Neosilba batesi Curran (Diptera: Lonchaeidae): Identification, Distribution, and Its Relationship with Avocado Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Taxonomic Identification of Insects
2.2. DNA Extraction and Sequencing
2.3. Observation and Description of Damages in Avocado Fruits
3. Results
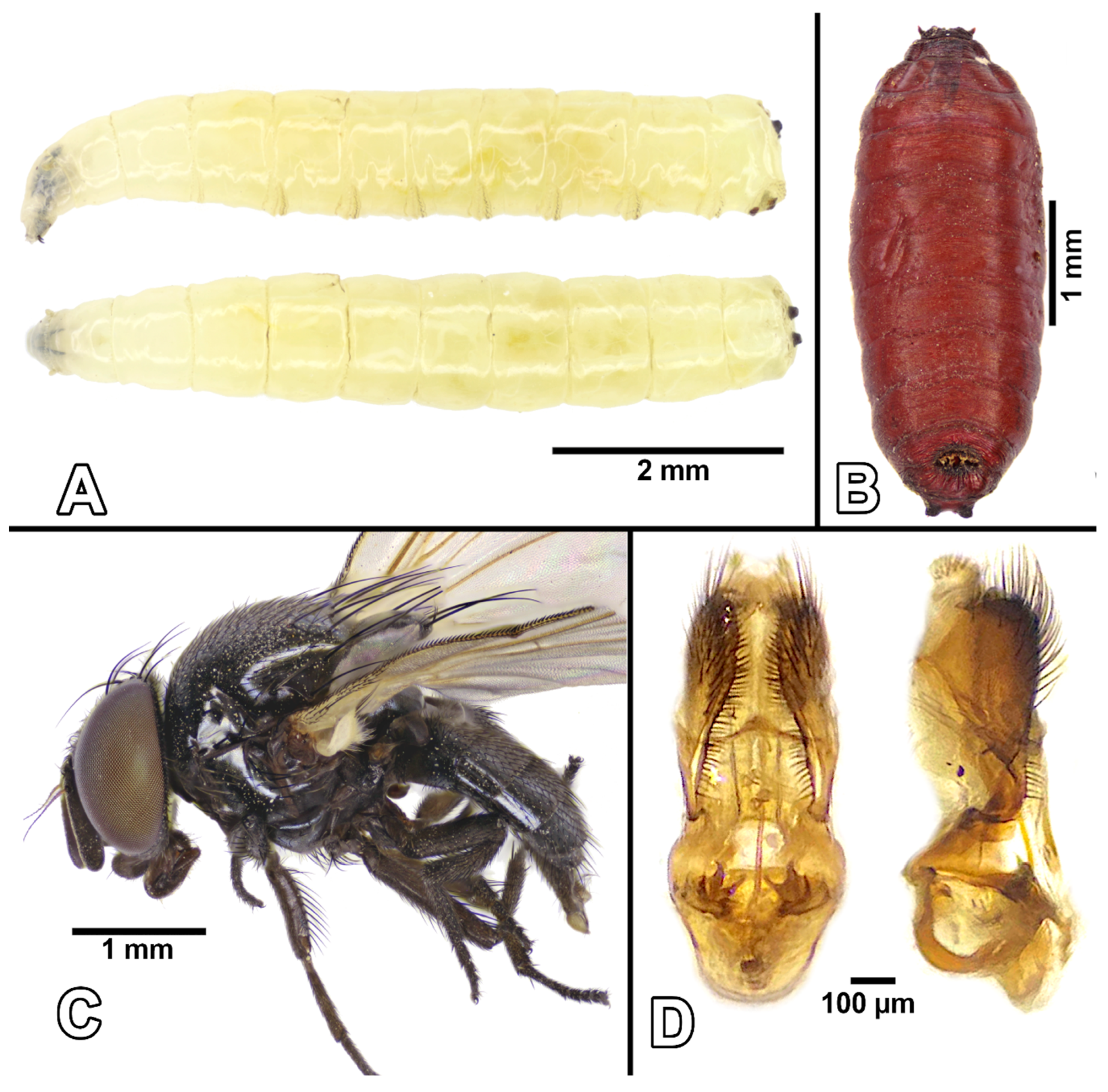
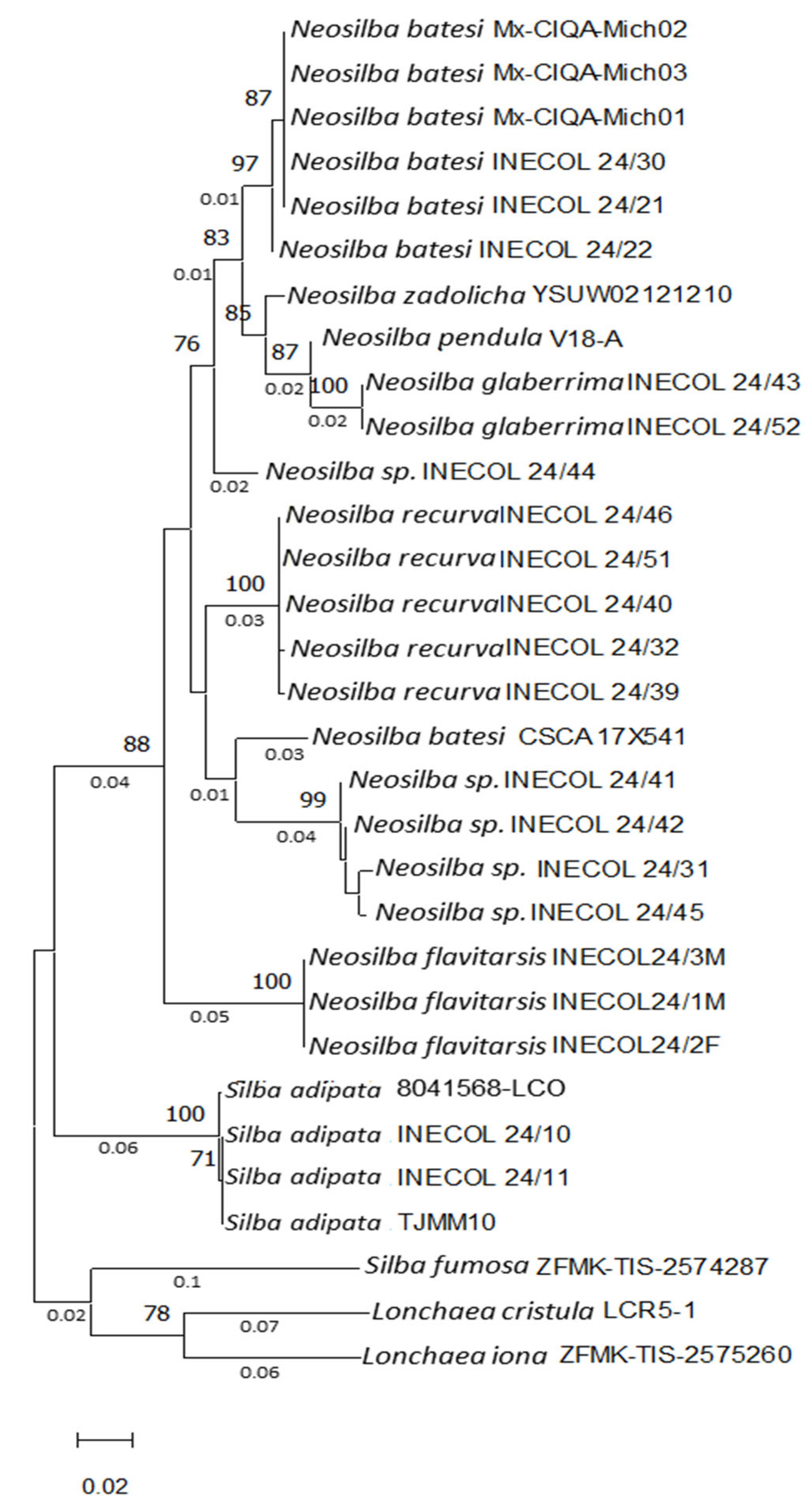
3.1. Identification and Phylogenetic Analyses
3.2. Distribution of Neosilba batesi in the Study Area
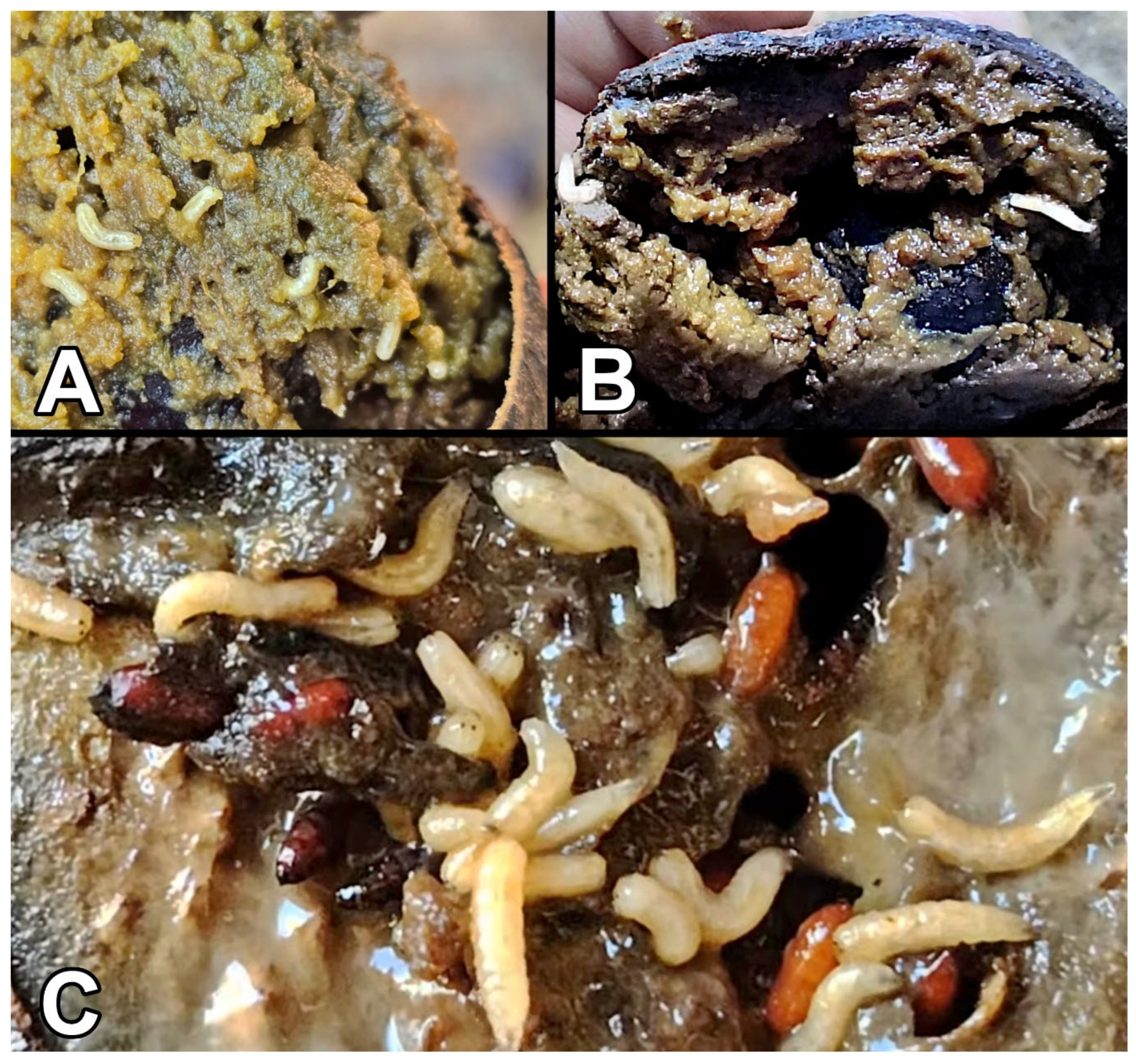
3.3. Neosilba batesi and Its Association with Avocado Fruits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jaagumagi, R. Lonchaeidae. In Manual of Nearctic Diptera; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., Wood, D.M., Eds.; Chapter 62; Research Branch, Agriculture Canada: Ottawa, ON, Canada, 1987; Volume 2, pp. 791–797. [Google Scholar]
- MacGowan, I.; Rotheray, G.E. Lonchaeidae (Lance flies). In Manual of Afrotropical Diptera. Volume 3. Brachycera—Cyclorrhapha, excluding Calyptratae; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2021; pp. 1587–1596. [Google Scholar]
- MacGowan, I. World Catalogue of the family Lonchaeidae (Diptera, Cyclorrhapha, Acalyptratae). Zootaxa 2023, 5307, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Norrbom, A.L.; Korytkowski, C.A. Lonchaeidae (Lance flies). In Manual of Central American Diptera; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbado, M.A., Eds.; NRC Research Press: Ottawa, ON, Canada, 2010; Volume 2, pp. 857–863. [Google Scholar]
