Morphological Diversity of Moroccan Honey Bees (Apis mellifera L. 1758): Insights from a Geometric Morphometric Study of Wing Venation in Honey Bees from Different Climatic Regions
Abstract
1. Introduction
2. Materials and Methods
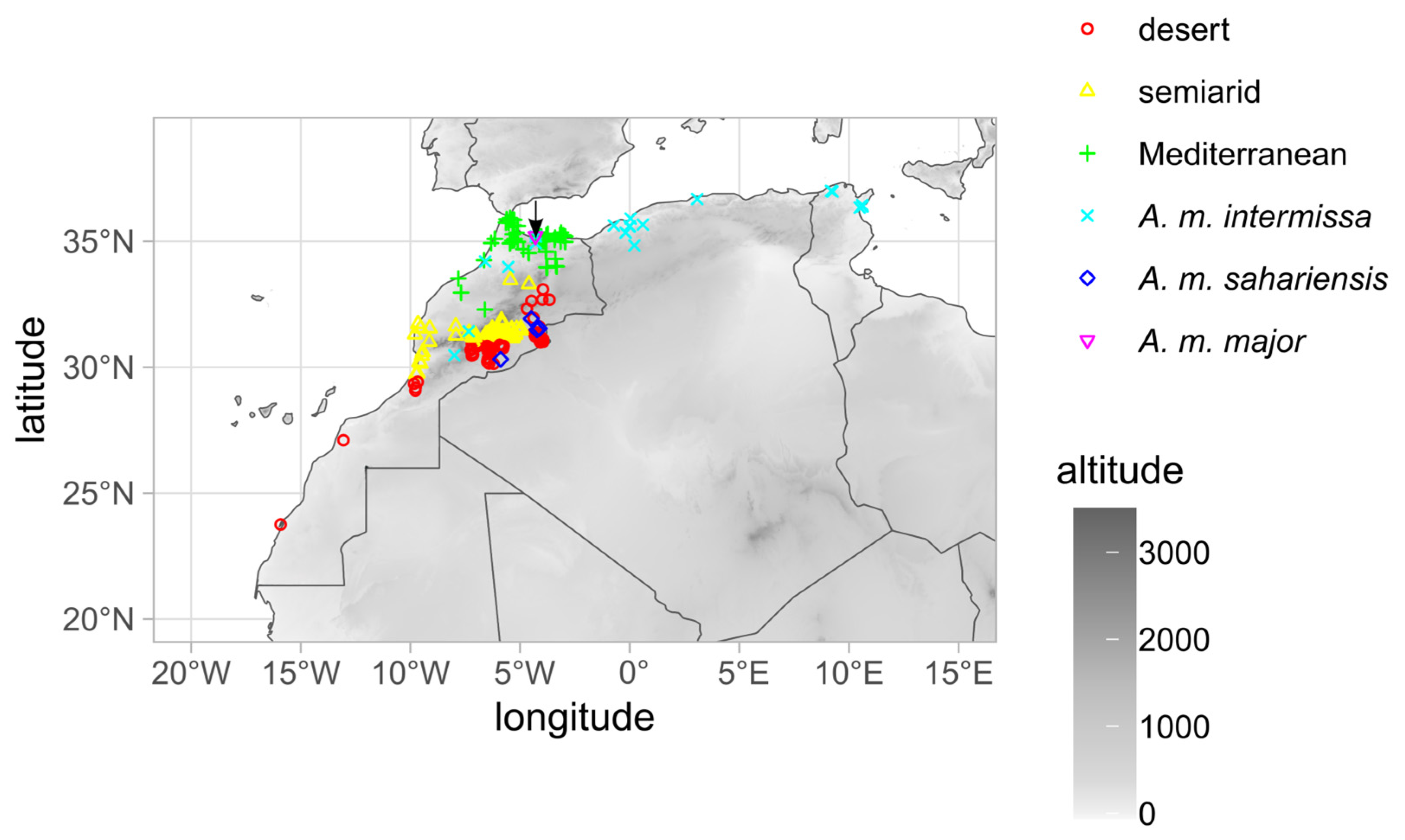
2.1. Sampling
2.2. Geometric Morphometric Analysis
2.3. Statistical Analysis
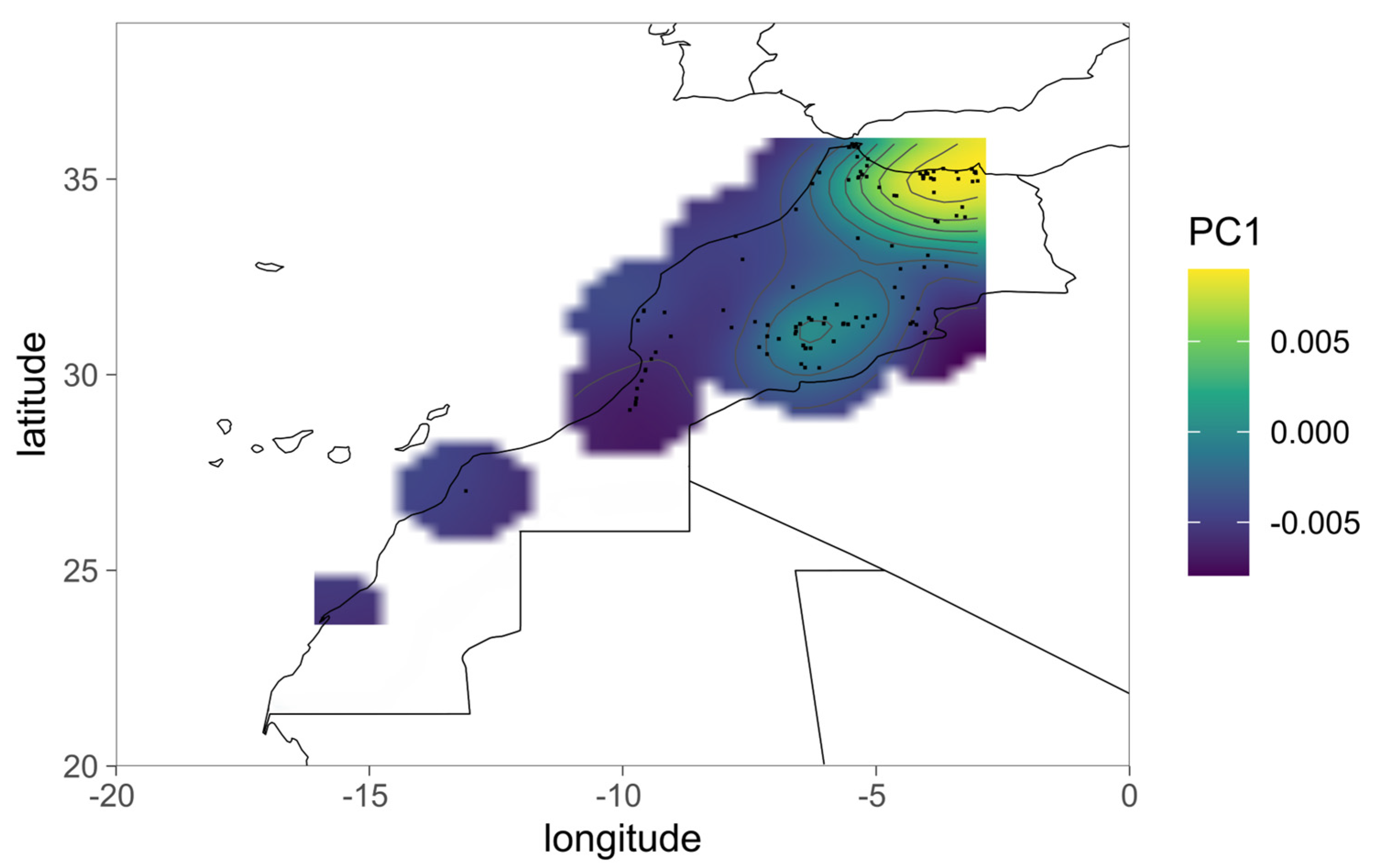
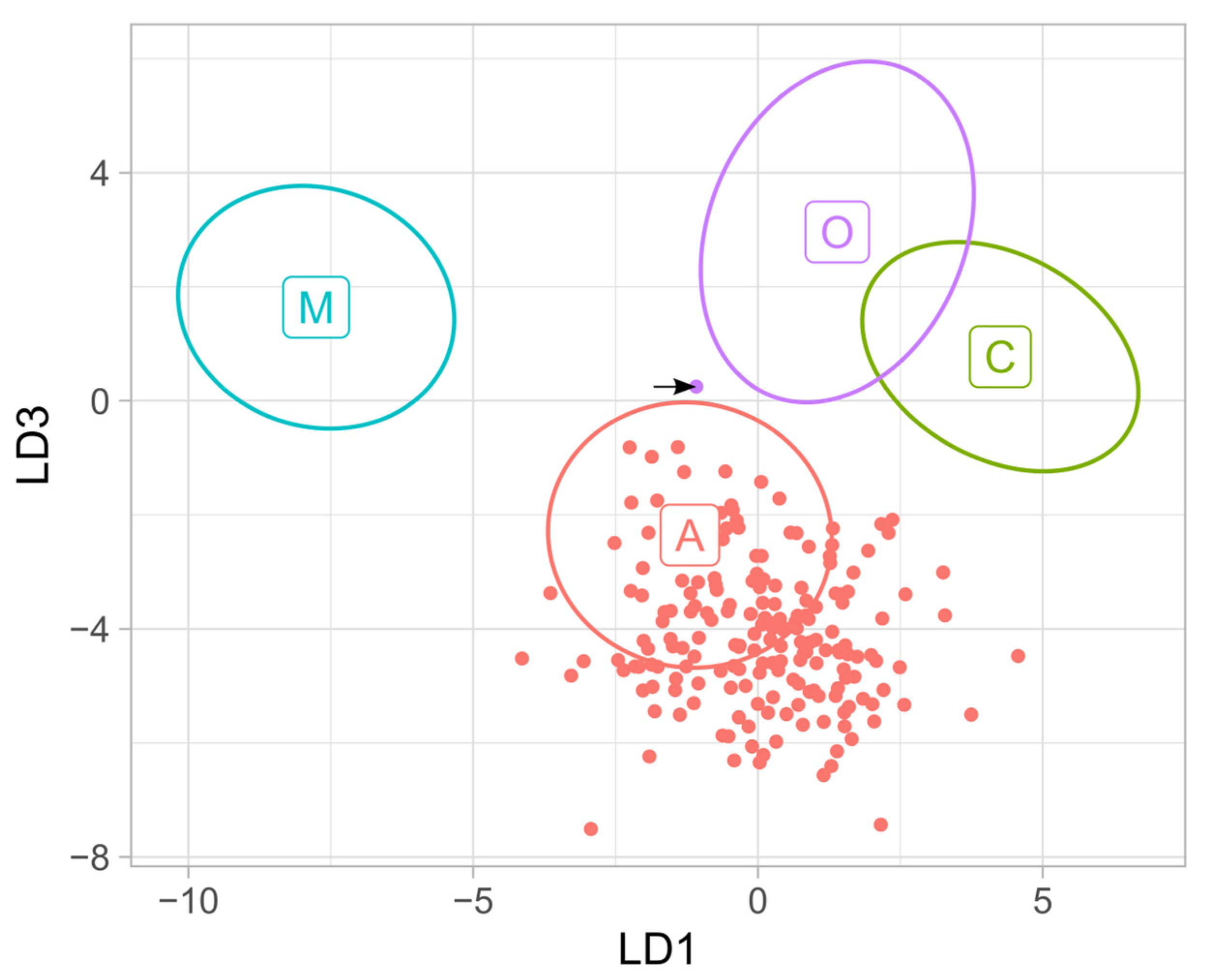
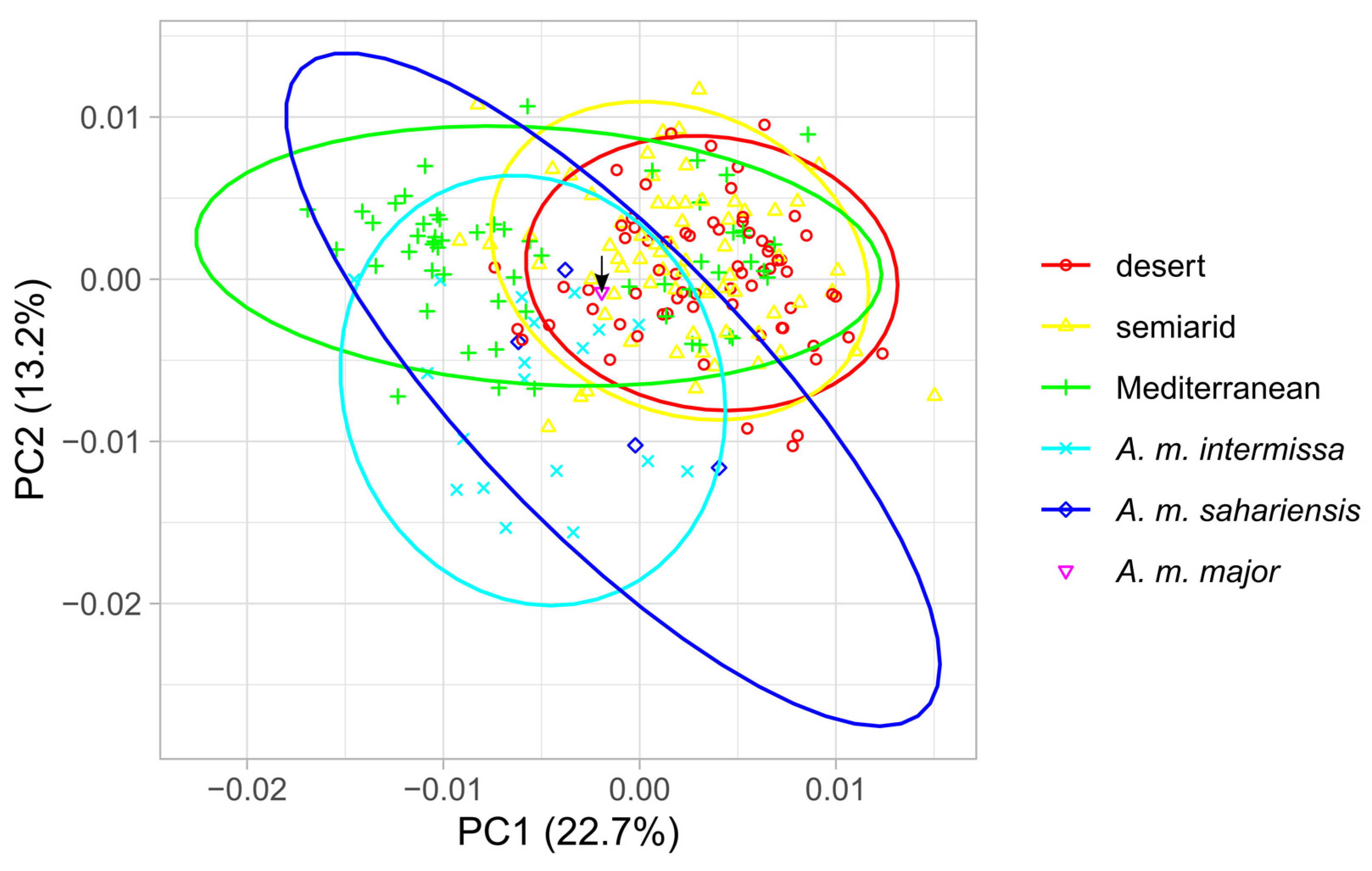
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MANOVA | multivariate analysis of variance |
| PCA | principal component analysis |
| MD | Mahalanobis distance |
| LDA | linear discriminant analysis |
| GPA | generalized Procrustes analysis |
References
- Lhomme, P.; Michez, D.; Christmann, S.; Scheuchl, E.; Abdouni, I.E.; Hamroud, L.; Ihsane, O.; Sentil, A.; Smaili, M.C.; Schwarz, M.; et al. The Wild Bees (Hymenoptera: Apoidea) of Morocco. Zootaxa 2020, 4892, 1–159. [Google Scholar] [CrossRef] [PubMed]
- El Abdouni, I.; Lhomme, P.; Christmann, S.; Dorchin, A.; Sentil, A.; Pauly, A.; Hamroud, L.; Ihsane, O.; Reverté, S.; Patiny, S.; et al. Diversity and Relative Abundance of Insect Pollinators in Moroccan Agroecosystems. Front. Ecol. Evol. 2022, 10, 866581. [Google Scholar] [CrossRef]
