The Impact of Different Agricultural Practices on Nematode Biodiversity on Tomato- and Lettuce-Growing Periods Across Two Consecutive Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site and Treatments Description
2.2. Soil Sampling Design
2.3. Soil Nematode Community Analysis
2.4. Statistical Analysis
3. Results
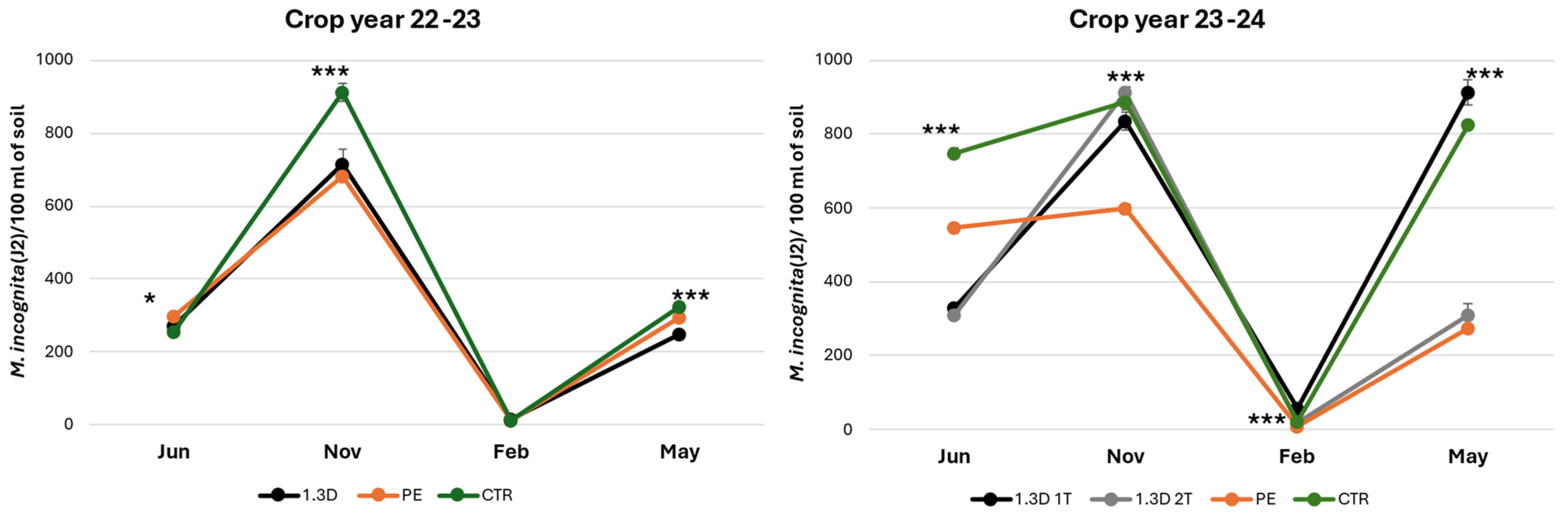
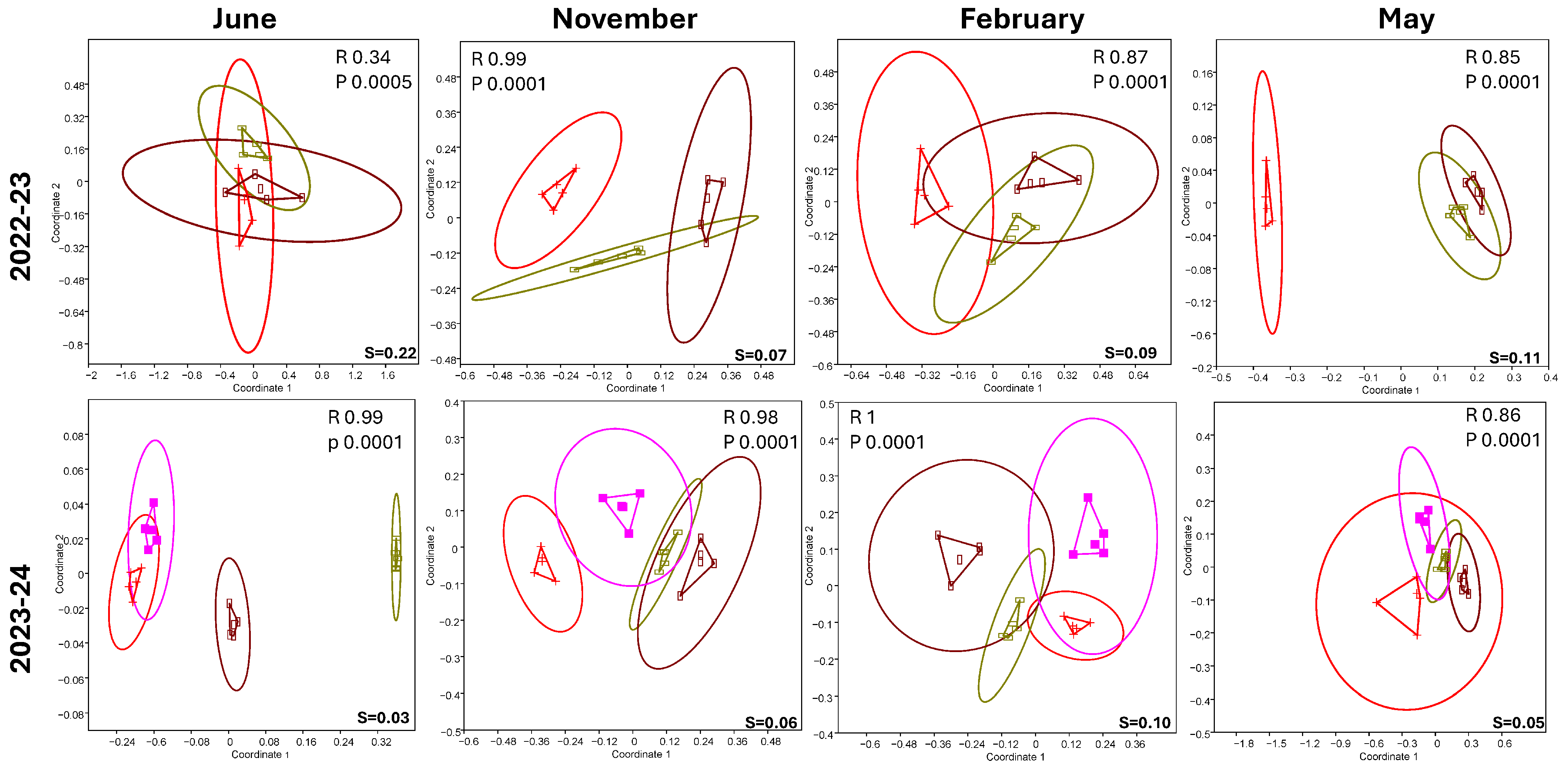
3.1. Soil Nematode Community Structure and Dynamic
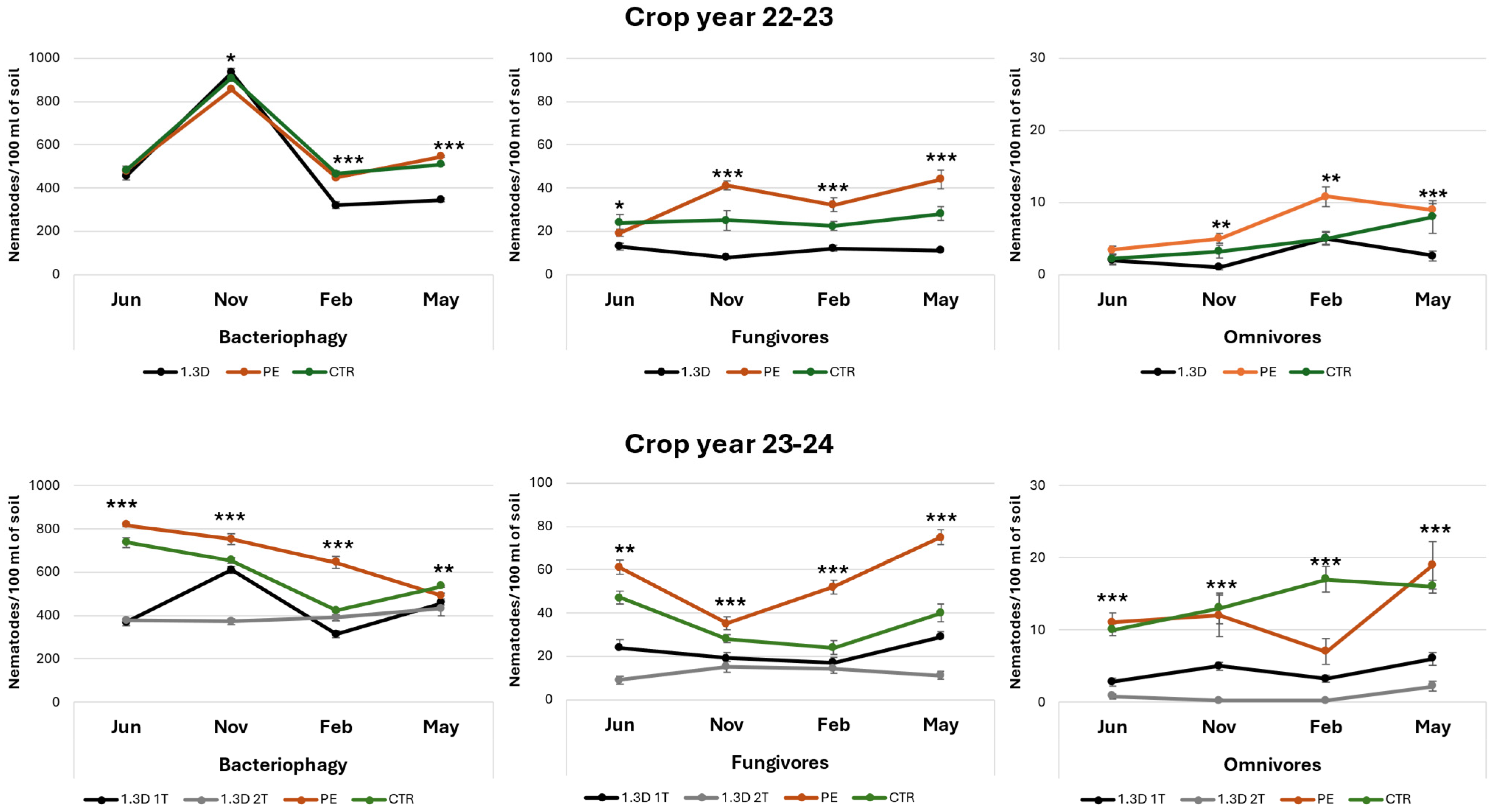
3.2. Soil Biological Indicators
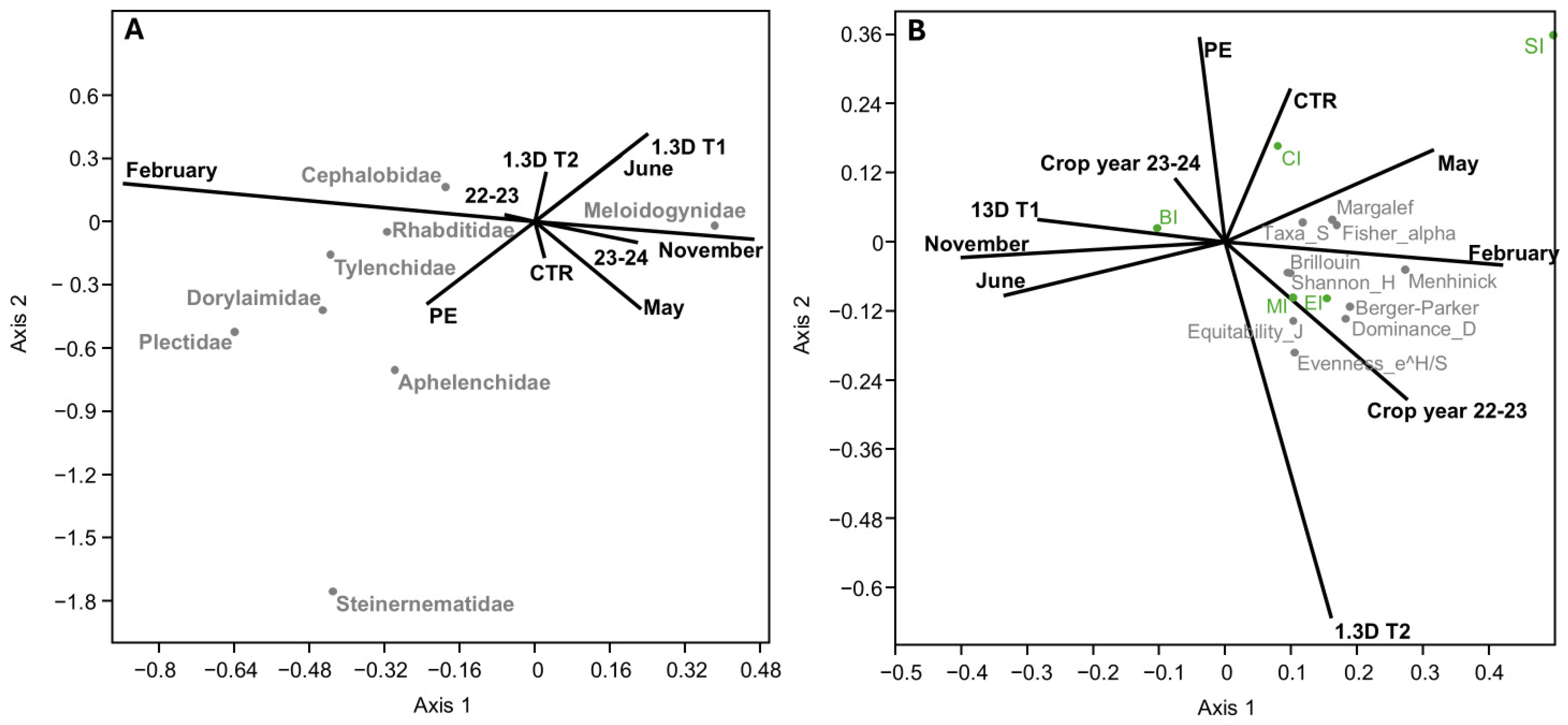
3.3. Relationship Between Environmental Variables and Community Structure and Nematode Community
4. Discussion
4.1. Meloidogyne Incognita Dynamic
4.2. Effects of Different Phytosanitary Management Methods on Soil Nematode Community Structure and Dynamics