- Strikis, P.C.; De Deus, E.G.; Silva, R.A.; Pereira, J.D.B.; Jesus, C.R.; Massaro-Júnior, A.L. Conhecimento sobre Lonchaeidae na Amazônia brasileira. In Moscas-das-Frutas na Amazónica Brasileira: Diversidade, Hospedeiros e Inimigos Naturais; Silva, R.A., Lemos, W.P., Zucchi, R.A., Eds.; Embrapa: Macapá, Brazil, 2011; pp. 206–215. [Google Scholar]
- MacGowan, I.; Okamoto, T. New species of Lonchaeidae (Diptera: Schizophora) from Japan and a re-evaluation of genus Setisquamalonchaea Morge. Entomol. Sci. 2013, 16, 196–202. [Google Scholar] [CrossRef]
- Juárez Maya, M.; Morales Galván, Ó.; Valdez Carrasco, J.; Illescas Salvador, D.; Gutiérrez Ruelas, J.; Illescas Riquelme, C.P. Morphology of immature stages of the black fig fly Silba adipata (Diptera, Lonchaeidae). Biodivers. Data J. 2024, 12, e137798. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.F.; Steyskal, G.C. A revision of Neosilba McAlpine with a key to the world genera of Lonchaeidae (Diptera). Can. Entomol. 1982, 114, 105–137. [Google Scholar] [CrossRef]
- Strikis, P.C.; Prado, A.P. Neosilba (Tephritoidea: Lonchaeidae) species reared from coffee in Brazil, with description of a new species. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance: From Basic to Applied Knowledge, Salvador, Brazil, 10–15 September 2006; pp. 187–193. [Google Scholar]
