“Winners” and “Losers” of the Bivalve Evolution
Abstract
1. Introduction
2. Materials and Methods
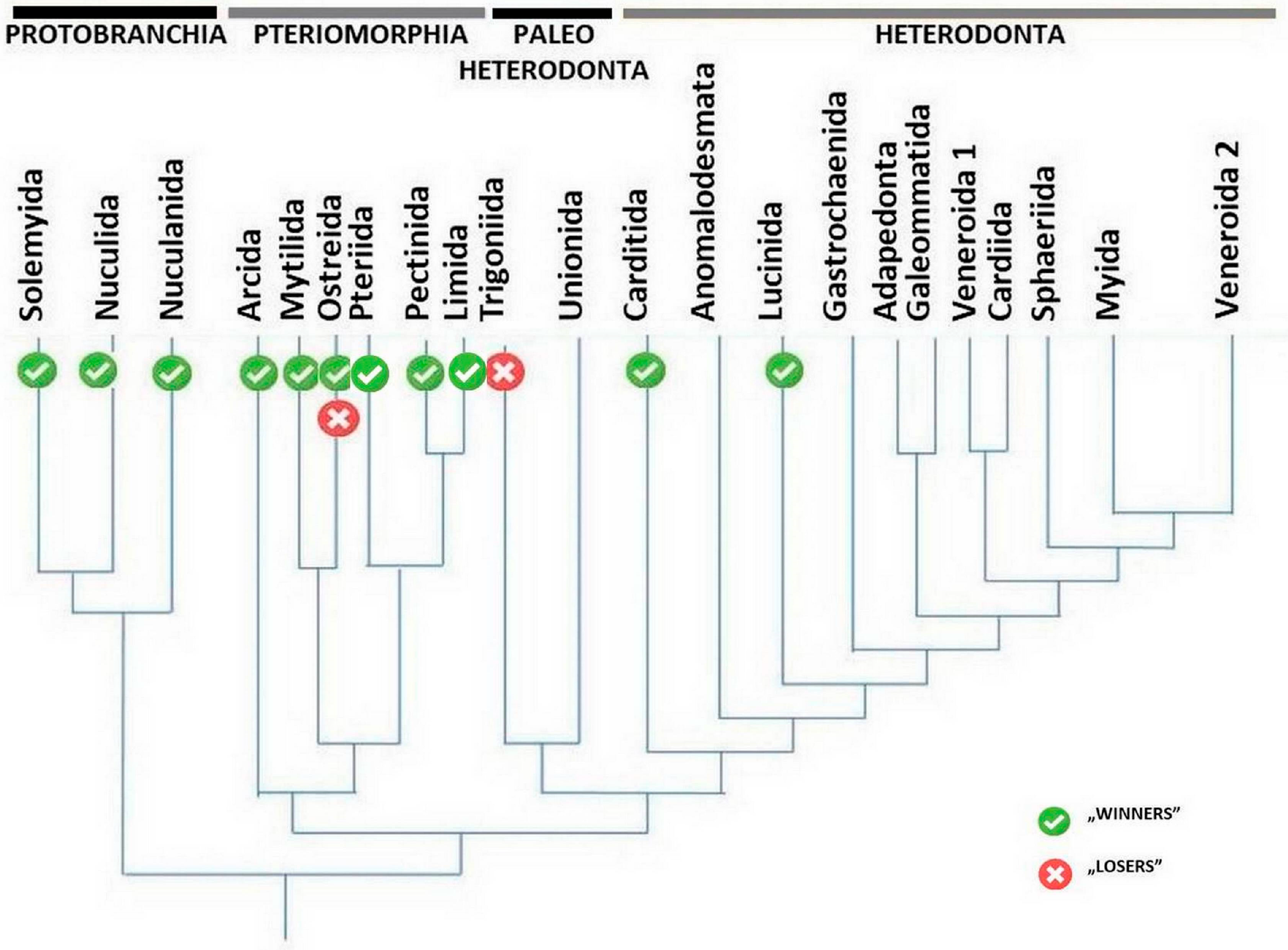
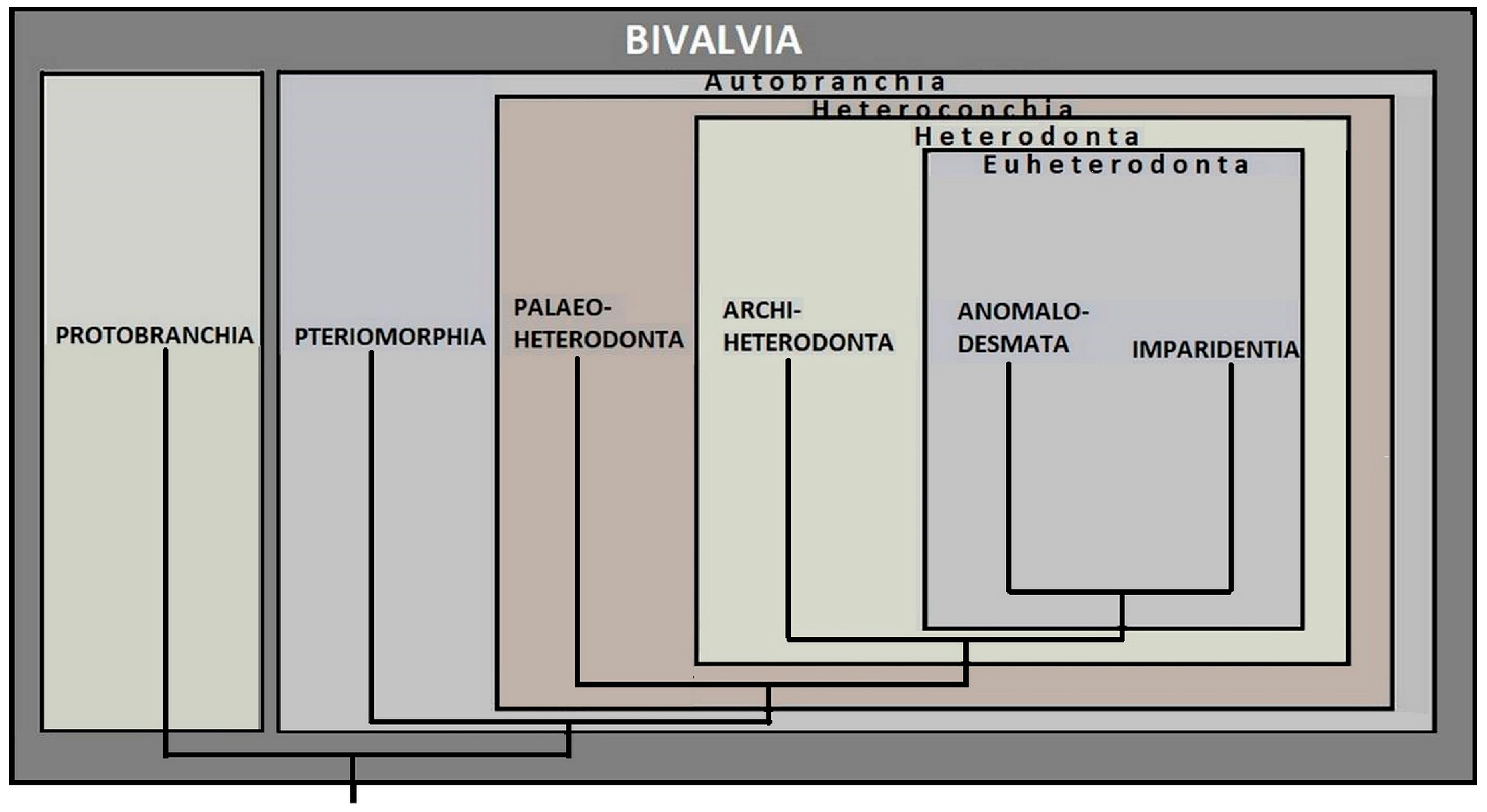
3. Bivalve Survivors
3.1. Order Nuculida Dall, 1889
3.2. Order Solemyida Dall, 1889
3.3. Order Nuculanida J. G. Carter, D. C. Campbell & M. R. Campbell, 2000
3.4. Order Arcida Stoliczka, 1871
3.5. Order Mytilida A. Férussac, 1822
3.6. Order Ostreida A. Férussac, 1822
3.7. Order Pectinida Gray, 1854
3.8. Order Limida Moore, 1952
3.9. Order Carditida Dall, 1889
3.10. Lucinida Gray, 1854
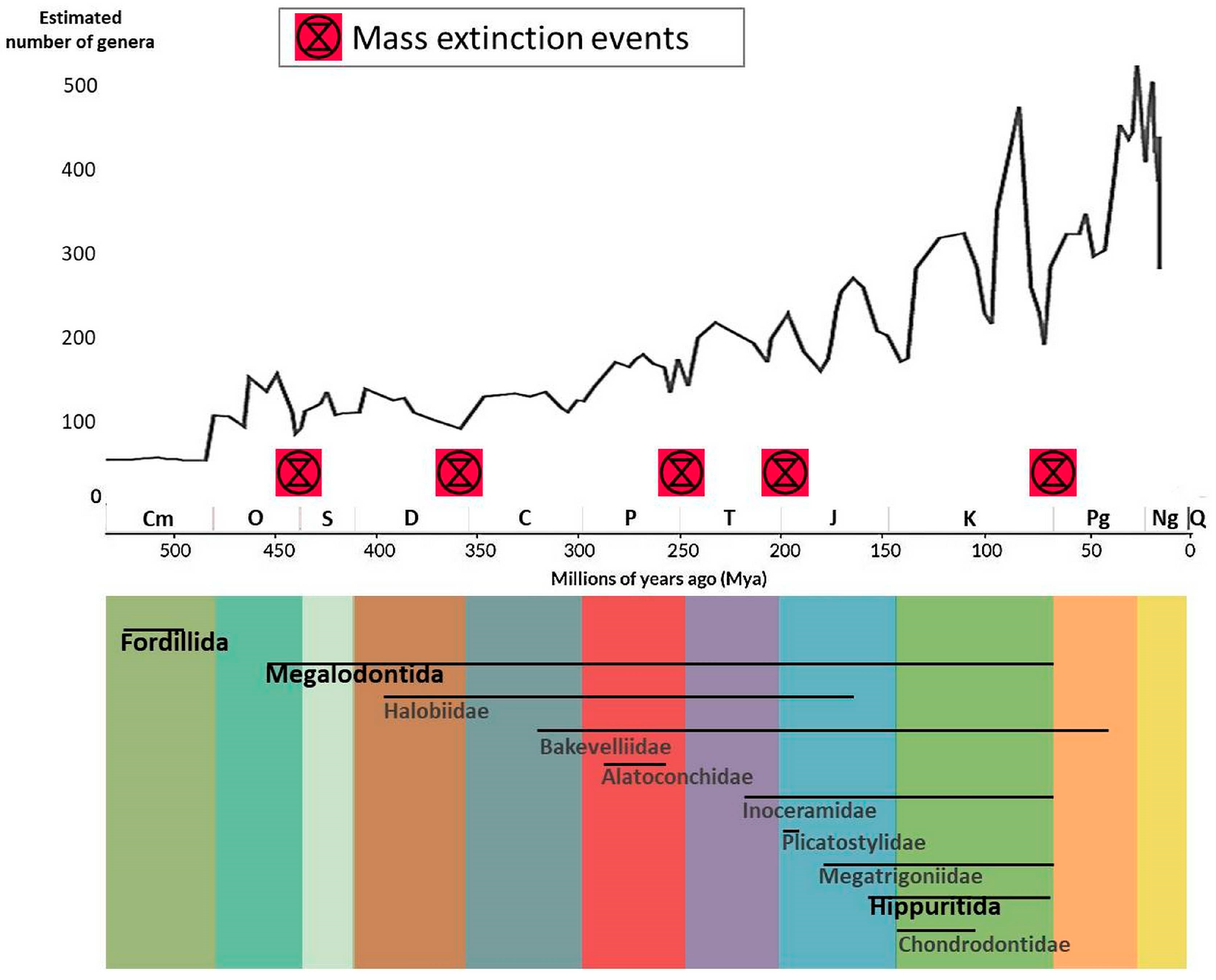
4. Extinct Bivalve Orders
4.1. Order Fordillida Pojeta, 1975
4.2. Order Megalodontida Starobogatov, 1992
4.3. Order Myalinida Paul, 1939
4.3.1. Family Alatoconchidae Termier et al., 1973
4.3.2. Family Inoceramidae Giebel, 1852
4.4. Order Hippuritida Newell, 1965
5. Extinct Bivalve Families from Still-Present Orders
5.1. Order Trigoniida Dall, 1889
Family Megatrigoniidae Van Hoepen, 1929
5.2. Order Ostreida A. Férussac, 1822
5.2.1. Family Halobiidae Kittl, 1912
5.2.2. Family Bakevelliidae King, 1850
5.2.3. Family Plicatostylidae Lupher & Packard, 1929 (Cochlearitidae Benini & Loriga, 1977)
5.2.4. Family Chondrodontidae Freneix, 1960
6. Discussion
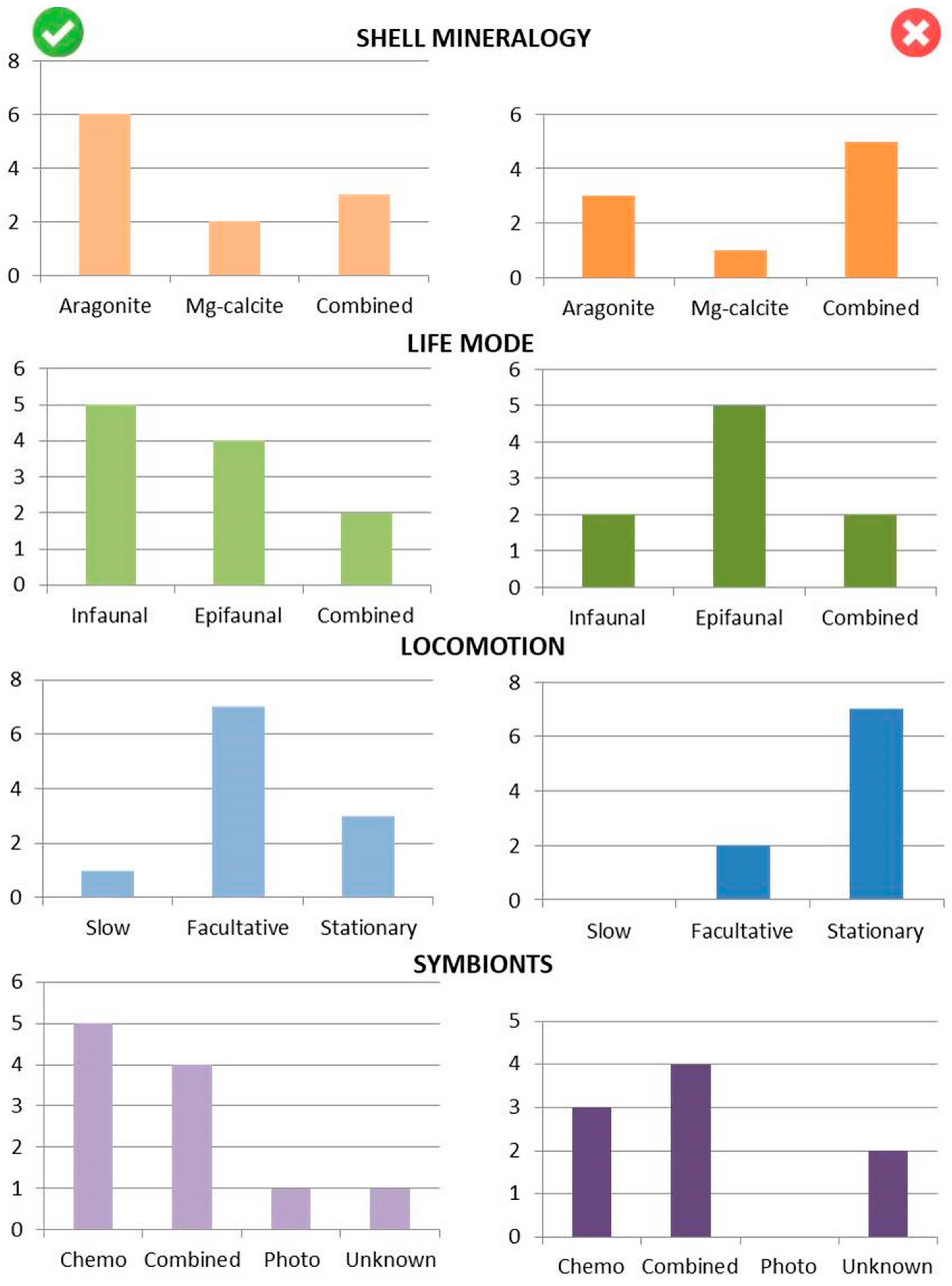
6.1. Mode of Life of the Successful vs. Extinct Bivalve Taxa
6.2. Extinctions and Their Victims
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linnaeus, C. Systema Naturae, Sive Regna Tria Naturae Systematice Proposita per Classes, Ordines, Genera, & Species; Haak: Leiden, The Netherlands, 1735; pp. 1–12. [Google Scholar]
- Linnaeus, C. Systema Naturæ per Regna Tria Naturæ, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis, 10th ed.; Impensis Direct. Laurentii Salvii; Holmiae: Stockholm, Sweden, 1758; pp. 1–824. [Google Scholar]
