Abstract
Spartina alterniflora invasion has posed severe ecological threats to coastal wetlands. Deep tillage is considered an effective physical method for ecological restoration in such wetlands; however, its effects on sediment nitrogen transformation processes remain unclear. In this study, we investigated the impacts of deep tillage on soil physicochemical properties and key nitrogen transformation pathways, including nitrification, denitrification, anammox, and DNRA, across different soil depths (0–10, 10–20, 20–30, 30–50, and 50–100 cm) in Spartina alterniflora-invaded coastal wetlands. Deep tillage significantly restructured the distribution of soil moisture (p < 0.05), pH (p > 0.05), electrical conductivity (p < 0.05), and nutrients, promoting NO3−-N accumulation in deeper layers while reducing NH4+-N concentrations in surface soils (p < 0.05). It markedly enhanced denitrification and DNRA rates (p < 0.05), suppressed surface nitrification (p < 0.05), and altered the vertical distribution of anammox activity. Correlation analysis revealed that NH4+-N and NO3−-N concentrations were the primary drivers of nitrogen transformation, with pH and electrical conductivity playing secondary roles. Overall, deep tillage stimulated nitrogen removal processes and affected net ammonium changes. These findings reveal that deep tillage can stimulate nitrogen removal processes by alleviating soil compaction and altering nitrogen transformation pathways, thus supporting biogeochemical recovery mechanisms after deep tillage. These insights provide scientific guidance for the ecological restoration of Spartina alterniflora-invaded coastal wetlands.
1. Introduction
Coastal wetlands serve as critical ecological buffer zones between terrestrial and marine ecosystems [1]. They play a pivotal role in maintaining the ecological balance of the land–sea interface, regulating regional climate, and facilitating biogeochemical cycles [2,3]. Biological invasions are a major threat to coastal wetland ecosystems, with Spartina alterniflora being one of the most aggressive invasive species worldwide [4,5,6]. By altering hydrology, sediment structure, and nutrient dynamics, the S. alterniflora invasion often disrupts native vegetation, reduces biodiversity, and fundamentally reshapes biogeochemical cycles, particularly nitrogen cycling processes [7,8,9].
Nitrogen transformation pathways, including nitrification, denitrification, anaerobic ammonium oxidation (Anammox), and dissimilatory nitrate reduction to ammonium (DNRA) [10,11], are highly sensitive to changes in soil physicochemical properties such as oxygen availability, redox potential, and organic matter distribution [12,13,14]. Nitrification converts ammonium (NH4+) into nitrate (NO3−) [15], whereas denitrification and Anammox remove reactive nitrogen from the system by producing gaseous nitrogen forms (N2, N2O) that eventually return to the atmosphere [11,16]. In contrast, DNRA retains nitrogen within the ecosystem by reducing nitrate to ammonium [17,18]. Together, these complementary processes maintain the dynamic balance of nitrogen cycling, determining both the nitrogen-buffering capacity of wetlands and their ability to mitigate nitrogen loss and accumulation, thereby safeguarding wetland ecological security [19,20].
However, the invasion of alien plants, particularly S. alterniflora, has been shown to degrade wetland ecological functions by altering soil structure and suppressing native species [21,22]. Specifically, S. alterniflora invasion significantly reduces denitrification rates [23], disrupts the balance between nitrification and Anammox processes, and weakens the nitrogen regulation capacity and water purification potential of wetlands. Additionally, it alters key soil properties, often resulting in enhanced nitrogen retention, shifts in greenhouse gas emissions, and changes in nutrient fluxes to adjacent coastal waters [24,25]. Similar patterns of ecosystem disruption have been documented for other invasive C4 grasses in coastal wetlands. For instance, Lopez Rosas et al. demonstrated that the invasive C4 grass Echinochloa pyramidalis significantly altered wetland structure and function in tropical freshwater marshes, with shade treatment proving effective in controlling its dominance and facilitating native species recovery [26]. These disruptions exacerbate the ecological vulnerability of coastal wetlands, highlighting the urgent need for effective restoration strategies to reverse such impacts and recover ecosystem functions.
