Twenty-One Mayfly Gynandromorphic Cases from China
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Description
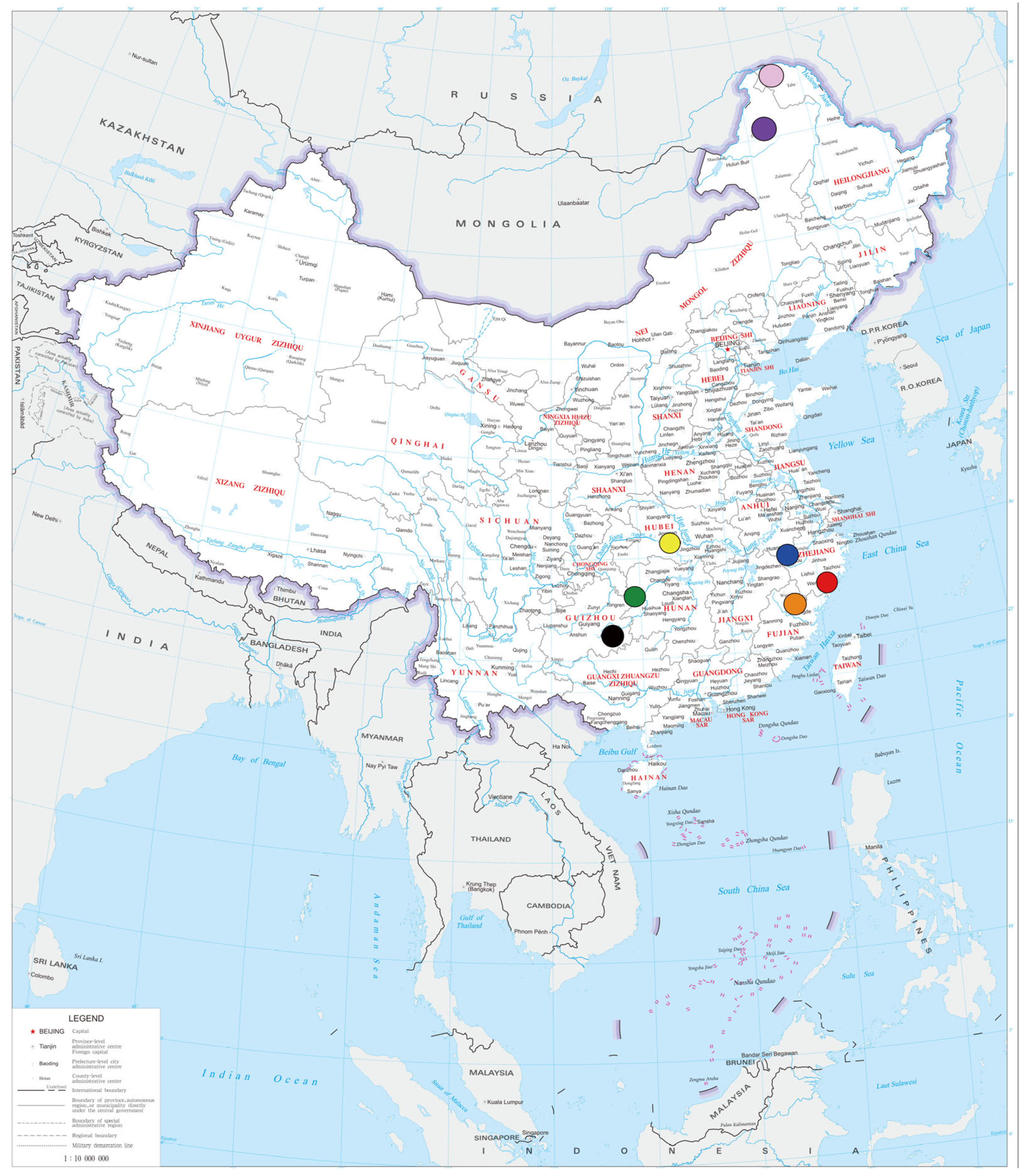
3.2. Summary Information
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soldán, T.; Landa, V. Gynandromorphism, intersexuality and teratology of external genitalia in the order Ephemeroptera. Věstník Ceskoslov. Společnosti Zool. 1981, 45, 189–203. [Google Scholar]
- Cui, J.-X.; Cai, W.-Z. Gynandromorphosis in Insects. Entomol. Knowl. 2003, 40, 565–570. [Google Scholar] [CrossRef]
- Santos, R.P.; Moreto, V.; Mariano, R. Intersexuality in Farrodes Peters, 1971 (Ephemeroptera: Leptophlebiidae: Atalophlebiinae) from Brazil. Rev. Bras. Entomol. 2019, 63, 202–204. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Li, J.; Zhou, C.-F. Report of intersex individuals from a southeastern Chinese Choroterpes facialis (Gillies, 1951) population (Ephemeroptera: Leptophlebiidae). Zootaxa 2023, 