Audouin’s Gull Colony Itinerancy: Breeding Districts as Units for Monitoring and Conservation
Abstract
1. Introduction
- The optimal scale is large enough to encompass multiple alternative breeding sites yet small enough to meaningfully represent the local population.
- Individuals in a given area gain an advantage in survival by remaining within rather than leaving the area.
- The emigration from this unit by an experienced breeder is a rare event.
2. Materials and Methods
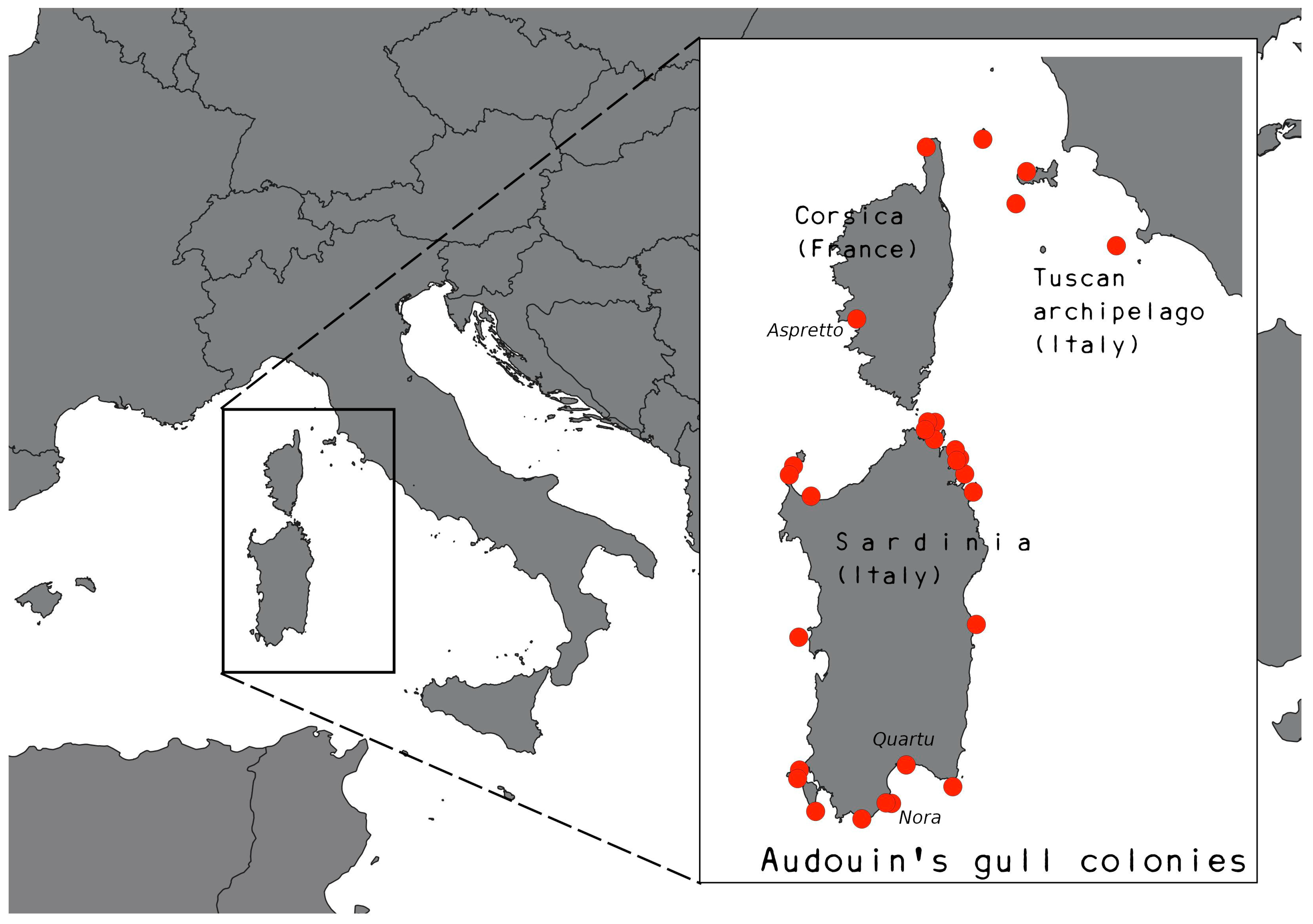
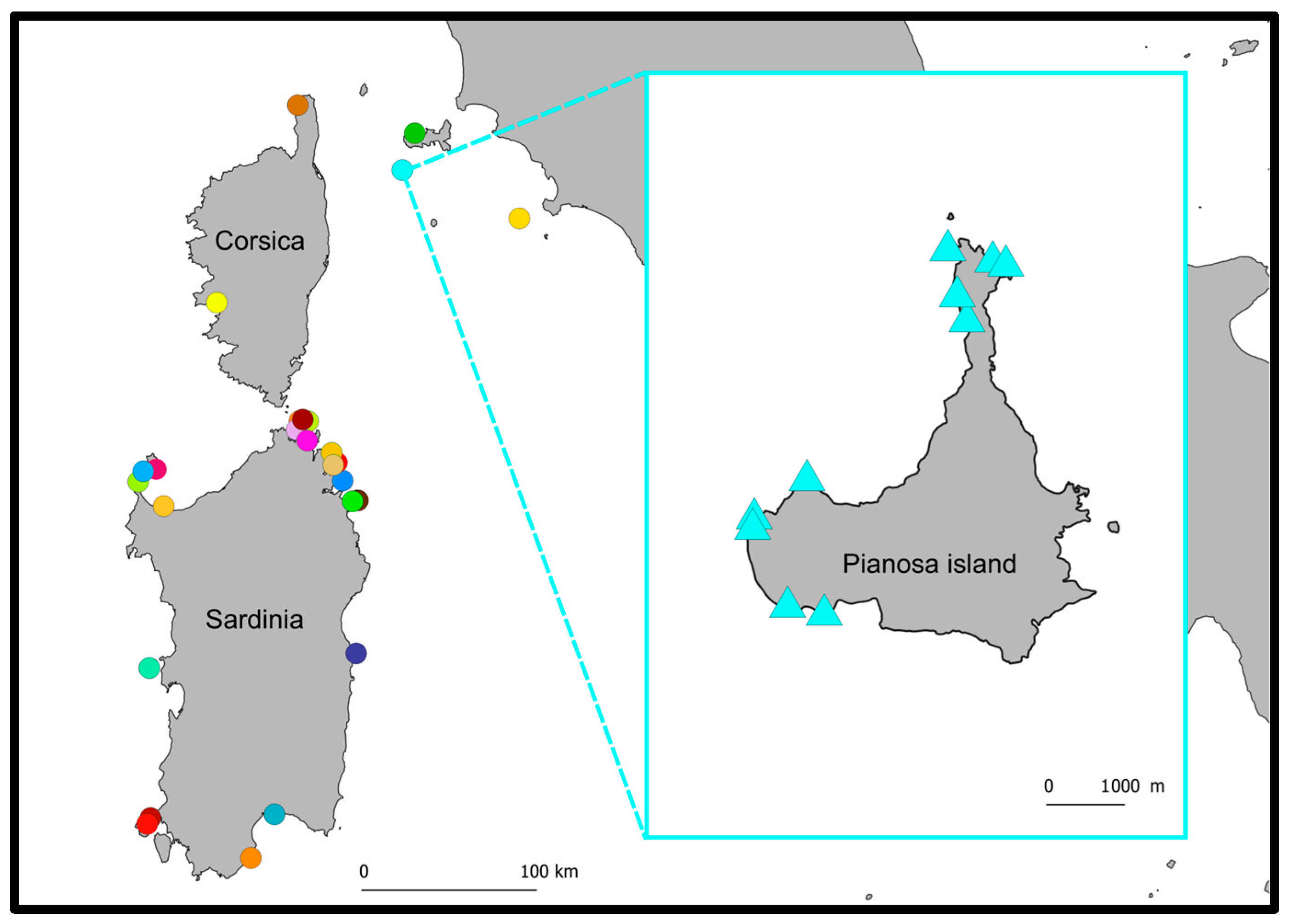
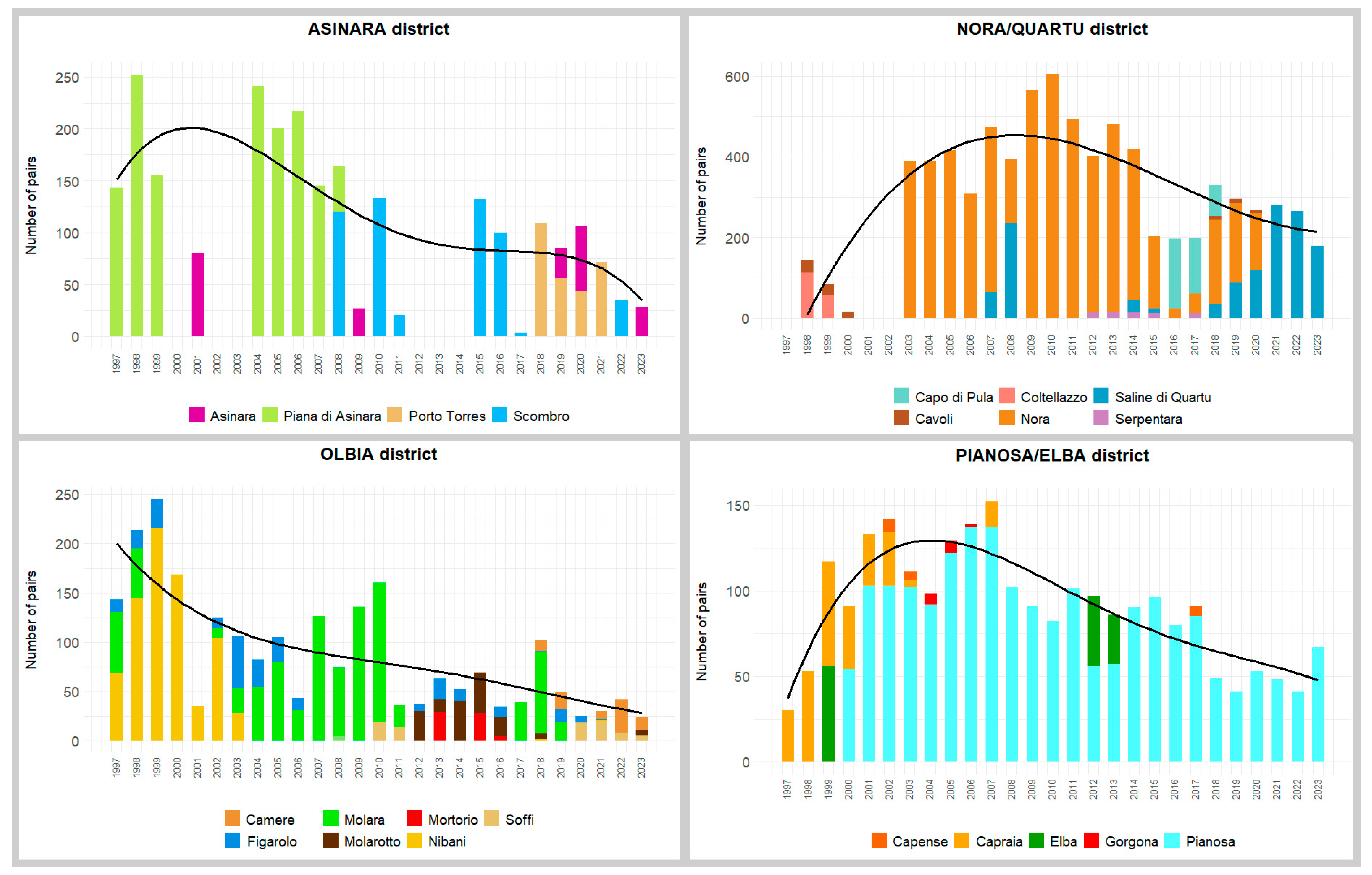
2.1. Study System and Data Collection
2.2. Statistical Analysis
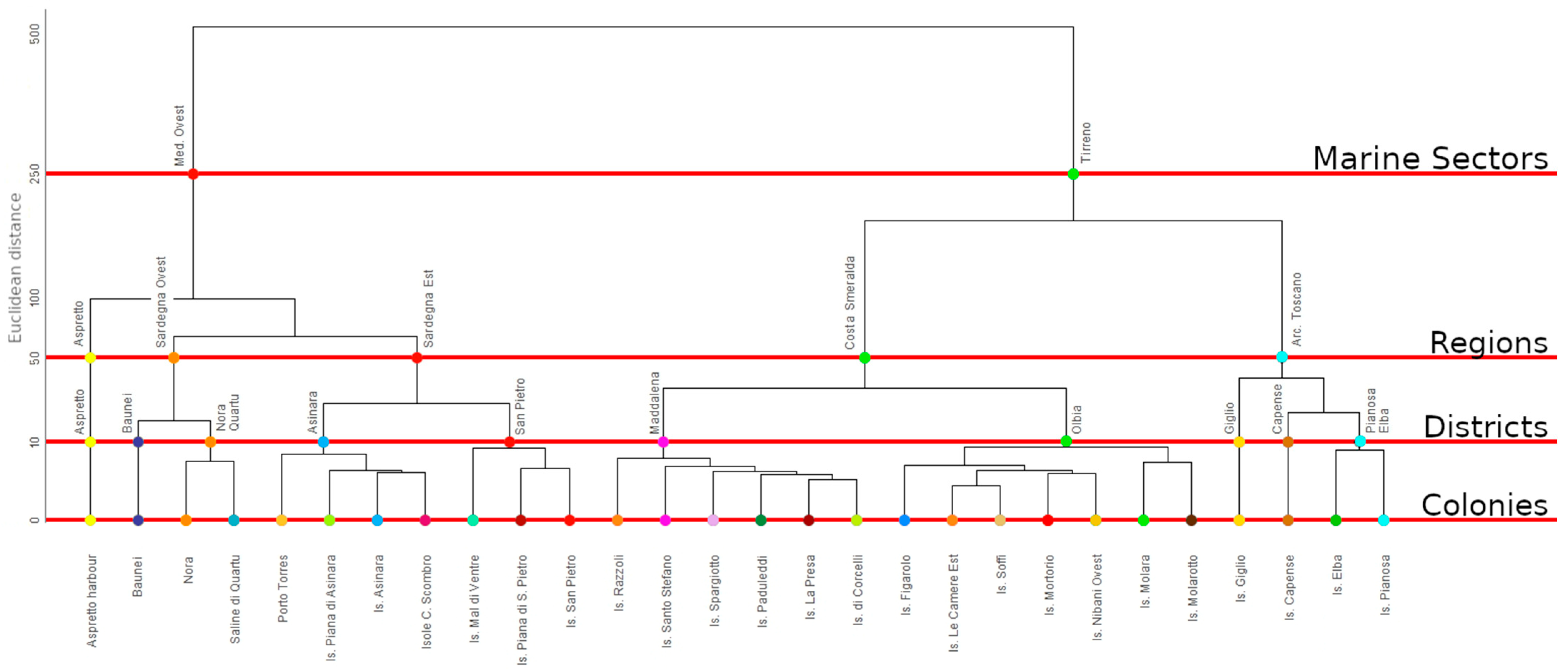
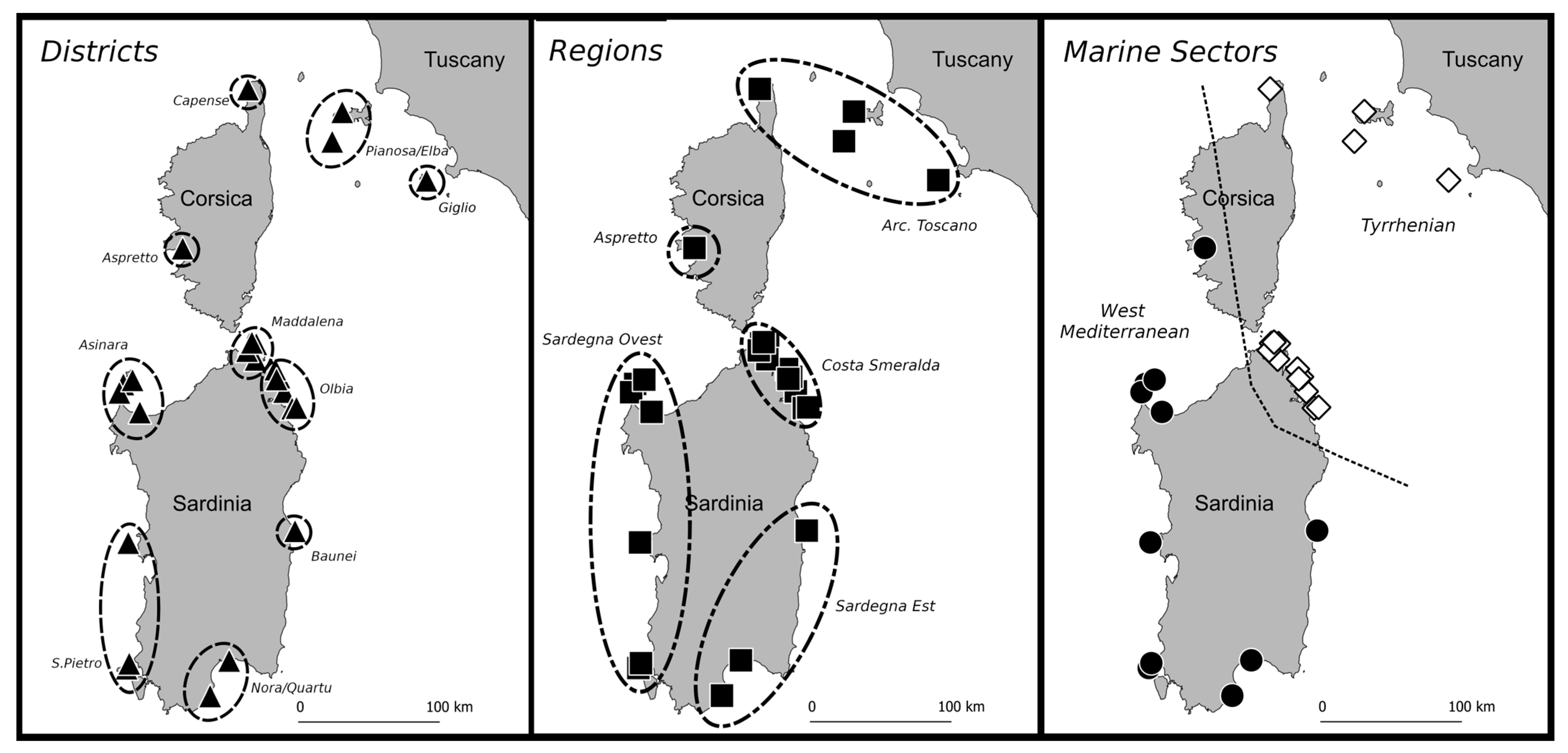
- Breeding Site level (BSI): Focuses on the exact locations where the local pairs established their annual breeding sites (e.g., a specific coastal section within an island). This level wasn’t included in the cluster analysis. Still, it was utilized in the subsequent analysis to verify whether information derived from the cluster analysis could also be confirmed at a highly detailed geographic scale. This represented the lowest geographic level.