- Skaou, A.; Aglagane, A.; Er-Rguibi, O.; El Mouden, E.H. Crops Dependency on Pollinators to Secure Pollination Success and Fruit Development: A Case Study of Almond Varieties from Morocco. J. Appl. Entomol. 2024, 148, 1068–1076. [Google Scholar] [CrossRef]
- Sabbahi, R. Economic Value of Insect Pollination of Major Crops in Morocco. Int. J. Trop. Insect Sci. 2021, 42, 1275–1284. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- De La Rúa, P.; Jaffé, R.; Muñoz, I.; Serrano, J.; Moritz, R.F.A.; Kraus, F.B. Conserving Genetic Diversity in the Honeybee: Comments on Harpur et al. (2012). Mol. Ecol. 2013, 22, 3208–3210. [Google Scholar] [CrossRef]
- Meixner, M.D.; Sheppard, W.S.; Poklukar, J. Asymmetrical Distribution of a Mitochondrial DNA Polymorphism between 2 Introgressing Honey Bee Subspecies. Apidologie 1993, 24, 147–153. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Lee, M.; Takahashi, J.; Kwon, H.W.; Nikolenko, A.G. A Revision of Subspecies Structure of Western Honey Bee Apis mellifera. Saudi J. Biol. Sci. 2020, 27, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Dogantzis, K.A.; Tiwari, T.; Conflitti, I.M.; Dey, A.; Patch, H.M.; Muli, E.M.; Garnery, L.; Whitfield, C.W.; Stolle, E.; Alqarni, A.S.; et al. Thrice out of Asia and the Adaptive Radiation of the Western Honey Bee. Sci. Adv. 2021, 7, eabj2151. [Google Scholar] [CrossRef] [PubMed]
- Garnery, L.; Mosshine, E.H.; Oldroyd, B.P.; Cornuet, J.M. Mitochondrial DNA Variation in Moroccan and Spanish Honey Bee Populations. Mol. Ecol. 1995, 4, 465–472. [Google Scholar] [CrossRef]
