2023, 89, 275–307. [Google Scholar] [CrossRef]
- Font, R.; Conesa, J.A.; Moltó, J.; Muñoz, M. Kinetics of pyrolysis and combustion of pine needles and cones. J. Anal. Appl. Pyrolysis 2009, 85, 276–286. [Google Scholar] [CrossRef]
- Sahin, H.A.L.İ.L.; Yalcin, O. Conifer cones: An alternative raw material for industry. Br. J. Pharm. Res. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Balaban Ucar, M.; Gonultas, O. Chemical characterization of cone and wood of Pinus pinea. Lignocellulose 2014, 2, 262–268. [Google Scholar]
- Ayrilmis, N.; Buyuksari, U.; Avci, E.; Koc, E. Utilization of pine (Pinus pinea L.) cone in manufacture of wood based composite. For. Ecol. Manag. 2009, 259, 65–70. [Google Scholar] [CrossRef]
- Talluto, L.; Benkman, C.W. Landscape-scale eco-evolutionary dynamics: Selection by seed predators and fire determine a major reproductive strategy. Ecology 2013, 94, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Su, C.X.; Lu, C.H. Cones structure and seed traits of four species of large-seeded pines: Adaptation to animal-mediated dispersal. Ecol. Evol. 2020, 10, 5293–5301. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Chang, S.S.; Lee, S.J. How the pine seeds attach to/detach from the pine cone scale? Front. Life Sci. 2017, 10, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, J.J.; Roques, A.; de Groot, P. Insect Fauna of Coniferous Seed Cones: Diversity, Host Plant Interactions, and Management. Annu. Rev. Entomol. 1994, 39, 179–212. [Google Scholar] [CrossRef]
- Siepielski, A.M.; Benkman, C.W. Interactions among Moths, Crossbills, Squirrels, and Lodgepole Pine in a Geographic Selection Mosaic. Evolution 2004, 58, 95–101. [Google Scholar]
- Micales, J.A.; Han, J.S.; Davis, J.L.; Joung, R.A. Chemical composition and fungitoxic activities of pine cone extractives. Biodeterior. Res. 1994, 4, 317–332. [Google Scholar]
- Kilic, A.; Hafizoglu, H.; Tumen, I.; Donmez, I.E.; Sivrikaya, H.; Sundberg, A.; Holmbom, B. Polysaccharides in cones of eleven coniferous species growing in Turkey. Wood Sci. Technol. 2010, 44, 523–529. [Google Scholar] [CrossRef]
- Xu, R.B.; Yang, X.; Wang, J.; Zhao, H.T.; Lu, W.H.; Cui, J.; Cheng, C.L.; Zou, P.; Huang, W.W.; Wang, P.; et al. Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. Int. J. Mol. Sci. 2012, 13, 14262–14277. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Han, J.S.; Micales, J.A.; Young, R.A. Decay Resistance in Conifer Seed Cones: Role of Resin Acids as Inhibitors of Decomposition by White-Rot Fungi. Holzforschung 1994, 48, 278–284. [Google Scholar] [CrossRef]
- Kasai, K.; Morinaga, T.; Horikoshi, T. Fungal Succession in the Early Decomposition Process of Pine Cones on the Floor of Pinus densiflora Forests. Mycoscience 1995, 36, 325–334. [Google Scholar] [CrossRef]
- Dawson, C.; Vincent, J.F.; Rocca, A.M. How pine cones open. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Worthy, F.R.; Hulme, P.E. Scale dependence shapes how plant traits differentially affect levels of pre-and post-dispersal seed predation in Scots pine. Eur. J. For. Res. 2019, 138, 653–672. [Google Scholar] [CrossRef]
- Ulrich, K.; Genter, L.; Schäfer, S.; Masselter, T.; Speck, T. Investigation of the resilience of cyclically actuated pine cone scales of Pinus jeffreyi. Bioinspir. Biomim. 2024, 19, 046009. [Google Scholar] [CrossRef]
- Lilja, A.; Hallaksela, A.M.; Heinonen, R. Fungi colonizing Scots-pine cone scales and seeds and their pathogenicity. Eur. J. For. Pathol. 1995, 25, 38–46. [Google Scholar] [CrossRef]