- Colony level (COL): Groups of geographically close sites representing traditionally recognized Colonies (Euclidean distance = 0).
- District level (DIS): Groups of close Colonies (Euclidean distance = 10).
- Region level (REG): Groups of multiple Districts extending over an area consistent with a regional scale (Euclidean distance = 50).
- Marine Sector (MSE): Aggregation that includes two or more Regions within an extensive macro-area (Euclidean distance = 250). This is the highest geographic level.
- SFA (Site-Faithful—Aware): Alive observed breeding in its first-ever known or last known site, “trap-aware”.
- FU (Site-Faithful—Unaware): Alive breeding in its first or last known site, “trap-unaware”.
- MA (Mover—Aware): Alive breeding in a different site than the last, “trap-aware”.
- MU (Mover—Unaware): Alive breeding in a different site than the last, “trap-unaware”.
- Dead: Individual dead or permanently emigrated.
- 0: Not detected.
- 1: Detected at its first site or at its previous known site.
- 2: Detected at a different site than the previous.
3. Results
4. Discussion
A District-Based Approach to Population Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittenberger, J.F.; Hunt, G.L. The Adaptive Significance of Coloniality in Birds. Avian Biol. 1985, VIII, 1–78. [Google Scholar] [CrossRef]
- Lack, D.L. Ecological Adaptations for Breeding in Birds; Methuen and Company: London, UK, 1968. [Google Scholar]
- Coulson, J.C. Colonial Breeding in Seabirds. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 87–113. [Google Scholar]
- Siegel-Causey, D.; Kharnitonov, S.P. The Evolution of Coloniality. In Current Ornithology; Current Ornithology; Power, D.M., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1990; Volume 7, pp. 285–330. [Google Scholar]
- Schippers, P.; Stienen, E.W.M.; Schotman, A.G.M.; Snep, R.P.H.; Slim, P.A. The Consequences of Being Colonial: Allee Effects in Metapopulations of Seabirds. Ecol. Modell. 2011, 222, 3061–3070. [Google Scholar] [CrossRef]
- Crespin, L.; Harris, M.P.; Lebreton, J.D.; Frederiksen, M.; Wanless, S. Recruitment to a Seabird Population Depends on Environmental Factors and on Population Size. J. Anim. Ecol. 2006, 75, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Pyk, T.M.; Weston, M.A.; Bunce, A.; Norman, F.I. Establishment and Development of a Seabird Colony: Long-Term Trends in Phenology, Breeding Success, Recruitment, Breeding Density and Demography. J. Ornithol. 2013, 154, 299–310. [Google Scholar] [CrossRef]
- Francesiaz, C.; Farine, D.; Laforge, C.; Béchet, A.; Sadoul, N.; Besnard, A. Familiarity Drives Social Philopatry in an Obligate Colonial Breeder with Weak Interannual Breeding Site Fidelity. Anim. Behav. 2017, 124, 125–133. [Google Scholar] [CrossRef]
- Sacchi, M.; Santoro, S.; Culina, A.; Pollonara, E.; Cozzo, M.; Pezzo, F.; Baccetti, N. Sex-Specific Fitness Consequences of Mate Change in Scopoli’ s Shearwater, Calonectris diomedea. Anim. Behav. 2023, 202, 87–98. [Google Scholar] [CrossRef]
- Ponchon, A.; Scarpa, A.; Bocedi, G.; Palmer, S.C.F.; Travis, J.M.J. Prospecting and Informed Dispersal: Understanding and Predicting Their Joint Eco-Evolutionary Dynamics. Ecol. Evol. 2021, 11, 15289–15302. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.; Ponchon, A.; Oro, D.; Amadesi, B.; Arizaga, J.; Baccetti, N.; Boulinier, T.; Cecere, J.G.; Corcoran, R.M.; Corman, A.M.; et al. Active Breeding Seabirds Prospect Alternative Breeding Colonies. Oecologia 2023, 201, 341–354. [Google Scholar] [CrossRef]
- Couzin, I.D. Collective Cognition in Animal Groups. Trends Cogn. Sci. 2009, 13, 36–43. [Google Scholar] [CrossRef]
- Lamb, J.S.; Satgé, Y.G.; Jodice, P.G.R. Influence of Density-Dependent Competition on Foraging and Migratory Behavior of a Subtropical Colonial Seabird. Ecol. Evol. 2017, 7, 6469–6481. [Google Scholar] [CrossRef] [PubMed]