- da Costa, E.M. Elements of Conchology: Or, an Introduction to the Knowledge of Shells; Benjamin White: London, UK, 1776. [Google Scholar]
- Deshayes, G.-P. Mollusques. In Expédition Scientifique de Morée. Section des Sciences Physiques. Tome III.—1.re Partie. Zoologie. Primière Section—Animaux Vertrébrés, Mollusques et Polypiers; Geoffroy Saint-Hilaire, I., Geoffroy Saint-Hilaire, E., Deshayes, G.-P., Bibron, G., Bory de Saint-Vincent, J.B.M.G., Eds.; F.G. Levrault: Paris, France, 1835. [Google Scholar]
- Gray, J.E. Additions and corrections to the arrangement of the families of bivalve shells. Ann. Mag. Nat. Hist. 1854, 14, 21–28. [Google Scholar] [CrossRef]
- Hörnes, M. Die fossilen Mollusken des Tertiär-Beckens von Wien. II. Bivalven; Abhandlungen der kaiserlich-königlichen geologischen Reichsanstalt; Aus der K.K.; Hof-und Staatsdruckerei: Wien, Austria, 1870; Volume 4, pp. 1–479. [Google Scholar]
- Bouchet, P.; Rocroi, J.P.; Bieler, R.; Carter, J.G.; Coan, E.V. Nomenclator of Bivalve Families with a Classification of Bivalve Families. Malacologia 2010, 52, 1–184. [Google Scholar] [CrossRef]
- Carter, J.G.; Altaba, C.R.; Anderson, L.R.; Araujo, R.; Biakov, A.S.; Bogan, A.E.; Campbell, D.C.; Campbell, M.; Chen, J.-h.; Cope, J.C.W.; et al. A synoptical classification of the Bivalvia (Mollusca). Paleontol. Contrib. 2011, 4, 1–47. [Google Scholar] [CrossRef]
- Rueda, J.; Urra, J.; Gofas, S.; Lopez-Gonzalez, N.; Fernandez-Salas, L.; Diaz-Del-Rio, V. New records of recently described chemosymbiotic bivalves for mud volcanoes within the European waters (Gulf of Cádiz). Mediterr. Mar. Sci. 2012, 13, 262–267. [Google Scholar] [CrossRef]