- Herard, F.; Mercadier, G. Natural enemies of Tomicus piniperda and Ips acuminatus (Coleoptera: Scolytidae) on Pinus sylvestris near Orléans, France: Temporal occurrence and relative abundance, and notes on eight predatory species. Entomophaga 1996, 41, 183–210. [Google Scholar] [CrossRef]
- Wermelinger, B. Development and distribution of predators and parasitoids during two consecutive years of an Ips typographus (Coleoptera, Scolytidae) infestation. J. Appl. Entomol. 2002, 126, 521–527. [Google Scholar] [CrossRef]
- Okamoto, T.; MacGowan, I.; Su, Z.H. Predation on the pollinating fig wasp of Ficus erecta by larvae of Silba sp. (Diptera: Lonchaeidae). Entomol. Sci. 2012, 15, 288–293. [Google Scholar] [CrossRef]
- Strikis, P.C.; Prado, A.P. A new species of genus Neosilba (Diptera: Lonchaeidae). Zootaxa 2005, 828, 1–4. [Google Scholar] [CrossRef]
- Nicácio, J.; Uchôa, M.A. Diversity of frugivorous flies (Diptera: Tephritidae and Lonchaeidae) and their relationship whit host plants (Angiospermae) in environments of south Pantanal region, Brazil. Fla. Entomol. 2011, 94, 443–466. [Google Scholar] [CrossRef]
