Abstract
Although the topic of trampling of alpine vegetation has been addressed by many authors in recent years, many unanswered questions still remain. The generalization of vegetation response patterns to trampling would be valuable, especially for problematic alpine areas, which are unsuitable for large hiking loads. Such an area is the limestone Belianske Tatras, which has been closed to tourists since 1978. Only one trail has been accessible in the area since 1993 as a one-way trail, and since 2008 it has been used as an educational two-way trail. Since there is renewed discussion about making the Belianske Tatras accessible to tourists, we sought answers to the following questions: (1) regenerated communities are more resistant to trampling than the native ones; (2) individual species in different communities react to trampling in the same way; and (3) some species can disappear or become extinct after being trampled. We conducted research in the form of an experiment according to the standard Cole and Bayfield protocol. We trampled three plant communities in 2008, treating them as native, and in 2022, treating them as regenerated. The regenerated communities appeared to be more resistant, although this came at the expense of some species disappearing or becoming extinct as a delayed response. Re-opening the area could be considered.
1. Introduction
Alpine protected areas have recently faced not only climate change but also a significant number of tourists. Therefore, understanding the impacts of human trampling on the alpine environment is essential for the use and management of recreational areas []. The impact of human trampling is extensive and can easily lead to the degradation of natural systems []. Several studies have shown that recreational trampling can have a range of effects on vegetation and soil in alpine ecosystems [,,]. Soil compaction leads to an increase in soil bulk density and a decrease in soil porosity, changing the water and temperature regime and altering the soil nutrient composition [,]. These effects on vegetation and soil may increase the surface runoff, which in turn contributes to soil erosion [,].
While we can study the impacts of trampling on abiotic components based on soil properties, geological conditions, and terrain characteristics, plants are living organisms, and their responses to trampling are much more complicated. There are no studies that provide a generalized understanding of how individual species, life forms, and the same communities in different parts of the world respond to trampling.
Early studies on trampling have focused on changes in vegetation cover [,]. Trampling causes a reduction in vegetation cover and height and changes in species diversity [,]. Pressure on vegetation also affects the number of flowers and the production of seeds []. These changes lead to reduced plant viability, less successful reproduction, and ultimately the disappearance or extinction of some less resistant plant species in affected communities [,].
Trampling can adversely affect natural habitats, leading to loss of vegetation and eventual soil degradation [,]. Tundra vegetation damaged for only a few seasons may take hundreds of years to recover or may not recover at all []. The impact of hiking contributes to uprooting, displacement of vegetation, and exposure of soil cover in the immediate vicinity of trails, leading to erosion [,,,].
Most researchers use experimental vegetation trampling methods that investigate the relationship between tourism intensity (trampling) and vegetation response [,]. Many experimental trampling methodologies are based on the standard protocol of Cole and Bayfield [], although authors often adapt them for local research needs. Initially, research was conducted mainly in the mountains []. Later, several researchers analyzed the experimental trampling of vegetation due to recreation in different areas [,]. However, only a few studies have focused on sensitive alpine environments [,,]. In this study, we focus on experimental trampling research in a high-altitude environment of the Tatra Mountains, the highest part of the Carpathians. This research was conducted at a very rare limestone site, namely the Belianske Tatras National Nature Reserve, which has been closed to tourists and scientists since 1978 due to damage by mass tourism. The studied communities, which were experimentally trampled, are located in the vicinity of a trail that was granted an exception and has been open to tourists as a one-way trail since 1993 and as a two-way nature trail since 2008. This research was conducted with the consent of the Ministry of the Environment of the Slovak Republic, the State Protection of the Slovak Republic, and the Tatra National Park Administration.
This research was carried out in the Juncetum trifidi Szafer et al. 1923 em. Krajina 1933 community. Although it does not belong to the endangered phytocenoses, as a pioneer community it has a significant protective function and contains endemic taxa. Another selected community was Junco trifidi-Callunetum vulgaris (Krajina 1933) Hadač ex Šibík et al. 2007, because it has a small-scale and rare occurrence in the Western Carpathians. The Seslerietum tatrae Domin 1929 cor. Kliment et al. 2005 community was selected because of its occurrence in a narrow altitude range of 1900–2000 m, with long-term high snow cover. Reopening the area could be considered; therefore, we needed to confirm or refute previously established claims about the impact of trampling on vegetation from work using the standard protocol of Cole and Bayfield []. However, we had to adapt the design of the research areas to the needs of investigating small-scale alpine communities in topographically diverse environments [], as very often, such areas are inhabited by several endangered or rare alpine communities.
The aim of this research is to answer the following questions: (1) regenerated communities are more resistant to trampling than the native ones; (2) all individual species in different communities react to trampling in the same way; and (3) some species may disappear or become extinct after trampling. Regenerated communities appeared to be more resistant, but this came at the expense of the disappearance or extinction of some species in the form of a delayed response. Individual species responded differently in different communities. We recommend monitoring vegetation recovery in the monitoring areas and verifying in the future whether there will be further delayed responses of vegetation to trampling and the extinction of other species.
2. Materials and Methods
2.1. The Area of Interest
The area of interest is the Tatra National Park (established in 1949), which has also been the Tatra Biosphere Reserve (since 1993). This research was conducted specifically in the Belianske Tatras National Nature Reserve (established in 1991) (Figure 1). Experimental human trampling was carried out in the following communities: Juncetum trifidi Szafer et al. 1923 em. Krajina 1933 (49 13.751 N; 20 13.179 E), Junco trifidi-Callunetum vulgaris (Krajina 1933) Hadač ex Šibík et al. 2007 (49 13.591 N; 20 13.313 E), and Seslerietum tatrae Domin 1929 cor. Climent et al. 2005 (49 23.467 N; 20 21.780 E). The valid names of the studied communities are given in accordance with the work of Kliment, Vlachovič [].
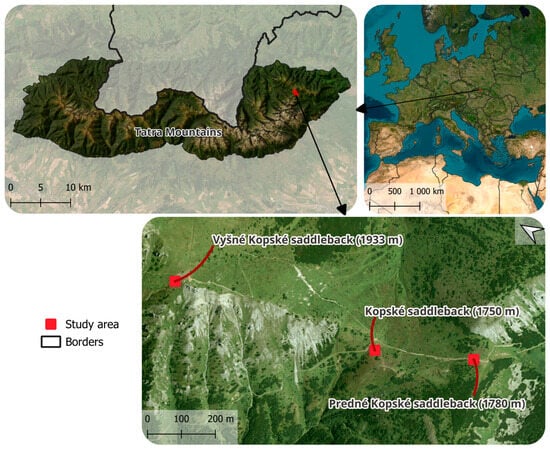
Figure 1.