- Sharma, P.P.; Zardus, J.D.; Boyle, E.E.; González, V.L.; Jennings, R.M.; McIntyre, E.; Wheeler, W.C.; Etterc, R.J.; Giribet, G. Into the deep: A phylogenetic approach to the bivalve subclass Protobranchia. Mol. Phylogenet. Evol. 2013, 69, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Bieler, R.; Mikkelsen, P.M.; Collins, T.M.; Glover, E.A.; González, V.L.; Graf, D.L.; Harper, E.M.; Healy, J.; Kawauchi, G.Y.; Sharma, P.P.; et al. Investigating the Bivalve Tree of Life—An exemplar-based approach combining molecular and novel morphological characters. Invertebr. Syst. 2014, 28, 32–115. [Google Scholar] [CrossRef]
- González, V.L.; Andrade, S.C.; Bieler, R.; Collins, T.M.; Dunn, C.W.; Mikkelsen, P.M.; Taylor, J.D.; Giribet, G. A phylogenetic backbone for Bivalvia: An RNA-seq approach. Proc. Biol. Sci. 2015, 282, 20142332. [Google Scholar] [CrossRef] [PubMed]
- Combosch, D.J.; Collins, T.M.; Glover, E.A.; Graf, D.L.; Harper, E.M.; Healy, J.M.; Kawauchi, G.Y.; Lemer, S.; McIntyre, E.; Strong, E.E.; et al. A family-level Tree of Life for bivalves based on a Sanger-sequencing approach. Mol. Phylogenet. Evol. 2017, 107, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Formaggioni, A.; Plazzi, F.; Passamonti, M. Mito-nuclear coevolution and phylogenetic artifacts: The case of bivalve mollusks. Sci. Rep. 2022, 12, 11040. [Google Scholar] [CrossRef] [PubMed]
- Giribet, G. Bivalvia. In Phylogeny and Evolution of the Mollusca; Ponder, W.F., Lindberg, D.R., Eds.; University of California Press: Oakland, CA, USA, 2008; pp. 105–142. [Google Scholar]
- Digital Atlas of Ancient Life. Available online: https://www.digitalatlasofancientlife.org/learn/mollusca/bivalvia/classification (accessed on 6 July 2025).
