Vertebrate Coprolites Reveal Diversity of Prey Fishes in the Oligocene Carpathian Basin of the Paratethys
Abstract
1. Introduction
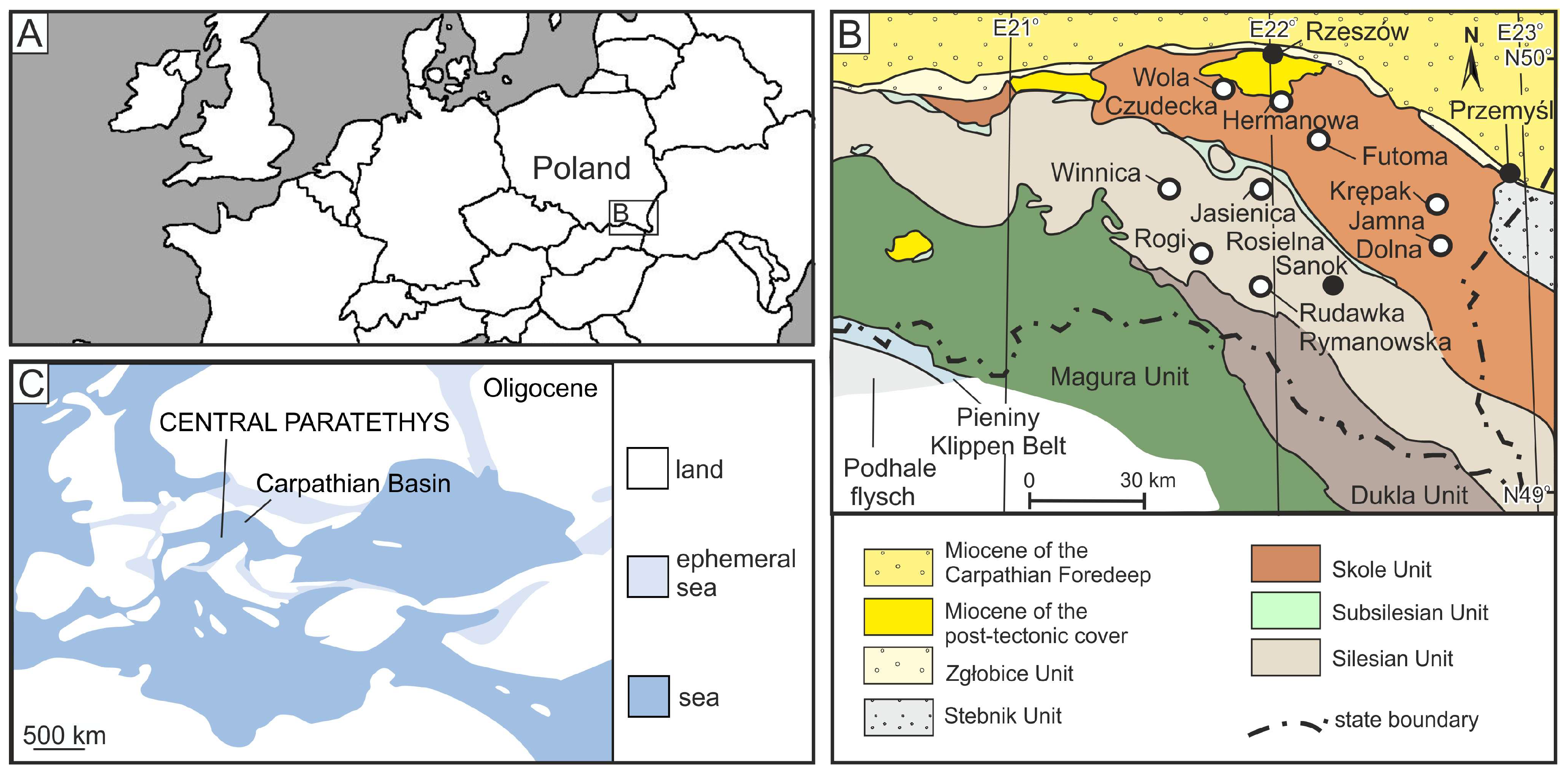
Geological Setting
2. Materials and Methods
2.1. Morphological Analyses of Coprolites
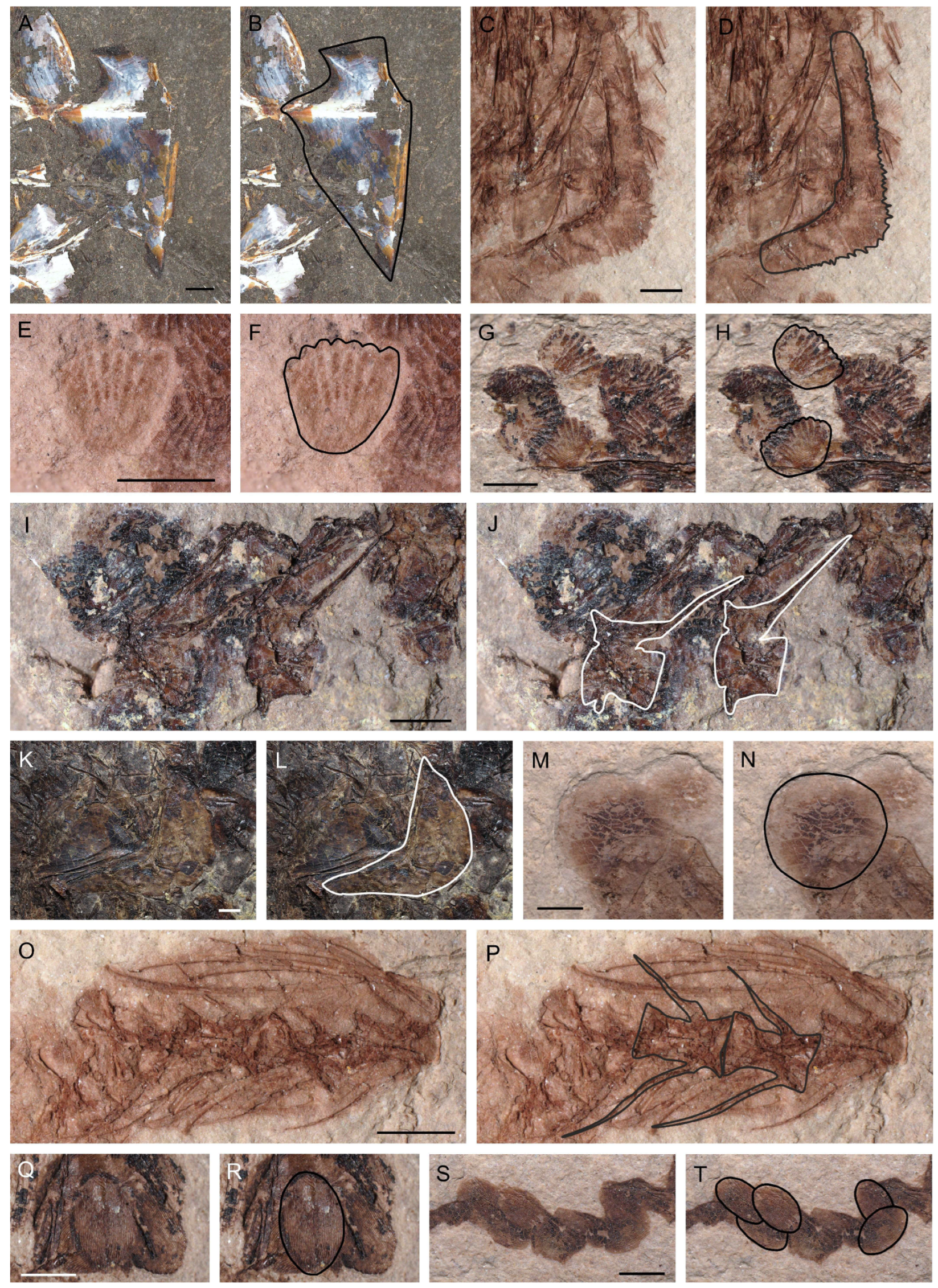
2.2. Inclusions in Coprolites
3. Results
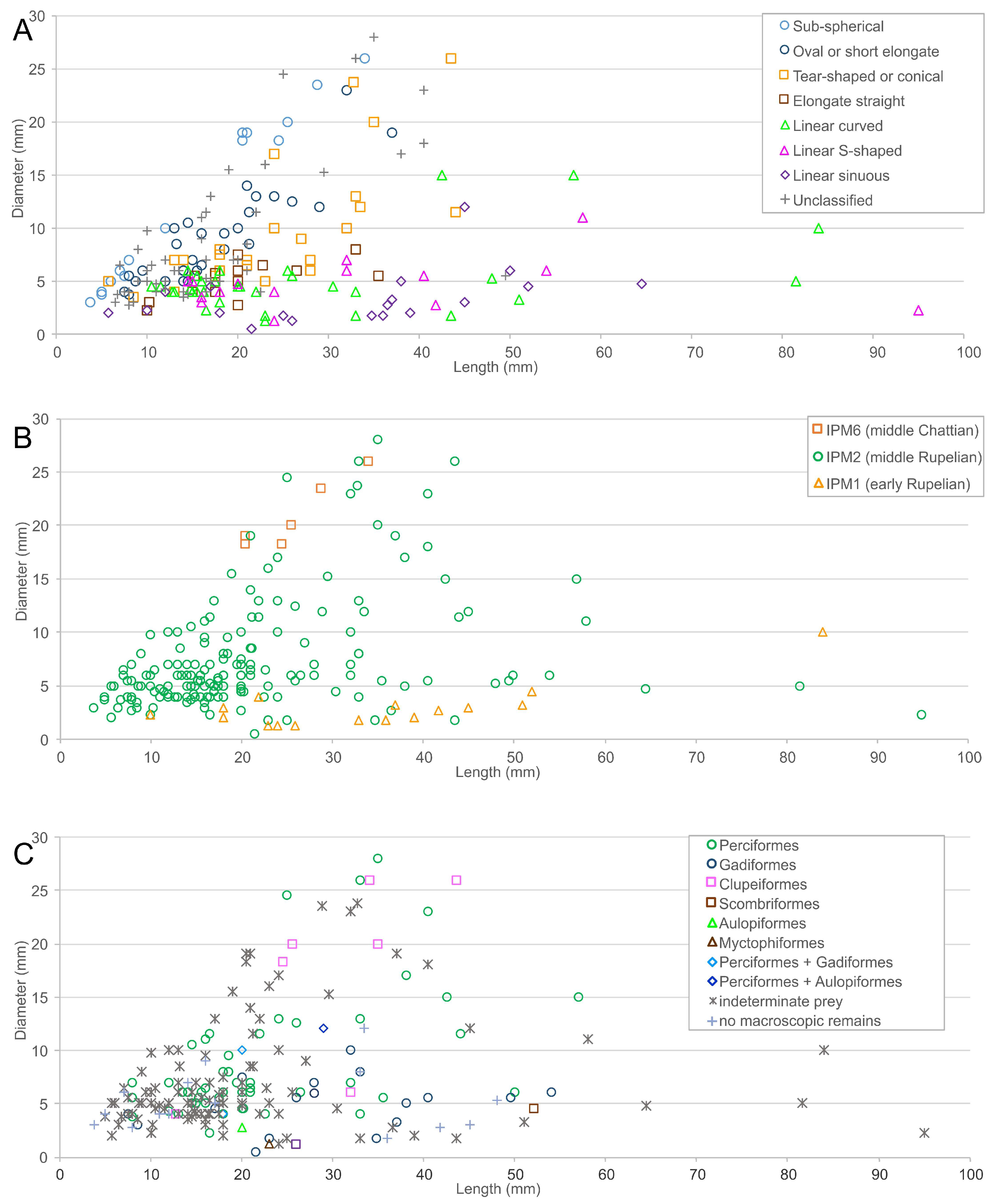
3.1. Morphological Diversity of Coprolites
3.2. Stratigraphic Distribution of Coprolite Shape Categories
3.3. Matrix Preservation
3.4. Inclusions
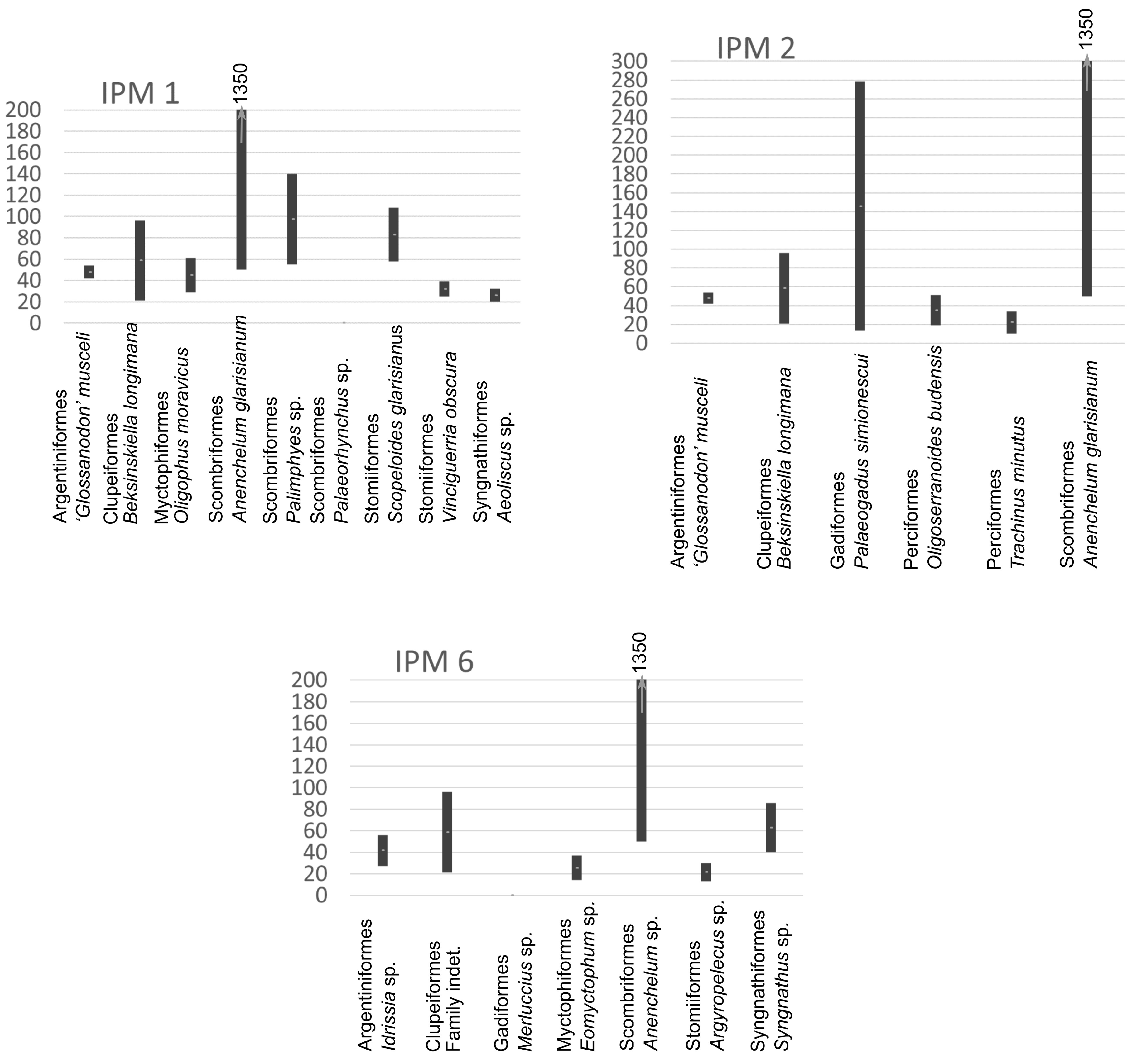
3.5. Sizes of Fishes in the Menilite Formation
3.6. Sizes of Preys from Coprolites
4. Discussion
4.1. Succession of Coprolite Shape Categories Throughout the Menilite Formation
4.2. Preservation of Coprolites in the Menilite Formation
4.3. Ecology of Fishes from the Menilite Formation
4.4. Relation of Prey Sizes to Skeletal Record of Fishes in the Menilite Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chin, K. The paleobiological implications of herbivorous dinosaur coprolites from the Upper Cretaceous Two Medicine Formation of Montana: Why eat wood? Palaios 2007, 22, 554–566. [Google Scholar] [CrossRef]
- Chin, K.; Bloch, J.; Sweet, A.; Tweet, J.; Eberle, J.; Cumbaa, S.; Witkowski, J.; Harwood, D. Life in a temperate Polar sea: A unique taphonomic window on the structure of a Late Cretaceous Arctic marine ecosystem. Proc. R. Soc. B Biol. Sci. 2008, 275, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.A.; Santucci, R.M.; De Oliveira, C.E.M.; De Andrade, M.B. Morphological and compositional analyses of coprolites from the Upper Cretaceous Bauru Group reveal dietary habits of notosuchian fauna. Lethaia 2021, 54, 664–686. [Google Scholar] [CrossRef]
- Román, Z.; Segesdi, M.; Sebe, K.; Földes, T.; Bakrač, K.; Virág, A.; Botfalvai, G. Palaeontological and taphonomical investigations of the exceptionally rich concentration of Miocene vertebrate coprolites from Pécs-Danitzpuszta (Hungary, Mecsek Mts.). Hist. Biol. 2023, 37, 663–678. [Google Scholar] [CrossRef]
- Bajdek, P.; Bienkowska-Wasiluk, M. Deep-sea ecosystem revealed by teleost fish coprolites from the Oligocene of Poland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 540, 109546. [Google Scholar] [CrossRef]
- Rakshit, N.; Ray, S. Bone-bearing coprolites from the Upper Triassic of India: Ichnotaxonomy, probable producers and predator–prey relationships. Pap. Palaeontol. 2022, 8, e1418. [Google Scholar] [CrossRef]
- Książkiewicz, M. Bathymetry of the Carpathian Flysch Basin. Acta Geol. Pol. 1975, 25, 309–367. [Google Scholar]
- Golonka, J.; Picha, F.J. The Carpathians and their foreland: Geology and hydrocarbon resources. Am. Assoc. Pet. Geol. Mem. 2006, 84, 1–848. [Google Scholar]