- Arias, M.C.; Sheppard, W.S. Molecular Phylogenetics of Honey Bee Subspecies (Apis mellifera L.) Inferred from Mitochondrial DNA Sequence. Mol. Phylogenet. Evol. 1996, 5, 557–566. [Google Scholar] [CrossRef]
- Franck, P.; Garnery, L.; Solignac, M.; Cornuet, J.-M. The origin of west European subspecies of honeybees (Apis mellifera): New insights from microsatellite and mitochondrial data. Evolution 1998, 52, 1119–1134. [Google Scholar] [CrossRef]
- Cornuet, J.-M.; Daoudi, A.; Mohssine, E.H.; Fresnaye, J. Étude biométrique de populations d’abeilles marocaines. Apidologie 1988, 19, 355–366. [Google Scholar] [CrossRef]
- Grissa, K.; Cornuet, J.M.; Msadda, K.; Fresnaye, J. Étude Biométrique de Populations d’abeilles Tunisiennes. Apidologie 1990, 21, 303–310. [Google Scholar] [CrossRef]
- Hepburn, H.R.; Radloff, S.E. Honeybees of Africa; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-64221-3. [Google Scholar]
- Aglagane, A.; Tofilski, A.; Er-Rguibi, O.; Laghzaoui, E.-M.; Kimdil, L.; El Mouden, E.H.; Fuchs, S.; Oleksa, A.; Aamiri, A.; Aourir, M. Geographical Variation of Honey Bee (Apis mellifera L. 1758) Populations in South-Eastern Morocco: A Geometric Morphometric Analysis. Insects 2022, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Aglagane, A.; Oleksa, A.; Er-Rguibi, O.; Tofilski, A.; El