- Stanley, S.M. Functional morphology and evolution of byssally attached bivalve Mollusks. J. Paleontol. 1972, 46, 165–212. [Google Scholar]
- Stanley, S.M. Aspects of the adaptive morphology and evolution of the Trigoniidae. Philos. Trans. R. Soc. Lond. 1978, B284, 247–258. [Google Scholar]
- Cope, J.C.W. The early evolution of the Bivalvia. In Origin and Evolutionary Radiation of the Mollusca; Taylor, J.D., Ed.; Oxford University Press: Oxford, UK, 1995; pp. 361–370. [Google Scholar]
- Zong-Jie, F. An introduction to Ordovician bivalves of southern China, with a discussion of the early evolution of the Bivalvia. Geol. J. 2006, 41, 303–328. [Google Scholar] [CrossRef]
- Ros, S.; De Renzi, M.; Damborenea, S.E.; Márquez-Aliaga, A. Treatise Online no. 39: Part N, Revised, Volume 1, Chapter 25: Early Triassic–Early Jurassic bivalve diversity dynamics. Treatise Online 2012, 1, 1–19. [Google Scholar] [CrossRef][Green Version]
- Zhou, S.; Stewart, M.E.; Collins, K.S.; Crousch, N.M.A.; Jablonski, D. Cambrian origin but no early burst in functional disparity for Class Bivalvia. Biol. Lett. 2023, 19, 20230157. [Google Scholar] [CrossRef] [PubMed]
- Echevarría, J.; Ros-Franch, S. Biogeographic response to major extinction events: The case of Triassic bivalves. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 638, 112053. [Google Scholar] [CrossRef]
- Seilacher, A. Der Beginn des Kambriums als biologische Wende. Neues Jahrb. Für Geol. Und Paläontologie 1956, 103, 155–180. [Google Scholar]
- Erwin, D.H. Metazoan phylogeny and the Cambrian radiation. Trends Ecol. Evol. 1991, 6, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, D. Current understanding on the Cambrian Explosion: Questions and answers. PalZ 2021, 95, 641–660. [Google Scholar] [CrossRef]
- Valentine, J.M. Patterns of taxonomic and ecological structure of the shelf benthos during Phanerozoic time. Palaeontology 1969, 12, 684–709. [Google Scholar]
- Sepkoski, J.J., Jr. A kinetic model for Phanerozoic taxonomic diversity: I. Analysis of marine orders. Paleobiology 1978, 4, 223–251. [Google Scholar] [CrossRef]
- Servais, T.; Harper, D.A.T. The Great Ordovician Biodiversification Event (GOBE): Definition, concept and duration. Lethaia 2018, 51, 151–164. [Google Scholar] [CrossRef]
- Skelton, P.W.; Crame, J.A.; Morris, N.J.; Harper, E.M. Adaptive divergence and taxonomic radiation in post-Palaeozoic bivalves. In Major Evolutionary Radiations; Taylor, P.D., Larwood, G.P., Eds.; Clarendon Press: Oxford, UK, 1990; pp. 91–117. [Google Scholar]
- Harnik, P.G. Direct and indirect effects of biological factors on extinction risk in fossil bivalves. Proc. Natl. Acad. Sci. USA 2011, 108, 13594–13599. [Google Scholar] [CrossRef] [PubMed]
- Ros, S.; Echevarría, J. Bivalves and evolutionary resilience: Old skills and new strategies to recover from the P/T and T/J extinction events. Hist. Biol. 2011, 23, 411–429. [Google Scholar] [CrossRef]
- Crisp, M.D.; Cook, L.G. Phylogenetic niche conservatism: What are the underlying evolutionary and ecological causes? New Phytol. 2012, 196, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Edie, S.M.; Collins, K.S.; Crouch, N.M.A.; Roy, K.; Jablonski, D. Diversity, distribution and intrinsic extinction vulnerability of exploited marine bivalves. Nat. Commun. 2023, 14, 4639. [Google Scholar] [CrossRef] [PubMed]
- Bretsky, P.W. Evolutionary patterns in the Paleozoic Bivalvia: Documentation and some theoretical considerations. Geol. Soc. Am. Bull. 1973, 84, 2079–2096. [Google Scholar] [CrossRef]
- MacLeod, K.G.; Hope, K.A. Evidence that Inoceramid bivalves were benthic and harbored chemosynthetic symbionts. Geology 1992, 20, 117–120. [Google Scholar] [CrossRef]
- Stewart, F.J.; Cavanaugh, C.M. Bacterial endosymbioses in Solemya (Mollusca: Bivalvia)-model systems for studies of symbiont-host adaptation. Antonie Van Leeuwenhoek 2006, 90, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Dubilier, N.; Bergin, C.; Lott, C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat. Rev. Microbiol. 2008, 6, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Duperron, S.; Halary, S.; Lorion, J.; Sibuet, M.; Gaill, F. Unexpected co-occurrence of six bacterial symbionts in the gills of the cold seep mussel Idas sp. (Bivalvia: Mytilidae). Environ. Microbiol. 2008, 10, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Glover, E.A. Chemosymbiotic Bivalves. In The Vent and Seep Biota; Kiel, S., Ed.; Topics in Geobiology; Springer: Dordrecht, The Netherlands, 2010; Volume 33, pp. 107–135. [Google Scholar] [CrossRef]
- Oliver, G.; Taylor, J.D. Bacterial symbiosis in the Nucinellidae (Bivalvia: Solemyida) with descriptions of two new species. J. Molluscan Stud. 2012, 78, 81–91. [Google Scholar] [CrossRef]
- Vermeij, G.J. The evolution of molluscan photosymbioses: A critical appraisal. Biol. J. Linn. Soc. 2013, 109, 497–511. [Google Scholar] [CrossRef]
- Kirkendale, L.; Paulay, G. Treatise Online no. 89: Part N, Revised, Volume 1, Chapter 9: Photosymbiosis in Bivalvia. Treatise Online 2017, 89, 1–39. [Google Scholar] [CrossRef]
- Hughes, I.V.; Girguis, P.R. A molluscan class struggle: Exploring the surprisingly uneven distribution of chemosymbiosis among two major mollusk groups. Front. Mar. Sci. 2023, 10, 1167803. [Google Scholar] [CrossRef]
- de Freitas, T.A.; Brunton, F.; Bernecker, T. Silurian megalodont bivalves of the Canadian Arctic and Australia: Paleoecological and evolutionary significance. Palaios 1993, 8, 450–464. [Google Scholar] [CrossRef]
- Fraser, N.M.; Bottjer, D.J.; Fischer, A.G. Dissecting “Lithiotis” Bivalves: Implications for the Early Jurassic Reef Eclipse. Palaios 2004, 19, 51–67. [Google Scholar] [CrossRef]
- Walliser, E.O.; Tanabe, K.; Hikida, Y.; Shirai, K.; Schöne, B.R. Sclerochronological study of the gigantic inoceramids Sphenoceramus schmidti and S. sachalinensis from Hokkaido, northern Japan. Lethaia 2019, 52, 410–428. [Google Scholar] [CrossRef]
- Chen, F.; Xue, W.; Yan, J.; Meng, Q. The implications of the giant bivalve family Alatoconchidae for the end-Guadalupian (Middle Permian) extinction event. Geol. J. 2021, 56, 6073–6087. [Google Scholar] [CrossRef]
- Simpson, C.; Harnik, P.G. Assessing the role of abundance in marine bivalve extinction over the post-Paleozoic. Paleobiology 2009, 35, 631–647. [Google Scholar] [CrossRef]
- Foote, M.; Edie, S.M.; Jablonski, D. Ecological structure of diversity-dependent diversification in Phanerozoic marine bivalves. Biol. Lett. 2024, 20, 20230475. [Google Scholar] [CrossRef] [PubMed]
- WoRMS Editorial Board. World Register of Marine Species. 2025. Available online: https://www.marinespecies.org (accessed on 6 July 2025). [CrossRef]
- The Paleobiology Database. Available online: https://paleobiodb.org/#/ (accessed on 6 July 2025).