- Żytko, K.; Gucik, S.; Oszczypko, N.; Zając, R.; Garlicka, I.; Nemčok, J.; Eliaš, M.; Menčik, E.; Dvorak, J.; Stranik, Z.; et al. Geological map of the Western Outer Carpathians and their foreland without Quaternary formations. In Geological Atlas of the Western Carpathians and Their Foreland 1: 500 000; Poprawa, D., Nemčok, J., Eds.; Państwowy Instytut Geologiczny: Warszawa, Poland, 1989. [Google Scholar]
- Popov, S.V.; Akhmetiev, M.A.; Bugrova, E.M.; Lopatin, A.V.; Amitrov, O.V.; Andreyeva-Grigorovich, A.; Zaporozhets, N.I.; Zherikhin, V.V.; Krasheninnikov, V.A.; Nikolaeva, I.A.; et al. Biogeography of the Northern Peri-Tethys from the Late Eocene to the Early Miocene: Part 2. Early Oligocene. Paleontol. J. 2002, 36 (Suppl. S3), 185–259. [Google Scholar]
- Kotlarczyk, J.; Jerzmańska, A.; Świdnicka, E.; Wiszniowska, T. A framework of ichthyofaunal ecostratigraphy of the Oligocene-Early Miocene strata of the Polish Outer Carpathian basin. Ann. Soc. Geol. Pol. 2006, 76, 1–111. [Google Scholar]
- Bienkowska-Wasiluk, M. Taphonomy of Oligocene teleost fishes from the Outer Carpathians of Poland. Acta Geol. Pol. 2010, 60, 479–533. [Google Scholar]
- Bienkowska, M. Taphonomy of ichthyofauna from an Oligocene sequence (Tylawa Limestones horizon) of the Outer Carpathians, Poland. Geol. Q. 2004, 48, 181–192. [Google Scholar]
- Martini, E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In Proceedings of the II. Planktonic Conference, Rome, Italy, 1970; Farinacci, A., Ed.; Tecnoscienza: Rome, Italy, 1971; Volume 2, pp. 739–785. [Google Scholar]
- Olszewska, B. Otwornice warstw menilitowych polskich Karpat zewnętrznych. Ann. Soc. Geol. Pol. 1985, 55, 201–250, (In Polish, with English Summary). [Google Scholar]
- Szydło, A.; Garecka, M.; Jankowski, L.; Malata, T. Paleogene microfossils from the submarine debris flows in the Skole Basin (Polish and Ukraine Outer Carpathians). Geol. Geophys. Environ. 2014, 40, 49–65. [Google Scholar] [CrossRef]
- Siemińska, A.; Starzec, K.; Waśkowska, A.; Wendorff, M. Sedimentary and diapiric mélanges in the Skrzydlna area (Outer Carpathians of Poland) as indicators of basinal and structural evolution. J. Geol. Soc. 2020, 177, 600–618. [Google Scholar] [CrossRef]
- Dziadzio, P.S.; Matyasik, I.; Garecka, M.; Szydło, A. Lower Oligocene Menilite Beds, Polish Outer Carpathians: Supposed deep-sea flysch locally reinterpreted as shelfal, based on new sedimentological, micropalaeontological and organic-geochemical data. Pr. Nauk. Inst. Naft. I Gazu 2016, 213, 1–120. [Google Scholar]
- Olszewska, B.; Szydło, A. Environmental stress in the northern Tethys during the Paleogene: A review of foraminiferal and geochemical records from the Polish Outer Carpathians. Geol. Q. 2017, 61, 682–695. [Google Scholar] [CrossRef]