The area of interest.
The Juncetum trifidi community Szafer et al. 1923 em. Krajina 1933 is not among the endangered phytocenoses, although it contains endemic taxa (Campanula tatrae, Leucanthemopsis tatrae, and Soldanella carpatica). The Junco trifidi-Callunetum vulgaris (Krajina 1933) Hadač ex Šibík et al. 2007 community, with a small-scale and rare occurrence in the Western Carpathians, is rare, but not yet threatened. The Seslerietum tatrae Domin 1929 cor. Climent et al. 2005 community is located in a narrow altitude range of 1900–2000 m ASL, with long-lasting high snow cover. The selection of plant associations for experimental research was carried out based on discussions with employees of the Tatra National Park Administration, as this is a destructive form of research that requires permits. Detailed information about individual study areas is provided in Table 1.

Table 1.
Detailed information about individual study areas.
The Kopské saddleback, with the Juncetum trifidi community, has a substrate that consists of limestone, dolomite, and slate. The soils are ranger cambisols and dystric cambisols. The experimental block was established on a NW-facing site with a slope of 22°, at an altitude of 1754 m ASL. The tourist trail passing near the experimental blocks has a low carrying capacity [].
The Predné Kopské saddleback, with the Junco trifidi-Callunetum vulgaris community, has a substrate that consists of limestone, dolomite, and slate, with rankers and dystric cambisols. The experimental block was established on a NE-facing site with a slope of 4° at an altitude of 1778 m ASL. The tourist trail passing near the experimental blocks has a moderate carrying capacity [].
The Vyšné Kopské, with the Seslerietum tatrae community, has a substrate that consists of limestone, dolomite, and slate. The soil is carbonate lithosoil. The community is spread on carbonate lithosoils. The experimental block was established on a SW-facing site with a slope of 39° at an altitude of 1924 m ASL. The tourist trail passing near the experimental blocks has a moderate carrying capacity [].
2.2. Experimental Block Design
Following the standard procedure of Cole and Bayfield [], we adapted the experimental block design for small-scale alpine plant communities and varied topographic relief []. One experimental block was established in each plant community. The experimental block consisted of three quadratic trampling plots with sides of 0.5 m separated by 0.5 m wide impact zones []. Each plot was divided into 25 quadratic subplots with sides of 0.1 m (Figure 2). The subplots were surveyed using a botanical grid (on the right side of the picture).
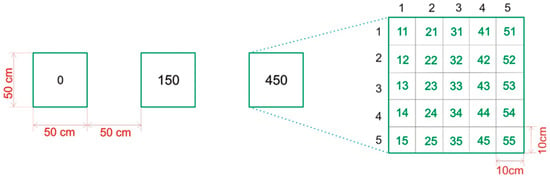
Figure 2.
Experimental block design. Quadrat 0 represents a control plot without trampling, quadrate 150 was trampled by 150 passes (75 tourists) and quadrat 450 was trampled by 450 passes (225 tourists). The numbers 11, 21, 31, etc., correspond to the row and column numbers.
One plot was a control plot and received no pedestrian pressure, while the other plots were exposed to progressive trampling intensities. The trampling treatment depended on the average traffic on the road in sunny and adverse weather. Each pass represented one footprint []. In the study area, we implemented a 150-passage and 450-passage traversal, i.e., a crossing area with 75 and 225 pedestrians on the same day []. The trampling direction should simulate a road, so trampling occurred in two directions. The trampling process was conducted over 1 day in June, July, August, and September of 2008 and 2022 [].
The parameters measured in each subplot are as follows:
- The cover (%) of vascular plant species (E1 layer), mosses, and lichens (E0 layer): Only green photosynthetic material was included in the cover estimates (visual estimates of the highest cover perpendicular to each subplot, and visual estimates of the cover of each species). Lichens and mosses were determined by a lichenologist and a bryologist.
- Bare ground cover (%): Bare ground in the form of either mineral or soil (visual estimates of the top cover of bare ground perpendicular to each subplot, and visual estimates of the ground cover of the surface).
- Litter cover (%): This included litter from recently trampled plants (visual estimates of the top litter cover perpendicular to each subplot, and visual estimates of litter cover per subplot).
To characterize the vulnerability of different vegetation types, we used the following formula to calculate the relative cover (RC) []:
where cf is the correction factor, calculated as follows:
In the absence of any change in coverage caused by trampling, the RC will be 100%. Therefore, we evaluated the resistance in the following ranges: 0.00–20.00%, very low; 20.01–40.00%, low; 40.01–60.00%, medium; 60.01–80.00%, high; and 80.01–100.00%, very high.
To describe changes in the relative coverage over time, we used linear regression models. The time variable represents the number of days since the first day of the first month (June) of each sampling session. Due to the nonlinear nature of some of the relationships, second-order polynomial regression models were used. The adjusted coefficient of determination (R2) was used to determine which model best and most simply described the collected data (i.e., linear or polynomial). All analyses were performed in R [] version 4.4.2.
3. Results
3.1. Question 1: Regenerated Plant Communities Are More Resistant to Trampling than Native Ones
When all three communities were considered, the resilience of the regenerated alpine communities to trampling was 6.47% higher than the resilience of the native, undisturbed ones. In the regenerated communities, we noted the absence of some moss, lichen, and hemicryptophyte species. They disappeared from the trampled areas between 2009 and 2022. This means that even though the regenerated communities appeared more resistant to repeated trampling after years, this was due to the absence of certain moss, lichen, or vascular plant species.
In response to trampling by 75 tourists per day, the Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities reacted with more resistance in the regenerated state, and the Seslerietum tatrae community in the native state (Table 2). In response to the trampling of 225 tourists per day, the Junco trifidi-Callunetum vulgaris and Seslerietum tatrae communities responded with more resistance than the regenerated ones, and the Juncetum trifidi community was more resistant than the native one. It was found that regenerated communities with less than 15% chamaephytes responded better to trampling by 75 tourists per day, and regenerated communities made up of almost one-third chamaephytes responded better to trampling by 225 tourists per day. It was also found that the regenerated communities whose chamaephytes covered a smaller area of the community (approximately up to 15%) responded better to trampling by 75 tourists per day, and regenerated communities whose chamaephytes covered a larger area (approximately 15% and more) responded better to trampling by 225 tourists per day.

Table 2.
Average RC values of individual communities in the native and regenerated states.
Juncetum trifidi response to 75 tourists per day: The regenerated community was more resistant to trampling (Figure 3). Although the E0 layer was significantly more resistant in the regenerated state (Figure 4), the lichen Thamnolia vermicularis was absent. The E1 layer was also more resistant in the regenerated state (Figure 4). However, the species Bistorta officinalis, Campanula tatrae, and Hieracium alpinum were absent in the regenerated state.