- SeaLifeBase. World Wide Web Electronic Publication, Version (04/2025); Palomares, M.L.D., Pauly, D., Eds.; SeaLifeBase: Stockholm, Sweden, 2025; Available online: www.sealifebase.org (accessed on 6 July 2025).
- Yang, M.; Li, B.; Gan, Z.; Dong, D.; Li, X. A new chemosymbiotic bivalve species of the genus Acharax Dall, 1908 (Bivalvia, Solemyida, Solemyidae) from the Haima cold seep of the South China Sea. ZooKeys 2024, 1198, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Cope, J.C.W. A new look at early bivalve phylogeny. Geol. Soc. Lond. Spec. Publ. 2000, 177, 81–95. [Google Scholar] [CrossRef]
- Pojeta, J.J. The origin and Paleozoic diversification of solemyoid pelecypods. New Mex. Bur. Mines Miner. Resour. 1988, 44, 201–271. [Google Scholar]
- Felbeck, H. Sulfide oxidation and carbon fixation by the gutless clam Solemya reidi: An animal-bacteria symbiosis. J. Comp. Physiol. 1983, 152, 3–11. [Google Scholar] [CrossRef]
- Taviani, M.; Angeletti, L.; Ceregato, A. Chemosynthetic bivalves of the family Solemyidae (Bivalvia, Protobranchia) in the Neogene of the Mediterranean Basin. J. Paleontol. 2011, 85, 1067–1076. [Google Scholar] [CrossRef]
- Taviani, M. Marine chemosynthesis in the Mediterranean Sea. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 69–83. [Google Scholar]
- Mikkelsen, P.M.; Bieler, R. Seashells of Southern Florida: Bivalves; Princeton University Press: Princeton, NJ, USA, 2008; pp. 1–503. [Google Scholar]
- Neogene Atlas of Ancient Life Southeastern United States. Available online: https://neogeneatlas.net/families/nuculanidae/ (accessed on 6 July 2025).
- Morton, B.S.; Prezant, R.S.; Wilson, B. Class Bivalvia. In Mollusca: The Southern Synthesis. Fauna of Australia; Beesley, P.L., Ross, G.J.B., Wells, A., Eds.; CSIRO Publishing: Melbourne, Australia, 1998; Volume 5, pp. 195–234. [Google Scholar]
- Thomas, R.D.K. Shell form and the ecological range of living andextinct Arcoida. Paleobiology 1978, 4, 181–194. [Google Scholar] [CrossRef]
- Morton, B.; Peharda, M. The biology and functional morphology of Arca noae (Bivalvia: Arcidae) from the Adriatic Sea, Croatia, with a discussion on the evolution of the bivalve mantle margin. Acta Zool. 2008, 89, 19–28. [Google Scholar] [CrossRef]
- Morton, B. Chapter 2. The evolution and success of the heteromyarian form in the Mytiloida. In The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E., Ed.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1992; pp. 21–52. [Google Scholar]
- Peterson, C.H.; Black, R. Experimental tests of the advantages and disadvantages of high density for two coexisting cockles in a Southern Ocean lagoon. J. Anim. Ecol. 1993, 62, 614–633. [Google Scholar] [CrossRef]
- Casey, M.M.; Chattopadhyay, D. Clumping behavior as a strategy against drilling predation: Implications for the fossil record. J. Exp. Mar. Biol. Ecol. 2008, 367, 174–179. [Google Scholar] [CrossRef]
- Seed, R. The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. Oecologia 1969, 3, 277–316. [Google Scholar] [CrossRef] [PubMed]
- Creese, R.; Hooker, S.; de Luca, S.; Wharton, Y. Ecology and environmental impact of Musculista senhousia (Mollusca: Bivalvia:Mytilidae) in Tamaki Estuary, Auckland, New Zealand. N. Z. J. Mar. Freshw. Res. 1997, 31, 225–236. [Google Scholar] [CrossRef]
- Stenzel, H.B. Part N Bivalvia—Oysters. In Treatise on Invertebrate Paleontology, 1st ed.; Moore, R.C., Ed.; The Geological Society of America, Inc.: Boulder, CO, USA; The University of Kansas: Lawrence, KS, USA, 1971; Volume 3 (of 3), pp. N953–N1224. [Google Scholar]
- Bošnjak, M.; Sremac, J.; Petricioli, D.; Bakran-Petricioli, T.; Prlj Šimić, N.; Vrsaljko, D. Neopycnodonte Stenzel, 1971 (Bivalvia: Ostreida: Pycnodonteinae)—An interesting grypheid fossil oyster from the Croatian Natural History Museum collections. Nat. Croat. 2024, 33, 337–350. [Google Scholar] [CrossRef]
- Angeletti, L.; Taviani, M. Offshore Neopycnodonte Oyster Reefs in the Mediterranean Sea. Diversity 2020, 12, 92. [Google Scholar] [CrossRef]
- Cox, L.R.; Newell, N.D.; Boyd, D.W.; Branson, C.C.; Casey, R.; Chavan, A.; Coogan, A.H.; Dechaseaux, C.; Fleming, C.A.; Haas, F.; et al. Part N, Mollusca 6, Bivalvia. In Treatise on Invertebrate Paleontology; Moore, R.C., Ed.; The Geological Society of America, Inc.: Boulder, CO, USA; The University of Kansas: Lawrence, KS, USA, 1969; Volume 1, pp. N471–N489. [Google Scholar]
- Ralph, J.; Von Bargen, D.; Martynov, P.; Zhang, J.; Que, X.; Prabhu, A.; Morrison, S.M.; Li, W.; Chen, W.; Ma, X. Mindat.org: The open access mineralogy database to accelerate data-intensive geoscience research. Am. Mineral. 2025, 110, 833–844. [Google Scholar] [CrossRef]
- iNaturalist. Available online: https://www.inaturalist.org (accessed on 6 July 2025).