4.3. Environmental Parameters Influencing Soil Nematode Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schownholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Timper, P. Conserving and Enhancing Biological Control of Nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar] [PubMed]
- Kauschuk, G.; Alberton, O.; Hungria, M. Quantifying effects of different agricultural land uses on soil microbial biomass and activity in Brazilian biomes: Inferences to improve soil quality. Plant Soil 2011, 338, 467–481. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T. Nematode diversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 113–135. [Google Scholar]
- Lee, K.E. The biodiversity of soil organisms. Appl. Soil Ecol. 1994, 1, 251–254. [Google Scholar] [CrossRef]
- FAO Statistical Databases. Statistics Division. 2020. Available online: https://www.fao.org/about/who-we-are/departments/statistics-division (accessed on 1 June 2025).
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.H.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sharma, H.K. Pankaj Nematodes problem in India. In Crop Pest and Disease Management—Challenges for the Millennium; Prasad, D., Puri, S.N., Eds.; Joyti Publishers: New Delhi, India, 2000; pp. 267–275. [Google Scholar]
- Mukhtar, T.; Hussain, M.A.; Kayani, M.Z.; Aslam, M.N. Evaluation of resistance to root-knot nematode (Meloidogyne incognita) in okra cultivars. Crop Protect. 2014, 56, 25–30. [Google Scholar] [CrossRef]
- Anamika, S.; Sobita, S. Variation in Life Cycle of Meloidogyne incognita in Different Months in Indian Condition. Intern. J. Sci. Res. 2012, 3, 2286–2288. [Google Scholar]
- Sikora, R.A.; Fernandez, E. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI: Wallingford, UK, 2005; pp. 319–322. [Google Scholar]
- Ragozzino, A.; d’Errico, G. Interactions between nematodes and fungi: A concise review. Redia 2011, 94, 123–125. [Google Scholar]
- Zhang, Y.; Li, S.; Li, H.; Wang, R.; Zhang, K.-Q.; Xu, J. Fungi–Nematode Interactions: Diversity, Ecology, and Biocontrol Prospects in Agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Greco, N.; Minuto, A.; d’Errico, F.P.; d’Errico, G. Synthetic nematicides in Italy past and present role in the management of plant parasitic nematodes and future perspectives of control. Redia 2024, 107, 107–130. [Google Scholar] [CrossRef]
- UE. CropLife Europe’s Statement on Farm to Fork Targets Trends for 2022. 2024. Available online: https://croplifeeurope.eu/croplife-europes-statement-on-farm-to-fork-targets-trends-for-2022/ (accessed on 1 May 2025).