5258, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Lestage, J.A. Two cases of gynanders: In a larva of Perla abdominalis Burm. (Plecoptera) and in an adult female of Baetis rhodani Pictet (Ephemeroptera). Ann. Limnol. Biol. 1922, 11, 85–87. [Google Scholar]
- Wu, X.-Y.; Gui, H.; Su, C.-R. On the first mayfly gynandromorph described in China. Acta Zootaxonomica Sin. 1993, 36, 382–384. [Google Scholar] [CrossRef]
- Kluge, N.J. Central Asian mountain Rhithrogenini (Ephemeroptera: Heptageniidae) with pointed and ephemeropteroid claws in the winged stages. Zootaxa 2015, 3994, 301–353. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Sivaruban, T.; Barathy, S.; Rajasekaran, I. First report of gynandromorph mayfly, Centroptella ghatensis Kluge, 2021, from India. Aquat. Insects 2023, 45, 314–318. [Google Scholar] [CrossRef]
- Domínguez, E. On the discovery of a gynandromorph of the genus Dactylobaetis (Ephemeroptera: Baetidae) in Uruguay. Acta Zool. 1984, 37, 317. [Google Scholar]
- McCafferty, W.P.; Bloodgood, D.W. Gynandromorphism and differential molting in a mayfly (Ephemeroptera). Entomol. News 1986, 97, 57–60. [Google Scholar]
- Grant, P.M.; Masteller, E.C. Intersexuality in Ephemeroptera: Description of four specimens and comments on its occurrence in a parthenogenetic species. Can. J. Zool. 1987, 65, 1985–1988. [Google Scholar] [CrossRef]
- Pohe, S.R. Macroecology of New Zealand Ephemeroptera. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2019. [Google Scholar]
- Nascimento, J.M.C.D.; Castelaci, L.C.; Hamada, N. More about Thraulodes Ulmer, 1920 (Ephemeroptera: Leptophlebiidae) from the Brazilian Amazonia: Three new species, a new record and a gynandromorph report. Zootaxa 2021, 5076, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-F.; Su, C.-R.; Gui, H. An Overview of Ephemeroptera in China; Science Press: Beijing, China, 2015; p. 310. [Google Scholar]
