Combined Soil Inoculation with Mycorrhizae and Trichoderma Alleviates Nematode-Induced Decline in Mycorrhizal Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Inocula Preparation
2.2. In Vitro and in Plant Experiments
2.3. Root and Soil Sampling
2.4. Molecular Fingerprinting of AMF Diversity from Root DNA
2.5. Trichoderma Abundance
2.6. Root Infestation Levels by M. javanica
2.7. Plant Growth Parameters
2.8. Data Analysis
3. Results
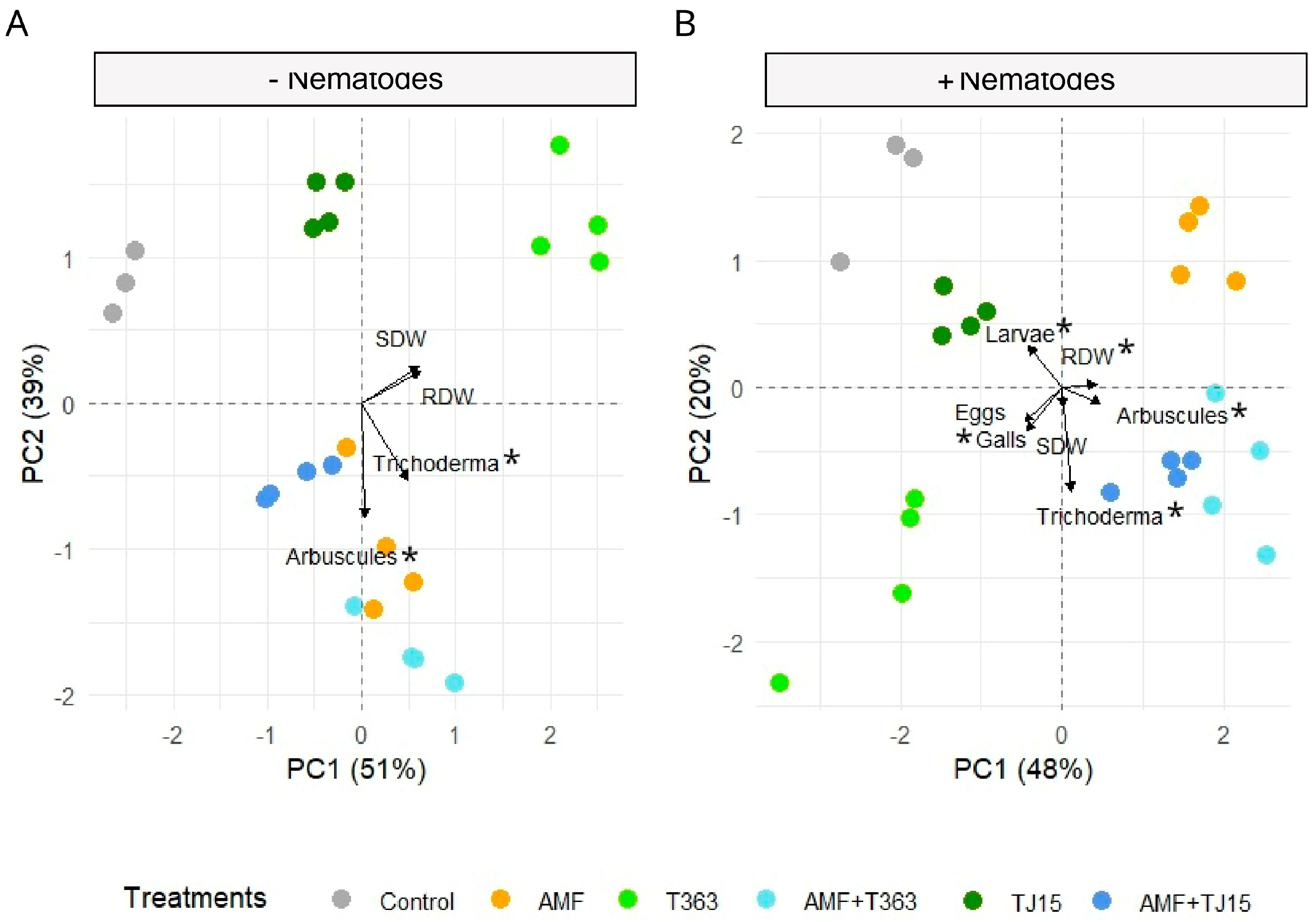
3.1. Strain-Specific Effects of AMF and Trichoderma on Tomato Growth Under Nematode Infection
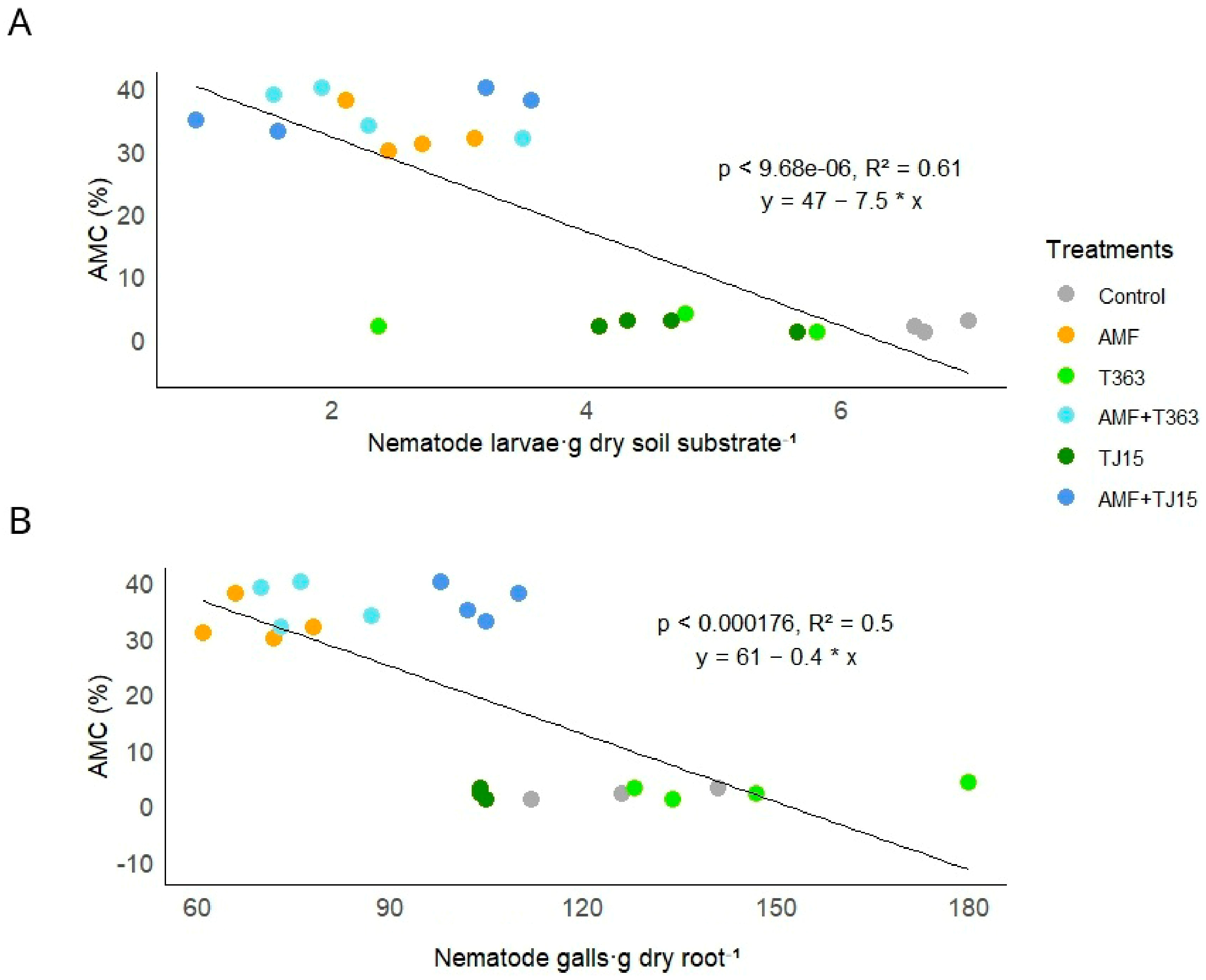
3.2. Trichoderma Exhibits Antagonism Toward Nematodes Independently of Their Abundance
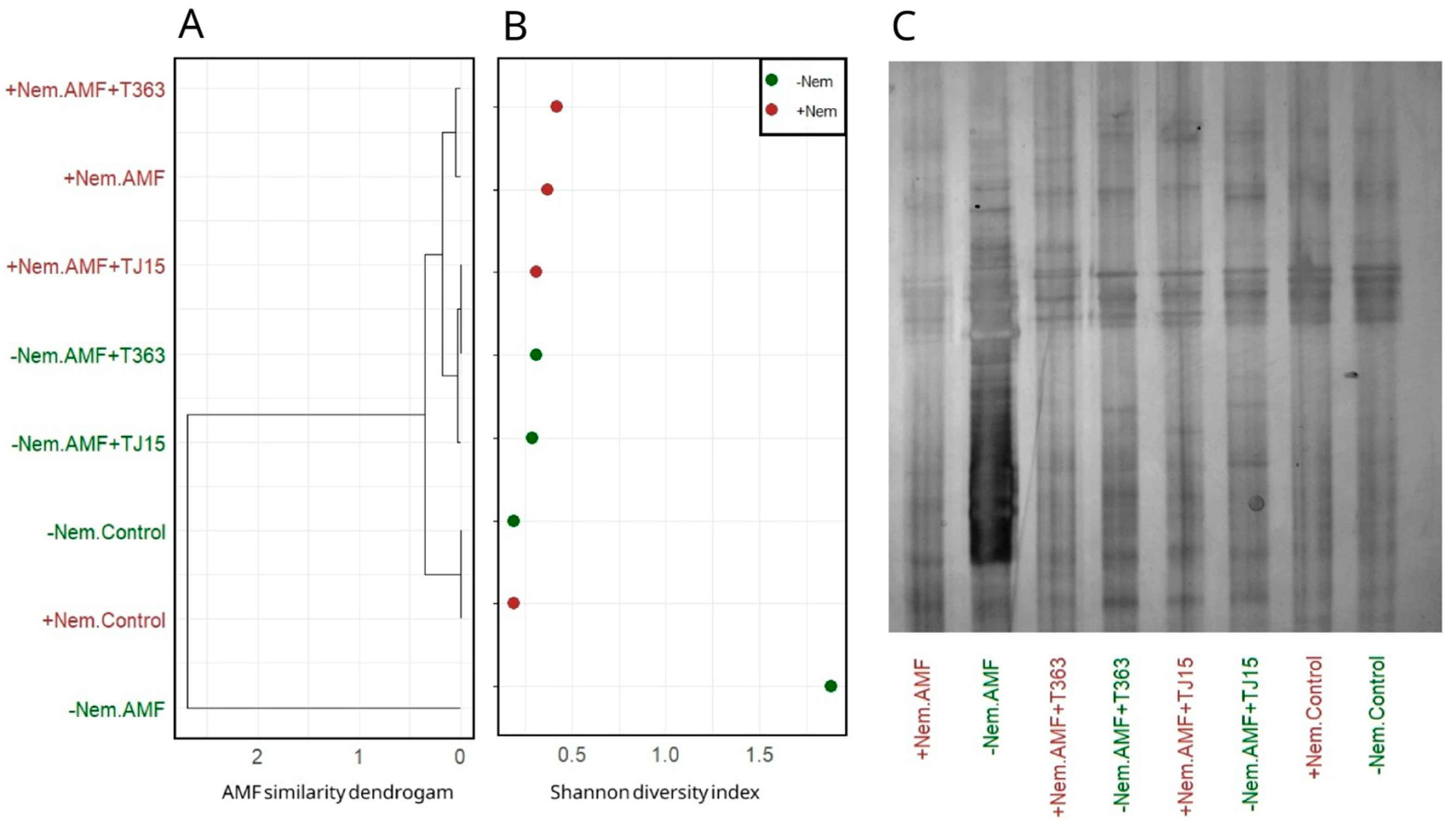
3.3. Trichoderma Mitigates Nematode-Induced Declines in AMF Diversity and Colonization
4. Discussion
4.1. Interactions Between Plant Beneficial Fungi and Nematodes in Soil Ecosystems
4.2. AMF Diversity: Impact of M. javanica and the Role of Trichoderma