- Ngabirano, H.; Birungi, G. Pesticide Residues in Vegetables Produced in Rural South-Western Uganda. Food Chem. 2022, 370, 130972. [Google Scholar] [CrossRef] [PubMed]
- Desaeger, J.A.; Wram, C.; Zasada, I. New reduced-risk agricultural nematicides—Rationale and review. J. Nematol. 2020, 52, e2020-91. [Google Scholar] [CrossRef] [PubMed]
- Keating, B.A.; Carberry, P.S.; Bindraban, P.S.; Asseng, S.; Meinke, H.; Dixon, J. Eco-efficient Agriculture: Concepts, Challenges, and Opportunities. Crop Sci. Soc. Am. 2010, 50, 109–119. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L. Global food security—The impact of veterinary parasites and parasitologists. Vet. Parasitol. 2013, 195, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Katan, J.; Greenberger, A.; Alon, H.; Crinstein, A. Solar heating by polyethylene mulching for the control of diseases caused by soil-borne pathogens. Phytopathology 1976, 66, 683–688. [Google Scholar] [CrossRef]
- Candido, V.; D’Addabbo, T.; Basile, M.; Castronuovo, D.; Miccolis, V. Long Time Effect of Soil Solarization Integrated with Dazomet or Chicken Manure on Yield, Weeds and Root-Knot Nematodes in Tomato and Melon. Ital. J. Agron. 2008, 3, 233–298. [Google Scholar] [CrossRef]
- Soppelsa, O.; Giacometti, R.; d’Errico, G.; D’Alessio, M. Effectiveness of soil solarization combined with a plant-derived formulation for the control of the root-knot nematode Meloidogyne incognita (Kofoid et White) Chitw. in greenhouse tomato. Redia 2011, 94, 163–166. [Google Scholar]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Ann. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Mocali, S.; Landi, S.; Curto, G.; Dallavalle, E.; Infantino, A.; Colzi, C.; d’Errico, G.; Roversi, P.F.; D’Avino, L.; Lazzeri, L. Resilience of soil microbial and nematode communities after biofumigant treatment with defatted seed meals. Ind. Crops Prod. 2015, 75, 79–90. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Brennan, R.J.B.; Glaze-Corcoran, S.; Wick, R.; Hashemi, M. Biofumigation: An alternative strategy for the control of plant parasitic nematodes. J. Integr. Agric. 2020, 19, 1680–1690. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Curto, G.; Santi, R.; Carella, A. Control of root-knot nematode Meloidogyne incognita by Quillaja saponaria extracts. In Atti Giornate Fitopatologiche; Brunelli, A., Canova, A., Collina, M., Eds.; Università di Bologna: Bologna, Italy, 2006; pp. 239–242. [Google Scholar]
- d’Errico, G.; Giacometti, R.; Soppelsa, O.; D’Alessio, M. Effectiveness of plant-derived formulations against the root-knot nematode Meloidogyne incognita (Kofoid et White) Chitw. in a protected tomato crop. Redia 2011, 94, 167–169. [Google Scholar]
- d’Errico, G.; Woo, S.L.; Lombardi, N.; Mangabiello, G.; Roversi, P.F. Activity of chestnut tannins against the southern root-knot nematode Meloidogyne incognita. Redia 2018, 101, 53–59. [Google Scholar] [CrossRef]
- d’Errico, G.; Sasanelli, N.; Guastamacchia, F.; Stillittano, V.; D’Addabbo, T. Efficacy of Azadirachtin in the Integrated Management of the Root Knot Nematode Meloidogyne incognita on Short- and Long-Cycle Crops. Plants 2023, 12, 1362. [Google Scholar] [CrossRef] [PubMed]
- Mwamula, A.O.; Kabir, M.F.; Lee, S.W. A Review of the Potency of Plant Extracts and Compounds from Key Families as an Alternative to Synthetic Nematicides: History, Efficacy, and Current Developments. Plant Pathol. J. 2022, 38, 53–77. [Google Scholar] [CrossRef] [PubMed]