- Lemos, L.N.; Adaime, R.; Costa Neto, S.V.; Deus, E.G.; Jesus-Barros, C.R.; Strikis, P.C. New findings on Lonchaeidae (Diptera: Tephritoidea) in the Brazilian Amazon. Fla. Entomol. 2015, 98, 1227–1237. [Google Scholar] [CrossRef]
- Sousa, E.M.; Louzeiro, L.R.F.; Strikis, P.C.; Souza-Filho, M.F.; Raga, A. Host plants and distribution records of lance flies (Diptera: Lonchaeidae) in São Paulo State, Brazil. EntomoBrasilis 2021, 14, e942. [Google Scholar] [CrossRef]
- Martins, D.S.; Fornazier, M.L.; Uramoto, K.; Guimarães, J.A.; Ferreira, P.S.F.; Ventura, J.A.; Guarçoni, R.C.; Culik, M.P.; Zanuncio Junior, J.S.; Fornazier, M.J. Frugivorous flies (Diptera: Tephritidae; Lonchaeidae) associated with guava tree: Species diversity, parasitoids and population fluctuation in the Espírito Santo state, Brazil. Pesqui. Agropecu. Trop. 2024, 54, e77329. [Google Scholar] [CrossRef]
- Ahlmark, K.; Steck, G.J. A new U.S. record for a secondary fruit infester Neosilba batesi (Curran) (Diptera: Lonchaeidae). Insecta Mundi 1997, 11, 116. [Google Scholar]
- Imbachi, K.; Quintero, E.; Manrique, M.; Kondo, T. Evaluación de tres proteínas hidrolizadas para la captura de adultos de la mosca del botón floral de la pitaya amarilla, Dasiops saltans Townsend (Diptera: Lonchaeidae). Rev. Corp. Cienc. Tecnol. Agropecu. 2012, 13, 159–166. [Google Scholar] [CrossRef]
- Curran, C.H. New American Diptera No. 534. Am. Mus. Novit. 1932, 534, 1–15. Available online: https://www.biodiversitylibrary.org/bibliography/91295 (accessed on 14 June 2025).