- Taylor, J.D.; Glover, E.A. Lucinidae (Bivalvia)—The most diverse group of chemosymbiotic molluscs. Zool. J. Linn. Soc. 2006, 148, 421–438. [Google Scholar] [CrossRef]
- Kiel, S.; Peckmann, J. Chemosymbiotic bivalves and stable carbon isotopes indicate hydrocarbon seepage at four unusual Cenozoic fossil localities. Lethaia 2007, 40, 345–357. [Google Scholar] [CrossRef]
- Kiel, S.; Sami, M.; Taviani, M. Unusual Miocene hydrocarbon-seep faunas from the Brisighella area in northern Italy: Embedded in clastics and first records of the lucinid bivalves Megaxinus and Miltha. Acta Palaeontol. Pol. 2023, 68, 127–132. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.; Harper, D.A.; Zhang, Y.; Wei, X.; Wang, G.; Zhan, R. New Ordovician bivalves from the Indochina Palaeoplate in Dali, western Yunnan, Southwest China and their palaeogeographic significance. Palaeoworld 2025, 34, 100883. [Google Scholar] [CrossRef]
- Liljedahl, L. Contrasting feeding strategies in bivalves from the Silurian of Gotland. Palaeontology 1991, 34, 219–235. [Google Scholar]
- Lee, Y.; Kwak, H.; Shin, J.; Kim, S.-C.-; Kim, T.; Park, J.-K. A mitochondrial genome phylogeny of Mytilidae (Bivalvia: Mytilida). Mol. Phylogenet. Evol. 2019, 139, 106533. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, E.; Venier, P.; Figueras, A.; Novoa, B.; Rosani, U. Evolutionary insights on a novel mussel-specific foot protein-3α gene family. Invertebr. Surviv. J. 2021, 18, 277–288. [Google Scholar]
- Pojeta, J. Fordilla troyensis Barrrande and early Pelecypod phylogeny. Bull. Am. Paleontol. 1975, 67, 363–384. [Google Scholar]
- Végh-Neubrandt, E. Triassische Megalodontaceae: Entwicklung, Stratigraphie, und Paläontologie; Akadémiai Kiadó: Budapest, Hungary, 1982; pp. 1–526. [Google Scholar]
- Aberhan, M.; Alroy, J.; Fursich, F.T.; Kiessling, W.; Kosnik, M.; Madin, J.; Patzkowsky, M.; Wagner, P.; Ecological attributes of Marine Invertebrates. PaleoDB. 2004. Available online: www.paleodb.org (accessed on 7 July 2025).
- Kochansky-Devidé, V. Tanchintongia—Eine aberrante permische Bivalve in Europa. Paläontologische Z. 1978, 52, 213–218. [Google Scholar] [CrossRef]
- Yancey, T.E. The alatoconchid bivalves: Permian analogs of modern tridacnid clams. Paleontol. Conv. Proc. 1982, 2, 589–592. [Google Scholar]
- Aljinović, D.; Isozaki, Y.; Sremac, J. The occurrence of giant bivalve Alatoconchidae from the Yabeina zone (Upper Guadalupian, Permian) in European Tethys. Gondwana Res. 2008, 13, 275–287. [Google Scholar] [CrossRef]
- Isozaki, Y.; Aljinović, D. End-Guadalupian extinction of the Permian gigantic bivalve Alatoconchidae: End of gigantism in tropical seas by cooling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 284, 11–21. [Google Scholar] [CrossRef]
- Asato, K.; Kase, T.; Ono, T.; Sashida, K.; Agematsu, S. Morphology, systematics and paleoecology of Shikamaia, aberrant Permian bivalves (Alatoconchidae: Ambonychioidea) from Japan. Paleontol. Res. 2017, 21, 358–379. [Google Scholar] [CrossRef]
- Harries, P.J.; Kauffman, E.G.; Crampton, J.S.; Bengtson, P.; Cech, S.; Crame, J.A.; Dhondt, A.V.; Ernst, G.; Hilbrecht, H.; Lopez Mortimore, G.R.; et al. Lower Turonian Euramerican Inoceramidae: A morphologic, taxonomic, and biostratigraphic overview. Mitteilungen Aus Dem Geol. Paläontologischen Mus. Der Univ. Hambg. 1996, 77, 641–671. Available online: http://www.fuhrmann-hilbrecht.de/Heinz/geology/InoIntro/InoIntro.html (accessed on 6 July 2025).
- Vrije Universiteit Amsterdam. Available online: https://www.geo.vu.nl/~smit/inoceramus/musling.htm (accessed on 7 July 2025).
- Ros-Franch, S.; Márquez-Aliaga, A.; Damborenea, S.E. Comprehensive database on Induan (Lower Triassic) to Sinemurian (Lower Jurassic) marine bivalve genera and their paleobiogeographic record. Paleontol. Contrib. 2014, 8, 3–219. [Google Scholar]
- Skelton, P.W. Treatise Online no. 104: Part N, Volume 1, Chapter 26A: Introduction to the Hippuritida (rudists): Shell structure, anatomy, and evolution. Treatise Online 2018, 104, 1–37. [Google Scholar] [CrossRef]
- Gould, S.J. Trigonia and the origin of species. J. Hist. Biol. 1968, 1, 41–56. [Google Scholar] [CrossRef]
- Stanley, S.M. Coadaptation in the Trigoniidae, a remarkable family of burrowing bivalves. Palaeontology 1977, 20, 869–899. [Google Scholar]
- Francis, A.O.; Hallam, A. Ecology and evolution of Jurassic trigoniid bivalves in Europe. Lethaia 2003, 36, 287–304. [Google Scholar] [CrossRef]
- Villamil, T.; Kauffman, E.G.; Leanza, H.A. Epibiont habitation patterns and their implications for life habits and orientation among trigoniid bivalves. Lethaia 2007, 31, 43–56. [Google Scholar] [CrossRef]
- McRoberts, C.A. Diversity dynamics and evolutionary ecology of Middle and Late Triassic halobiid and monotid bivalves. In The Global Triassic; Lucas, S.G., Spielmann, J.A., Eds.; New Mexico Museum of Natural History and Science Bulletin; New Mexico Museum of Natural History: Albuquerque, NM, USA, 2007; Volume 41, p. 272. [Google Scholar]
- McRoberts, C.A. Biochronology of Triassic bivalves. In The Triassic Timescale; Lucas, S.G., Ed.; Geological Society London, Special Publications: Bath, UK, 2010; Volume 334, pp. 201–219. [Google Scholar]
- Bakke, N. The Evolution of the Triassic Bivalve Daonella into Halobia in the Botneheia Formation on Svalbard. Master’s Thesis, NTNU, Trondheim, Norway, 2017; 185p. [Google Scholar]
- Del Piero, N.; Riguad, S.; Takahashi, S.; Poulton, S.W.; Martini, R. Unravelling the paleoecology of flat clams: New insights from an Upper Triassic halobiid bivalve. Glob. Planet. Change 2020, 190, 103195. [Google Scholar] [CrossRef]