- Wang, Y.-Y.; Qin, J.-Z.; Chen, P.; Zhou, C.-F. A New Species of Baetis from Zijin Hill of Nanjing (Eastern China) (Ephemeroptera: Baetidae). Orient. Insects 2011, 45, 72–79. [Google Scholar] [CrossRef]
- Gillies, M.T. Further notes on Ephemeroptera from India and South East Asia. Proc. R. Soc. Lond. B 1951, 20, 121–130. [Google Scholar] [CrossRef]
- Eaton, A.E. On some new British species of Ephemeridae. Trans. Entomol. Soc. Lond. 1870, 1870, 1–8. [Google Scholar] [CrossRef]
- Poulin, R. “Adaptive” changes in the behaviour of parasitized animals: A critical review. Int. J. Parasitol. 1995, 25, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Vance, S.A. Morphological and behavioural sex reversal in mermithid-infected mayflies. Proc. R. Soc. London. Ser. B Biol. Sci. 1996, 263, 907–912. [Google Scholar] [CrossRef]
- Agnew, J.D. Gynanders and sex determination in Ephemeroptera. Eatonia 1979, 24, 1–2. [Google Scholar]
- Funk, D.H.; Sweeney, B.W.; Jackson, J.K. Why stream mayflies can reproduce without males but remain bisexual: A case of lost genetic variation. J. N. Am. Benthol. Soc. 2010, 29, 1258–1266. [Google Scholar] [CrossRef]