- d’Errico, G.; Marra, R.; Crescenzi, A.; Davino, S.W.; Fanigliulo, A.; Woo, S.L.; Lorito, M. Integrated management strategies of Meloidogyne incognita and Pseudopyrenochaeta lycopersici on tomato using a Bacillus firmus-based product and two synthetic nematicides in two consecutive crop cycles in greenhouse. Crop Prot. 2019, 122, 159–164. [Google Scholar] [CrossRef]
- d’Errico, G.; Mormile, P.; Malinconico, M.; Bolletti Censi, S.; Laudonia, S.; Lanzuise, A.; Crasto, A.; Woo, S.L.; Marra, R.; Lorito, M.; et al. Trichoderma spp. and a carob (Ceratonia siliqua) galactomannan to control the root-knot nematode Meloidogyne incognita on tomato plants. Can. J. Plant Pathol. 2021, 43, 267–274. [Google Scholar] [CrossRef]
- d’Errico, G.; Greco, N.; Vinale, F.; Marra, R.; Stillittano, V.; Davino, S.W.; Woo, S.L.; D’Addabbo, T. Synergistic Effects of Trichoderma harzianum, 1,3 Dichloropropene and Organic Matter in Controlling the Root-Knot Nematode Meloidogyne incognita on Tomato. Plants 2022, 11, 2890. [Google Scholar] [CrossRef] [PubMed]
- D’Addabbo, T.; Landi, S.; Palmieri, D.; Piscitelli, L.; Caprio, E.; Esposito, V.; d’Errico, G. Potential Role of the Yeast Papiliotrema terrestris Strain PT22AV in the Management of the Root-Knot Nematode Meloidogyne incognita. Horticulturae 2024, 10, 472. [Google Scholar] [CrossRef]
- Linford, M.B.; Yap, F.; Oliveira, J.M. Reduction of soil populations of the root-knot nematode during decomposition of organic matter. Soil Sci. 1938, 45, 127–142. [Google Scholar] [CrossRef]
- Akhtar, M.; Alam, M.M. Utilization of waste materials in nematode control: A review. Bioresour. Technol. 1993, 45, 1–7. [Google Scholar] [CrossRef]
- D’Addabbo, T. The nematicidal effect of organic amendments: A review of the literature, 1982–1994. Nematol. Mediterr. 1994, 23, 299–305. [Google Scholar]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Matisic, M.; Dugan, I.; Bogunovic, I. Challenges in sustainable agriculture—The role of organic amendments. Agriculture 2024, 14, 643. [Google Scholar] [CrossRef]
- Jaffee, B.A.; Ferris, H.; Stapleton, J.J.; Norton, M.V.K.; Muldoon, A.E. Parasitism of nematodes by the fungus Hirsutella rhossiliensis as affected by certain organic amendments. J. Nematol. 1994, 26, 152–161. [Google Scholar] [PubMed]
- Kimpinski, J.; Gallant, C.F.; Henry, R.; Macleod, J.A.; Sanderson, J.B.; Sturz, A.V. Effect of compost and manure soil amendments on nematodes and yields of potato and barley: A 7-year study. J. Nematol. 2003, 35, 289–293. [Google Scholar] [PubMed]
- Barker, K.R.; Koenning, S.R. Developing sustainable systems for nematode management. Annu. Rev. Phytopathol. 1998, 36, 165–205. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Pennacchio, F.; Papini, R.; d’Errico, G.; Torrini, G.; Strangi, A.; Barabaschi, D.; Roversi, P.F. Evaluation of sheep grazing effects on nematode community, insect infes-tation and soil fertility in sweet chestnut orchards: A case of study. Redia 2016, 99, 117–126. [Google Scholar]