- Prinoth, H.; Posenato, R. Bivalves from the Changshingian (upper Permian) Bellerophon Formation of the Dolomites (Italy): Ancestors of Lower Triassic post-extinction benthic communities. Pap. Palaeontol. 2023, 9, e1486. [Google Scholar] [CrossRef]
- Chinzei, K. Morphological and structural adaptations to soft substrates in the Early Jurassic monomyarians Lithiotis and Cochlearites. Lethaia 1982, 15, 179–197. [Google Scholar] [CrossRef]
- Nauss, A.L.; Smith, P.L. Lithiotis (Bivalvia) bioherms in the Lower Jurassic of east-central Oregon, U.S.A. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988, 65, 253–268. [Google Scholar] [CrossRef]
- Savazzi, E. Preserved ligament in the Jurassic bivalve Lithiotis: Adaptive and evolutionary significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 120, 281–289. [Google Scholar] [CrossRef]
- Posenato, R.; Crippa, G.; de Winter, N.J.; Frijia, G.; Kaskes, P. Microstructures and sclerochronology of exquisitely preserved Lower Jurassic lithiotid bivalves: Paleobiological and paleoclimatic significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 602, 111162. [Google Scholar] [CrossRef]
- Posenato, R.; Frijia, G.; Morsilli, M.; Moro, A.; Del Viscio, G.; Mezga, A. Paleoecology and proliferation of the bivalve Chondrodonta joannae (Choffat) in the upper Cenomanian (Upper Cretaceous) Adriatic Carbonate Platform of Istria (Croatia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 548, 109703. [Google Scholar] [CrossRef]
- Del Viscio, G.; Morsilli, M.; Posenato, R.; Frijia, G.; Moro, A.; Mezga, A. Proliferation of Chondrodonta in upper Cenomanian shallow-water limestones of the Adriatic Carbonate Platform (Croatia) as a proxy of environmental instability. Cretac. Res. 2022, 134, 105151. [Google Scholar] [CrossRef]
- de Winter, N.J.; Goderis, S.; Van Malderen, S.J.M.; Sinnesael, M.; Vansteenberge, S.; Snoeck, C.; Belza, J.; Vanhaecke, F.; Claeys, P. Subdaily-scale chemical variability in a Torreites sanchezi rudist shell: Implications for rudist paleobiology and the Cretaceous day-night cycle. Paleoceanogr. Paleoclimatol. 2020, 35, e2019PA003723. [Google Scholar] [CrossRef]
- Vogel, K. Endosymbiotic algae in rudists? Palaeogeogr. Palaeoclimatol. Palaeoecol. 1975, 17, 327–332. [Google Scholar] [CrossRef]
- Skelton, P.W.; Wright, V.P. A Caribbean rudist bivalve in Oman-island-hopping across the Pacific in the Late Cretaceous. Palaeontology 1987, 30, 505–529. [Google Scholar]
- Steuber, T. Isotopic and chemical intra-shell variations in low-Mg calcite of rudist bivalves (Mollusca-Hippuritacea): Disequilibrium fractionations and late Cretaceous seasonality. Int. J. Earth Sci. 1999, 88, 551–570. [Google Scholar] [CrossRef]
- Johnson, C. The rise and Fall of Rudist Reefs. Am. Sci. 2002, 90, 148–153. [Google Scholar] [CrossRef]
- Kouchinsky, A.; Alexander, R.; Bengtson, S.; Bowyer, F.; Clausen, S.; Holmer, L.E.; Kolesnikov, K.A.; Korovnikov, I.V.; Pavlov, V.; Skovsted, C.B.; et al. Early–middle Cambrian stratigraphy and faunas from northern Siberia. Acta Palaeontol. Pol. 2022, 67, 341–464. [Google Scholar] [CrossRef]
- Gill, B.; Lyons, T.; Young, S.; Kump, L.R.; Knoll, A.H.; Saltzman, M.R. Geochemical evidence for widespread euxinia in the Later Cambrian Ocean. Nature 2011, 469, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Posenato, R.; Crippa, G.; de Winter, N.J.; Claeys, P.; Goderis, S.; Frijia, G.; Brombin, V. Microstructures and sclerochronology of the Lithiotis Facies bivalves (Lower Jurassic): Paleobiological and paleoclimatic significance and their resilience to the early Toarcian extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 649, 112329. [Google Scholar] [CrossRef]
- Buatois, L.A.; Carmona, N.B.; Curran, H.A.; Netto, R.G.; Mángano, M.G.; Wetzel, A. The Mesozoic Marine Revolution. In The Trace-Fossil Record of Major Evolutionary Events; Mángano, M., Buatois, L., Eds.; Topics in Geobiology; Springer: Dordrecht, The Netherlands, 2016; Volume 40. [Google Scholar] [CrossRef]
- Steuber, T.; Scott, R.W.; Mitchell, S.F.; Skelton, P.W. Part N, Revised, Volume 1, Chapter 26C: Stratigraphy and diversity dynamics of Jurassic–Cretaceous Hippuritida (rudist bivalves). Treatise Online 2016, 81, 1–17. [Google Scholar] [CrossRef]
- Skelton, P.W.; Gili, E. Rudists and carbonate platforms in the Aptian: A case study on biotic interactions with ocean chemistry and climate. Sedimentology 2012, 59, 81–117. [Google Scholar] [CrossRef]
- Philip, J.; Airaud-Crumière, C. The demise of the rudist bearing carbonate platforms at the Cenomanian/Turonian boundary: A global control. Coral Reefs 1991, 10, 115–125. [Google Scholar] [CrossRef]
- Carannante, G.; Graziano, R.; Pappone, G.; Ruberti, D.; Simone, L. Depositional system and response to sea level oscillations of the Senonian rudist-bearing carbonates helves. Examples from Central Mediterranean areas. Facies 1999, 40, 1–24. [Google Scholar] [CrossRef]
- Simone, L.; Carannante, G.; Ruberti, D.; Sirna, M.; Sirna, G.; Laviano, A.; Tropeano, M. Development of rudist lithosomes in the Coniacian-Lower Campanian carbonate shelves of central-southern Italy: High-energy vs low-energy settings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 200, 5–29. [Google Scholar] [CrossRef]
- Steuber, T. Plate tectonic control on the evolution of Cretaceous platform-carbonate production. Geology 2002, 30, 259–262. [Google Scholar] [CrossRef]
- Sha, J.; Cestari, R.; Fabbi, S. Paleobiogeographic distribution of rudist bivalves (Hippuritida) in the Oxfordian–early Aptian (Late Jurassic–Early Cretaceous). Cretac. Res. 2020, 108, 104289. [Google Scholar] [CrossRef]
- Digital Atlas of Ancient Life. Available online: https://www.digitalatlasofancientlife.org/learn/mollusca/bivalvia/evolutionary-history/ (accessed on 6 July 2025).