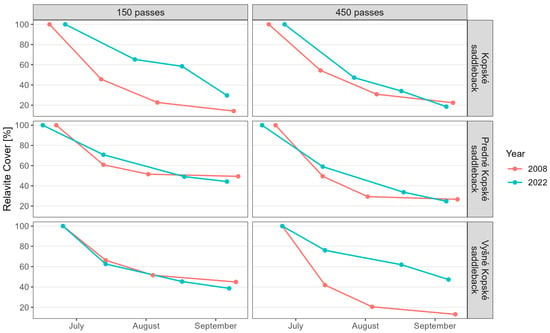
Figure 3.
Relative cover of the communities Juncetum trifidi (Kopské saddleback), Junco trifidi-Callunetum vulgaris (Predné Kopské saddleback), and Seslerietum tatrae (Vyšné Kopské saddleback) in 2008 and 2022. The values for relative cover, regression equations, R2, and the mean difference in relative cover per month are available in Supplementary Table S1a,b.
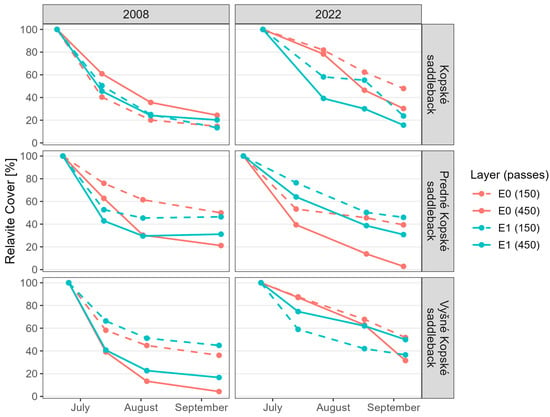
Figure 4.
Relative cover of the E1 and E0 layers of the communities Juncetum trifidi (Kopske saddleback), Junco trifidi-Callunetum vulgaris (Predne Kopske saddleback), and Seslerietum tatrae (Vysne Kopske saddleback) in 2008 and 2022. The values for relative cover, regression equations, R2, and the mean difference in relative cover per month are available in Supplementary Table S2a,b.
Juncetum trifidi response to 225 tourists per day: Although the resulting community response to trampling was approximately the same in the native and regenerated plots (Figure 3), the E0 and E1 layer resistance varied. The resistance of the E0 layer in the regenerated state was higher (Figure 4). However, the lichens Alectoria ochroleuca and Thamnolia vermicularis were absent. The resistance of the E1 layer in the regenerated state decreased (Figure 4). The species Bistorta officinalis, Campanula tatrae, and Hieracium alpinum were absent in the regenerated state.
Junco trifidi-Callunetum vulgaris response to 75 tourists per day: The regenerated community was approximately as resistant to trampling as the native one (Figure 3). However, the resistance of the E0 layer was reduced in the regenerated community (Figure 4), and the lichen Alectoria ochroleuca and the mosses Pleurozium schreberi and Polytrichasrum alpinum were absent. However, the E1 layer appears to have been more resistant in the regenerated state (Figure 4), but even here, species were absent, namely Bistorta officinalis, Campanula alpina, C. tatrae, Hieracium alpinum, Juncus trifidus, and Pulsatilla alpina subsp. alba.
Junco trifidi-Callunetum vulgaris response to 225 tourists per day: The resistance of the regenerated and native vegetation was almost the same (Figure 3). However, the resistance of the E0 layer in the regenerated community decreased (Figure 4), and the lichen Thamnolia vermicularis, the mosses Pleurozium schreberi and Polytrichasrum alpinum were absent. Layer E1 appears to have been more resistant in the regenerated state (Figure 4), but the species Bistorta officinalis, Campanula alpina, C. tatrae, Hieracium alpinum, Juncus trifidus, and Pulsatilla alpina subsp. alba were absent.
Seslerietum tatrae response to 75 tourists per day: The regenerated state was approximately as resistant to trampling as the native state (Figure 3). The resistance of the E0 layer in the regenerated community increased (Figure 4). The E1 layer appeared to be less resistant in the regenerated state (Figure 4), and Thymus pulcherrimus was absent.
Seslerietum tatrae response to 225 tourists per day: The regenerated state was significantly more resistant to trampling than the native state (Figure 3). The resistance of the E0 layer in the regenerated state increased (Figure 4). The E1 layer also appeared to be more resistant in the regenerated state (Figure 4), but the species Thymus pulcherrimus was absent.
3.2. Question 2: Individual Species Found in Different Communities Defend Themselves with the Same Resistance to Trampling
Each species creates a certain life form, i.e., its buds are located in different positions on the plant at different heights from the ground. Therefore, we assumed that these species would resist trampling with at least approximately the same resistance in different communities. However, this hypothesis was not confirmed. This means that it depended on the composition of the community, i.e., the combination of different life forms and the coverage of individual life forms. It also depended on the intensity of trampling.
Bistorta officinalis Delarbre is a perennial herb with a serpentine, coiled rhizome. It forms a geophyte/hemicryptophyte life form. Its seeds are spread by autochory, epichory (e.g., on animal fur), endochory (through the digestive tracts of animals), or hemerochory (through human contamination). In the native Juncetum trifidi community trampled by 75 tourists per day, this species was less resistant to trampling than in the Junco trifidi-Callunetum vulgaris community. However, it responded similarly to trampling by 225 tourists per day (Figure 5).
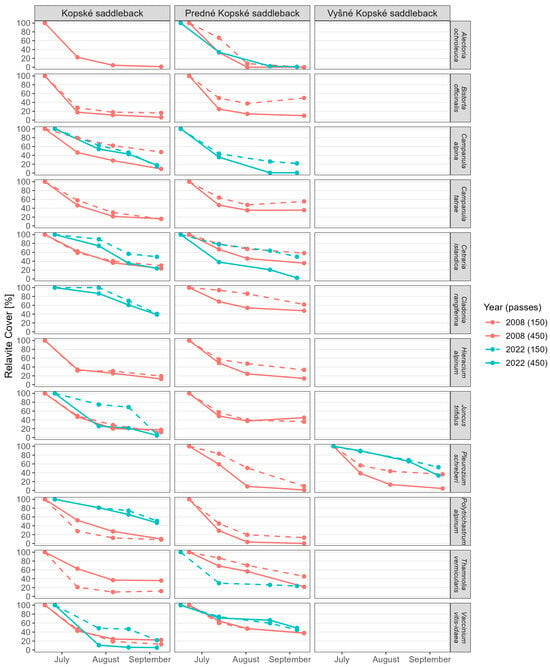
Figure 5.