- Suchocka, A.; Barski, M.; Bienkowska-Wasiluk, M. Dinoflagellate cyst stratigraphy of the Popiele Member and Menilite Formation from the Boryslav-Pokuttya Nappe (Aksmanice, SE Poland). Geol. Q. 2019, 63, 539–557. [Google Scholar]
- Rögl, F. Palaeogeographic considerations for Mediterranean and Paratethys sea ways (Oligocene to Miocene). Ann. Naturhist. Mus. Wien 1998, 99A, 279–310. [Google Scholar]
- Rögl, F. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview). Geol. Carpath. 1999, 50, 339–349. [Google Scholar]
- Schulz, H.-M.; Bechtel, A.; Sachsenhofer, R.F. The birth of the Paratethys during the early Oligocene: From Tethys to an ancient Black Sea analogue? Glob. Planet. Change 2005, 49, 163–176. [Google Scholar] [CrossRef]
- Sótak, J. Paleoenvironmental changes across the Eocene-Oligocene boundary: Insights from the Central-Carpathian Paleogene Basin. Geol. Carpath. 2010, 61, 393–418. [Google Scholar] [CrossRef]
- Sachsenhofer, R.F.; Popov, S.V.; Coric, S.; Mayer, J.; Misch, D.; Morton, M.T.; Pupp, M.; Rauball, A.; Tari, G. Paratethyan Petroleum Source Rocks: An Overview. J. Pet. Geol. 2018, 41, 219–245. [Google Scholar] [CrossRef]
- Krhovský, J.; Adamová, J.; Hladiková, J.; Maslowská, H. Paleoenvironmental changes across the Eocene/Oligocene boundary in the Ždánice and Pouzdřany units (western Carpathians, Czechoslovakia): The long-term trend and orbitality forced changes in nannofossils assemblages. Knih. ZPN (Zemn. Plyn A Naft.) 1992, 14, 105–187. [Google Scholar]
- Garecka, M. Record of changes in the Oligocene-Miocene sediments of the Menilite-Krosno series of the Skole Unit based on calcareous nannoplankton studies-biostratigraphy and palaeogeographical implications (Polish Outer Carpathians). Biul. PIG 2012, 453, 1–22. [Google Scholar]
- Studencka, B.; Popov, S.V.; Bienkowska-Wasiluk, M.; Wasiluk, R. Oligocene bivalve faunas from Silesian Nappe, Polish Outer Carpathians: Evidence of the early history of the Paratethys. Geol. Q. 2016, 60, 317–340. [Google Scholar] [CrossRef][Green Version]
- Bienkowska-Wasiluk, M. The fish fauna of the Dynow Marl Member (Menilite Formation, Poland): Paleoenvironment and paleobiogeography of the early Oligocene Paratethys. Bull. Geosci. 2021, 96, 493–511. [Google Scholar] [CrossRef]
- Pickering, K.; Stow, D.; Watson, M.; Hiscott, R. Deep-water facies, processes and models: A review and classification scheme for modern and ancient sediments. Earth-Sci. Rev. 1986, 23, 75–174. [Google Scholar] [CrossRef]
- Schieber, J.; Miclăuș, C.; Seserman, A.; Liu, B.; Teng, J. When a mudstone was actually a “sand”: Results of a sedimentological investigation of the bituminous marl formation (Oligocene), Eastern Carpathians of Romania. Sed. Geol. 2019, 384, 12–28. [Google Scholar] [CrossRef]
- Górniak, K. Origin of marls from the Polish Outer Carpathians: Lithological and sedimentological aspects. Mineralogia 2012, 42, 165–297. [Google Scholar] [CrossRef]