- Liegeois, M.; Sartori, M.; Schwander, T. Extremely widespread parthenogenesis and a trade-off between alternative forms of reproduction in mayflies (Ephemeroptera). J. Hered. 2021, 112, 45–57. [Google Scholar] [CrossRef] [PubMed]

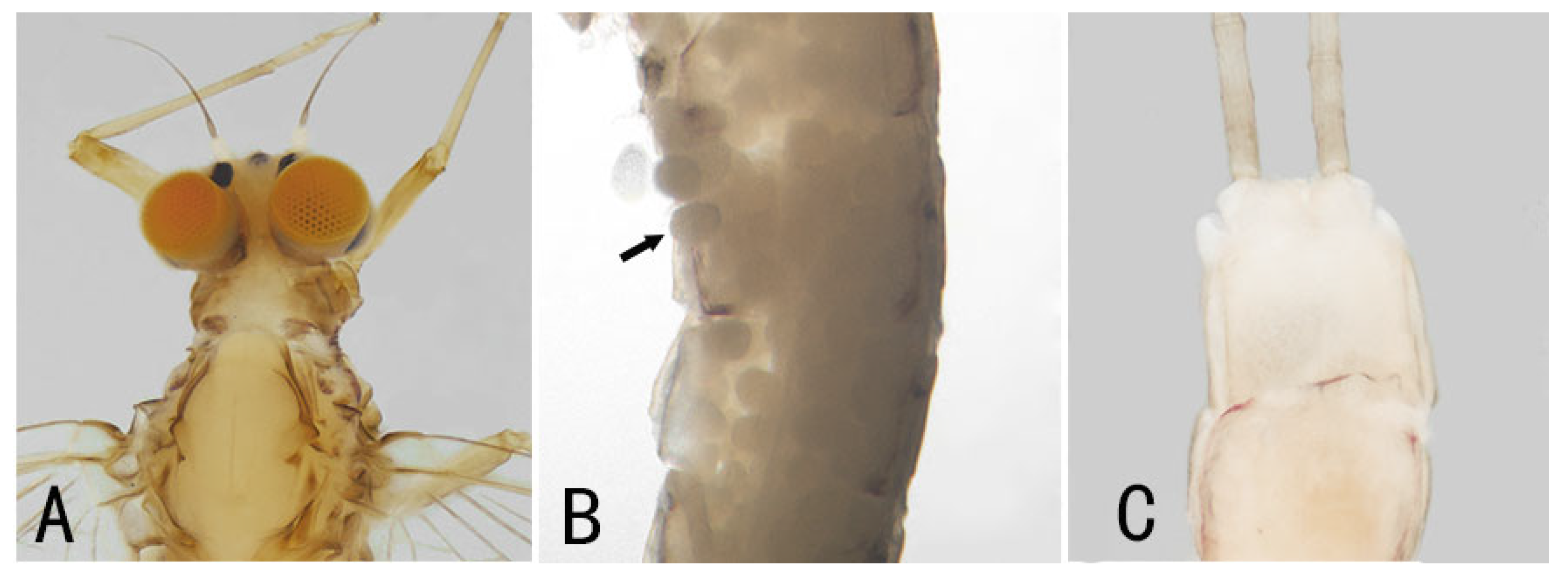
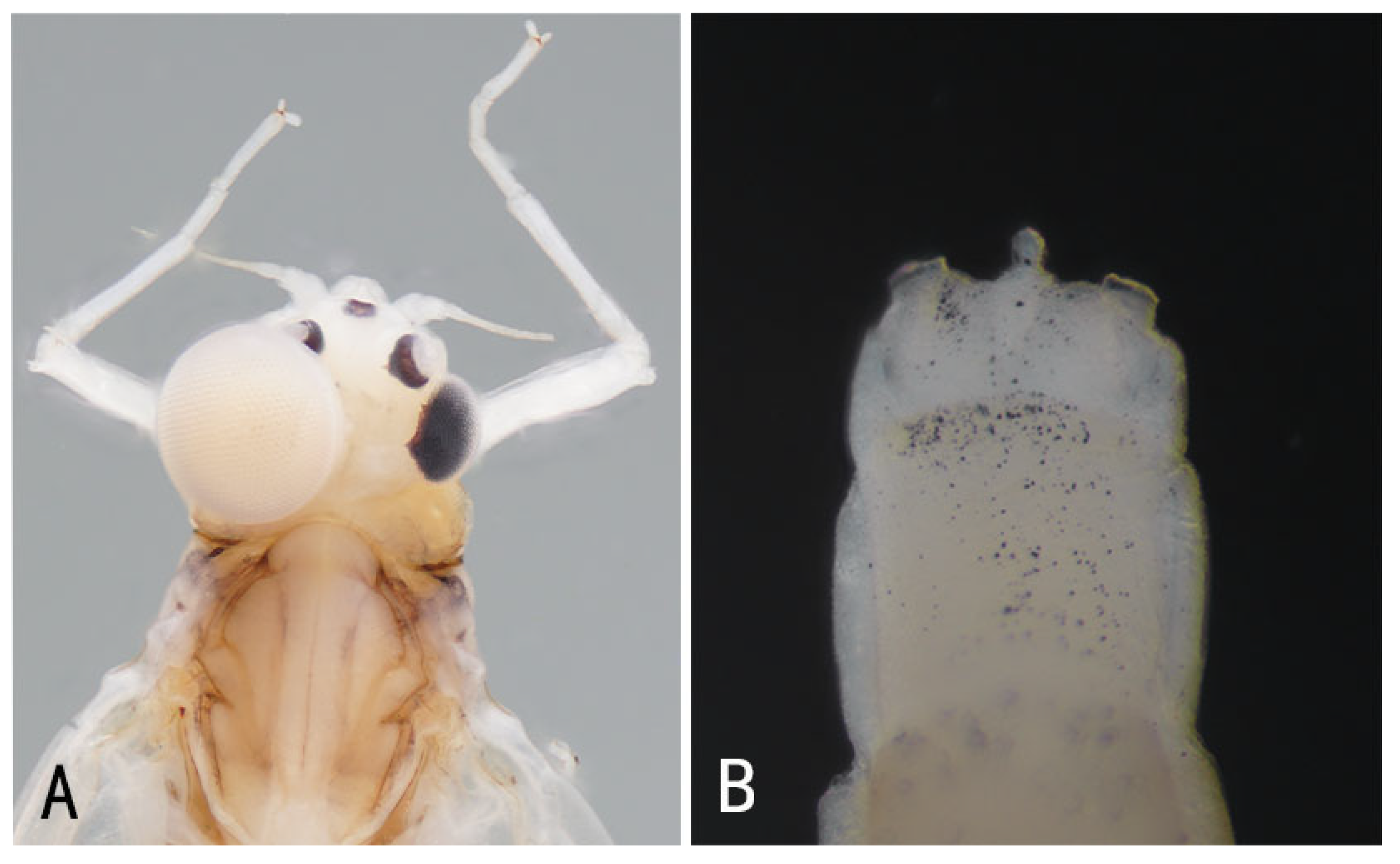

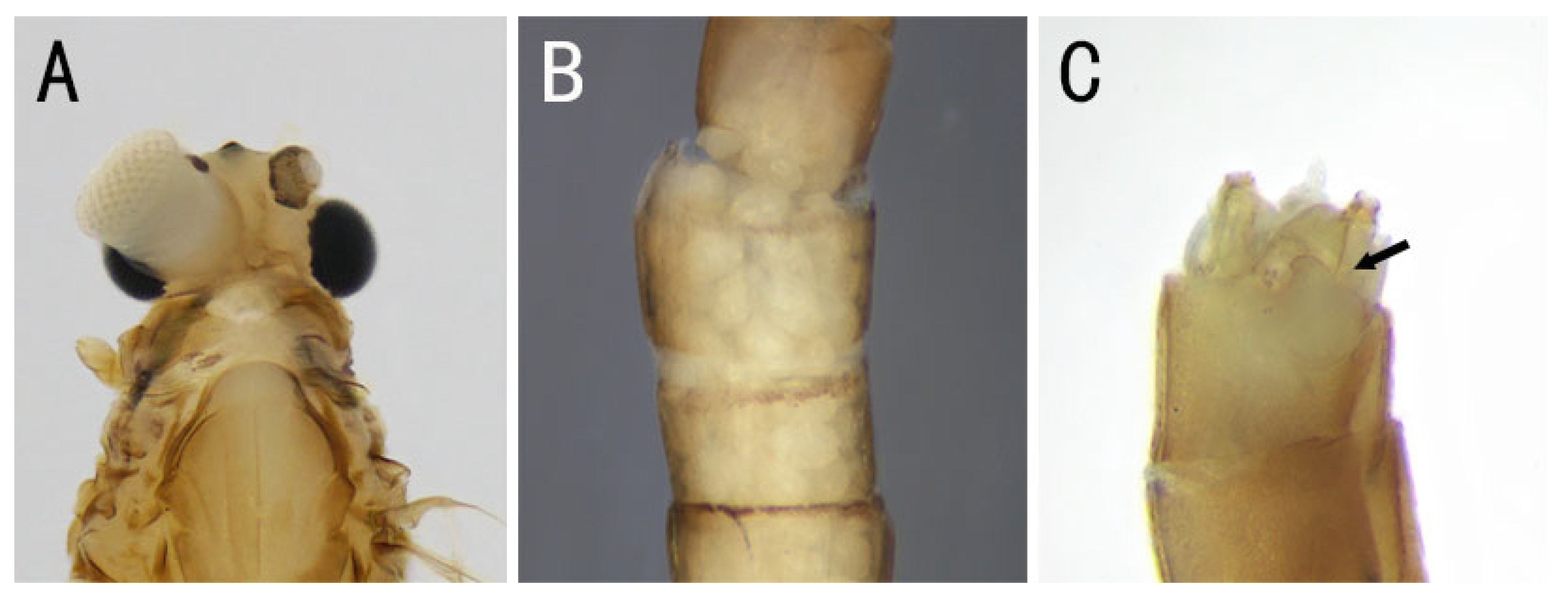
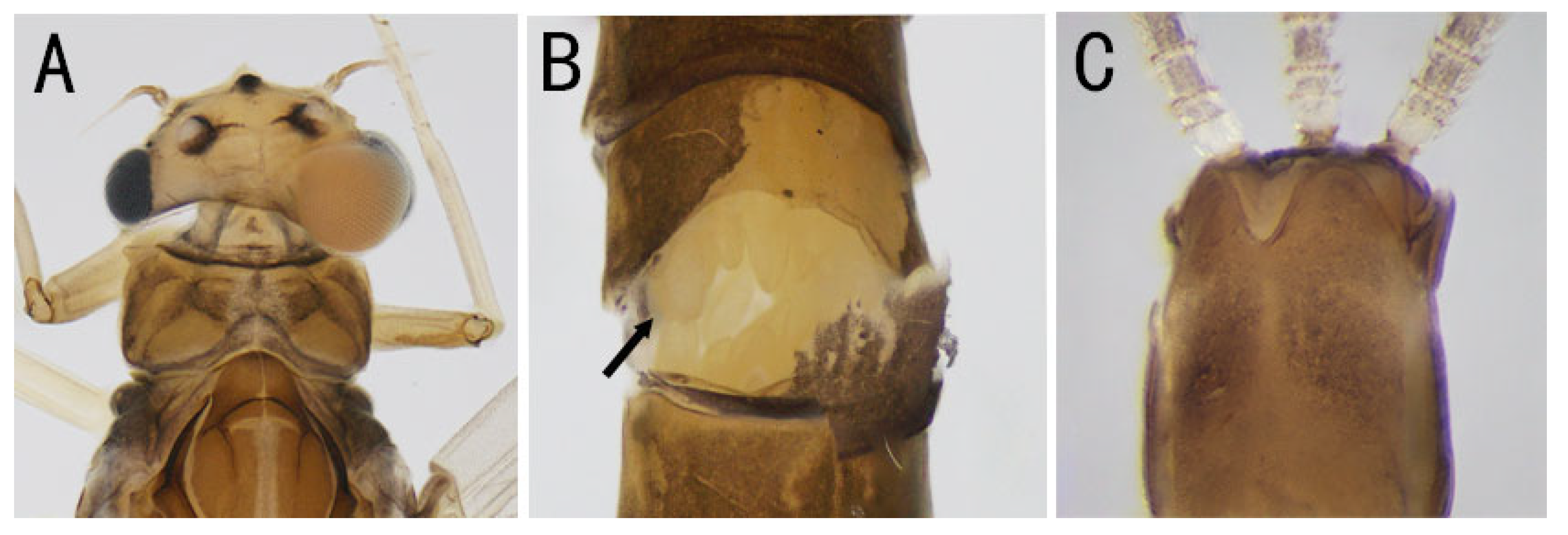
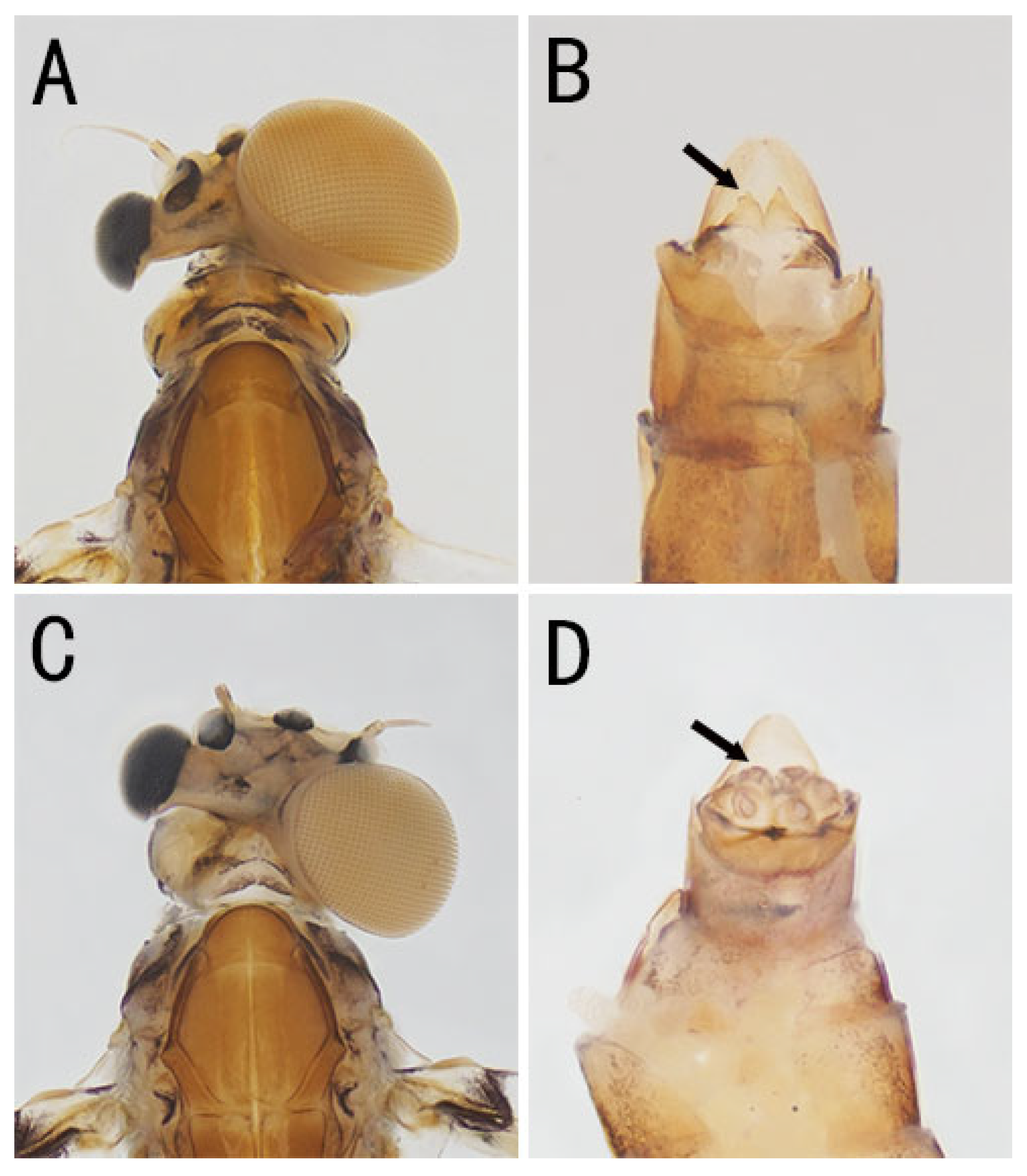


| Individual | Stage | Name | Family | Collected Province | Sex (Judging from the Content in Abdomen) | Main Feature |
|---|---|---|---|---|---|---|
| 1 | imago | Baetis rutilocylindratus | Baetidae | Zhejiang | female | Predominantly female with male compound eyes |
| 2 | subimago | Baetis sp. | Baetidae | Hunan | male | Predominantly male with right female eye, left foreleg shorter than right |
| 3 | subimago | Baetis sp. | Baetidae | Hubei | female | Predominantly female with right male eye |