- Irshad, U.; Villenave, C.; Brauman, A.; Plassard, C. Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol. Biochem. 2011, 43, 2121–2126. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Oka, Y. From old-generation to next-generation nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Huang, P.-M.; Wang, M.-K.; Chiu, C.-Y. Soil mineral–organic matter–microbe interactions: Impacts on biogeochemical processes and biodiversity in soils. Pedobiologia 2005, 49, 609–635. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Bloem, J.; de Vries, F.T.; Kalbitz, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 2012, 4, 523–528. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, J.; Li, Y.; Koziol, L.; Podzikowski, L.; Delgado-Baquerizo, M.; Wang, G.; Zhang, J. Relationships between soil biodiversity and multifunctionality in croplands depend on salinity and organic matter. Geoderma 2023, 49, 116273. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Mai, W.F.; Lyon, H.H. Pictorial Key to Genera of Plant Parasitic Nematodes. In Pictorial Key to Genera of Plant Parasitic Nematodes; Art Craft of Ithaca, Inc.: Ithaca, NY, USA, 1962. [Google Scholar]
- Bongers, T. De Nematoden van Nederland; KNNV: Zeist, The Netherlands, 1988. [Google Scholar]
- Marinari-Palmisano, A.; Vinciguerra, M. Classificazione dei nematodi. In Nematologia Agraria Generale e Applicata; Ambrogioni, L., d’Errico, F.P., Greco, N.A., Marinari-Palmisano, A., Roversi, P.F., Eds.; Società Italiana di Nematologia: Bari, Italy, 2014; pp. 23–42. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.; Freckman, D.; Georgieva, S. Feeding habits in soil nematode families and genera in outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar] [PubMed]
- Okada, H.; Harada, H.; Kadota, I. Fungal-feeding habits of six nematode isolates in the genus Filenchus. Soil Biol. Biochem. 2005, 37, 1113–1120. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T.; De Goede, R. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST—Palaeontological statistics, ver. 1.89. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of currently used pesticides in soils and earthworms: A silent threat. Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Nicholson, P.S.; Hirsch, P.R. The effects of pesticides on the diversity of culturable soil bacteria. J. Appl. Microbiol. 1988, 84, 551–558. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; McSorley, R.; Kokalis-Burelle, N. Effects of cover cropping, solarization, and soil fumigation on nematode communities. Plant Soil 2006, 286, 229–243. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Jiméenez, L.; Alonso-Prados, J.L.; García-Baudí, J.M. Nematodes as indicators of fumigant effects on soil food webs in strawberry crops in Southern Spain. Ecol. Indic. 2010, 10, 148–156. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Mauldin, M.D.; Habteweld, A.; Carter, E.T. Nematicide efficacy at managing Meloidogyne arenaria and non-target effects on free-living nematodes in peanut production. J. Nematol. 2020, 52, e2020-28. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Carletti, B.; Binazzi, F.; Cacini, S.; Nesi, B.; Resta, E.; Roversi, P.F.; Simoni, S. Impact of phytosanitary treatments with natural extractions on soil nematode community in pot farming. Redia 2024, 107, 53–64. [Google Scholar] [CrossRef]
- Hodson, A.K.; Milkereit, J.; John, G.C.; Doll, D.A.; Duncan, R.A. The effect of fumigation on nematode communities in California almond orchards. Nematology 2019, 21, 899–912. [Google Scholar] [CrossRef]
- Thoden, T.C.; Korthals, G.M.; Termorshuizen, A.J. Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management? Nematology 2011, 13, 133–153. [Google Scholar] [CrossRef]
