Do Adult Frogs Remember Their Lives as Tadpoles and Behave Accordingly? A Consideration of Memory and Personality in Anuran Amphibians
Abstract
1. Introduction
1.1. Memory
1.2. Personality
1.3. Anuran Diversity
1.4. Anuran Life History Diversity
2. Anuran Memory
3. The Nature of Our Knowledge of Memory
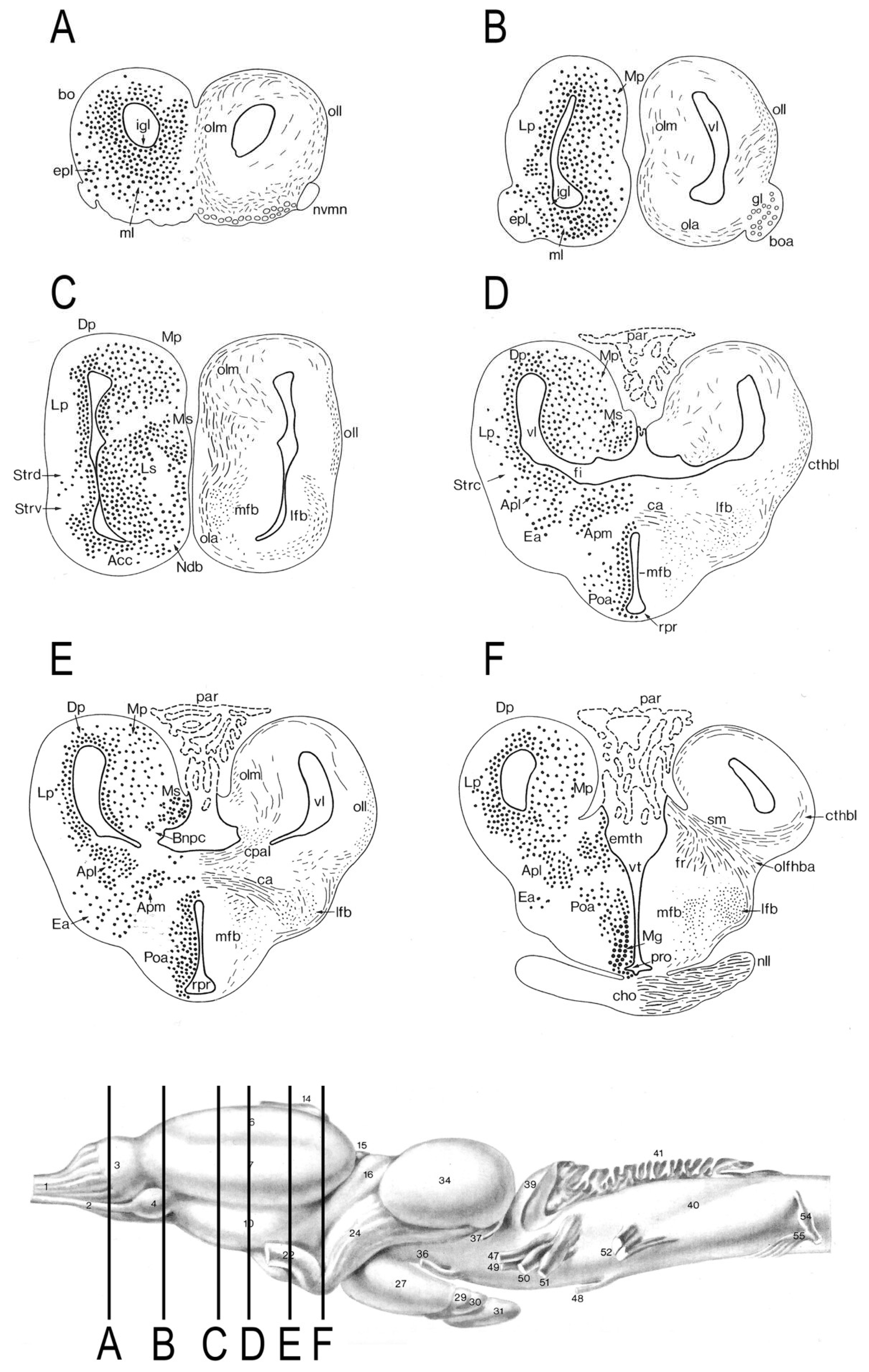
4. The Amphibian Neural Substrate for Memory
- -
- -
- While the mammalian hippocampus contains specialized place cells [4], as well as landmark-vector cells [117], grid cells, boundary cells, and head-direction cells that affect the activity of place cells [118,119], the amphibian medial pallium is composed of neurons that are relatively homogeneous morphologically [6,88,90]. It has yet to be determined if they have specialized functions.
- -
- While mammals have direct perforant and indirect alvear pathways through the hippocampal formation that form complex connections with heteromodal association cortex, amphibians have relatively simple reciprocal connections between the medial pallium and other forebrain regions both ipsilaterally and contralaterally [88,90].