Relative cover (RC) of individual species occurring in different communities. The values for relative cover, regression equations, R2, and the mean difference in relative cover per month are available in Supplementary Table S3a,b.
Campanula alpina Jacq. is an endangered species in Slovakia. It forms a hemicryptophyte life form. It is a biennial to perennial herb. Its seeds are spread by boleochory (wind blast). Its reactions to trampling were surprising. We recorded the regeneration of this species in September 2008 before trampling in the Juncetum trifidi community, on an area trampled by 225 tourists per day (Figure 6). In 2013 and 2014, this species was completely absent during the monitoring of regeneration. In 2022, this species occurred in the community again. Based on the botanical grid, we found that these were new plants. Their resistance was higher, precisely in the area trampled by 225 tourists per day in the regenerated Junco trifidi-Callunetum vulgaris community. No other species reacted this way. We recorded this species in the regenerated Junco trifidi-Callunetum vulgaris community. Looking at the surroundings, it was mainly found in areas trampled in 2008. It appeared to be more resistant to 75 tourists than to 225 tourists per day (Figure 5).
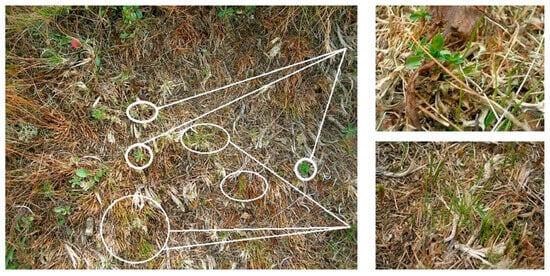
Figure 6.
Regeneration of Campanula alpina herb (detail top right) and Juncus trifidus grass (down right) on a trampled plot (left) by 225 tourists per day in the Juncetum trifidi community during a trampling experiment, September 2008 (Source: Piscová et al. []).
Campanula tatrae Borbás is an endemic species of the Western Carpathians, a perennial herb whose fruit is a drooping capsule. It has a hemicryptophyte life form. Its seeds are spread by boleochory (wind gusts) and endochory (through the digestive tracts of animals). In both native communities of Juncetum trifdi and Junco trifidi-Callunetum vulgaris, it was more resistant to trampling in areas trampled by 75 tourists compared to those trampled by 225 tourists per day (Figure 5).
Hieracium alpinum L. is a perennial plant whose seeds are spread by trichometeochory (they have flying devices). It has a hemicryptophyte life form. This hieracium occurred in the native Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities, where it appeared to be more resistant to trampling by 75 tourists per day (Figure 5).
Juncus trifidus L. is a perennial, densely tufted herb with a creeping rhizome. Its seeds are spread by cystometeorochory (they are very tiny, but swollen). It has the hemicryptophyte life form. This grass grew in the native Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities. In both communities, it was more resistant to trampling by 75 tourists per day in July and August, but in September, it appeared stronger against trampling by 225 tourists per day (Figure 5). The species Juncus trifidus is the second species (apart from Campanula alpina) whose regeneration we recorded in the process of experimental trampling in 2008, specifically before trampling in September (Figure 6). However, it was absent in the regenerated Junco trifidi-Callunetum vulgaris community. In the regenerated Juncetum trifidi community, it resisted trampling by 75 tourists more than by 225 tourists per day (Figure 5).
Vaccinium vitis-idaea L. is an evergreen and densely branched shrub, the fruit of which is a berry. This species is a woody chamaephyte, and its seeds are spread through the digestive tracts of animals. We recorded the occurrence of this species in both the native and regenerated Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities. In both the native and the regenerated Junco trifidi-Callunetum vulgaris communities, the species responded similarly, but was slightly more resistant to 225 tourists per day (Figure 5). In the regenerated Juncetum trifidi community, the species was more resistant to trampling by 75 tourists per day (Figure 5).
Alectoria ochroleuca (Hoffm.) Massal. is a fruticose lichen. We recorded this lichen species in the native Juncetum trifidi community, on an area trampled by 225 tourists per day. It was absent in the regenerated community. The species was recorded until 2014, later disappearing due to a delayed response to trampling. Its resistance to trampling was already very low in August and September 2008 (Figure 5). In the Junco trifidi-Callunetum vulgaris community, this species was present on both trampled areas. It did not survive in the area trampled by 75 tourists per day. However, it survived in the area trampled by 225 tourists per day and even responded with higher resistance in July (native community with fewer than 75 tourists per day) (Figure 5).
Cetraria islandica (L.) Ach. is a fruticose lichen, which was more resistant to trampling in the regenerated Juncetum trifidi community than in the native one, in all months and all plots. However, it was less resistant to trampling in the regenerated Junco trifidi-Callunetum vulgaris community than in the native one (Figure 5).
Cladonia rangiferina (L.) F. H. Wigg. is a fruticose cup lichen. This species was recorded in the regenerated Juncetum trifidi community and in the native Junco trifidi-Callunetum vulgaris community (Figure 4). In both cases, it was less resistant to trampling by 225 tourists per day (Figure 5).
Thamnolia vermicularis (Swartz) Ach. Ex Schaerer is a fruticose lichen. We recorded its presence in the native Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities. While in the Juncetum trifidi community it reacted more resistant to trampling by 225 tourists per day, in the Junco trifidi-Callunetum vulgaris community it was more resistant to 75 tourists per day (Figure 5). After regeneration, we recorded it only in the Junco trifidi-Callunetum vulgaris community, in a plot trampled by 75 tourists per day. However, its resistance in July, August, and September was only low (Figure 5).
Pleurozium schreberi (Brid.) Mitt. is a moss, a rarely fertile dioecious species. We recorded its presence in the native Junco trifidi-Callunetum vulgaris community and in the native and regenerated Seslerietum tatrae communities. In the native communities, it reacted more resistantly in the Seslerietum tatrae community, especially in August and September. After the regeneration of the communities, it was more resistant to trampling in the Seslerietum tatrae community (Figure 5). This species was absent in the regenerated Junco trifidi-Callunetum vulgaris community.
Polytrichastrum alpinum (Hedw.) G. L. Sm. is a moss, a dioecious species that bears fruit fairly often, with spores maturing in summer. We recorded its occurrence in the Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities (Figure 5). In the native communities, in the plots trampled by 75 tourists per day, it appeared to be more resistant in the Junco trifidi-Callunetum vulgaris community. In response to trampling by 225 tourists per day, it was restored in both communities approximately equally. In the regenerated Juncetum trifidi community, the moss was resistant to both numbers of tourists. This species was absent in the regenerated Junco trifidi-Callunetum vulgaris community.