- Kotlarczyk, J.; Uchman, A. Integrated ichnology and ichthyology of the Oligocene Menilite Formation, Skole and Subsilesian nappes, Polish Carpathians: A proxy to oxygenation history. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 331–332, 104–118. [Google Scholar] [CrossRef]
- Haczewski, G. Coccolith limestone horizons in the Menilite-Krosno Series (Oligocene, Carpathians): Identification, correlation, and origin. Ann. Soc. Geol. Pol. 1989, 59, 435–523, (In Polish, with English summary). [Google Scholar]
- Ciurej, A.; Haczewski, G. The Tylawa Limestones—A regional marker horizon in the Lower Oligocene of the Paratethys: Diagnostic characteristics from the type area. Geol. Q. 2012, 56, 833–844. [Google Scholar] [CrossRef]
- Leonowicz, P.; Bienkowska-Wasiluk, M.; Ochmanski, T. Benthic microbial mats from deep-marine flysch deposits (Oligocene Menilite Formation from S Poland): Palaeoenvironmental controls on the MISS types. Sed. Geol. 2021, 417, 105881. [Google Scholar] [CrossRef]
- Bojanowski, M.; Ciurej, A.; Haczewski, G.; Jokubauskas, P.; Schoutene, S.; Tyszka, J.; Bijl, P.K. The Central Paratethys during Oligocene as an ancient counterpart of the present-day Black Sea: Unique records from the coccolith limestones. Mar. Geol. 2018, 403, 301–328. [Google Scholar] [CrossRef]
- Čech, M.; Čech, P.; Kubečka, J.; Prchalová, M.; Draštík, V. Size selectivity in summer and winter diets of great cormorant (Phalacrocorax carbo): Does it reflect season-dependent difference in foraging efficiency? Waterbirds 2008, 31, 438–447. [Google Scholar] [CrossRef]
- Čech, M.; Vejřík, L. Winter diet of great cormorant (Phalacrocorax carbo) on the River Vltava: Estimate of size and species composition and potential for fish stock losses. Fol. Zool. 2011, 60, 129–142. [Google Scholar] [CrossRef]
- Granadeiro, J.P.; Silva, M.A. The use of otoliths and vertebrae in the identification and size-estimation of fish in predator-prey studies. Cybium 2000, 24, 383–393. [Google Scholar]
- Tarkan, A.S.; Gursoy Gaygusuz, C.; Gaygusuz, Ö.; Acipinar, H. Use of bone and otolith measures for size-estimation of fish in predator-prey studies. Fol. Zool. 2007, 56, 328–336. [Google Scholar]
- Wise, M.H. The use of fish vertebrae in scats for estimating prey size of otters and mink. J. Zool. 1980, 192, 25–31. [Google Scholar] [CrossRef]
- Bienkowska-Wasiluk, M.; Paldyna, M. Taxonomic revision of the Oligocene percoid fish Oligoserranoides budensis (Heckel, 1856), from the Paratethys and paleobiogeographic comments. Geol. Acta 2018, 16, 75–92. [Google Scholar]
- Gregorová, R. Isolated fish scales (Teleostei) from the Paleogene and Neogene sediments of the Central Paratethys (Czech Republic, Slovak Republic). Acta Mus. Mor. Sci. Geol. 2023, 108, 283–308. [Google Scholar]