5. Behavioral Evidence for Amphibian Memory
6. Anuran Personality
7. A Few Considered Hypotheses
8. Factors Inhibiting Our Understanding of Anuran Personality
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ortega-de San Luis, C.; Ryan, T.J. Understanding the physical basis of memory: Molecular mechanisms of the engram. J. Biol. Chem. 2022, 298, 101866. [Google Scholar] [CrossRef]
- Josselyn, S.A.; Tonegawa, S. Memory engrams: Recalling the past and imagining the future. Science 2020, 367, eaaw4325. [Google Scholar] [CrossRef]
- Bisaz, R.; Travaglia, A.; Alberini, C.M. The neurobiological bases of memory formation: From physiological conditions to psychopathology. Psychopathy 2014, 47, 347–365. [Google Scholar] [CrossRef]
- Murray, E.A.; Wise, S.P.; Graham, K.S. The Evolution of Memory Systems: Ancestors, Anatomy, and Adaptations; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Nieuwenhuys, R.; ten Donkelaar, H.J.; Nicholson, C. The Central Nervous System of Vertebrates; Springer: Berlin, Germany, 1998. [Google Scholar]
- Striedter, G.F.; Northcutt, R.G. Brains Through Time: A Natural History of Vertebrates; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Koenig, A.M.; Ousterhout, B.H. Behavioral syndrome persists over metamorphosis in a pond-breeding amphibian. Behav. Ecol. Sociobiol. 2018, 72, 184. [Google Scholar] [CrossRef]
- Sih, A.K.; Mathot, K.J.; Moiroón, M.; Montiglio, P.O.; Wolf, M.; Dingemanse, N.J. Animal personality and state-behavior feedbacks: A review and guide for empiricists. Trends Ecol. Evol. 2015, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Urszán, T.J.; Török, J.; Hettyey, A.; Garamszegi, L.Z.; Herczeg, G. Behavioural consistency and life history of Rana dalmatina tadpoles. Oecologia 2015, 178, 129–140. [Google Scholar] [CrossRef]
- Castellano, S.; Friard, O. Environmental effects on the ontogenesis of tadpole personality. Anim. Behav. 2021, 175, 153–161. [Google Scholar] [CrossRef]
- Bégué, L.; Tschirren, N.; Peignier, M.; Szabo, B.; Ringler, E. Behavioural consistency across metamorphosis in a neotropical poison frog. Evol. Ecol. 2024, 38, 157–174. [Google Scholar] [CrossRef]
- Cabrera, D.; Nilsson, J.R.; Griffen, B.D. The development of animal personality across ontogeny: A cross-species review. Anim. Behav. 2021, 173, 137–144. [Google Scholar] [CrossRef]
- Plaskonka, B.; Zaborowska, A.; Mikulski, A.; Pietrzak, B. Predation risk experienced by tadpoles shapes personalities before but not after metamorphosis. Ecol. Evol. 2024, 14, e70532. [Google Scholar] [CrossRef]
- Wilson, A.D.M.; Krause, J. Personality and metamorphosis: Is behavioral variation consistent across ontogenetic niche shifts? Behav. Ecol. 2012, 23, 1316–1323. [Google Scholar] [CrossRef]
- Cortazar-Chinero, M.; Corral-Lopez, A.; Lüdtke, D.; Luquet, E.; Laurila, A. Metamorphosis reverses the risk-taking behavioral phenotype in Moor Frog along a latitudinal gradient. bioRXiv 2023. [Google Scholar] [CrossRef]
- Adler, A. Understanding Human Nature; Oneworld Publications: London, UK, 1927; Reprinted in 1992. [Google Scholar]
- Lannoo, M.J. (Ed.) Status and Conservation of Midwestern Amphibians; University of Iowa Press: Iowa City, IA, USA, 1998. [Google Scholar]
- Lannoo, M.J. (Ed.) Amphibian Declines: The Conservation Status of United States Species; University of California Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Lannoo, M.J.; Stiles, R.M. The Call of the Crawfish Frog; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Sloan Wilson, D.; Clark, A.B.; Coleman, K.; Dearstyne, T. Shyness and boldness in human and other animals. Trends Ecol. Evol. 1994, 9, 426–442. [Google Scholar] [CrossRef]
- AmphibiaWeb. Available online: https://amphibiaweb.org/amphibian/speciesnums.html (accessed on 2 May 2025).
- ASM Mammal Diversity Database. Available online: https://www.mammaldiversity.org (accessed on 30 April 2025).