3.3. Question 3: Trampling Can Cause Species Disappearance or Extinction
Several species of hemicryptophytes, bryophytes, and lichens in the communities disappeared as a delayed response to trampling. This hypothesis was confirmed, although we only considered it in the case of mosses and lichens. The reasons for the disappearance of specific species are not known. We discuss possible reasons in the discussion section.
In the regenerated Juncetum trifidi community, the lichens Alectoria ochroleuca and Thamnolia vermicularis and the hemicryptophytes Bistorta officinalis, Campanula tatrae, and Hieracium alpinum were absent. These species were absent in the area trampled by 75 and 225 tourists per day. A new species, the lichen Cladonia rangiferina, was also added to both trampled areas (Figure 7).
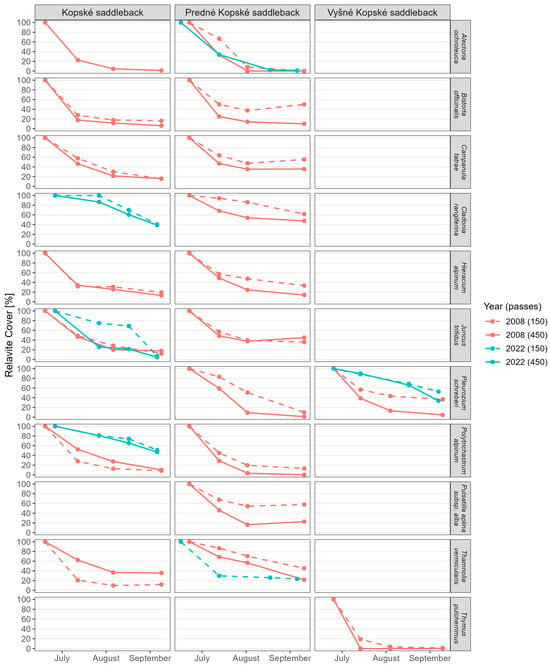
Figure 7.
RC values in June, July, August, and September in 2008 and 2022 of selected species that disappeared in trampled areas as a delayed response to experimental trampling of vegetation. The values for relative cover, regression equations, R2, and the mean difference in relative cover per month are available in Supplementary Table S3a,b.
In the regenerated Junco trifidi-Callunetum vulgaris community, the lichen Cladonia rangiferina, the mosses Pleurozium schreberi and Polytrichastrum alpinum, and the hemicryptophytes Bistorta officinalis, Campanula tatrae, Hieracium alpinum, Juncus trifidus, and Pulsatilla alpina subsp. alba were absent. These species were absent in the plot trampled by 75 tourists as well as in the plot trampled by 225 tourists per day. The lichen Alectoria ochroleuca was absent in the regenerated area trodden by 75 tourists per day, and the lichen Thamnolia vermicularis was absent in the area trodden by 225 tourists per day (Figure 7).
The dwarf semishrub chamaephyte Thymus pulcherrimus was absent in the regenerated Seslerietum tatrae community (Figure 7).
To quantify the relationship between relative cover (RC) and time under trampling pressure, we applied regression analysis for each area and trampling intensity (150 and 450 passes) in both 2008 and 2022 (Supplementary Table S1b), E0 and E1 layers (Supplementary Table S2b), and every species (Supplementary Table S3b). These regression equations provide a functional model describing how vegetation cover changes over time as a result of repeated disturbance. By including both linear and quadratic terms where appropriate, the models capture potential non-linear trends in vegetation degradation. The high adjusted R2 values (0.64–0.99) indicate strong explanatory power. These equations are not only valuable for interpreting past patterns of vegetation response but also offer a predictive framework that can be applied in future monitoring, restoration planning, or comparative assessments in similar alpine environments experiencing trampling pressure.
4. Discussion
We searched the literature for various claims that would confirm our conclusions. However, it seems that claims about the effects of trampling cannot be generalized. We realize that certain patterns of assumptions can be found when researching the effects of trampling on the abiotic components of the environment. When examining the biotic component, some general claims are only beginning to emerge.
Pescott and Stewart [] conducted a systematic review focused on assessing the impact of human trampling on vegetation. The study argues that vegetation dominated by hemicryptophytes and geophytes, life forms with greater protection for their permanent buds [], recovers to a greater extent than vegetation dominated by other life forms. Therefore, these could potentially be trampled more intensively, provided that monitoring is carried out to provide early warning of deterioration or unsustainable use. This statement is probably not generalizable, and it would be necessary to consider the intensity of visitation. In the case of the regenerated state of the Juncetum trifidi and Junco trifidi-Callunetum vulgaris communities, which were dominated by hemicryptophytes and contained less than 15% chamaephytes, they were more resistant to 75 tourists per day. However, this was not the case when 225 tourists per day passed through. Although the Junco trifidi-Callunetum vulgaris community remained more resistant to trampling in the regenerated state, the regenerated Juncetum trifidi community was less resistant than in the native state. The Junco trifidi-Callunetum vulgaris community responded to the intensity of trampling by 75 and 225 tourists per day similarly in both the native and regenerated states. However, when regenerated, it achieved higher resistance. In contrast to these two communities, the Seslerietum tatrae community, dominated by hemicryptophytes, but with woody and herbaceous chamaephytes making up a quarter of the vegetation, reacted differently. While it responded to trampling by 75 tourists per day more resistantly in its native state, it responded to trampling by 225 tourists per day significantly more resistantly in its regenerated state. This implies that if the vegetation is dominated by hemicryptophytes and less than a quarter is made up of chamaephytes, these communities may regenerate well with lower numbers of tourists. However, when vegetation is dominated by hemicryptophytes but at least a quarter is made up of chamaephytes, it responds better to higher numbers of tourists. However, this statement needs to be verified with a larger number of communities. In reality, however, these changes in resistance are related to decreases in the resistance of several species and, in particular, the likely disappearance of some species of mosses, lichens, and hemicryptophytes.
Pescott and Stewart [] also argued that even mild disturbances can have significant impacts on plant communities. Simple indicators, such as the life form of the dominant community, can then be useful for quickly assessing a community’s vulnerability to recreational pressure. We do not consider this statement to be general either. When we looked closely at the behavior of some hemicryptophytes in different communities, their reactions were not consistent. For example, these communities included the hemicryptophytes Campanula alpina and Juncus trifidus, as well as the woody chamaephyte Vaccinium vitis idaea. In particular, some species of mosses and lichens behave differently in different communities (Alectoria ochroleuca, Cestraria islandica, Pleurozium schreberi, Polytrichastrum alpinum, and Thamnolia vermicularis). Therefore, dividing recreation into high- and low-intensity zones with “honey-pots” located away from vulnerable vegetation, which, according to [], may be a more effective conservation strategy than encouraging moderately intensive but more widespread recreational use, is probably not feasible.