- Bräger, Z.; Moritz, T. A scale atlas for common Mediterranean teleost fishes. Vert. Zool. 2016, 66, 275–386. [Google Scholar] [CrossRef]
- Patterson, R.T.; Wright, C.; Chang, A.S.; Taylor, L.A.; Lyons, P.D.; Dallimore, A.; Kumar, A. Atlas of common squamatological (fish scale) material in coastal British Columbia, and an assessment of the utility of various scale types in paleofisheries reconstruction. Palaeontol. Electron. 2002, 4, 1–88. [Google Scholar]
- Jerzmańska, A. Ichtyofaune des couches a menilite (flysch des Karpathes). Acta Pal. Pol. 1968, 13, 379–487. [Google Scholar]
- Bienkowska-Wasiluk, M.; Granica, M.; Kovalchuk, O. A new extinct shad from Poland in the light of clupeiform diversity and distribution within the Paratethys during the Oligocene. Acta Geol. Pol. 2024, 74, e23. [Google Scholar] [CrossRef]
- Granica, M.; Bienkowska-Wasiluk, M.; Pałdyna, M. A new clupeoid genus from the Oligocene of Central Paratethys (Menilite Formation, Poland). Acta Geol. Pol. 2024, 74, e5. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Baykina, E.; Świdnicka, E.; Stefaniak, K.; Nadachowski, A. A systematic revision of herrings (Teleostei, Clupeidae, Clupeinae) from the Oligocene and early Miocene from the Eastern Paratethys and the Carpathian Basin. J. Vertebr. Paleontol. 2020, 40, e1778710. [Google Scholar] [CrossRef]
- Platt, S.G.; Elsey, R.M.; Bishop, N.D.; Rainwater, T.R.; Thongsavath, O.; Labarre, D.; McWilliam, A.G. Using scat to estimate body size in crocodilians: Case studies of the Siamese crocodile and American alligator with practical applications. Herpet. Conserv. Biol. 2020, 15, 325–334. [Google Scholar]
- Gaeta, J.W.; Ahrenstorff, T.D.; Diana, J.S.; Fetzer, W.W.; Jones, T.S.; Lawson, Z.J.; McInerny, M.C.; Santucci, V.J., Jr.; Vander Zanden, M.J. Go big or… don’t? A field-based diet evaluation of freshwater piscivore and prey fish size relationships. PLoS ONE 2018, 13, e0194092. [Google Scholar] [CrossRef]
- Scharf, F.S.; Juanes, F.; Rountree, R.A. Predator size-prey size relationships of marine fish predators: Interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Progr. Ser. 2000, 208, 229–248. [Google Scholar] [CrossRef]
- Cohen, J.E.; Jonsson, T.; Carpenter, S.R. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA 2003, 100, 1781–1786. [Google Scholar] [CrossRef]
- Přikryl, T.; Kania, I.; Krzemiński, W. Synopsis of fossil fish fauna from the Hermanowa locality (Rupelian; Central Paratethys; Poland): Current state of knowledge. Swiss J. Geosci. 2016, 38, 429–443. [Google Scholar] [CrossRef]
- Gregorová, R. Osteological and morphological analysis of the scabbardfish Anenchelum glarisianum Blainville, 1818 (Trichiuridae) from the Menilitic Formation of the Moravian, part of West Carpathians (Oligocene, Rupelian). Acta Musei Morav. Sci. Geol. 2010, 95, 141–149. [Google Scholar]