- Liao, W.B.; Lou, S.L.; Zeng, Y.; Merilä, J. Evolution of anuran brains: Disentangling ecological and phylogenetic sources of variation. J. Evol. Biol. 2015, 28, 1986–1996. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Halliday, T. The Book of Frogs: A Life-Size Guide to Six Hundred Species from Around the World; University of Chicago Press: Chicago IL, USA, 2015. [Google Scholar]
- Orton, G.L. The systematics of vertebrate larvae. Syst. Zool. 1953, 2, 63–75. [Google Scholar] [CrossRef]
- Altig, R.; Johnson, G.F. Guilds of anuran larvae: Relationships among developmental modes, morphologies, and habitats. Herpetol. Monogr. 1989, 3, 81–109. [Google Scholar] [CrossRef]
- McDiarmid, R.W.; Altig, R. Research: Materials and techniques. In Tadpoles: The Biology of Anuran Larvae; McDiarmid, R.W., Altig, R., Eds.; University of Chicago Press: Chicago, IL, USA, 1999; pp. 7–23. [Google Scholar]
- Roelants, K.; Haas, A.; Bossuyt, F. Anuran radiations and the evolution of tadpole morphospace. Proc. Natl. Acad. Sci. USA 2011, 108, 8731–8736. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.M.; Womble, M.; Ledon-Rettig, C.; Hull, M.; Dickinson, A.; Nascone-Yoder, N. Budgett’s Frog (Leipdobatrachus laevis): New amphibian embryo for developmental biology. Dev. Biol. 2015, 405, 291–303. [Google Scholar] [CrossRef]
- Fabrezi, M.; Quinzio, S.I.; Goldberg, J.; Cruz, J.C.; Pereyra, M.C.; Wassersug, R.J. Developmental changes and novelties in ceratophryid frogs. Evodevo 2016, 7, 5. [Google Scholar] [CrossRef]
- Altig, R.; McDiarmid, R.W. Diversity: Familial and generic characterizations. In Tadpoles: The Biology of Anuran Larvae; McDiarmid, R.W., Altig, R., Eds.; University of Chicago Press: Chicago, IL, USA, 1999; pp. 295–337. [Google Scholar]
- Townsend, D.S.; Stewart, M.M. Reproductive ecology of the Puerto Rican frog Eleutherodactylus coqui. J. Herpetol. 1994, 28, 34–40. [Google Scholar] [CrossRef]
- Tyler, M. Gastric Brooding Frog; Croom Helm: London, UK, 1983. [Google Scholar]
- Deban, S.M.; Olson, W.M. Suction feeding by a tiny predator tadpole. Nature 2002, 420, 41–42. [Google Scholar] [CrossRef]
- Haas, A.; Pohlmeyer, J.; McLeod, D.S.; Kleinteich, T.; Hertwig, S.; Das, I.; Buchholz, D.R. Extreme tadpoles II: The highly derived larval anatomy of Occidozyga baluensis (Boulenger, 1896), an obligate carnivorous tadpole. Zoomorphism 2014, 133, 321–342. [Google Scholar] [CrossRef]
- Wassersug, R.J.; Heyer, W.R. A survey of internal oral features of Leptodactyloid larvae (Amphibia: Anura). Smithson. Contrib. Zool. 1988, 457, 1–99. [Google Scholar] [CrossRef]
- Savage, J.M. The tadpole of the Costa Rican Fringe-limbed Treefrog, Hyla fimbrimembra. Proc. Biol. Soc. Washing. 1981, 93, 1177–1183. [Google Scholar]
- Petranka, J.W.; Kats, L.B.; Sih, A. Predator- prey interactions among fish and larval amphibians: Use of chemical cues to detect predatory fish. Anim. Behav. 1987, 35, 420–425. [Google Scholar] [CrossRef]
- Hews, D.K. Alarm response in larval western toads, Bufo boreas: Release of larval chemicals by a natural predator and its effect on predator capture efficiency. Anim. Behav. 1988, 36, 125–133. [Google Scholar] [CrossRef]
- Hagman, M.; Shine, R. Understanding the toad code: Behavioural responses of Cane Toad (Chaunus marinus) larvae and metamorphs to chemical cues. Aust. Ecol. 2008, 33, 37–44. [Google Scholar] [CrossRef]
- Hagman, M.; Hayes, R.A.; Capon, R.J.; Shine, R. Alarm cues experienced by Cane Toad tadpoles affect post-metamorphic morphology and chemical defences. Func. Ecol. 2009, 23, 126–132. [Google Scholar] [CrossRef]
- Wilson, D.J.; Lefcort, H. The effect of predator diet on the alarm response of Red-legged Frog, Rana aurora, tadpoles. Anim. Behav. 1993, 46, 1017–1019. [Google Scholar] [CrossRef]
- Fraker, M.E.; Hu, F.; Vindhya, C.; McCollum, S.A.; Relyea, R.A.; Hempel, J.; Denver, R.J. Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm. Behav. 2009, 55, 520–529. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Chivers, D.P. Temporal variability, threat sensitivity and conflicting information about the nature of risk: Understanding the dynamics of tadpole antipredator behaviour. Anim. Behav. 2009, 78, 11–16. [Google Scholar] [CrossRef]
- Chivers, D.P.; Ferrari, M.C.O. Tadpole antipredator responses change over time: What is the role of learning and generalization? Behav. Ecol. 2013, 24, 1114–1121. [Google Scholar] [CrossRef]
- Chivers, D.P.; Ferrari, M.C.O. Social learning of predators by tadpoles: Does food restriction alter the efficacy of tutors as information sources? Anim. Behav. 2014, 89, 93–97. [Google Scholar] [CrossRef]
- Pollo-Cavia, N.; Gomez-Mestre, I. Learned recognition of introduced predators determines survival of tadpole prey. Func. Ecol. 2014, 28, 432–439. [Google Scholar] [CrossRef]