Pescott and Stewart [] tested a positive correlation between recovery time and resilience, which showed that after dieback, limited recovery may occur if there is a period without further disturbances. We agree with this statement. However, we also specify that such recovery is probably not long-term. We saw this in species that were absent from the regenerated communities in 2022. The community as a whole showed RC values close to complete regeneration, but this was accompanied by the disappearance of some species, e.g., in the Juncetum trifidi community, including hemicryptophytes Bistorta officinalis, Campanula tatrae and Hieracium alpinum, and lichens Alectoria ochroleuca and Thamnolia vermicularis.
Pescott and Stewart further argued [] that the negative correlation of recovery time for the hemicryptophyte subgroup is due to the fact that where recovery is not observed in the short term, other factors, such as changes in soil characteristics, may reduce the potential for full recovery. We agree with this statement because, for example, the hemicryptophyte Juncus trifidus regenerated during trampling in the native Juncetum trifidi community and was also present in the regenerated community. However, although it survived trampling in the native Junco trifidi-Callunetum vulgaris community, its regeneration was weaker compared to Juncetum trifidi, and it eventually disappeared.
Another claim by these and other authors [,] is that hemicryptophytes and geophytes will be more resistant to the impacts of trampling compared to other life forms. In contrast, chamaephyte-dominated communities die after trampling, despite their initially high resistance [,]. However, this statement cannot be generalized. As mentioned above, many species of hemicryptophytes react differently to trampling in different communities. In addition, while, e.g., in the Juncetum trifidi community, the hemicryptophytes Campanula aplina, C. tatrae, Hieracium alpinum, and Bistorta officinalis (hemicryptophyte/geophyte) did not survive trampling, the creeping willows Salix. kitaibeliata and S. retuculata were recorded in the regenerated community that was trampled again.
We found that individual species found in different communities do not respond with the same resistance to trampling. This may be related to the transformation of the ecological niche of individual species in different communities at different trampling intensities. In this consideration, we base our reasoning on the work of Odling-Smee et al. [], according to which niche construction is the process by which organisms modify their own and/or foreign niches. Since organisms and the way they interact with their environment are what cause niche construction, any or several abiotic and biotic, direct and indirect interactions can be the source of environmental modification and niche construction []. Older works have suggested that herbs and graminoids show little response to short-term trampling, and their recovery rate is linked to photosynthesis and growth rate, which is higher in graminoids, herbs, and deciduous dwarf shrubs than in evergreens [,]. However, under prolonged trampling, herbs completely disappear [], suggesting that their tolerance is reduced by their low resistance to trampling. Based on our observations, we agree with this statement.
Jahns [] and Törn et al. [] argued that lichens tolerate trampling quite well, and their coverage increases above their native level, which may be a result of the lichen tissue breaking down into smaller particles. After drying, lichens break very easily into small pieces, which have the ability to grow. We cannot generalize this statement either, as it does not apply to all lichen species. For example, while Cetraria islandica tolerates trampling well, species such as Cladonia rangiferina in the Junco trifidi-Callunetum vulgaris community or Thamnolia vermicularis in the Juncetum trifidi community did not survive trampling.
According to [,,,], bryophytes showed a delayed response to trampling. This is probably due to their slower growth [,] compared to vascular plants. This claim is debatable because, as mentioned above, many species of both hemicryptophytes and lichens have disappeared as a result of a delayed response to trampling.
There are many unresolved questions in the field of trampling. For example, the hypothesis that vegetation vulnerability to trampling may be related to primary productivity [] was not confirmed by Pescott and Stewart [].
We consider the detection of a delayed reaction of some plants to be very important because, in rare small-scale communities with the last individuals of some species, trampling can cause the extinction of such a species. Therefore, we recommend continuing long-term research with repeated experimental trampling. The results of long-term work can be used by managers of protected areas.
By reopening the hiking trails in the Belianske Tatras, a relatively small part of the area would be trampled. However, in this area, the very existence of the trail seems to be problematic. In the vicinity of the trails on steep slopes, large-scale erosion occurs, and the trails often slide down due to avalanches and intense rainfall []. This erosion also threatens valuable communities.
5. Conclusions
There has been much discussion recently about the reopening of hiking trails in the Belianske Tatras National Nature Reserve. The limestone NPP Belianske Tatras has been closed since 1978, with the exception of the trail leading through Monková valley from the Široké saddleback to the Kopské saddleback, due to destruction caused by mass tourism. Therefore, in this study, we address three basic questions.
The first question was that regenerated plant communities would be more resistant to trampling than native ones. This hypothesis was not confirmed. When all three communities were considered, the resistance of the regenerated alpine communities to trampling was 6.47% higher than the resistance of the native, undisturbed ones. In the regenerated communities, we noted the absence of some moss, lichen, and hemicryptophyte species before the repeated trampling experiment in 2022. These disappeared years after the trampling in 2008. This means that even though regenerated communities may have appeared to be more resistant to repeated trampling after years, this was due to the absence of some moss, lichen, or vascular plant species.
The second question was that individual species found in different communities defend themselves with the same resistance to trampling. Each species belongs to a certain life form, i.e., its buds are located in different positions on the plant at different heights from the ground. Therefore, we assumed that these species resist trampling with at least approximately the same resistance in different communities. However, this hypothesis was not confirmed. This means that it depends on the composition of the community, i.e., the combination of different life forms and the coverage of individual life forms. In some cases, however, the intensity of trampling also matters.
The third question was that some plant species could disappear or become extinct as a result of the trampling of vegetation. Unfortunately, this hypothesis was confirmed. Several species of hemicryptophytes, bryophytes, and lichens in the communities disappeared as a result of a delayed response to trampling. This hypothesis was confirmed, although we considered it only in the case of mosses and lichens. The reasons for the disappearance of specific species are not known.