- Hernández-Ortiz, V. Diptera. In Catálogo de Insectos y Ácaros Plaga de los Cultivos Agrícolas de México; Deloya-López, A.C., Valenzuela-González, J.E., Eds.; Sociedad Mexicana de Entomología A.C.: Xalapa, México, 1999; pp. 69–82. [Google Scholar]
- Aluja, M.; Díaz-Fleischer, F.; Arredondo, J. Non host status of commercial Persea americana “Hass” to Anastrepha ludens, Anastrepha obliqua, Anastrepha serpentina and Anastrepha striata (Diptera: Tephritidae) in México. J. Econ. Entomol. 2004, 97, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Riquelme, C.P.; González-Hernández, H.; Valdez-Carrasco, J.M.; Llanderal-Cázares, C.; Ruiz-Montiel, C. Lonchaeidae (Diptera: Tephritoidea) associated with the genus Annona in Mexico. Southwest. Entomol. 2015, 40, 121–130. [Google Scholar] [CrossRef]
- Herrera, A.M.; Canal, N.A.; Agudelo-Martínez, J.C.; Pérez-Buitrago, N. Diversidad y ecología de Tephritoidea (Insecta: Diptera) en el norte de la Orinoquía colombiana. Rev. Biol. Colomb. 2022, 70, 423–436. [Google Scholar] [CrossRef]
- APEAM. Neosilba batesi. Origen de la Especie y Cuidados Para Evitar su Incidencia en Los Huertos de Aguacate. Unidad de Investigación y Desarrollo de la APEAM. 2024. Available online: https://fliphtml5.com/dpfeq/morn/neosilba_batesi/ (accessed on 28 February 2025).