Mouden, E.H.; Aamiri, A.; Aourir, M. Genetic Diversity and Population Structure of the Saharan Honey Bee Apis mellifera sahariensis from Southeastern Morocco: Introgression Assessment and Implications for Conservation. Apidologie 2023, 54, 31. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Nawrocka, A.; Kandemir, İ.; Fuchs, S.; Tofilski, A. Computer Software for Identification of Honey Bee Subspecies and Evolutionary Lineages. Apidologie 2018, 49, 172–184. [Google Scholar] [CrossRef]
- Bookstein, F.L. Combining the Tools of Geometric Morphometrics. In Advances in Morphometrics; Marcus, L.F., Corti, M., Loy, A., Naylor, G.J.P., Slice, D.E., Eds.; Springer: Boston, MA, USA, 1996; pp. 131–151. ISBN 978-1-4757-9085-6. [Google Scholar]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; Wiley series in probability and statistics; Wiley: Chichester, UK; New York, NY, USA; Weinheim, Germany, 1998; ISBN 978-0-471-95816-1. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Introduction. In Geometric Morphometrics for Biologists; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–20. ISBN 978-0-12-778460-1. [Google Scholar][Green Version]
- Whitfield, C.W.; Behura, S.K.; Berlocher, S.H.; Clark, A.G.; Johnston, J.S.; Sheppard, W.S.; Smith, D.R.; Suarez, A.V.; Weaver, D.; Tsutsui, N.D. Thrice Out of Africa: Ancient and Recent Expansions of the Honey Bee, Apis mellifera. Science 2006, 314, 642–645. [Google Scholar] [CrossRef]