The results of the experimental trampling research in the NPP Belianske Tatras, which we had the opportunity to carry out in both native and regenerated alpine communities, indicate that some previous statements in other studies cannot be generalized. The research opens up many other unresolved questions. The research results will be submitted to the Tatra National Park Administration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17070474/s1: Table S1a. Relative cover (%) of regenerated (2022) and native (2008) plant communities under trampling by 75 (150 passes) and 225 (450 passes) tourists in three study areas: Kopské saddleback (Juncetum trifidi). Predné Kopské saddleback (Junco trifidi-Callunetum vulgaris) and Vyšné Kopské saddleback (Seslerietum tatrae). Table S1b. Regression equations describing the change in relative cover (RC) as a function of the number of passes for each area in 2008 and 2022. The table includes the number of passes, adjusted R2 values, and the mean monthly decrease in RC. Table S2a. Relative cover (%) of E0 and E1 layers in regenerated (2022) and native (2008) plant communities under trampling 75 (150 passes) and 225 (450 passes) tourists across three study areas: Kopské saddleback (Juncetum trifidi), Predné Kopské saddleback (Junco trifidi-Callunetum vulgaris) and Vyšné Kopské saddleback (Seslerietum tatrae). Table S2b. Regression equations describing the change in relative cover (RC) as a function of the number of passes for each area in 2008 (native) and 2022 (regenerated), for E0 and E1 veg. layer. The table includes the number of passes, adjusted R2 values, and the mean monthly decrease in RC. Table S3a. Relative cover (%) of regenerated (2022) and native (2008) plant communities under trampling by 75 (150 passes) and 225 (450 passes) tourists across three study areas: Kopské saddleback (Juncetum trifidi), Predné Kopské saddleback (Junco trifidi-Callunetum vulgaris) and Vyšné Kopské saddleback (Seslerietum tatrae). Table S3b. Regression equations describing the change in relative cover (RC) as a function of the number of passes for each area in 2008 (native) and 2022 (regenerated), for each species. The table includes the number of passes, adjusted R2 values, and the mean monthly decrease in RC.
Author Contributions
All authors contributed meaningfully to this study. V.P., research topic; V.P., M.Š., A.S. and J.H., methodology, data acquisition, and analysis; F.P., methodology support; V.P., writing—original draft preparation; M.Š., J.H. and F.P., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic, and the Slovak Academy of Sciences, grant number VEGA 2/0031/23: Analysis and evaluations of the environmental history of selected types of Slovak landscape from the early prehistory to the present; by project KEGA 037UKF-4/2025: Integration of modern technologies and procedures into the teaching of spatial ecology; by Project APVV-20-0108: Implementation of Agenda 2030 through biosphere reserves.
Institutional Review Board Statement
This study did not require ethical approval.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was conducted with the consent of the Ministry of the Environment of the Slovak Republic, the State Protection of the Slovak Republic, and the Tatra National Park Administration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NPP | National nature reserve |
| RC | Relative cover |
References
- Li, Y.; Chen, K.; Liu, Z.; Cao, G. Short-term impacts of trampling on selected soil and vegetation properties of alpine grassland in Qilian Mountain National Park, China. Glob. Ecol. Conserv. 2022, 36, e02148. [Google Scholar] [CrossRef]
- Hill, W.; Pickering, C.M. Vegetation associated with different walking track types in the Kosciuszko alpine area, Australia. J. Environ. Manag. 2006, 78, 24–34. [Google Scholar] [CrossRef]
- Growcock, A.J. Impacts of Camping and Trampling on Australian Alpine and Subalpine Vegetation 2005. Ph.D. Thesis, Griffith University, Gold Coast, Australia, 2006. [Google Scholar]
- Pickering, C.M.; Growcock, A.J. Impacts of experimental trampling on tall alpine herbfields and subalpine grasslands in the Australian Alps. J. Environ. Manag. 2009, 91, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Siemann, E.; Deng, B.; Wang, S.; Zhang, L. Soil microbial community responses to soil chemistry modifications in alpine meadows following human trampling. Catena 2020, 194, 104717. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Yüksek, Y. Effect of visitor activities on surface soil environmental conditions and aboveground herbaceous biomass in Ayder natural park. Clean Soil Air Water 2009, 37, 170–175. [Google Scholar] [CrossRef]
- Marion, J.L.; Cole, D.N. Spatial and temporal variation in soil and vegetation impacts on campsites. J. Appl. Ecol. 1996, 6, 520–530. [Google Scholar] [CrossRef]
- Arocena, J.M.; Arocena, S.K.; Nepal, M. Rutherford Visitor-induced changes in the chemical composition of soils in backcountry areas of Mt Robson Provincial Park, British Columbia, Canada. J. Environ. Manag. 2006, 79, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Meinecke, E.P. A Report on the Effect of Excessive Tourist Travel on the California Redwood Parks; California State Printing Office: Sacramento, CA, USA, 1928.
- Bates, G.H. The vegetation of footpaths, sidewalks, cart-tracks and gateways. J. Ecol. 1935, 23, 470–487. [Google Scholar] [CrossRef]
- Kutiel, P.; Zhevelev, Y. Recreational use impact on soil and vegetation at picnic sites in Aleppo pine forests on Mount Carmel, Israel. Isr. J. Plant Sci. 2001, 49, 49–56. [Google Scholar] [CrossRef]
- Roovers, P.; Verheyen, K.; Hermy, M.; Gulinck, H. Experimental trampling and vegetation recovery in some forest and heathland communities. Appl. Veg. Sci. 2004, 7, 111–118. [Google Scholar] [CrossRef]
- Speight, M.C. Outdoor Recreation and Its Ecological Effects: A Bibliography and Review; University College: London, UK, 1973; Volume 4. [Google Scholar]
- Dale, D.; Weaver, T. Trampling effects on vegetation of the trail corridors of north Rocky Mountain forests. J. Appl. Ecol. 1974, 11, 767–772. [Google Scholar] [CrossRef]
- Cole, D.N. Minimizing conflict between recreation and nature conservation. In Ecology of Greenways: Design and Function of Linear Conservation Areas; Smith, D.S., Hellmund, P.C., Eds.; University of Minnesota Press: Minneapolis, MN, USA, 1993; pp. 105–122. [Google Scholar]