4.3. Interaction of AMF and Trichoderma for Plant Growth-Promoting and Nematode Biocontrol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| T | Trichoderma |
| RKN | Root-knot nematodes |
| PGPM | Plant growth-promoting microorganisms |
| AMC | Arbuscular mycorrhizal colonization |
| Nem | Nematode |
| SSCP | Single-strand conformation polymorphism |
| IR | Inoculation response |
| CFU | Colony-forming units |
References
- Garbeva, P.; Hol, W.H.G.; Termorshuizen, A.J.; Kowalchuk, G.A.; De Boer, W. Fungistasis and general soil biostasis—A new synthesis. Soil Biol. Biochem. 2011, 43, 469–477. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A.H. An arbuscular mycorrhizal inoculum enhances root proliferation in, but not nitrogen capture from, nutrient-rich patches in soil. New Phytol. 2000, 145, 575–584. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52 (Suppl. 1), 487–511. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P. Root-knot nematodes. Nematology 2010, 12, 483–484. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Rius, J.E.P.; Castillo, P. Systematics of root-knot nematodes (Nematoda: Meloidogynidae). In Nematology Monographs and Perspectives; Hunt, D.J., Perry, R.N., Eds.; Brill Publishing Netherlands: Leiden, The Netherlands, 2021; Volume 14, pp. 31–39. ISBN 9789004366343. [Google Scholar]
- Seid, A.; Fininsa, C.; Mekete, T.; Decraemer, W.; Wesemael, W.M.L. Tomato (Solanum lycopersicum) and root-knot nematodes (Meloidogyne spp.)—A century-old battle. Nematology 2015, 17, 995–1009. [Google Scholar] [CrossRef]
- Almohithef, A.H.; Al-Yahya, F.A.; Al-Hazmi, A.S.; Dawabah, A.A.M.; Lafi, H.A. Prevalence of plant-parasitic nematodes associated with certain greenhouse vegetable crops in Riyadh region, Saudi Arabia. J. Saudi Soc. Agric. Sci. 2018, 19, 22–25. [Google Scholar] [CrossRef]
- Hajihassani, A.; Davis, R.F.; Timper, P. Evaluation of selected nonfumigant nematicides on increasing inoculation densities of Meloidogyne incognita on cucumber. Plant Dis. 2019, 103, 3161–3165. [Google Scholar] [CrossRef]
- Gullino, M.L.; Garibaldi, A.; Gamliel, A.; Katan, J. Soil disinfestation: From soil treatment to soil and plant health. Plant Dis. 2022, 106, 1541–1554. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, K.; Hyde, K.D. Fungi–nematode interactions: Diversity, ecology, and biocontrol prospects in agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef]
- Manteghi, A.; Danesh, Y.R.; Hesar, A.M.; Demir, S.; Boyno, G.; Catani, L.; Semprucci, F. Is the Arbuscular Mycorrhizal Fungus Funneliformis mosseae a suitable agent to control Criconematid populations? Diversity 2022, 14, 898. [Google Scholar] [CrossRef]
- Mondino, E.A.; Thougnon Islas, A.J.; Covacevich, F. Potencialidad en el control de infección de nematodos en tomate por hongos micorrícicos arbusculares nativos. Hortic. Argent. 2019, 8, 20–29. Available online: https://www.horticulturaar.com.ar/es/articulos/potencialidad-en-el-control-de-infeccion-de-nematodos-en-tomate-por-hongos-micorricicos-arbusculares-nativos.html (accessed on 1 October 2024). (In Spanish).