- Heemeyer, J.L.; Lannoo, M.J. Breeding migrations in Crawfish Frogs (Lithobates areolatus): Long-distance movements, burrow philopatry, and mortality in a near-threatened species. Copeia 2012, 2012, 440–450. [Google Scholar] [CrossRef]
- Heemeyer, J.L.; Williams, P.J.; Lannoo, M.J. Obligate crayfish burrow use and core habitat requirements of Crawfish Frogs. J. Wildl. Manag. 2012, 76, 1081–1091. [Google Scholar] [CrossRef]
- Bee, M.A.; Marshall, V.T.; Humfield, S.C.; Gerhardt, H.C. The role of learning in the formation of frog choruses. Integr. Comp. Biol. 2002, 42, 1193. [Google Scholar]
- Lannoo, M.J.; Townsend, D.S.; Wassersug, R.J. Larval life in the leaves: Arboreal tadpole types, with special attention to the morphology, ecology, and behavior of the oophagous Osteopilus brunneus (Hylidae) larva. Fieldiana (Zool.) Ser. 1987, 38, 1–31. [Google Scholar]
- Morey, S.R. Age and Size at Metamorphosis in Spadefoot Toads: A Comparative Study of Adaptation to Certain Environments. Ph.D. Thesis, University of California, Riverside, CA, USA, 1994. [Google Scholar]
- Morey, S.R. Scaphiopus couchii, Couch’s Spadefoot. In Amphibian Declines: The Conservation Status of United States Species; Lannoo, M.J., Ed.; University of California Press: Berkeley, CA, USA, 2005; pp. 508–511. [Google Scholar]
- Tinsley, R.C.; Tocque, K. The population dynamics of a desert anuran. Scaphiopus couchii. Aust. J. Ecol. 1995, 20, 376–384. [Google Scholar] [CrossRef]
- Cory, B.L. Life-history and behavior differences between ranids in isolated populations in the Sierra Nevada. Am. Zool. 1962, 2, 515. [Google Scholar]
- Bradford, D.F. Winterkill, oxygen relations, and energy-metabolism of a submerged dormant amphibian. Rana Muscosa Ecol. 1983, 64, 1171–1183. [Google Scholar] [CrossRef]
- Matthews, K.R.; Miaud, C. A skeletochronology study of the age structure, growth, and longevity of the Mountain Yellow-legged Frog, Rana muscosa, in the Sierra Nevada, California. Copeia 2007, 2007, 986–993. [Google Scholar] [CrossRef]
- Fellers, G.M.; Kleeman, P.M.; Miller, D.A.W.; Halstead, B.J.; Link, W.A. Population size, survival, growth, and movements of Rana sierrae. Herpetology 2013, 69, 147–162. [Google Scholar] [CrossRef]
- Feder, M.E.; Burggren, W.W. (Eds.) Environmental Physiology of the Amphibians; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Ewert, J.-P. The visual system of the toad: Behavioral and physiological studies on a pattern recognition system. In The Amphibian Visual System; Fite, K.V., Ed.; Academic Press: New York, NY, USA, 1976; pp. 142–202. [Google Scholar]
- Lannoo, M.J. Integration: Nervous and sensory systems. In Tadpoles: The Biology of Anuran Larvae; McDiarmid, R., Altig, R., Eds.; University of Chicago Press: Chicago, IL, USA, 1999; pp. 149–169. [Google Scholar]
- Roberts, A.; Hill, N.A.; Hicks, R. Simple mechanisms organize orientation of escape swimming in embryos and hatchlings of Xenopus laevis. J. Exp. Biol. 2000, 203, 1869–1885. [Google Scholar] [CrossRef]
- Bullock, T.H. Comparative neuroethology of startle rapid escape, and giant-fiber-mediated responses. In Neural Mechanisms of Startle Behavior; Eaton, R.C., Ed.; Springer: Berlin, Germany, 1978; pp. 1–13. [Google Scholar] [CrossRef]
- Rock, M.K. Functional properties of the Mauthner cell in the tadpole Rana catesbeiana. J. Neurophysiol. 1980, 44, 135–150. [Google Scholar] [CrossRef]
- Will, U. Amphibian Mauthner cells. Brain Behav. Evol. 1991, 37, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Zottoli, S.J.; Feiner, D.G.; Hering, J.R.; Faber, D.S. Behavioral recovery of fast startle responses after spinal cord crush in goldfish can occur in the absence of Mauthner cells. Soc. Neurosci. Abstr. 1998, 24, 309. [Google Scholar]
- Hughes, A. Metamorphic changes in the brain and spinal cord. In Frog Neurobiology: A Handbook; Llinás, R., Precht, W., Eds.; Springer: Berlin, Germany, 1976; pp. 856–863. [Google Scholar]
- Rugh, R. The Frog: Reproduction and Development; The Blakiston Company: Toronto, ON, Canada, 1951. [Google Scholar]
- Capranica, R.R. Auditory system. 17. Morphology and physiology of the auditory system. In Frog Neurobiology: A Handbook; Llinás, R., Precht, W., Eds.; Springer: Berlin, Germany, 1976; pp. 551–575. [Google Scholar]
- Boatwright-Horowitz, S.; Simmons, A.M. Transient “deafness” accompanies auditory development during metamorphosis from tadpole to frog. Proc. Natl. Acad. Sci. USA 1997, 94, 14877–14882. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.M. Brain development in anuran larvae after thyroid or pituitary removal. Endocrinology 1924, 8, 639–651. [Google Scholar] [CrossRef]
- Karpicke, J.D.; Coverdale, M.E. The adaptive value of forgetting. J. Appl. Res. Mem. Cogn. 2020, 9, 33–36. [Google Scholar] [CrossRef]
- Fawcett, J.M.; Hulbert, J.C. The many faces of forgetting: Toward a constructive view of forgetting in everyday life. J. Appl. Res. Mem. Cogn. 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Nørby, S. Why forget? On the adaptive value of memory loss. Perspect. Psychol. Sci. 2015, 10, 551–578. [Google Scholar] [CrossRef]