- Wojczulanis-Jakubas, K.; Jakubas, D.; Øigarden, T.; Lifjeld, J.T. Extrapair Copulations Are Frequent but Unsuccessful in a Highly Colonial Seabird, the Little Auk, Alle alle. Anim. Behav. 2009, 77, 433–438. [Google Scholar] [CrossRef]
- Ludwig, S.C.; Becker, P.H. Immigration Prevents Inbreeding in a Growing Colony of a Long-Lived and Philopatric Seabird. Ibis 2012, 154, 74–84. [Google Scholar] [CrossRef]
- Sin, S.Y.W.; Hoover, B.A.; Nevitt, G.A.; Edwards, S.V. Demographic History, Not Mating System, Explains Signatures of Inbreeding and Inbreeding Depression in a Large Outbred Population. Am. Nat. 2021, 197, 658–676. [Google Scholar] [CrossRef]
- Cairns, D.K. Population Regulation of Seabird Colonies. In Current Ornithology; Current Ornithology; Power, D.M., Ed.; Springer: New York, NY, USA, 1992; Volume 9, pp. 37–61. [Google Scholar] [CrossRef]
- Crawford, R.J.M.; Dyer, B.M.; Brooke, R.K. Breeding Nomadism in Southern African Seabirds-Constraints, Causes and Conservation. Ostrich 1994, 65, 231–246. [Google Scholar] [CrossRef]
- Oro, D. Breeding Biology and Population Dynamics of Slender-Billed Gulls at the Ebro Delta (Northwestern Mediterranean). Waterbirds 2002, 25, 67–77. [Google Scholar] [CrossRef]
- Acker, P.; Francesiaz, C.; Béchet, A.; Sadoul, N.; Lessells, C.M.; Pijl, A.S.; Besnard, A. Insights on Dispersal and Recruitment Paradigms: Sex- and Age-Dependent Variations in a Nomadic Breeder. Oecologia 2018, 186, 85–97. [Google Scholar] [CrossRef]
- Kharitonov, S.P.; Siegel-Causey, D. Colony Formation in Seabirds. In Current Ornithology; Current Ornithology; Johnston, R.F., Ed.; Springer: New York, NY, USA, 1988; Volume 5. [Google Scholar] [CrossRef]
- Burger, J. The Role of Reproductive Success in Colony-Site Selection and Abandonment in Black Skimmers (Rynchops niger). Auk 1982, 99, 109–115. [Google Scholar] [CrossRef]
- Evans, R.M. Colony Desertion and Reproductive Synchrony of Black-Billed Gulls Larus bulleri. Ibis 1982, 124, 491–501. [Google Scholar] [CrossRef]
- Poslavskii, A.N.; Krivonosov, G.A. Ecology of Sandwich Tern (Thalasseus sandvicensis Lath) at Boundary of Distribution Range. Sov. J. Ecol. 1976, 7, 232–236. [Google Scholar]
- Bradley, P.M. The Breeding Biology and Conservation of Audouin’s Gull Larus audouinii on the Chafarinas Islands. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 1988; p. 284. [Google Scholar]
- Muntaner, J. Situaciòn de la gaviota de Audouin Larus audouinii en las islas Baleares en 1998–1999. Anu. Ornitològic De Les Balear. 1999, 14, 19–25. [Google Scholar]
- Muntaner, J.; Cardona, E.; Escandell, R. Situación de La Gaviota de Audouin Larus audouinii En Las Islas Baleares En El Trienio 2000–2002. Anu. Ornitològic Les Balear. Rev. d’observació Estud. Conserv. Dels Aucells 2002, 20, 71–85. [Google Scholar]
- Martínez-Abraín, A.; Oro, D.; Forero, M.G.; Conesa, D. Modeling Temporal and Spatial Colony-Site Dynamics in a Long-Lived Seabird. Popul. Ecol. 2003, 45, 133–139. [Google Scholar] [CrossRef]
- Baccetti, N.; Giunti, M.A.; Leone, L.M.; Sposimo, P.; Baccetti, N.; Leone, L.M.; Sposimo, P. Piano per la tutela dei potenziali siti di nidificazione del gabbiano Corso (Larus audouinii). In I Quaderni del Parco, Documenti Tecnici n. 1 “Progetto Life Natura, Isole di Toscana: Nuove Azioni per Uccelli Marini e Habitat”; Parco Nazionale Arcipelago Toscano: Portoferraio, Italy, 2008; Volume 45–52, p. 68. [Google Scholar]
- Bécares, J.; Arcos, J.M.; Oro, D. Migración y Ecología Espacial de la Gaviota de Audouin en el Mediterráneo Occidental y Noroeste Africano; Monografía no. 1 del programa Migra; SEO/BirdLife: Madrid, Spain, 2016. [Google Scholar]