- Franck, P.; Garnery, L.; Loiseau, A.; Oldroyd, B.P.; Hepburn, H.R.; Solignac, M.; Cornuet, J.M. Genetic Diversity of the Honeybee in Africa: Microsatellite and Mitochondrial Data. Heredity 2001, 86, 420–430. [Google Scholar] [CrossRef]
- Puškadija, Z.; Kovačić, M.; Raguž, N.; Lukić, B.; Prešern, J.; Tofilski, A. Morphological Diversity of Carniolan Honey Bee (Apis mellifera carnica) in Croatia and Slovenia. J. Apic. Res. 2021, 60, 326–336. [Google Scholar] [CrossRef]
- Oliveira, W.P., Jr.; Brandeburgo, M.A.M.; Marcolino, M.T. Morphometrics and Adaptatives Aspects in Africanized Honeybees (Apis mellifera). Rev. Bras. Biol. 2000, 60, 307–314. [Google Scholar] [CrossRef]
- Tofilski, A.; Căuia, E.; Siceanu, A.; Vișan, G.O.; Căuia, D. Historical Changes in Honey Bee Wing Venation in Romania. Insects 2021, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.A.; Henriques, D.; Chávez-Galarza, J.; Kryger, P.; Garnery, L.; Van Der Zee, R.; Dahle, B.; Soland-Reckeweg, G.; De La Rúa, P.; Dall’ Olio, R.; et al. Genetic Integrity of the Dark European Honey Bee (Apis mellifera mellifera) from Protected Populations: A Genome-Wide Assessment Using SNPs and mtDNA Sequence Data. J. Apic. Res. 2014, 53, 269–278. [Google Scholar] [CrossRef]