- Blumenfeld, H. Neuroanatomy Through Clinical Cases; Sinauer, Associates: Sunderland, MA, USA, 2021. [Google Scholar]
- Semon, R. Die Mneme als erhaltendes Prinzip in Wechsel des organischen Geschehens; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1904; We consulted the 1920 reissue. [Google Scholar]
- Calderon, J.; Perry, R.J.; Erzinclioglu, S.W.; Berrios, G.E.; Dening, T.R.; Hodges, J.R. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bussè, C.; Mitolo, M.; Mozzetta, S.; Venneri, A.; Cagnin, A. Impact of Lewy bodies disease on visual skills and memory abilities: From prodromal stages to dementia. Front. Psychiatry 2024, 15, 1461620. [Google Scholar] [CrossRef]
- Rainville, P.; Duncan, G.H.; Price, D.D.; Carrier, B.; Bushnell, M.C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997, 277, 968–971. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kensinger, E.A.; Schacter, D.L. Memory and emotion. In Handbook of Emotions; Lewis, M., Haviland-Jones, J.M., Barrett, L.F., Eds.; Guilford Press: New York, NY, USA, 2008; pp. 601–617. [Google Scholar]
- Brox, A.; Puelles, L.; Ferreiro, B.; Medina, L. Expression of the genes Emx1, Tbr1, and Eomes (Tbr2) in the telencephalon of Xenopus laevis confirms the existence of ventral pallial division in all tetrapods. J. Comp. Neurol. 2004, 474, 562–577. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Kicliter, E.E. Organization of the amphibian telencephalon. In Comparative Neurology of the Telencephalon; Ebbesson, S.O.E., Ed.; Plenum Press: New York, NY, USA, 1980; pp. 203–255. [Google Scholar]
- ten Donkelaar, H.J. Chapter 19: Anurans. In The Central Nervous System of Vertebrates; Nieuwenhuys, R., ten Donkelaar, H.J., Nicholson, C., Eds.; Springer: Berlin, Germany, 1998; Volume 2, pp. 1151–1314. [Google Scholar]
- Roth, G. The Long Evolution of Brains and Minds; Springer: Berlin, Germany, 2013. [Google Scholar]
- Northcutt, R.G.; Ronan, M. Afferent and efferent connections of the bullfrog medial pallium. Brain Behav. Evol. 1992, 40, 1–16. [Google Scholar] [CrossRef]
- Papini, M.R.; Muzio, R.N.; Segura, E.T. Instrumental learning in toads (Bufo arenarum): Reinforcer magnitude and medial pallium. Brain Behav. Evol. 1995, 46, 61–71. [Google Scholar] [CrossRef]
- Westhoff, G.; Roth, G. Morphology and projection pattern of medial and dorsal pallial neurons in the frog Discoglossus pictus and the salamander Plethodon jordani. J. Comp. Neurol. 2002, 445, 97–121. [Google Scholar] [CrossRef]
- Sotelo, M.I.; Daneri, M.F.; Bingman, V.P.; Muzio, R.N. Amphibian spatial cognition, medial pallium and other supporting telencephalic structures. Neurosci. Biobehav. Rev. 2024, 163, 105739. [Google Scholar] [CrossRef]
- Roth, G.; Laberge, F.; Mühlenbrock-Lenter, S.; Grunwald, W. Organization of the pallium in the fire-bellied toad Bombina orientalis. I. Morphology and axonal projection pattern of neurons revealed by intracellular biocytin labeling. J. Comp. Neurol. 2007, 501, 443–464. [Google Scholar] [CrossRef]
- Bachy, I.; Berthon, J.; Reótaux, S. Defining pallial and subpallial divisions in the developing Xenopus forebrain. Mech. Dev. 2002, 117, 163–172. [Google Scholar] [CrossRef]
- Endepols, H.; Roden, K.; Walkowiak, W. Hodological characterization of the septum in anuran amphibians: II. Efferent connections. J. Comp. Neurol. 2005, 483, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Roden, K.; Endepols, H.; Walkowiak, W. Hodological characterization of the septum in anuran amphibians: I. Afferent connections. J. Comp. Neurol. 2005, 483, 415–436. [Google Scholar] [CrossRef] [PubMed]
- Lanuza, E.; Martiónez-Garcióa, F. Evolution of septal nuclei. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin, Germany, 2009; pp. 1270–1278. [Google Scholar]
- Vesselkin, N.P.; Ermakova, N.B.; Kenigfest, N.B.; Goikovic, M. The striatal connections in frog, Rana temporaria. An HRP study. J. Für Hirnforsch. 1980, 26, 365–383. [Google Scholar]
- Endepols, H.; Roden, K.; Luksch, H.; Dicke, U.; Walkowiak, W. Dorsal striatopallidal system in anurans. J. Comp. Neurol. 2004, 468, 299–310. [Google Scholar] [CrossRef]
- Maier, S.; Walkowiak, W.; Luksch, H.; Endepols, H. An indirect basal ganglia pathway in anuran amphibians? J. Chem. Neuroanat. 2010, 40, 21–35. [Google Scholar] [CrossRef]
- Stephenson-Jones, M.; Samuelsson, E.; Ericsson, J.; Robertson, B.; Grillner, S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol. 2011, 21, 1081–1091. [Google Scholar] [CrossRef]
- Loonen, A.J.M.; Ivanova, S.A. Circuits regulating pleasure and happiness: The evolution of the amygdalar-hippocampal- habenular connectivity in vertebrates. Front. Neurosci. 2016, 10, 00539. [Google Scholar] [CrossRef]