- Přikryl, T. Notes on development of the Oligocene trachinid Trachinus minutus (Jonet, 1958). Palaeontogr. Abt. A Palaeozool. 2017, 308, 69–87. [Google Scholar] [CrossRef]
- Jerzmańska, A. Ichthyofauna from the Jasło Shales at Sobniów (Poland). Acta Pal. Pol. 1960, 5, 367–419, (In Polish, with English Summary). [Google Scholar]




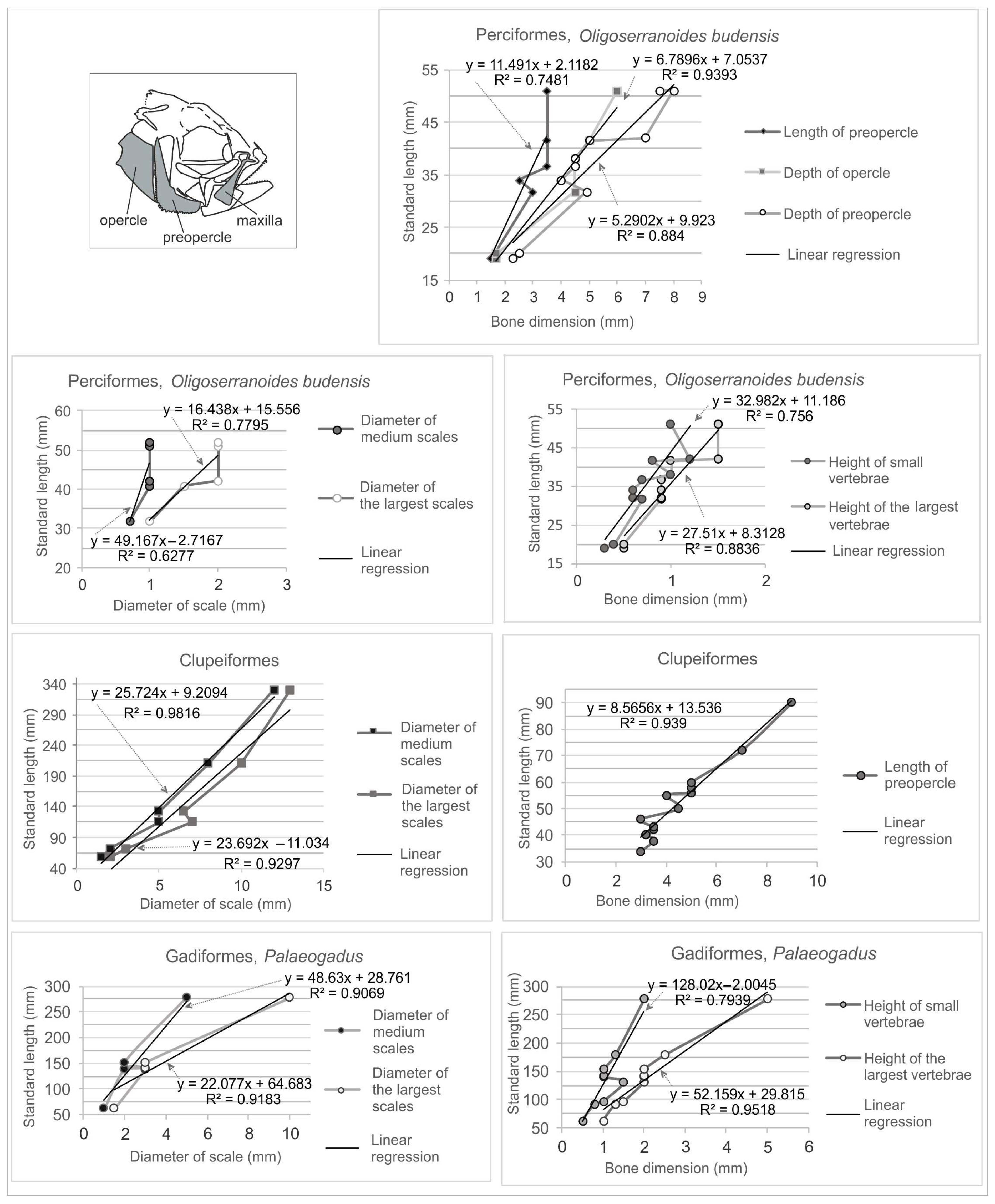
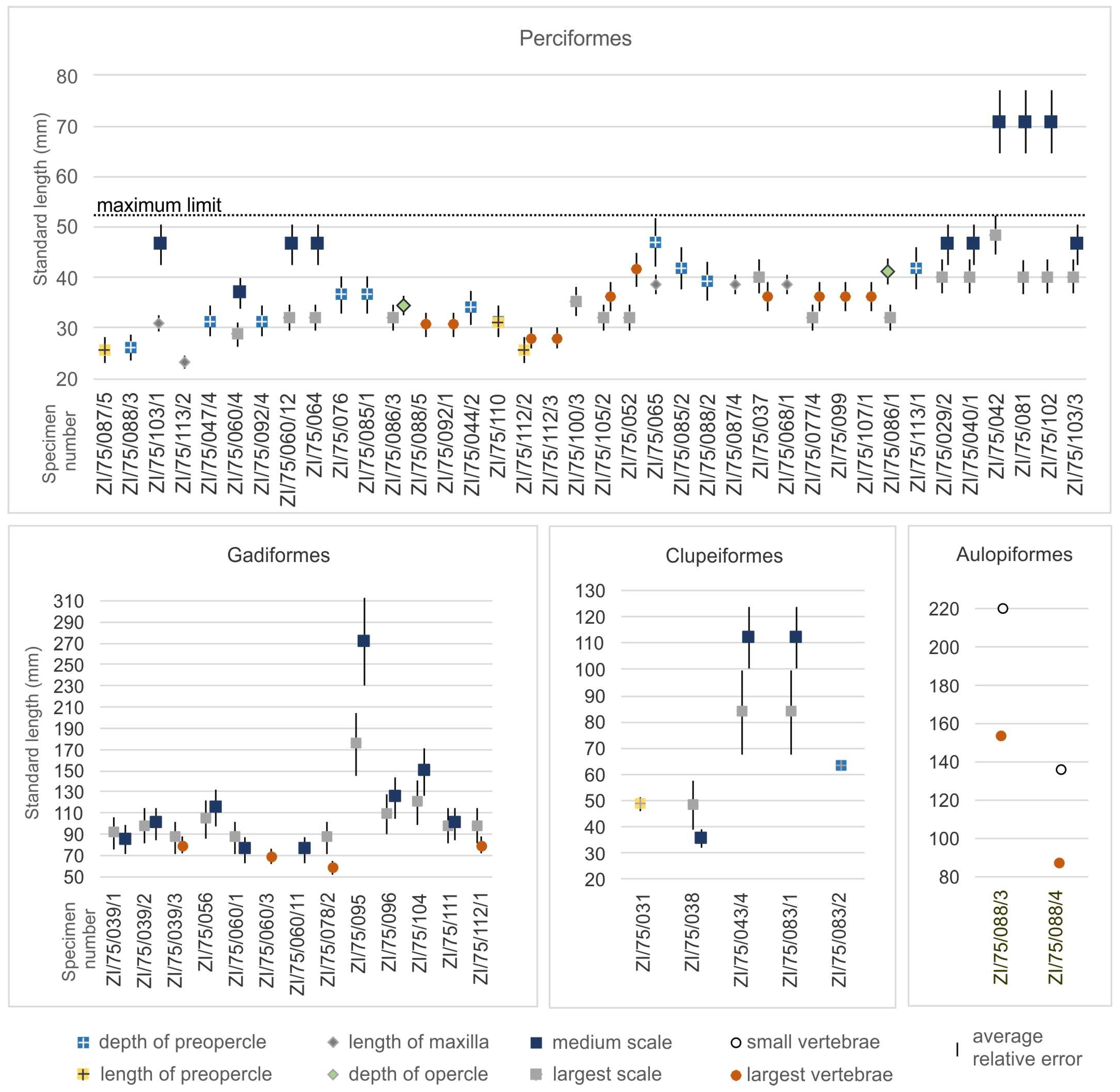
| Specimen Number | Inclusions | Taxonomy of Inclusions | Size (mm) | Estimated Prey Size (SL in mm) |
|---|---|---|---|---|
| ZI/75/029/2/a, ZI/75/029/2/b | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1–1.5 | 40–46 |
| ZI/75/031 | Preopercle of Clupeiformes | Clupeiformes | Length of preopercle l’pop 4 | 48 |
| ZI/75/037 | Perciform-like scales similar to Oligoserranoides budensis, vertebrae | Perciformes | Height of vertebra 1, maximum diameter of scale 1.5 | 36–40 |
| ZI/75/038 | Vertebrae and scales of Clupeiformes | Clupeiformes | Height of vertebra 1, maximum diameter of scale 2.5 | 35–48 |
| ZI/75/039/1/a, ZI/75/039/1/b, | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus, bones | Gadiformes | Maximum diameter of scale 1.2 | 87 |
| ZI/75/039/2/a, ZI/75/039/2/b, | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus, bones | Gadiformes | Maximum diameter of scale 1.5 | 98–102 |
| ZI/75/039/3/a, ZI/75/039/3/b, | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus, bones | Gadiformes | Maximum diameter of scale 1, height of vertebra 1 | 82–88 |
| ZI/75/040/1 | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1–1.5 | 40–46 |
| ZI/75/042 | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1.5–2 | >48 |
| ZI/75/043/4 | Scales of Clupeiformes | Clupeiformes | Maximum diameter of scale 4 | 84–112 |
| ZI/75/044/2/a, ZI/75/044/2/b, | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 4.5 | 34 |
| ZI/75/047/4/a, ZI/75/047/4/b | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 4 | 31 |
| ZI/75/052 | Perciform-like scales similar to Oligoserranoides budensis, bones, vertebrae | Perciformes | Height of vertebra 1.2, maximum diameter of scale 1 | 32–41 |
| ZI/75/056 | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Maximum diameter of scale 1.8 | 104–116 |
| ZI/75/060/1 | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Maximum diameter of scale 1 | 77–88 |
| ZI/75/060/3/a, ZI/75/060/3/b | Vertebrae of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Height of vertebra 0.8 | 72 |
| ZI/75/060/4/a, ZI/75/060/4/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Maximum diameter of scale 0.8 | 29–37 |
| ZI/75/060/11 | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Maximum diameter of scale 1 | 77 |