- Reynoso, L.F. Llega Plaga Desde Colombia a Huertas de Aguacate en Uruapan: Investigador. Quadrantin Michoacán. 2024. Available online: https://www.quadratin.com.mx/principal/llega-plaga-desde-colombia-a-huertas-de-aguacate-en-uruapan-investigador/ (accessed on 30 February 2024).
- Cruz-López, D.F.; Caamal-Cauich, I.; Pat-Fernández, V.G.; Gómez-Gómez, A.A.; Espinoza-Torres, L.E. Posicionamiento internacional del aguacate (Persea americana L.) producido en México. Rev. Mex. Agronegocios 2020, 47, 561–571. [Google Scholar]
- SIAP. Aguacate Mexicano. Servicio de Información Agroalimentaria y Pesquera. 2024. Available online: https://www.gob.mx/cms/uploads/attachment/file/885638/Brochure_aguacate_2024.pdf (accessed on 14 June 2025).
- Lasa, R.; Navarro-de-la-Fuente, L.; MacGowan, I.; Williams, T. A Complex of Lance Flies (Diptera: Lonchaeidae) Infesting Figs in Veracruz, Mexico, with the Description of a New Species. Insects 2025, 16, 458. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-Y.; Ro, K.-E. Molecular phylogeny of the superfamily Tephritoidea (Insecta: Diptera) reanalysed based on expanded taxon sampling and sequence data. J. Zool. Syst. Evol. Res. 2016, 54, 276–288. [Google Scholar] [CrossRef]
- Talamonte de Oliveira, T.C.; Pereira Gomes, L.R.; Egan, S.P.; Brandão-Dias, P.F.P.; Riverón, A.Z.; Del Bianco Faria, L. Diversity of Diptera and their parasitoids associated with Inga vera Willd. (1806) (Fabaceae) with new host record in Minas Gerais, Brazil. Rev. Agrogeoambient. 2024, 16, e20241918. [Google Scholar] [CrossRef]
- Reimann, A.; Rulik, B. The Lonchaeidae (Diptera) of the GBOL project, with the description of a new Priscoearomyia species. Contrib. Entomol. 2024, 74, 165–179. [Google Scholar] [CrossRef]
- Balseiro Teheran, F.J.; Uribe Soto, S.I. Códigos de barra de ADN para identificación de especies del género Lonchaea fallen 1820 (Diptera: Lonchaeidae) de Antioquia. Rev. Fac. Cienc. 2021, 10, 51–66. [Google Scholar] [CrossRef]
- Prosser, S.W.; deWaard, J.R.; Miller, S.E.; Hebert, P.D. DNA barcodes from century-old type specimens using next-generation sequencing. Mol. Ecol. Resour. 2016, 16, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Hall, T. BioEdit Sequence Alignment Editor. GitHub Pages. 2021. Available online: https://thalljiscience.github.io/ (accessed on 14 June 2025).