- Ozkan, K.; Yapan, B.C. Census of Breeding Avifauna and the Status of Audouin’s Gull (Ichthyaetus audouinii) along the Eastern Mediterranean Coast of Turkey. J. Field Ornithol. 2024, 95, 2. [Google Scholar] [CrossRef]
- Lambertini, M. The Ecology and Conservation of Audouin’s Gull (Larus audouinii) at the Northern Limit of Its Breeding Range. In Status and Conservation of Seabirds; Aguilar, J.S., Monbailliu, X., Paterson, A.M., Eds.; Sociedad Española de Ornitologia/Birdlife/Medmaravis: Madrid, Spain, 1993; pp. 261–272. [Google Scholar]
- Serra, G.; Melega, L.; Baccetti, N. Piano d’Azione Nazionale per il Gabbiano Corso (Larus audouinii); Ministero dell’Ambiente e Istituto Nazionale Fauna Selvatica: Roma, Italy, 2001; Volume 6, pp. 7–46. [Google Scholar]
- Oro, D. Larus audouinii Audouin’s Gull. BWP Update 1998, 2, 47–61. [Google Scholar]
- Hanski, I.; Gyllenberg, M. Two General Metapopulation Models and the Core-Satellite Species Hypothesis. Am. Nat. 1993, 142, 17–41. [Google Scholar] [CrossRef]
- Genovart, M.; Bécares, J.; Igual, J.M.; Martínez-Abraín, A.; Escandell, R.; Sánchez, A.; Rodríguez, B.; Arcos, J.M.; Oro, D. Differential Adult Survival at Close Seabird Colonies: The Importance of Spatial Foraging Segregation and Bycatch Risk during the Breeding Season. Glob. Change Biol. 2018, 24, 1279–1290. [Google Scholar] [CrossRef]
- Oliveira, N.; Nascimento, T.; Vulcano, A. Report of the Action E5, Project LIFE Ilhas Barreira; SPEA—Portuguese Society for the Study of Birds: Lisbon, Portugal, 2024. [Google Scholar]
- Oro, D.; Martinez-Villalta, A. The Colony of the Audouin’s Gull at the Ebro Delta. Avocetta 1992, 16, 98–101. [Google Scholar]
- Vilaplana, A.F.; Afán, I.; Oro, D.; Bécares, J.; Illa, M.; Gil, M.; Bertolero, A.; Forero, M.G.; Ramírez, F.F. Distribution and habitat use by the Audouin’s Gull (Ichthyaetus audouinii) in anthropized environments. Sci. Total Environ. 2024, 954, 176555. [Google Scholar] [CrossRef]
- Amadesi, B.; Baccetti, N.; Cavaliere, V.; Corbi, F.; Ientile, C.; Nissardi, S.; Zenatello, M. An Analysis of the Italian Breeding Population Trend of Audouin’s Gull (1998–2022). In Proceedings of the XXI CIO 2023, Varese, Italy, 2–9 September 2023. [Google Scholar]
- European Commission. MSFD CIS Guidance Document No. 19, Article 8 MSFD, May 2022; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Lebreton, J.D.; Pradel, R. Multistate Recapture Models: Modelling Incomplete Individual Histories. J. Appl. Stat. 2002, 29, 353–369. [Google Scholar] [CrossRef]
- Pradel, R. Multievent: An Extension of Multistate Capture-Recapture Models to Uncertain States. Biometrics 2005, 61, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Choquet, R.; Rouan, L.; Pradel, R. Program E-SURGE: A Software Application for Fitting Multievent Models. In Modeling Demographic Processes in Marked Populations; Thomson, D.L., Cooch, E.G., Conroy, M.J., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; pp. 845–865. [Google Scholar]
- Choquet, R.; Reboulet, A.; Lebreton, J.-D.; Gimenez, O.; Pradel, R. U-CARE 3.3 User’s Manual; Centre d’Ecologie Fonctionnelle et Evolutive (CEFE): Montpellier, France, 2020. [Google Scholar]
- Oro, D.; Pradel, R.; Lebreton, J.D. Food Availability and Nest Predation Influence Life History Traits in Audouin’s Gull, Larus audouinii. Oecologia 1999, 118, 438–445. [Google Scholar] [CrossRef]
- Pradel, R.; Sanz-Aguilar, A. Modeling Trap-Awareness and Related Phenomena in Capture-Recapture Studies. PLoS ONE 2012, 7, e32666. [Google Scholar] [CrossRef]
- Doherty, P.F.; White, G.C.; Burnham, K.P. Comparison of Model Building and Selection Strategies. J. Ornithol. 2012, 152, 317–323. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Anderson, D.R. A Practical Information-Theoretic Approach. In Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002; pp. 70–71. [Google Scholar]