| ZI/75/060/12 | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1 | 32–46 |
| ZI/75/064/a, ZI/75/064/b | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1 | 32–46 |
| ZI/75/065 | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 7, length of maxilla Lp 5 | 39–47 |
| ZI/75/068/1 | Bones and scales of perciform Oligoserranoides budensis | Perciformes | Length of maxilla Lp 5 | 39 |
| ZI/75/076/a, ZI/75/076/b | Bones and scales of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 5 | 36 |
| ZI/75/077/4 | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Height of vertebra 1, maximum diameter of scale 1 | 32–36 |
| ZI/75/078/2 | Bones (including vertebrae) and scales of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Height of vertebra 0.6 | 61–87 |
| ZI/75/081 | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Maximum diameter of scale 1.5 | 40–51 |
| ZI/75/083/1 | Scales of Clupeiformes | Clupeiformes | Maximum diameter of scale 4 | 84–112 |
| ZI/75/083/2 | Bones of Clupeiformes | Clupeiformes | Depth of preopercle h’pop 10 | 63 |
| ZI/75/085/1 | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 5 | 36 |
| ZI/75/085/2 | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 6 | 42 |
| ZI/75/086/1/a, ZI/75/086/1/b | Bones and scales of perciform Oligoserranoides budensis | Perciformes | Depth of opercle 5, scales 1–1.8 | 32–41 |
| ZI/75/086/3 | Bones and scales of perciform Oligoserranoides budensis | Perciformes | Depth of opercle 4, scales 1–1.5 | 32–34 |
| ZI/75/087/4 | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Length of maxilla Lp 5 | 39 |
| ZI/75/087/5 | Bones and scales of perciform Oligoserranoides budensis | Perciformes | Length of preopercle l’pop 2 | 25 |
| ZI/75/088/2/a, ZI/75/088/2/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Depth of preopercle h’pop 5.5 | 39 |
| ZI/75/088/3/a, ZI/75/088/3/b | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 3 | 26 |
| ZI/75/088/3/a, ZI/75/088/3/b | Bones of Holosteus | Aulopiformes | Height of vertebra 2 | 153–220 |
| ZI/75/088/4/a, ZI/75/088/4/b | Bones of Holosteus | Aulopiformes | Height of vertebra 1 | 86–136 |
| ZI/75/088/5/a, ZI/75/088/5/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Height of vertebra 0.8 | 30 |
| ZI/75/092/1/a, ZI/75/092/1/b, | Bones of perciform Oligoserranoides budensis | Perciformes | Height of vertebra 0.8 | 30 |
| ZI/75/092/4/a, ZI/75/092/4/b, | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 4 | 31 |
| ZI/75/095/a, ZI/75/095/b | Scales of Gadiformes | Gadiformes | Maximum diameter of scale 5 | 175–272 |
| ZI/75/096 | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Maximum diameter of scale 2 | 109–126 |
| ZI/75/099/a, ZI/75/099/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Height of vertebra 1 | 36 |