- Moreno, N.; Gonzaólez, A. Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio-amniotic transition. J. Anat. 2007, 211, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Gonzaólez, A. Hodological characterization of the medial amygdala in anuran amphibians. J. Comp. Neurol. 2003, 466, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Gonzalez, A. Localization and connectivity of the lateral amygdala in anuran amphibians. J. Comp. Neurol. 2004, 479, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Gonzalez, A. Central amygdala in anuran amphibians: Neurochemical organization and connectivity. J. Comp. Neurol. 2005, 489, 69–91. [Google Scholar] [CrossRef]
- Loópez, J.M.; Morona, R.; Gonzaólez, A. Immunohistochemical localization of DARPP-32 in the brain of two lungfishes: Further assessment of its relationship with the dopaminergic system. Brain Behav. Evol. 2017, 90, 289–310. [Google Scholar] [CrossRef]
- Jackson, M.E.; Moghaddam, B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. Neurosci. J. 2001, 21, 676–681. [Google Scholar] [CrossRef]
- Marión, O.; Gonzaólez, A.; Smeets, W.J. Basal ganglia organization in amphibians: Afferent connections of the striatum and the nucleus accumbens. J. Comp. Neurol. 1997, 378, 16–49. [Google Scholar] [CrossRef]
- Marín, O.; González, A.; Smeets, W.J. Basal ganglia organization in amphibians: Efferent connections of the striatum and the nucleus accumbens. J. Comp. Neurol. 1997, 380, 23–50. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion: Clues from the brain. Ann. Rev. Psych. 1995, 46, 209–235. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Ann. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Burmeister, S.S. Brain-behavior relationships of cognition in vertebrates: Lessons from amphibians. Chapter 3. Adv. Study Behav. 2022, 54, 109–127. [Google Scholar] [CrossRef]
- Burmeister, S.S. Ecology, cognition, and the hippocampus: A tale of two frogs. Brain Behav. Evol. 2022, 97, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Laberge, F.; Mühlenbrock-Lenter, S.; Grunwald, W.; Roth, G. Evolution of the amygdala: New insights from studies in amphibians. Brain Behav. Evol. 2006, 67, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Laberge, F.; Roth, G. Is there a structure equivalent to the mammalian basolateral amygdaloid complex in amphibians? J. Anat. 2007, 211, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Palyanov, A.; Koutsikou, S.; Li, W.; Soffe, S.; Roberts, A.; Borisyuk, R. From decision to action: Detailed modelling of frog tadpoles reveals neuronal mechanisms of decision-making and reproduces unpredictable swimming movements in response to sensory signals. PLoS Comput. Biol. 2021, 17, e1009654. [Google Scholar] [CrossRef]
- O’KEefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Knierim, J.J. Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus 2013, 23, 253–267. [Google Scholar] [CrossRef]
- Knierim, J.J.; Hamilton, D.A. Framing spatial cognition: Neural representations of proximal and distal frames of reference and their roles in navigation. Physiol Rev. 2011, 91, 1245–1279. [Google Scholar] [CrossRef]
- Deshe, N.; Eliezer, Y.; Hoch, L.; Itskovits, E.; Bokman, E.; Ben-Ezra, S.; Zaslaver, A. Inheritance of associative memories and acquired cellular changes in C. elegans. Nat. Commun. 2023, 14, 4232. [Google Scholar] [CrossRef]
- Williams, J.T., Jr. A test for dominance of cues during maze learning by toads. Psychon. Sci. 1967, 9, 259–260. [Google Scholar] [CrossRef]
- Miller, R.R.; Berk, A.M.; Springer, A.D. Acquisition and retention of active avoidance in Xenopus lavevis. Psychon. Bull. Rev. 1974, 3, 139–141. [Google Scholar] [CrossRef]
- Harvey, C.B.; Ellis, C.; Tate, M. Inhibition of the righting reflex in the Common Bullfrog (Rana catesbiana) employing an operant-avoidance procedure. Psychon. Bull. Rev. 1976, 7, 57–58. [Google Scholar] [CrossRef]
- Alberini, C. (Ed.) Memory Reconsolidation; Academic Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Sotelo, M.I.; Florencia Daneri, M.; Bingman, V.P.; Muzio, R.N. 2016. Telencephalic neuronal activation associated with spatial memory in the terrestrial toad Rhinella arenarum: Participation of the medial pallium during navigation by geometry. Brain Behav. Evol. 2016, 88, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Shaykevich, D.A.; Pareja-Mejía, D.; Golde, C.; Pašukonis, A.; O’Connell, L.A. Neural and sensory basis of homing behavior in the invasive Cane Toad, Rhinella marina. Proc. Biol. Sci. 2025, 292, 20250045. [Google Scholar] [CrossRef]
- Crane, A.L.; Mathis, A. Landmark learning by the Ozark Zigzag Salamander, Plethodon angusticlavius. Curr. Zool. 2025, 57, 485–490. [Google Scholar] [CrossRef]
- Crump, M.L. Parental care among the Amphibia. Adv. Study Behav. 1996, 25, 109–144. [Google Scholar] [CrossRef]
- AmphibiaWeb. Available online: https://amphibiaweb.org/amphibian/facts.html (accessed on 2 May 2025).