- Santini, A.; Pepori, A.; Ghelardini, L.; Capretti, P. Persistence of some pine pathogens in coarse woody debris and cones in a Pinus pinea forest. For. Ecol. Manag. 2008, 256, 502–506. [Google Scholar] [CrossRef]
- Simijaca, D.; Moncada, B.; Lücking, R. Bosque de roble o plantación de coníferas,¿ qué prefieren los líquenes epífitos? Colomb. For. 2018, 21, 123–141. [Google Scholar] [CrossRef]
- Käffer, M.I.; Marcelli, M.P.; Ganade, G. Distribution and composition of the lichenized mycota in a landscape mosaic of southern Brazil. Acta Bot. Bras. 2010, 24, 790–802. [Google Scholar] [CrossRef]
- Churchill, S.P.; Linares, C.E. Prodromus Bryologiae Novo-Granatensis: Introduccion a la Flora de Musgos de Colombia; Instituto de Ciencias Naturales: Bogota, Colombia, 1995. [Google Scholar]
- Gradstein, S.R.; da Costa, D.P. The Hepaticae and Anthocerotae of Brazil; New York Botanical Garden Press: New York, NY, USA, 2003; Volume 87, pp. 1–318. [Google Scholar]
- Gradstein, S.R. The Liverworts and Hornworts of Colombia and Ecuador; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Brodo, I.M. Lichens of North America; Yale University Press: New Haven, CT, USA, 2001; Volume 828. [Google Scholar]
- Nash, T.H., III; Ryan, B.D.; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichens Unlimited: Tempe, AZ, USA, 2002. [Google Scholar]
- Nash, T.H., III; Ryan, B.D.; Diederich, P.; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichens Unlimited: Tempe, AZ, USA, 2004. [Google Scholar]
- Nash, T.H., III; Gries, C.; Bungartz, F. Lichen Flora of the Greater Sonoran Desert Region; Lichens Unlimited: Tempe, AZ, USA, 2007. [Google Scholar]
- Aptroot, A.; Bungartz, F. The Lichen Genus Ramalina on the Galapagos. Lichenologist 2007, 39, 519–542. [Google Scholar] [CrossRef]
- Lücking, R.; Archer, A.W.; Aptroot, A. A world-wide key to the genus Graphis (Ostropales: Graphidaceae). Lichenologist 2009, 41, 363–452. [Google Scholar] [CrossRef]
- Moberg, R. The Lichen Genus Heterodermia (Physciaceae) in South America–A Contribution Including Five New Species. Nord. J. Bot. 2011, 29, 129–147. [Google Scholar] [CrossRef]
- Aptroot, A. A world key to the species of Anthracothecium and Pyrenula. Lichenologist 2012, 44, 5–53. [Google Scholar] [CrossRef]
- Bungartz, F.; Benatti, M.N.; Spielmann, A.A. The Genus Bulbothrix (Parmeliaceae, Lecanoromycetes) in the Galapagos Islands: A Case Study of Superficially Similar, but Overlooked Macrolichens. Bryologist 2013, 116, 358–372. [Google Scholar] [CrossRef]
- Bungartz, F.; Spielmann, A.A. The Genus Parmotrema (Parmeliaceae, Lecanoromycetes) in the Galapagos Islands. Plant Fungal Syst. 2019, 64, 199–221. [Google Scholar] [CrossRef][Green Version]
- Bungartz, F.; Elix, J.A.; Printzen, C. Lecanoroid lichens in the Galapagos Islands: The genera Lecanora, Protoparmeliopsis, and Vainionora (Lecanoraceae, Lecanoromycetes). Phytotaxa 2020, 431, 1–85. [Google Scholar] [CrossRef]
- Söderström, L.; Hagborg, A.; Von Konrat, M.; Bartholomew-Began, S.; Bell, D.; Briscoe, L.; Zhu, R.L. World checklist of hornworts and liverworts. PhytoKeys 2016, 59, 1–828. [Google Scholar] [CrossRef]
- Yánez-Ayabaca, A.; Benítez, Á.; Batallas Molina, R.; Naranjo, D.; Etayo, J.; Prieto, M.; Cevallos, G.; Caicedo, E.; Scharnagl, K.; McNerlin, B.; et al. Towards a Dynamic Checklist of Lichen-Forming, Lichenicolous and Allied Fungi of Ecuador—Using the Consortium of Lichen Herbaria to Manage Fungal Biodiversity in a Megadiverse Country. Lichenologist 2023, 55, 203–222. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 7 July 2023).