| ZI/75/100/3 | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1.2 | 35 |
| ZI/75/102/a, ZI/75/102/b | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1.5 | 40–51 |
| ZI/75/103/1/a, ZI/75/103/1/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Length of maxilla Lp 4, maximum diameter of scale 1 | 31–46 |
| ZI/75/103/3 | Perciform-like scales similar to Oligoserranoides budensis | Perciformes | Maximum diameter of scale 1–1.5 | 40–46 |
| ZI/75/104/a, ZI/75/104/b | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus | Gadiformes | Maximum diameter of scale 2.5 | 120–150 |
| ZI/75/105/2 | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Height of vertebra 1, maximum diameter of scale about 1 | 32–36 |
| ZI/75/107/1/a, ZI/75/107/1/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Height of vertebra 1 | 36 |
| ZI/75/110/a, ZI/75/110/b | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 4, length of preopercle l’pop 2.5 | 31 |
| ZI/75/111/a, ZI/75/111/b | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus, bones | Gadiformes | Maximum diameter of scale 1.5 | 98–102 |
| ZI/75/112/1/a, ZI/75/112/1/b | Scales of Gadiformes, family Merlucciidae, similar to Palaeogadus, bones | Gadiformes | Maximum diameter of scale 1.5, height of vertebra 1 | 82–98 |
| ZI/75/112/2/a, ZI/75/112/2/b | Bones of perciform Oligoserranoides budensis | Perciformes | Height of vertebra 0.7, length of preopercle l’pop 2 | 25–28 |
| ZI/75/112/3 | Bones of perciform Oligoserranoides budensis | Perciformes | Height of vertebra 0.7 | 28 |
| ZI/75/113/1/a, ZI/75/113/1/b | Bones of perciform Oligoserranoides budensis | Perciformes | Depth of preopercle h’pop 6 | 42 |
| ZI/75/113/2/a, ZI/75/113/2/b | Perciform-like scales similar to Oligoserranoides budensis, bones | Perciformes | Length of maxilla Lp 3 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienkowska-Wasiluk, M.; Bajdek, P.; Granica, M. Vertebrate Coprolites Reveal Diversity of Prey Fishes in the Oligocene Carpathian Basin of the Paratethys. Diversity 2025, 17, 507. https://doi.org/10.3390/d17080507
Bienkowska-Wasiluk M, Bajdek P, Granica M. Vertebrate Coprolites Reveal Diversity of Prey Fishes in the Oligocene Carpathian Basin of the Paratethys. Diversity. 2025; 17(8):507. https://doi.org/10.3390/d17080507
Chicago/Turabian StyleBienkowska-Wasiluk, Malgorzata, Piotr Bajdek, and Mateusz Granica. 2025. "Vertebrate Coprolites Reveal Diversity of Prey Fishes in the Oligocene Carpathian Basin of the Paratethys" Diversity 17, no. 8: 507. https://doi.org/10.3390/d17080507
APA StyleBienkowska-Wasiluk, M., Bajdek, P., & Granica, M. (2025). Vertebrate Coprolites Reveal Diversity of Prey Fishes in the Oligocene Carpathian Basin of the Paratethys. Diversity, 17(8), 507. https://doi.org/10.3390/d17080507