| 4 | subimago | Baetis sp. | Baetidae | Guizhou | female | Predominantly female with left male eye and a relic forceps |
| 5 | subimago | Neoleptophlebia sp. | Leptophlebiidae | Anhui | female | Predominantly female with right male eye |
| 6 | imago | Choroterpes facialis | Leptophlebiidae | Fujian | male | Predominantly male with left female eye, without forceps but with slightly degenerated penes |
| 7 | imago | Choroterpes facialis | Leptophlebiidae | Fujian | female | Predominantly female with right male eye and degenerated penes |
| 8–21 | imago | Siphlonurus lacustris | Siphlonuridae | Inner Mongolia and Heilongjiang | male | Predominantly male with female eyes, forceps degenerated in 13 individuals and missing in one |
| Information | Data |
|---|---|
| Family | 3 (Baetidae, Leptophlebiidae, Siphlonuridae) |
| Number of species | 7 (Baetidae 4, Leptophlebiidae 2, Siphlonuridae 1) |
| Number of individuals | 21 (Baetidae 4, Leptophlebiidae 3, Siphlonuridae 14) |
| sex | Male and female in Baetidae and Leptophlebiidae, male in Siphlonuridae |
| stage | Subimago and imago |
| type | Dominant male (17) or dominant female (4) |
| Distribution | North to South China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, M.; Deng, M.; Qiang, X.; Zhou, C. Twenty-One Mayfly Gynandromorphic Cases from China. Diversity 2025, 17, 509. https://doi.org/10.3390/d17080509
Li J, Li M, Deng M, Qiang X, Zhou C. Twenty-One Mayfly Gynandromorphic Cases from China. Diversity. 2025; 17(8):509. https://doi.org/10.3390/d17080509
Chicago/Turabian StyleLi, Jing, Mengyao Li, Muhe Deng, Xinhe Qiang, and Changfa Zhou. 2025. "Twenty-One Mayfly Gynandromorphic Cases from China" Diversity 17, no. 8: 509. https://doi.org/10.3390/d17080509
APA StyleLi, J., Li, M., Deng, M., Qiang, X., & Zhou, C. (2025). Twenty-One Mayfly Gynandromorphic Cases from China. Diversity, 17(8), 509. https://doi.org/10.3390/d17080509