- Landi, S.; Valboa, G.; Vignozzi, N.; d’Errico, G.; Pellegrini, S.; Simoncini, S.; Torrini, G.; Roversi, P.F.; Priori, S. Response of nematode community structure to different restoration practices in two vineyard soils in Tuscany (Italy). Biol. Agric. Hortic. 2023, 39, 149–169. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bezemer, M.T.; Bloem, J.; Bonkowski, M.; Christensen, S.; Dubois, D.; Ekelund, F.; Fliessbach, A.; Gunst, L.; Hedlund, K.; et al. Long-term organic farming forests below and above-ground biota: Implications for soil quality, biological control and productivity. Soil Biol. Biochem. 2008, 40, 2297–2308. [Google Scholar] [CrossRef]
- Hu, C.; Cao, Z.P. Nematode community structure under compost and chemical fertilizer management practice, in the North China plain. Expl Agric. 2008, 44, 485–496. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar] [PubMed]
| MI | BI | EI | SI | CI | |
|---|---|---|---|---|---|
| Mgmt Method | |||||
| 1.3D T2 | 1.42 ± 0.01 b | 148.00 ± 9.09 c | 84.99 ± 0.69 a | 4.64 ± 1.08 b | 1.37 ± 0.12 c |
| 1.3D T1 | 1.61 ± 0.02 a | 210.40 ± 0.02 a | 73.73 ± 1.13 b | 5.62 ± 0.44 b | 3.14 ± 0.34 a |
| PE | 1.44 ± 0.01 b | 217.56 ± 10.77 a | 85.40 ± 0.55 a | 13.22 ± 1.25 a | 2.83 ± 0.19 ab |
| CTR | 1.43 ± 0.01 b | 194.06 ± 8.61 b | 85.70 ± 0.61 a | 13.48 ± 1.25 a | 2.40 ± 0.28 b |
| Years | |||||
| 2022–23 | 1.42 ± 0.01 b | 185.23 ± 9.33 b | 85.04 ± 0.65 a | 8.83 ± 0.89 b | 1.96 ± 0.14 b |
| 2023–24 | 1.48 ± 0.01 a | 193.49 ± 7.33 a | 82.68 ± 0.78 b | 10.45 ± 1.15 a | 2.61 ± 0.21 a |
| Season | |||||
| June | 1.51 ± 0.01 a | 212.91 ± 9.16 b | 79.98 ± 0.99 b | 5.70 ± 0.70 b | 2.76 ± 0.32 a |
| November | 1.42 ± 0.01 b | 242.47 ± 10.53 a | 84.69 ± 0.64 a | 6.43 ± 1.00 b | 1.71 ± 0.19 b |
| February | 1.45 ± 0.02 b | 141.07 ± 6.03 d | 84.88 ± 1.10 a | 12.63 ± 1.59 a | 2.21 ± 0.17 b |
| May | 1.44 ± 0.02 b | 163.34 ± 7.51 c | 85.22 ± 0.85 a | 14.26 ± 0.14 a | 2.65 ± 0.25 a |
| p value | |||||
| Main effect | |||||
| M | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00001 |
| Y | 0.00001 | 0.50 | 0.18 | 0.0001 | 0.025 |
| S | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.0005 |
| Interaction | |||||
| M + Y | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.06 |
| M + S | 0.002 | 0.00001 | 0.00001 | 0.0014 | 0.002 |
| T + S | 0.00001 | 0.00001 | 0.00001 | 0.0001 | 0.22 |
| M + Y + S | 0.00001 | 0.00001 | 0.0005 | 0.00001 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Errico, G.; Landi, S. The Impact of Different Agricultural Practices on Nematode Biodiversity on Tomato- and Lettuce-Growing Periods Across Two Consecutive Years. Diversity 2025, 17, 501. https://doi.org/10.3390/d17080501
d’Errico G, Landi S. The Impact of Different Agricultural Practices on Nematode Biodiversity on Tomato- and Lettuce-Growing Periods Across Two Consecutive Years. Diversity. 2025; 17(8):501. https://doi.org/10.3390/d17080501
Chicago/Turabian Styled’Errico, Giada, and Silvia Landi. 2025. "The Impact of Different Agricultural Practices on Nematode Biodiversity on Tomato- and Lettuce-Growing Periods Across Two Consecutive Years" Diversity 17, no. 8: 501. https://doi.org/10.3390/d17080501
APA Styled’Errico, G., & Landi, S. (2025). The Impact of Different Agricultural Practices on Nematode Biodiversity on Tomato- and Lettuce-Growing Periods Across Two Consecutive Years. Diversity, 17(8), 501. https://doi.org/10.3390/d17080501