- Muntaner, J. Itinerancia de Las Colonias Reproductoras de Gaviota de Audouin (Larus audouinii) En Las Islas Baleares (España) y Efectos Sobre Su Conservación. In Resúmenes del 6° Simposio Mediterráneo Sobre Aves Marinas, Benidorm (Alicante), 11–15 de Octubre de 2000; Medmaravis-Sociedad Española de Ornitología: Madrid, Spain, 2000; Volume 46. [Google Scholar]
- Oro, D.; Pradel, R. Recruitment of Audouin’s Gull to the Ebro Delta Colony at Metapopulation Level in the Western Mediterranean. Mar. Ecol. Prog. Ser. 1999, 180, 267–273. [Google Scholar] [CrossRef]
- Faggio, G.; Charrier, J.; Gwenaelle, D.; Linossier, J.; Recorbet, B. Rythme d’Activité et Utilisation de l’Espace chez le Goéland d’Audouin Ichthyaetus audouinii sur une Colonie de Reproduction en Corse. Alauda 2022, 90, 193–214. [Google Scholar]
- Recorbet, B.; Ledru, A.; Travichon, S.; Jolin, C.; Faggio, G.; Baccetti, N.; Besnard, A. Premières Données Biologiques Sur Le Goéland d’Audouin Larus audouinii de La Colonie d’Aspretto-Ajaccio (Corse). Alauda 2011, 79, 187–198. [Google Scholar]
- Danchin, E. The Incidence of the Tick Parasite Ixodes Uriae in Kittiwake Rissa Tridactyla Colonies in Relation to the Age of the Colony, and a Mechanism of Infecting New Colonies. Ibis 1992, 134, 134–141. [Google Scholar] [CrossRef]
- Cam, E.; Oro, D.; Pradel, R.; Jimenez, J. Assessment of Hypotheses about Dispersal in a Long-Lived Seabird Using Multistate Capture-Recapture Models. J. Anim. Ecol. 2004, 73, 723–736. [Google Scholar] [CrossRef]
- Tavecchia, G.; Pradel, R.; Genovart, M.; Oro, D. Density-Dependent Parameters and Demographic Equilibrium in Open Populations. Oikos 2007, 116, 1481–1492. [Google Scholar] [CrossRef]
- Fernández-Chacón, A.; Genovart, M.; Pradel, R.; Tavecchia, G.; Bertolero, A.; Piccardo, J.; Forero, M.G.; Afán, I.; Muntaner, J.; Oro, D. When to Stay, When to Disperse and Where to Go: Survival and Dispersal Patterns in a Spatially Structured Seabird Population. Ecography 2013, 36, 1117–1126. [Google Scholar] [CrossRef]
- Payo-Payo, A.; Sanz-Aguilar, A.; Genovart, M.; Bertolero, A.; Piccardo, J.; Camps, D.; Ruiz-Olmo, J.; Oro, D. Predator Arrival Elicits Differential Dispersal, Change in Age Structure and Reproductive Performance in a Prey Population. Sci. Rep. 2018, 8, 1971. [Google Scholar] [CrossRef] [PubMed]
- Oro, D.; Alsedà, L.; Hastings, A.; Genovart, M.; Sardanyes, J. Social Copying Drives a Tipping Point for Nonlinear Population Collapse. Proc. Natl. Acad. Sci. USA 2023, 120, e2214055120. [Google Scholar] [CrossRef]
- McNicholl, M.K. Larid Site Tenacity and Group Adherence in Relation to Habitat. Auk 1975, 92, 98–104. [Google Scholar] [CrossRef]
- Switzer, P.V. Site Fidelity in Predictable and Unpredictable Habitats. Evol. Ecol. 1993, 7, 533–555. [Google Scholar] [CrossRef]
- Péron, G.; Lebreton, J.D.; Crochet, P.A. Breeding Dispersal in Black-Headed Gull: The Value of Familiarity in a Contrasted Environment. J. Anim. Ecol. 2010, 79, 317–326. [Google Scholar] [CrossRef]
- Sacchi, M.; Zenatello, M.; Pezzo, F.; Cozzo, M.; Pollonara, E.; Gotti, C.; De Faveri, A.; Baccetti, N. Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis. Diversity 2023, 15, 718. [Google Scholar] [CrossRef]
- Oro, D.; Ruxton, G.D. The Formation and Growth of Seabird Colonies: Audouin’s Gull as a Case Study. J. Anim. Ecol. 2001, 70, 527–535. [Google Scholar] [CrossRef]
- Oro, D.; Bécares, J.; Bartumeus, F.; Arcos, J.M. High Frequency of Prospecting for Informed Dispersal and Colonisation in a Social Species at Large Spatial Scale. Oecologia 2021, 197, 395–409. [Google Scholar] [CrossRef]
- Tenan, S.; Pradel, R.; Tavecchia, G.; Igual, J.M.; Sanz-Aguilar, A.; Genovart, M.; Oro, D. Hierarchical Modelling of Population Growth Rate from Individual Capture-Recapture Data. Methods Ecol. Evol. 2014, 5, 606–614. [Google Scholar] [CrossRef]