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Masadeh, B.; von Alten, H.; Grunewaldt-Stoecker, G.; Sikora, R.A. Biocontrol of root-knot nematodes using the arbuscular mycorrhizal fungus Glomus intraradices and the antagonist Trichoderma viride in two tomato cultivars differing in their suitability as hosts for the nematodes. J. Plant Dis. Prot. 2004, 111, 322–333. [Google Scholar]
- Fernandez-Gnecco, G.A.; Gégu, L.; Covacevich, F.; Consolo, V.F.; Bouffaud, M.L.; Buscot, F.; Smalla, K.; Babin, D. Alone as effective as together: AMF and Trichoderma inoculation boost maize performance but differentially shape soil and rhizosphere microbiota. J. Sustain. Agric. Environ. 2024, 3, e12091. [Google Scholar] [CrossRef]
- INVAM. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi; West Virginia University: Morgantown, WV, USA, 2019; Available online: http://invam.wvu.edu/the-fungi/species-descriptions (accessed on 1 March 2024).
- Brundrett, M.C. Mycorrhizal Associations: The Web Resource. Section 4. Arbuscular Mycorrhizas. In Version 2 © Mark Brundrett; Web: Perth, Australia, 2008; Available online: https://www.mycorrhizas.info/vam.html#fungi (accessed on 2 April 2024).
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ.-Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. Comparison of methods for collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Kaur, T.; Jasrotia, S.; Ohri, P.; Manhas, R.K. Evaluation of in vitro and in vivo nematicidal potential of a multifunctional streptomycete, Streptomyces hydrogenans strain DH16 against Meloidogyne incognita. Microbiol. Res. 2016, 192, 247–252. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Cordo, C.A.; Monaco, C.I.; Segarra, C.I.; Simon, M.R.; Mansilla, A.Y.; Perelló, A.E.; Kripelz, N.I.; Bayo, D.; Conde, R.D. Trichoderma spp. as elicitors of wheat plant defense responses against Septoria tritici. Biocontrol Sci. Technol. 2007, 17, 687–698. [Google Scholar] [CrossRef]
- Kjøller, R.; Rosendahl, S. Detection of arbuscular mycorrhizal fungi (Glomales) in roots by nested PCR and SSCP (Single Stranded Conformation Polymorphism). Plant Soil 2000, 226, 189–196. [Google Scholar] [CrossRef]
- Rosendahl, S.; Stukenbrock, E.H. Community structure of arbuscular mycorrhizal fungi in undisturbed vegetation revealed by analyses of LSU rDNA sequences. Mol. Ecol. 2004, 13, 3179–3186. [Google Scholar] [CrossRef]
- Covacevich, F.; Hernández Guijarro, K.; Crespo, E.M.; Lumini, E.; Rivero Mega, M.S.; Lugo, M.A. Arbuscular Mycorrhizal Fungi from Argentinean Highland Puna Soils Unveiled by Propagule Multiplication. Plants 2021, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Benbouza, H.; Jacquemin, J.M.; Baudoin, J.P.; Mergeai, G. Optimization of a reliable, fast, cheap and sensitive silver staining method to detect SSR markers in polyacrylamide gels. Biotechnol. Agron. Soc. Environ. 2006, 10, 77–81. [Google Scholar]
- Elad, Y.; Chet, I.; Henis, Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 1981, 9, 59–67. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Van Wees, S.C. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef]
- Gil, S.V.; Meriles, J.; Conforto, C.; Figoni, G.; Basanta, M.; Lovera, E.; March, G.J. Field assessment of soil biological and chemical quality in response to crop management practices. World J. Microbiol. Biotechnol. 2009, 25, 439–448. [Google Scholar]
- Atamian, H.S.; Roberts, P.A.; Kaloshian, I. High and Low Throughput Screens with Root-knot Nematodes Meloidogyne spp. J. Vis. Exp. 2012, 12, e3629. [Google Scholar] [CrossRef]
- Jenkins, W.R.B. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Cavagnaro, T.R.; Smith, F.A.; Ayling, S.M.; Smith, S.E. Growth and phosphorus nutrition of a Paris-type arbuscular mycorrhizal symbiosis. New Phytol. 2003, 157, 127–134. [Google Scholar] [CrossRef]
- Zulfarina; Rusmana, I.; Mubarik, N.R.; Santosa, D.A. Application of Denaturing Gradient Gel Electrophoresis (Dgge) to Study the Diversity of Nitrification Bacteria in Tropical Rain Forest and Oil Palm Plantation SEMIRATA-International Conference on Science and Technology; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.J.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions (Version 2.1.3). CRAN. 2022. Available online: https://cran.r-project.org/package=cluster (accessed on 2 December 2024).