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova+ for Primer: Guide to Software and Statistical Methods; The University of Chicago Press: Plymouth, MI, USA, 2008. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, versión 2.5-6; 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 February 2025).
- R Development Core Team. A language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2024; Available online: http://www.R-project.org (accessed on 20 May 2024).
- Gosselin, M.; Fourcin, D.; Dumas, Y.; Gosselin, F.; Korboulewsky, N.; Toïgo, M.; Vallet, P. Influence of forest tree species composition on bryophytic diversity in mixed and pure pine (Pinus sylvestris L.) and oak (Quercus petraea (Matt.) Liebl.) stands. For. Ecol. Manag. 2017, 406, 318–329. [Google Scholar] [CrossRef]
- Notov, A.A.; Zhukova, L.A. Epiphytic lichens and bryophytes at different ontogenetic stages of Pinus sylvestris. Wulfenia 2015, 22, 245–260. [Google Scholar]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Sillett, S.C.; McCune, B.; Peck, J.E.; Rambo, T.R.; Ruchty, A. Dispersal limitations of epiphytic lichens result in species dependent on old-growth forests. Ecol. Appl. 2000, 10, 789–799. [Google Scholar] [CrossRef]
- Dettki, H.; Klintberg, P.; Esseen, P.A. Are epiphytic lichens in young forests limited by local dispersal? Ecoscience 2000, 7, 317–325. [Google Scholar] [CrossRef]
- Fritz, Ö.; Niklasson, M.; Churski, M. Tree age is a key factor for the conservation of epiphytic lichens and bryophytes in beech forests. Appl. Veg. Sci. 2009, 12, 93–106. [Google Scholar] [CrossRef]
- Lie, M.H.; Arup, U.; Grytnes, J.A.; Ohlson, M. The importance of host tree age, size and growth rate as determinants of epiphytic lichen diversity in boreal spruce forests. Biodivers. Conserv. 2009, 18, 3579–3596. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Culmsee, H. Bryophyte diversity on tree trunks in montane forests of Central Sulawesi, Indonesia. Trop. Bryol. 2010, 31, 95–105. [Google Scholar]
- Benítez, Á.; Prieto, M.; Aragón, G. Large trees and dense canopies: Key factors for maintaining high epiphytic diversity on trunk bases (bryophytes and lichens) in tropical montane forests. J. For. Res. 2015, 88, 521–527. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Brockway, D.G. Power laws in cone production of longleaf pine across its native range in the United States. Sust. Agri. Res. 2017, 4, 64–73. [Google Scholar] [CrossRef][Green Version]
- Patterson, T. The Percentage of Trees Bearing Cones as a Predictor for Annual Longleaf Pine Cone Production. Front. For. Glob. Change 2021, 4, 718218. [Google Scholar] [CrossRef]
- Hågvar, S. From Litter to Humus in a Norwegian Spruce Forest: Long-Term Studies on the Decomposition of Needles and Cones. Forests 2016, 7, 186. [Google Scholar] [CrossRef]
- Cha, J.S.; Um, B.H. Delignification of Pinecone and Extraction of Formic Acid in the Hydrolysate Produced by Alkaline Fractionation. Appl. Biochem. Biotechnol. 2020, 192, 103–119. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, Á. Pine Cones in Plantations as Refuge and Substrate of Lichens and Bryophytes in the Tropical Andes. Diversity 2025, 17, 548. https://doi.org/10.3390/d17080548
Benítez Á. Pine Cones in Plantations as Refuge and Substrate of Lichens and Bryophytes in the Tropical Andes. Diversity. 2025; 17(8):548. https://doi.org/10.3390/d17080548
Chicago/Turabian StyleBenítez, Ángel. 2025. "Pine Cones in Plantations as Refuge and Substrate of Lichens and Bryophytes in the Tropical Andes" Diversity 17, no. 8: 548. https://doi.org/10.3390/d17080548
APA StyleBenítez, Á. (2025). Pine Cones in Plantations as Refuge and Substrate of Lichens and Bryophytes in the Tropical Andes. Diversity, 17(8), 548. https://doi.org/10.3390/d17080548