- Tomczyk, A.M.; Ewertowski, M. Quantifying short-term surface changes on recreational trails: The use of topographic surveys and ‘digital elevation models of differences’ (DODs). Geomorphology 2013, 183, 58–72. [Google Scholar] [CrossRef]
- Tomczyk, A.M.; Ewertowski, M. Planning of recreational trails in protected areas: Application of regression tree analysis and geographic information systems. Appl. Geogr. 2013, 40, 129–139. [Google Scholar] [CrossRef]
- Komarkova, V. Alpine Vegetation of the Indian Peaks Area, Front Range, Colorado Rocky Mountains. Ph.D. Dissertation, University of Colorado, Boulder, CO, USA, 1976; 655p. [Google Scholar]
- Zachar, D. Soil Erosion; Elsevier: New York, NY, USA, 2011. [Google Scholar]
- Fidelus, J. Slope transformations within tourist footpaths in the northern and southern parts of the Western Tatra Mountains (Poland, Slovakia). Z. Für Geomorphol. 2016, 60, 139–162. [Google Scholar] [CrossRef]
- Klug, B.; Scharfetter-Lehrl, G.; Scharfetter, E. Effects of trampling on vegetation above the timberline in the eastern Alps, Austria. Arct. Antarct. Alp. Res. 2002, 34, 377–388. [Google Scholar] [CrossRef]
- Hill, R.; Pickering, C.M. Differences in resistance of three subtropical vegetation types to experimental trampling. J. Environ. Manag. 2009, 90, 1305–1312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cole, D.N. Impacts of hiking and camping on soils and vegetation: A review. In Environmental Impacts of Ecotourism: Ecotourism Series; Buckley, R., Ed.; CABI Publishing: New York, NY, USA, 2004; pp. 41–60. [Google Scholar]
- Pescott, O.L.; Stewart, G.B. Assessing the impact of human trampling on vegetation: A systematic review and meta-analysis of experimental evidence. PeerJ 2014, 2, e360. [Google Scholar] [CrossRef]
- Cole, D.N.; Bayfield, N.G. Recreational trampling of vegetation: Standard experimental procedures. Biol. Conserv. 1993, 63, 209–215. [Google Scholar] [CrossRef]
- Kycko, M.; Zagajewski, B.; Lavender, S.; Romanowska, E.; Zwijacz-Kozica, M. The impact of tourist traffic on the condition and cell structures of alpine swards. Remote Sens. 2018, 10, 220. [Google Scholar] [CrossRef]
- Hertlová, B.; Popelka, O.; Zeidler, M.; Banaš, M. Alpine plant communities responses to simulated mechanical disturbances of tourism, case study from the High Sudetes Mts. J. Environ. Eng. Landsc. Manag. 2016, 7, 16–21. [Google Scholar]
- Willard, B.E.; Cooper, D.J.; Forbes, B.C. Natural regeneration of alpine tundra vegetation after human trampling: A 42-year data set from Rocky Mountain National Park, Colorado, USA. Arct. Antarct. Alp. Res. 2007, 39, 177–183. [Google Scholar]
- Chardon, N.I.; Rixen, C.; Wipf, S.; Doak, D.F. Human trampling disturbance exerts different ecological effects at contrasting elevational range limits. J. Appl. Ecol. 2019, 56, 1389–1399. [Google Scholar] [CrossRef]
- Piscová, V.; Ševčík, M.; Hreško, J.; Petrovič, F. Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia. Sustainability 2021, 13, 2750. [Google Scholar] [CrossRef]
- Kliment, J.; Valachovič, M. Plant Communities of Slovakia 4.—Alpine Vegetation (Rastlinné Spoločenstvá Slovenska 4.—Vysokohorská Vegetácia, in Slovak Language); VEDA: Bratislava, Slovakia, 2007; 388p. [Google Scholar]
- Piscová, V. Changes in the Vegetation of the Tatras at Selected Locations Influenced by Humans; VEDA, Slovak Academy of Sciences: Bratislava, Slovakia, 2011; 300p, ISBN 978-80-224-1220-9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.4.2; R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://www.R-project.org/ (accessed on 3 March 2025).
- Kent, M. Vegetation Description and Data Analysis: A practical Approach, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2012; Available online: http://www.amazon.co.uk/dp/0471490938 (accessed on 13 March 2025).
- Cole, D.N. Experimental trampling of vegetation. II. Predictors of resistance and resilience. J. Appl. Ecol. 1995, 32, 215–224. [Google Scholar] [CrossRef]
- Cole, D.N. Trampling disturbance of high-elevation vegetation, Wind river mountains, Wyoming, USA. Arct. Antarct. Alp. Res. 2002, 34, 365–376. [Google Scholar] [CrossRef]
- Odling-Smee, J.F.; Erwin, D.H.; Palkovacs, E.P.; Feldman, M.W.; Laland, K.N. Niche construction theory: A practical guide for ecologists. Q. Rev. Biol. 2013, 88, 3–28. [Google Scholar] [CrossRef]
- Odling-Smee, F.J.; Laland, K.N.; Feldman, M.W. Niche Construction: The Neglected Process in Evolution; Princeton University Press: Oxfordshire, UK, 2003. [Google Scholar]
- Chapin, F.S.; Chapin, M.C. Revegetation of an arctic disturbed site by native tundra species. J. Appl. Ecol. 1980, 17, 449–456. [Google Scholar] [CrossRef]
- Karlsson, P.S. In situ photosynthetic performance of four coexsisting dwarf shrubs in relation to light in a subarctic woodland. Funct. Ecol. 1989, 3, 485–487. [Google Scholar] [CrossRef]
- Pesonen, E.M. Kokeellisen Virkistyskäytön Kasvillisuusvaikutukset Oulangan Kansallispuistossa. Master’s Thesis, Department of Biology, University of Oulu, Oulu, Finland, 2003. [Google Scholar]
- Jahns, H.M. Sanikkaiset, Sammalet Ja Jäkälät; Otava: Helsinki, Finland, 1996. [Google Scholar]
- Törn, A.; Rautio, J.; Norokorpi, Y.; Tolvanen, A. Revegetation after shortterm trampling a subalpine heath vegetation. Ann. Bot. Fenn. 2006, 43, 129–138. [Google Scholar]
- Callaghan, T.V.; Emanuelsson, U. Population structure and processes of tundra plants and vegetation. In The Population Stucture of Vegetation; White, J., Ed.; Junk: Dordrecht, The Netherlands, 1985; pp. 399–439. [Google Scholar]
- Ukkola, R. Trampling tolerance of plants and ground cover in finnish lapland, with an example from the pyhätunturi national park. In Environmental Aspects of the Timberline in Finland and in the Polish Carpathians; Heikkinen, H., Obrebska-Starkel, B., Tuhkanen, S., Eds.; Uniwersytet Jagiellonski: Kraków, Poland, 1995; pp. 91–110. [Google Scholar]
- Rydgren, K.; Økland, R.H.; Økland, T. Population biology of the clonal moss Hylocomium splendens in Norwegian boreal spruce forests. IV. Effects of experimental fine-scale disturbance. Oikos 1998, 82, 5–19. [Google Scholar] [CrossRef]
- Liddle, M.J. A theoretical relationship between the primary productivity of vegetation and its ability to tolerate trampling. Biol. Conserv. 1975, 8, 251–255. [Google Scholar] [CrossRef]
- Hreško, J.; Bugár, G.; Petrovič, F. Changes of vegetation and soil cover in alpine zone due to anthropogenic and geomorphological processes. Landf. Anal. 2009, 18, 39–43. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).