- Lannoo, M.J.; Stiles, R.M. Uncovering shifting amphibian ecological relationships in a world of environmental change. Herpetologica 2020, 76, 144–152. [Google Scholar] [CrossRef]
- Fabbro, F.; Aglioti, S.N.; Bergamasca, M.; Clarici, A.; Panksepp, J. Evolutionary aspects of self- and world consciousness in vertebrates. Front. Hum. Neurosci. 2015, 9, 157. [Google Scholar] [CrossRef]
- Lannoo, M.J.; Stiles, R.M. The use of cognition by amphibians confronting environmental change: Examples from the behavioral ecology of Crawfish Frogs (Rana areolata). Animals 2025, 15, 736. [Google Scholar] [CrossRef]
- Wilczynski, W.; Chu, J. Acoustic communication, endocrine control, and the neurochemical systems of the brain. In Anuran Communication; Ryan, M.J., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 23–25. [Google Scholar]
- Zornik, E.; Kelly, D.B. A neuroendocrine basis for the hierarchical control of frog courtship vocalizations. Front. Neuroendocrinol. 2011, 32, 353–366. [Google Scholar] [CrossRef]
- Kelley, D.B. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science 1980, 207, 553–555. [Google Scholar] [CrossRef]
- Liu, Q.; Debski, E.A. Origins of serotonin-like immunoreactivity in the optic tectum of Rana pipiens. J. Comp. Neurol. 1995, 352, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Adli, D.S.; Rosenthal, B.M.; Yuen, G.L.; Ho, R.H.; Cruce, W.L. Immunohistochemical localization of substance P, somatostatin, enkephalin, and serotonin in the spinal cord of the northern leopard frog, Rana pipiens. J. Comp. Neurol. 1988, 275, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Marín, O.; Smeets, W.J.A.J.; González, A. Basal ganglia organization in amphibians: Chemoarchitecture. J. Comp. Neurol. 1998, 392, 285–312. [Google Scholar] [CrossRef]
- Zhao, Y.; Debski, E.A. Serotonergic reticular formation cells in Rana pipiens: Categorization, development, and tectal projections. J. Comp. Neurol. 2005, 487, 441–456. [Google Scholar] [CrossRef]
- Sanchez-Camancho, C.; Marín, O.; Smeets, W.J.; ten Donkelaar, H.J.; González, A. Descending supraspinal pathways in amphibians. II. Distribution and origin of the catecholaminergic innervation of the spinal cord. J. Comp. Neurol. 2001, 434, 209–232. [Google Scholar] [CrossRef]
- Beyts, C.; Cella, M.; Colegrave, N.; Downie, R.; Martin, J.G.A.; Walsh, P. The effect of heterospecific and conspecific competition on inter-individual differences in Tungara Frog tadpole (Engystomps pustulosus) behavior. Behav. Ecol. 2023, 34, 210–222. [Google Scholar] [CrossRef]
- Mettke-Hofmann, C. 2014. Cognitive ecology: Ecological factors, life-styles, and cognition. WIREs Cogn. Sci. 2014, 5, 345–360. [Google Scholar] [CrossRef]
- Roberts, A.; Li, W.-C.; Soffe, S.R. How neurons generate behavior in a hatchling amphibian tadpole: An outline. Front. Behav. Neurosci. 2010, 4, 1535. [Google Scholar] [CrossRef]
- Van Buskirk, J.; McCollum, S.A. Functional mechanisms of an inducible defence in tadpoles: Morphology and behavior influence mortality risk from predation. J. Evol. Biol. 2000, 13, 336–347. [Google Scholar] [CrossRef]
- Relyea, R.A. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 2001, 82, 523–540. [Google Scholar] [CrossRef]
- Eterovick, P.C.; Kloh, J.S.; Figueredo, C.C.; Macaório Viana, P.I.; Marcella Goulart, M.; Milan, D.T.; Fonseca, M.B.; Martins, ó.M.; Pinheiro, L.T.; QuintaÃo, R.P.; et al. Background choice and immobility as context dependent tadpole responses to perceived predation risk. Sci. Rep. 2020, 10, 13577. [Google Scholar] [CrossRef]
- Engbrecht, N.J.; Heemeyer, J.L.; Murphy, C.G.; Stiles, R.M.; Swan, J.W.; Lannoo, M.J. Upland calling behavior in Crawfish Frogs (Lithobates areolatus) and calling triggers caused by noise pollution. Copeia 2015, 103, 1046–1057. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Gans, C. [Review of] Frog Neurobiology: A Handbook. Edited by R. Llinás & W. Precht. Quart. Rev. Biol. 1977, 52, 450. [Google Scholar]