- Genovart, M.; Doak, D.F.; Igual, J.M.; Sponza, S.; Kralj, J.; Oro, D. Varying Demographic Impacts of Different Fisheries on Three Mediterranean Seabird Species. Glob. Change Biol. 2017, 23, 3012–3029. [Google Scholar] [CrossRef]
- Lambertini, M. International Action Plan for Audouin’s Gull (Larus audouinii); Lega Italiana Protezione Uccelli: Rome, Italy, 1996; pp. 1–26. [Google Scholar]
- Gallo-Orsi, U. Species Action Plans for the Conservation of Seabirds in the Mediterranean Sea: Audouin’s Gull, Balearic Shearwater and Mediterranean Shag. Sci. Mar. 2003, 67, 47–55. [Google Scholar] [CrossRef]


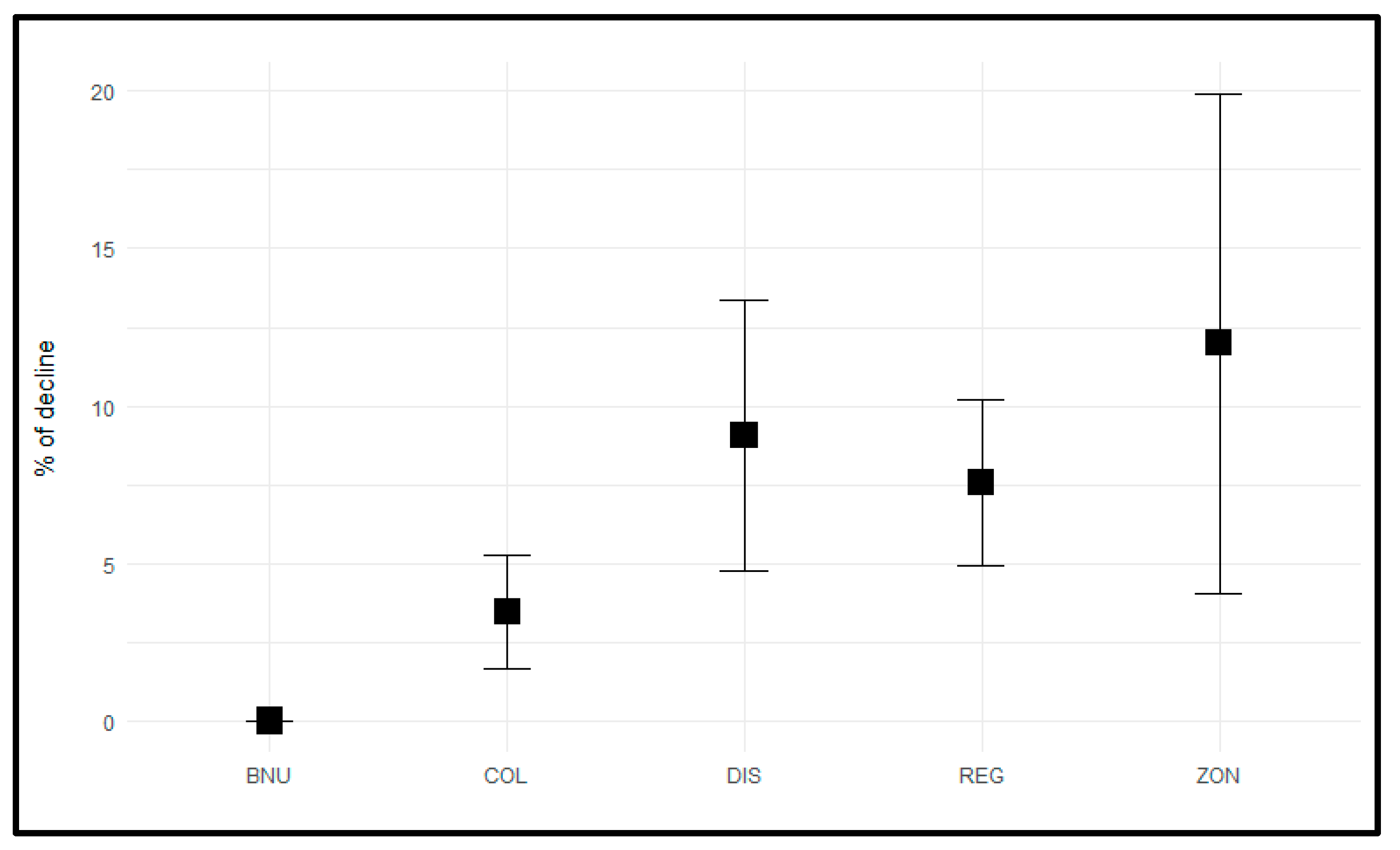
| Unit | Φ | Ψ | Γ | np | Dev | QAICc | w_AIC | W_ϕ | W_ψ | W_δ |
|---|---|---|---|---|---|---|---|---|---|---|
| BSI | constant | state | state + time | 27 | 8701.631 | 8756.307 | 0.690 | 0.690 | 1.000 | 1.000 |
| COL | state + age | state | state + time | 29 | 8399.533 | 8458.311 | 0.530 | 0.530 | 1.000 | 1.000 |
| DIS | state | state | state + time | 28 | 7046.145 | 7102.872 | 0.789 | 0.794 | 0.991 | 1.000 |
| REG | state | state | state + time | 28 | 7586.539 | 7643.266 | 0.782 | 0.782 | 1.000 | 1.000 |
| MSE | state + age | state | state + time | 29 | 7037.907 | 7096.686 | 0.534 | 0.536 | 0.996 | 1.000 |
| Unit | S/M | Survival | CI95% | SE | F Test |
|---|---|---|---|---|---|
| BSI | S and M | 0.854 | 0.841–0.867 | 0.006 | - |
| COL | Newly marked | 0.800 | 0.744–0.846 | 0.026 | |
| S | 0.875 | 0.856–0.891 | 0.009 | F = 4.225, p-value < 0.001 | |
| M | 0.840 | 0.805–0.870 | 0.017 | ||
| DIS | S | 0.860 | 0.845–0.873 | 0.007 | F = 24.715, p-value < 0.001 |
| M | 0.769 | 0.676–0.842 | 0.042 | ||
| REG | S | 0.862 | 0.847–0.876 | 0.008 | F = 7.4, p-value < 0.001 |
| M | 0.805 | 0.751–0.849 | 0.047 | ||
| MSE | Newly marked | 0.790 | 0.743–0.831 | 0.023 | |
| S | 0.872 | 0.855–0.887 | 0.008 | F = 87.042, p-value < 0.001 | |
| M | 0.752 | 0.570–0.874 | 0.079 |
| Units | Site Change (ψ) | CI95% | SE |
|---|---|---|---|
| BSI1 → BSI2 | 0.238 | 0.205–0.276 | 0.018 |
| COL1 → COL2 | 0.146 | 0.121–0.176 | 0.014 |
| DIS1 → DIS2 | 0.035 | 0.024–0.052 | 0.007 |
| REG1 → REG2 | 0.054 | 0.039–0.073 | 0.009 |
| MSE1 → MSE2 | 0.034 | 0.022–0.050 | 0.007 |
| Unit | % Philopatry | CI95% | SE |
|---|---|---|---|
| BSI | 43.0 | 39.6–46.6 | 1.8 |
| COL | 48.5 | 44.9–52.0 | 1.8 |
| DIS | 54.0 | 50.4–57.6 | 1.8 |
| REG | 62.6 | 59.1–66.0 | 1.8 |
| MSE | 83.3 | 80.5–85.8 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchi, M.; Amadesi, B.; De Faveri, A.; Faggio, G.; Gotti, C.; Ledru, A.; Nissardi, S.; Recorbet, B.; Zenatello, M.; Baccetti, N. Audouin’s Gull Colony Itinerancy: Breeding Districts as Units for Monitoring and Conservation. Diversity 2025, 17, 526. https://doi.org/10.3390/d17080526
Sacchi M, Amadesi B, De Faveri A, Faggio G, Gotti C, Ledru A, Nissardi S, Recorbet B, Zenatello M, Baccetti N. Audouin’s Gull Colony Itinerancy: Breeding Districts as Units for Monitoring and Conservation. Diversity. 2025; 17(8):526. https://doi.org/10.3390/d17080526
Chicago/Turabian StyleSacchi, Massimo, Barbara Amadesi, Adriano De Faveri, Gilles Faggio, Camilla Gotti, Arnaud Ledru, Sergio Nissardi, Bernard Recorbet, Marco Zenatello, and Nicola Baccetti. 2025. "Audouin’s Gull Colony Itinerancy: Breeding Districts as Units for Monitoring and Conservation" Diversity 17, no. 8: 526. https://doi.org/10.3390/d17080526
APA StyleSacchi, M., Amadesi, B., De Faveri, A., Faggio, G., Gotti, C., Ledru, A., Nissardi, S., Recorbet, B., Zenatello, M., & Baccetti, N. (2025). Audouin’s Gull Colony Itinerancy: Breeding Districts as Units for Monitoring and Conservation. Diversity, 17(8), 526. https://doi.org/10.3390/d17080526