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3029. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Uchôa, M.A.; Oliveira, I.; Molina, R.M.S.; Zucchi, R.A. Species diversity of frugivorous flies (Diptera: Tephritoidea) from hosts in the cerrado of the state of Mato Grosso do Sul, Brazil. Neotrop. Entomol. 2002, 31, 515–524. [Google Scholar] [CrossRef]
- Raga, A.; Souza-Filho, M.F.; Strikis, P.C.; Montes, S.M.N.M. Lance fly (Diptera: Lonchaeidae) host plants in the State of São Paulo, Southeast Brazil. Entomotropica 2015, 30, 57–68. [Google Scholar]
- López-Bautista, E.; García-Sánchez, A.N.; Sierra-Gómez, U.A.; Tejeda-Reyes, M.A.; Illescas-Riquelme, C.P. Efecto nulo de trampas y cebos en la captura de la mosca negra del higo, Silba adipata. In Avances en Agricultura Sostenible y Cambio Climático; Soto-Ortiz, R., Avilés-Marín, S.M., Brígido-Morales, J.G., Escobosa-García, M.I., Eds.; Astra: Jalisco, México, 2024; pp. 441–447. [Google Scholar]


| Species/Voucher Code | Estate, Country | Accession GenBank | Reference |
|---|---|---|---|
| Neosilba batesi Mx-CIQA-Mich01 | Michoacan, Mexico | PV243309 | Nucleotide sequences generated by this work |
| Neosilba batesi Mx-CIQA-Mich02 | Michoacan, Mexico | PV243310 | |
| Neosilba batesi Mx-CIQA-Mich03 | Michoacan, Mexico | PV243311 | |
| Neosilba batesi INECOL_24/21 | Veracruz, Mexico | PV504763 | [29] |
| Neosilba batesi INECOL_24/22 | Veracruz, Mexico | PV504764 | [29] |
| Neosilba batesi INECOL_24/30 | Veracruz, Mexico | PV504765 | [29] |
| Neosilba batesi CSCA 17X541 | FL, USA | MW283302.1 | unpublished |
| Neosilba zadolicha YSUW02121210 | Brazil | KR262649.1 | [30] |
| Neosilba pendula IV18-A | Brazil | OQ160349.1 | [31] |
| Neosilba glaberrima INECOL_24/43 | Veracruz, Mexico | PV504766 | [29] |
| Neosilba glaberrima INECOL_24/52 | Veracruz, Mexico | PV504767 | [29] |
| Neosilba recurva INECOL_24/32 | Veracruz, Mexico | PV522074 | [29] |
| Neosilba recurva INECOL_24/39 | Veracruz, Mexico | PV522075 | [29] |
| Neosilba recurva INECOL_24/40 | Veracruz, Mexico | PV522076 | [29] |
| Neosilba recurva INECOL_24/46 | Veracruz, Mexico | PV522077 | [29] |
| Neosilba recurva INECOL_24/51 | Veracruz, Mexico | PV522078 | [29] |
| Neosilba sp. INECOL_24/31 | Veracruz, Mexico | PV504768 | [29] |
| Neosilba sp. INECOL_24/41 | Veracruz, Mexico | PV504769 | [29] |
| Neosilba sp. INECOL_24/42 | Veracruz, Mexico | PV504770 | [29] |
| Neosilba sp. INECOL_24/42 | Veracruz, Mexico | PV504771 | [29] |
| Neosilba sp. INECOL_24/44 | Veracruz, Mexico | PV504772 | [29] |
| Neosilba flavitarsis INECOL24/1M | Veracruz, Mexico | PQ834830 | [29] |
| Neosilba flavitarsis INECOL24/2F | Veracruz, Mexico | PQ834831 | [29] |
| Neosilba flavitarsis INECOL24/3M | Veracruz, Mexico | PQ834832 | [29] |
| Silba adipata 8041568-LCO | Turkey | MK450115 | unpublished |
| Silba adipata INECOL_24/10 | Veracruz, Mexico | PV504773 | [29] |
| Silba adipata INECOL_24/11 | Veracruz, Mexico | PV504774 | [29] |
| Silba adipata TJMM10 | Morelos, Mexico | OM949837 | unpublished |