- de Vries, E.G.; Ripley, B.D. ggdendro: Visualizing Dendrograms (Version 0.1.3). CRAN. 2022. Available online: https://cran.r-project.org/package=ggdendro (accessed on 2 December 2024).
- Schouteden, N.; De Waele, D.; Panis, B.; Vos, C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed]
- Hol, W.G.; Cook, R. An overview of arbuscular mycorrhizal fungi–nematode interactions. Basic Appl. Ecol. 2005, 6, 489–503. [Google Scholar] [CrossRef]
- Lee, D.H.; Zo, Y.G.; Kim, S.J. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR single strand conformation polymorphism. Appl. Environ. Microbiol. 1996, 62, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Z.; Zhang, Y.; Zhao, Z.-W. Molecular diversity of arbuscular mycorrhizal fungi and their distribution patterns related to host-plants and habitats in a hot and arid ecosystem, southwest China. FEMS Microbiol. Ecol. 2010, 71, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Florez, V.A.; Carvalhais, L.C.; Schenk, P.M. Culture-independent molecular tools for soil and rhizosphere microbiology. Diversity 2013, 5, 581–612. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Roldán, A.; Torres, M.P. Assessing the diversity of AM fungi in arid gypsophilous plant communities. Environ. Microbiol. 2009, 11, 2649–2659. [Google Scholar] [CrossRef]
- Sreenayana, B.; Vinodkumar, S.; Nakkeeran, S.; Muthulakshmi, P.; Poornima, K. Multitudinous potential of Trichoderma species in imparting resistance against F. oxysporum f. sp. cucumerinum and Meloidogyne incognita disease complex. J. Plant Growth Regul. 2021, 41, 1187–1206. [Google Scholar] [CrossRef]
- Naserinasab, F.; Sahebani, N.; Etebarian, H.R. Biological control of Meloidogyne javanica by Trichoderma harzianum BI and salicylic acid on tomato. Afr. J. Food Sci. 2011, 5, 276–280. [Google Scholar]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]