- Liu, Y.; Day, L.B.; Summers, K.; Burmeister, S.S. A cognitive map in a Poison Frog. J. Exp. Biol. 2019, 222, jeb197467. [Google Scholar] [CrossRef] [PubMed]
- Manzano, A.S.; Herrel, A.; Fabre, A.-C.; Abdala, V. Variation in brain anatomy in frogs and its possible bearing on their locomotor ecology. J. Anat. 2017, 231, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.M.; Nol, E.; Boire, D. Brain regions and encephalization in anurans: Adaptations or stability. Brain Behav. Evol. 1995, 45, 96–109. [Google Scholar] [CrossRef]
- Tinbergen, N. The Study of Instinct; Oxford University Press: Oxford, UK, 1953. [Google Scholar]
- von Uexküll, V.J. Umwelt und Innenwelt der Tiere; Springer: Berlin, Germany, 1909; pp. 259. Science 1909, 31, 303–305. [Google Scholar]
- Lorenz, K. Vergleichende Verhaltensforschung. Zool. Anz. Suppl. Bd. 1939, 12, 69–102. [Google Scholar]
- Marler, P.R.; Hamilton, W.J. Mechanisms of Animal Behavior; John Wiley and Sons: New York, NY, USA, 1966. [Google Scholar]
- Ewert, J.-P. The neural basis of visually guided behavior. Sci. Am. 1974, 230, 34–42. [Google Scholar] [CrossRef]
- Eysel, U.T.; Grüsser, O.-J. Neurophysiological basis of pattern recognition in the cat’s visual system. In Zeichenerkennung durch biologische und technische Systeme/Pattern Recognition in Biological and Technical Systems; Grüsser, O.J., Klinke, R., Eds.; Springer: Berlin, Germany, 1971. [Google Scholar] [CrossRef]
- James, W. Principles of Psychology; Pantianos Classics: Columbia, SC, USA, 1980. [Google Scholar]
- Simmons, A.M.; Buxbaum, R.C. Neural codes for “pitch” processing in a unique auditory system. In Neuroethological Studies of Cognitive and Perceptual Processes; Moss, C.F., Shettleworth, S.J., Eds.; Westview Press: Boulder, CO, USA, 1996; pp. 185–228. [Google Scholar]
- Howard, R.D. The evolution of mating strategies in Bullfrogs, Rana catesbeiana. Evolution 1978, 32, 850–871. [Google Scholar] [CrossRef]
- Howard, R.D. Alternative mating behaviors of young American Bullfrogs. Am. Zool. 1984, 24, 397–406. [Google Scholar] [CrossRef]
- Perrill, S.A.; Gerhardt, H.C.; Daniel, R. Mating strategy shifts in male Green Treefrogs (Hyla cinerea): An experimental study. Anim. Behav. 1982, 30, 43–48. [Google Scholar] [CrossRef]
- Fritzsch, B.; Neary, T.J. The octavolateralis system of mechanosensory and electrosensory organs. In Amphibian Biology: Volume 3, Sensory Perception; Heatwole, H., Ed.; Surrey Beatty & Sons: New South Wales, Australia, 1998; pp. 878–922. [Google Scholar]
- Box, G.E.P.; Draper, R. Empirical Model-Building and Response Surfaces; John Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lannoo, M.J.; Stiles, R.M. Do Adult Frogs Remember Their Lives as Tadpoles and Behave Accordingly? A Consideration of Memory and Personality in Anuran Amphibians. Diversity 2025, 17, 506. https://doi.org/10.3390/d17080506
Lannoo MJ, Stiles RM. Do Adult Frogs Remember Their Lives as Tadpoles and Behave Accordingly? A Consideration of Memory and Personality in Anuran Amphibians. Diversity. 2025; 17(8):506. https://doi.org/10.3390/d17080506
Chicago/Turabian StyleLannoo, Michael J., and Rochelle M. Stiles. 2025. "Do Adult Frogs Remember Their Lives as Tadpoles and Behave Accordingly? A Consideration of Memory and Personality in Anuran Amphibians" Diversity 17, no. 8: 506. https://doi.org/10.3390/d17080506
APA StyleLannoo, M. J., & Stiles, R. M. (2025). Do Adult Frogs Remember Their Lives as Tadpoles and Behave Accordingly? A Consideration of Memory and Personality in Anuran Amphibians. Diversity, 17(8), 506. https://doi.org/10.3390/d17080506