- Muñoz, I.; Pinto, M.A.; De La Rúa, P. Effects of Queen Importation on the Genetic Diversity of Macaronesian Island Honey Bee Populations (Apis mellifera Linneaus 1758). J. Apic. Res. 2014, 53, 296–302. [Google Scholar] [CrossRef]
- Engel, M.S. The Taxonomy of Recent and Fossil Honey Bees (Hymenoptera: Apidae; Apis). J. Hymenopt. Res. 1999, 8, 165–196. [Google Scholar]


| Subspecies | Number of Colonies/Sites | Number of Honey Bees | Total Images |
|---|---|---|---|
| Mediterranean | 57 | 750 | 1641 |
| Semiarid | 68 | 624 | 900 |
| Desert | 68 | 974 | 925 |
| A. m. intermissa | 19 | 229 | 229 |
| A. m. major | 1 | 10 | 10 |
| A. m. sahariensis | 4 | 40 | 40 |
| Total | 217 | 2627 | 3745 |
| Desert | Semiarid | Mediterranean | |
|---|---|---|---|
| Desert | 0.00 | <0.0001 | <0.0001 |
| Semiarid | 1.58 | 0.00 | <0.0001 |
| Mediterranean | 3.51 | 3.22 | 0.00 |
| Desert | Semiarid | Mediterranean | A. m. intermissa | A. m. sahariensis | |
|---|---|---|---|---|---|
| Desert | - | 0.0053 | 0.0001 | 0.0001 | <0.0001 |
| Semiarid | 1.47 | - | 0.0001 | 0.0001 | <0.0001 |
| Mediterranean | 3.48 | 3.17 | - | <0.0001 | <0.0001 |
| A. m. intermissa | 6.01 | 6.00 | 5.56 | - | <0.0001 |
| A. m. sahariensis | 8.25 | 8.63 | 8.25 | 6.62 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhchou, S.; Aglagane, A.; Tofilski, A.; Mokrini, F.; Er-Rguibi, O.; El Mouden, E.H.; Machlowska, J.; Fellahi, S.; Mohssine, E.H. Morphological Diversity of Moroccan Honey Bees (Apis mellifera L. 1758): Insights from a Geometric Morphometric Study of Wing Venation in Honey Bees from Different Climatic Regions. Diversity 2025, 17, 527. https://doi.org/10.3390/d17080527