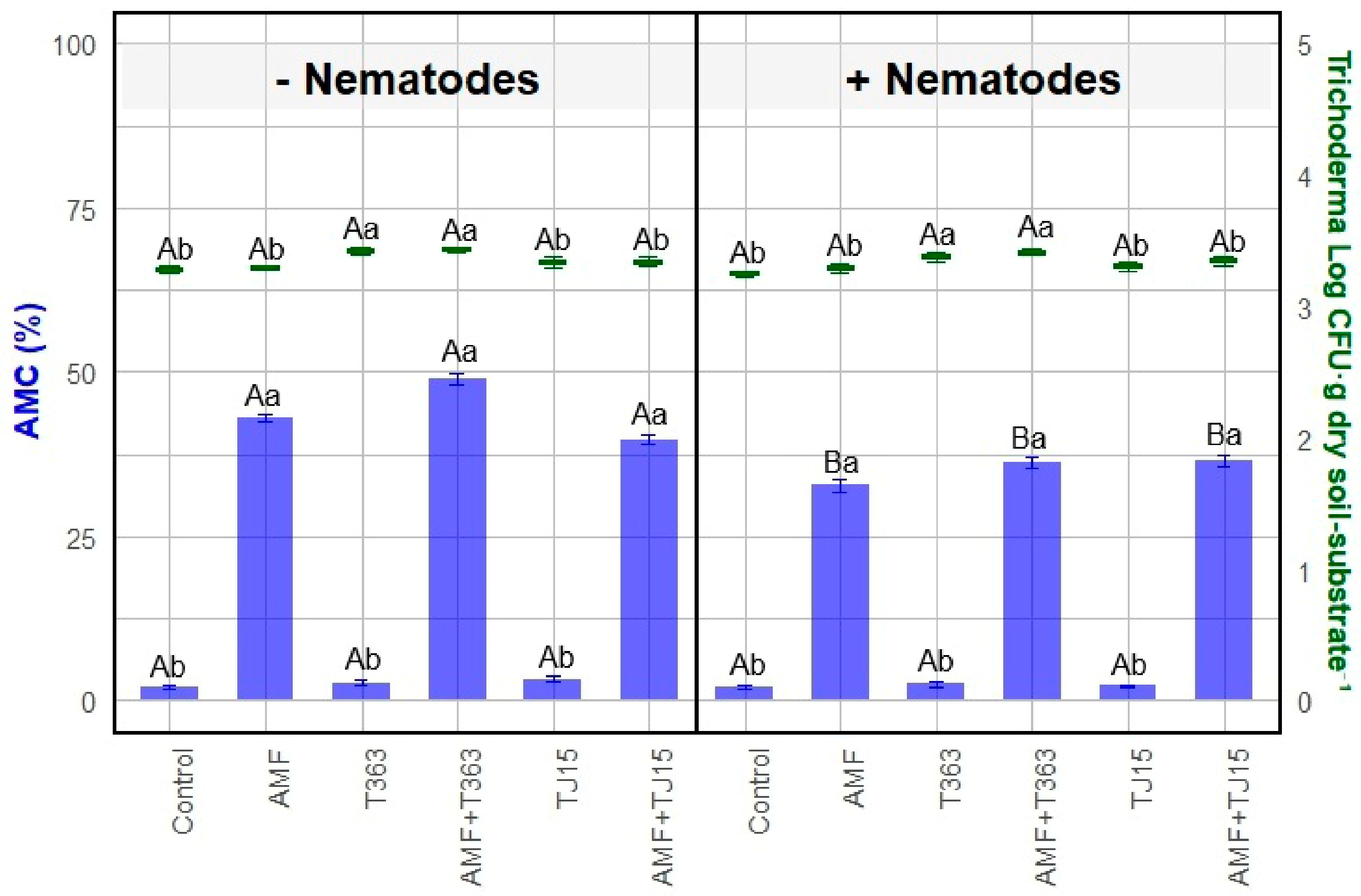
| Treatment | Galls.g dry root−1 | Eggs masses.g dry root−1 | J2 larvae.g dry soil–substrate−1 |
|---|---|---|---|
| Control | 126 ± 15 b | 147 ± 14 a | 7 ± 0.2 a |
| AMF | 62 ± 15 d | 69 ± 2 c | 3 ± 0.5 c |
| T363 | 147 ± 23 a | 125 ± 2 b | 5 ± 0.7 b |
| AMF + T363 | 73 ± 10 d | 75 ± 6 c | 3 ± 0.9 c |
| TJ15 | 104 ± 10 c | 121 ± 7 b | 3 ± 0.7 c |
| AMF + TJ15 | 102 ± 6 c | 95 ± 11 c | 5 ± 0.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covacevich, F.; Fernandez-Gnecco, G.; Consolo, V.F.; Burges, P.L.; Calo, G.F.; Mondino, E.A. Combined Soil Inoculation with Mycorrhizae and Trichoderma Alleviates Nematode-Induced Decline in Mycorrhizal Diversity. Diversity 2025, 17, 334. https://doi.org/10.3390/d17050334
Covacevich F, Fernandez-Gnecco G, Consolo VF, Burges PL, Calo GF, Mondino EA. Combined Soil Inoculation with Mycorrhizae and Trichoderma Alleviates Nematode-Induced Decline in Mycorrhizal Diversity. Diversity. 2025; 17(5):334. https://doi.org/10.3390/d17050334
Chicago/Turabian StyleCovacevich, Fernanda, Gabriela Fernandez-Gnecco, Verónica F. Consolo, Pablo L. Burges, Gonzalo F. Calo, and Eduardo A. Mondino. 2025. "Combined Soil Inoculation with Mycorrhizae and Trichoderma Alleviates Nematode-Induced Decline in Mycorrhizal Diversity" Diversity 17, no. 5: 334. https://doi.org/10.3390/d17050334
APA StyleCovacevich, F., Fernandez-Gnecco, G., Consolo, V. F., Burges, P. L., Calo, G. F., & Mondino, E. A. (2025). Combined Soil Inoculation with Mycorrhizae and Trichoderma Alleviates Nematode-Induced Decline in Mycorrhizal Diversity. Diversity, 17(5), 334. https://doi.org/10.3390/d17050334