| Silba fumosa ZFMK-TIS-2574287 | Germany | OP831869 | [32] |
| Lonchaea cristula LCR5-1 | Colombia | MZ189773 | [33] |
| Lonchaea iona ZFMK-TIS-2575260 | Germany | OP831877.1 | [32] |
| Site | Coordinates/ Height (m a.s.l.) | Date of Fruit Collection | (Number of Fruits)/Emerged Adults | |
|---|---|---|---|---|
| Fallen Fruits | Tree Fruits | |||
| Tingüindín, Mich. | 19°43′46″ N 102°27′7″ W/1882 | 22 December 2022 | (13)/5♂♂ 11♀♀ | (3)/1♂♂ 3♀♀ |
| Ario de los Rosales, Mich. | 19°11′10″ N 101°39′52″ W, 2206 | 10 January 2023 | (8)/4♂♂ 7♀♀ | (6)/4♂♂ 7♀♀ |
| Los Reyes 1, Mich. | 19°35′17.20″ N 102°23′4.82″ W/1592 | 10 November 2022 | (19)/10♂♂ 16♀♀ | (4)/2♂♂ 9♀♀ |
| Los Reyes 2, Mich. | 19°34′34″ N 102°19′30″ W, 2141 | 14 December 2022 | (8)/6♂♂ 10♀♀ | (5)/3♂♂ 5♀♀ |
| Los Reyes 3, Mich. | 19°39′50″ N 102°24′51″ W, 1620 | 14 December 2022 | (10)/8♂♂ 6♀♀ | (5)/5♂♂ 7♀♀ |
| Los Reyes 4, Mich | 19°38′50″ N 102°23′39″ W, 1780 | 14 December 2022 | (6)/4♂♂ 7♀♀ | (4)/6♂♂ 8♀♀ |
| Tancítaro, Mich. | 19°23′28”N 102°25′18”W, 2020 | 14 January 2023 | (14)/4♂♂ 6♀♀ | (6)/3♂♂ 5♀♀ |
| Tuxpan, Jal. | 19°32′5″ N 103°27′50″ W, 1301 | 28 December 2022 | (11)/6♂♂ 8♀♀ | (4)/3♂♂ 5♀♀ |
| Quitupan, Jal. | 19°48′2″ N 102°48′32″ W, 2040 | 29 December 2022 | (15)/5♂♂ 9♀♀ | (6)/4♂♂ 6♀♀ |
| Collect Date | Number of Fruits Collected | Average Sizes of Infested Avocado Trees (cm) | Average Number of Emerged Larvae/Fruit | Average Number of Adults | |
|---|---|---|---|---|---|
| Long | Width | ||||
| 27 January 2024 | 18 | 7.95 | 5.05 | 4.94 | 1.72 |
| 3 February 2024 | 65 | 4.57 | 3.4 | 5.07 | 1.06 |
| 5 March 2024 | 44 | 4.05 | 3.2 | 15.09 | 2.5 |
| 18 April 2024 | 30 | 5.04 | 3.93 | 13.34 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemus-Soriano, B.A.; Morales-Galván, O.; García-Gallegos, D.; García-Banderas, D.V.; Kassem, M.; Illescas-Riquelme, C.P. Neosilba batesi Curran (Diptera: Lonchaeidae): Identification, Distribution, and Its Relationship with Avocado Fruits. Diversity 2025, 17, 499. https://doi.org/10.3390/d17070499
Lemus-Soriano BA, Morales-Galván O, García-Gallegos D, García-Banderas DV, Kassem M, Illescas-Riquelme CP. Neosilba batesi Curran (Diptera: Lonchaeidae): Identification, Distribution, and Its Relationship with Avocado Fruits. Diversity. 2025; 17(7):499. https://doi.org/10.3390/d17070499
Chicago/Turabian StyleLemus-Soriano, Braulio Alberto, Oscar Morales-Galván, David García-Gallegos, Diana Vely García-Banderas, Mona Kassem, and Carlos Patricio Illescas-Riquelme. 2025. "Neosilba batesi Curran (Diptera: Lonchaeidae): Identification, Distribution, and Its Relationship with Avocado Fruits" Diversity 17, no. 7: 499. https://doi.org/10.3390/d17070499
APA StyleLemus-Soriano, B. A., Morales-Galván, O., García-Gallegos, D., García-Banderas, D. V., Kassem, M., & Illescas-Riquelme, C. P. (2025). Neosilba batesi Curran (Diptera: Lonchaeidae): Identification, Distribution, and Its Relationship with Avocado Fruits. Diversity, 17(7), 499. https://doi.org/10.3390/d17070499