Bakhchou S, Aglagane A, Tofilski A, Mokrini F, Er-Rguibi O, El Mouden EH, Machlowska J, Fellahi S, Mohssine EH. Morphological Diversity of Moroccan Honey Bees (Apis mellifera L. 1758): Insights from a Geometric Morphometric Study of Wing Venation in Honey Bees from Different Climatic Regions. Diversity. 2025; 17(8):527. https://doi.org/10.3390/d17080527
Chicago/Turabian StyleBakhchou, Salma, Abdessamad Aglagane, Adam Tofilski, Fouad Mokrini, Omar Er-Rguibi, El Hassan El Mouden, Julita Machlowska, Siham Fellahi, and El Hassania Mohssine. 2025. "Morphological Diversity of Moroccan Honey Bees (Apis mellifera L. 1758): Insights from a Geometric Morphometric Study of Wing Venation in Honey Bees from Different Climatic Regions" Diversity 17, no. 8: 527. https://doi.org/10.3390/d17080527
APA StyleBakhchou, S., Aglagane, A., Tofilski, A., Mokrini, F., Er-Rguibi, O., El Mouden, E. H., Machlowska, J., Fellahi, S., & Mohssine, E. H. (2025). Morphological Diversity of Moroccan Honey Bees (Apis mellifera L. 1758): Insights from a Geometric Morphometric Study of Wing Venation in Honey Bees from Different Climatic Regions. Diversity, 17(8), 527. https://doi.org/10.3390/d17080527