- de Graaff, S.J.; Percival, L.M.E.; Kaskes, P.; Déhais, T.; de Winter, N.J.; Jansen, M.N.; Smit, J.; Sinnesael, M.; Vellekoop, J.; Sato, H.; et al. Geochemical records of the end-Triassic Crisis preserved in a deep marine section of the Budva Basin, Dinarides, Montenegro. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 606, 111250. [Google Scholar] [CrossRef]
- Cox, L.R. Taxonomic notes on Isognomonidae and Bakevelliidae. Proc. Malacol. Soc. Lond. 1954, 31, 46–49. [Google Scholar]
- Lazo, D.G. Taxonomy, facies relationships and palaeobiology of bakevelliid bivalves from the Lower Cretaceous of west-central Argentina. Cretac. Res. 2003, 24, 765–788. [Google Scholar] [CrossRef]
- Fielding, C.R.; Bryan, S.E.; Crowley, J.L.; Frank, T.D.; Hren, M.T.; Mays, C.; McLoughlin, S.; Shen, J.; Wagner, P.J.; Winguth, A.; et al. A multidisciplinary approach to resolving the end-Guadalupian extinction. Evol. Earth 2023, 1, 100014. [Google Scholar] [CrossRef]
- Ozanne, C.R.; Harries, P.J. Role of predation and parasitism in the extinction of the inoceramid bivalves: An evaluation. Lethaia 2007, 35, 1–19. [Google Scholar] [CrossRef]
- Brame, H.M.R.; Martindale, R.C.; Ettinger, N.P.; Debeljak, I.; Vasseur, R.; Lathuilière, B.; Kabiri, L.; Bodin, S. Stratigraphic distribution and paleoecological significance of Early Jurassic (Pliensbachian-Toarcian) lithiotid-coral reefal deposits from the Central High Atlas of Morocco. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 514, 813–837. [Google Scholar] [CrossRef]
- Killam, D.; Clapham, M.E.; Posenato, R.; Franceschi, M. Sclerochronology of the early Jurassic lithiotid bivalves: Searching for symbiosis. In Proceedings of the Geological Society of America 2016 Annual Meeting, Denver, CO, USA, 25–28 September 2016. [Google Scholar] [CrossRef]
- Gulick, S.P.S. End of the Cretaceous. In Cretaceous Project 200 Volume 1: The Cretaceous World; Hart, M.B., Batenburg, S.J., Huber, B.T., Price, G.D., Thibault, N., Wagreich, M., Walaszczyk, I., Eds.; Special Publications; Geological Society, London: Bath, UK, 2025; Volume 544, pp. 549–570. [Google Scholar]
- Edie, S.M.; Collins, K.S.; Jablonski, D. The end-Cretaceous mass extinction restructured functional diversity but failed to configure the modern marine biota. Sci. Adv. 2025, 11, eadv1171. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.M.; Hansen, T.A. Detritus feeding as a buffer to extinction at the end of the Cretaceous. Geology 1986, 14, 868–870. [Google Scholar] [CrossRef]
- Robertson, D.S.; McKenna, M.C.; Toon, O.B.; Hope, S.; Lillegraven, J.A. Survival in the first hours of the Cenozoic. GSA Bull. 2004, 116, 760–768. [Google Scholar] [CrossRef]
- Jablonski, D. Body size and macroevolution. In Evolutionary Paleobiology; Jablonski, D., Erwin, D.H., Lipps, J.H., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; pp. 256–289. [Google Scholar]
- Twitchett, R.J. The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 252, 132–144. [Google Scholar] [CrossRef]
- Erwin, H. How Life on Earth Nearly Ended 250 Million Years Ago; Princeton University Press: Princeton, NJ, USA, 2008; pp. 1–320. [Google Scholar]
- Pincelli, H. Life in the Aftermath of Mass Extinctions. Curr. Biol. 2015, 25, R941–R952. [Google Scholar] [CrossRef] [PubMed]
| Order | Shell Mineralogy | Life Mode | Locomotion | Feeding | Microsymbionts |
|---|---|---|---|---|---|
| Nuculida | Aragonite | Infaunal | Slow | Detritivore | Chemosynthetic ? |
| Solemyida | Aragonite | Infaunal | Facultatively | Suspension- feeder | Chemosynthetic |
| Nuculanida | Aragonite | Infaunal | Facultatively | Combined | Chemosynthetic |
| Arcida | Aragonite | Epifaunal Infaunal | Facultatively | Suspension- feeder | Chemosynthetic |
| Mytilida | Aragonite Mg-calcite | Epifaunal Infaunal | Stationary | Suspension- feeder | Chemosynthetic, Photosynthetic ? |
| Ostreida | Mg-calcite | Epifaunal | Stationary | Suspension- feeder | Variable |
| Pectinida | Mg-calcite Aragonite | Epifaunal | Facultatively | Suspension- feeder | Chemosynthetic, Photosynthetic |
| Limida | Aragonite Mg-calcite | Epifaunal | Facultatively | Suspension- feeder | Not known |
| Carditida | Aragonite | Infaunal | Facultatively | Suspension- feeder | Chemosynthetic, Photosynthetic |
| Lucinida | Aragonite | Infaunal | Facultatively | Suspension- feeder | Chemosynthetic |
| Order Family | Shell Mineralogy | Life Mode | Locomotion | Feeding | Microsymbionts |
|---|---|---|---|---|---|
| Fordillida † Both families | Aragonite | Epifaunal | Stationary | Suspension- feeder | Chemosynthetic ? |
| Megalodontida † All families | Aragonite | Infaunal | Facultatively mobile | Suspension- feeder | Photosynthetic ? Chemosynthetic ? |
| Myalinida † Alatoconchidae † | Aragonite Low Mg-calcite | Semi-infaunal | Stationary | Suspension- feeder | Photosynthetic ? Chemosynthetic ? |
| Myalinida † Inoceramidae † | Low Mg-calcite | Epifaunal | Facultatively mobile | Suspension- feeder | Chemosynthetic |
| Hippuritida † all families | Low Mg-calcite Aragonite | Intermediate-level epifaunal, Gregarious | Stationary | Suspension- feeder | Chemosynthetic |
| Trigoniida Megatrigoniidae † | Aragonite | Infaunal | Facultatively mobile | Suspension- feeder | Not known |
| Ostreida Bakevelliidae † | Aragonite Low Mg-calcite | Epifaunal | Stationary | Suspension- feeder | Chemosynthetic, Photosynthetic |
| Ostreida Halobiidae † | Aragonite Low Mg-calcite | Epifaunal | Stationary | Suspension- feeder | Chemosynthetic |
| Ostreida Plicatostylidae † | Low Mg-calcite | Epifaunal | Stationary | Suspension-feeder, Photosymbiotic | Chemosynthetic, Photosynthetic |
| Ostreida Chondrodontidae † | Low Mg-calcite Aragonite | Epifaunal | Stationary | Suspension- feeder | Not known |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sremac, J.; Bošnjak, M. “Winners” and “Losers” of the Bivalve Evolution. Diversity 2025, 17, 500. https://doi.org/10.3390/d17070500
Sremac J, Bošnjak M. “Winners” and “Losers” of the Bivalve Evolution. Diversity. 2025; 17(7):500. https://doi.org/10.3390/d17070500
Chicago/Turabian StyleSremac, Jasenka, and Marija Bošnjak. 2025. "“Winners” and “Losers” of the Bivalve Evolution" Diversity 17, no. 7: 500. https://doi.org/10.3390/d17070500
APA StyleSremac, J., & Bošnjak, M. (2025). “Winners” and “Losers” of the Bivalve Evolution. Diversity, 17(7), 500. https://doi.org/10.3390/d17070500