Deep tillage, as a physical intervention, demonstrates several advantages, including ease of implementation and rapid results [27], and has been increasingly applied to coastal wetlands to disrupt dense root mats of S. alterniflora and improve soil aeration [28]. Previous studies suggest that deep tillage can promote organic matter mineralization and affect redox-sensitive processes [29]; however, its specific effects on sediment nitrogen cycling, especially the balance between nitrogen removal (denitrification, anammox) and nitrogen retention (DNRA), remain poorly understood. Therefore, understanding the dynamic relationship between soil physicochemical properties and nitrogen transformation functions under deep tillage treatment is of significant scientific value for optimizing coastal wetland restoration technologies. Yet, existing studies on how deep tillage specifically influences the balance between nitrogen removal and nitrogen retention remain insufficiently explored.
This study will examine the impact of deep tillage-induced disturbance on soil physicochemical properties and nutrient distribution and how deep tillage influences the dynamic relationship between nitrogen removal processes and nitrogen retention. We investigate how deep tillage treatment alters soil physicochemical properties and key nitrogen transformation processes across soil profiles in S. alterniflora-invaded coastal wetlands. We hypothesize that deep tillage enhances denitrification and DNRA processes by restructuring soil oxygen availability and nutrient distribution. The findings aim to provide mechanistic insights into the role of soil disturbance in wetland nitrogen cycling and to inform effective strategies for coastal wetland restoration.
2. Materials and Methods
2.1. Study Area
The study was conducted in the coastal wetland of southern Hangzhou Bay, located in the eastern part of Zhejiang Province, China. The area is bordered by the Qiantang River to the west and the East China Sea to the east, featuring a funnel-shaped estuarine geomorphology that is concave in the north and convex in the south [30]. The southern shoreline is mainly composed of muddy tidal flats and shallow intertidal zones, with a predominance of depositional convex coastlines formed by tidal flat accretion. The region experiences a subtropical monsoon climate, characterized by mild and humid conditions with distinct seasonal variation. The mean annual temperature is 16.1 °C, the average annual precipitation is 1351.1 mm, and the region receives approximately 2038 h of sunshine annually, with a frost-free period of 244 days [31]. The tidal regime is irregular semidiurnal, and the dominant soil type is saline soil. The geographic coordinates of the sampling area are 30°17′–30°19′ N and 121°5′–121°7′ E (Figure 1).

Figure 1.
Location of S. alterniflora control area in the Hangzhou Bay Zhejiang Province, China.
2.2. Experimental Design and Soil Sampling
The study was conducted in Yuyao, located along the southern coast of Hangzhou Bay, where the invasion of S. alterniflora has severely degraded the structure and ecological functions of coastal wetlands. As a commonly used restoration approach in this region, deep tillage to a depth of 0–100 after the tillage method effectively breaks up the dense belowground rhizome mats of S. alterniflora, disrupts its clonal propagation, and improves soil aeration and hydrological conditions, thereby inhibiting regrowth and promoting habitat recovery. Based on topographic elevation gradients, three sampling sites (SA1, SA2, and SA3) were established. SA1 was designated as the untreated control (CK), while SA2 and SA3 received deep tillage treatment (hereafter referred to as DT). Each treatment included three replicates to ensure statistical robustness and experimental reliability.
Soil samples were collected in April 2023 using a five-point sampling method. At each plot, five soil cores (5 cm diameter) were randomly extracted, and five cores per depth were composited into one representative sample. Sampling covered five depth intervals: 0–10 cm, 10–20 cm, 20–30 cm, 30–50 cm, and 50–100 cm. Samples were placed in coolers and immediately transported to the laboratory. After passing through a 2 mm sieve, subsamples were divided: one portion was stored at 4 °C for nitrogen transformation analysis, and the other was air-dried in a ventilated room for soil property measurements.
2.3. Soil Physicochemical Analysis
Soil moisture was measured using the gravimetric oven-drying method. Soil pH was determined in a 1:2.5 soil-to-water suspension using a pH meter (PHSJ-3F, INESA Scientific Instrument Co., Ltd., Shanghai, China) [32]. Electrical conductivity (EC) and salinity were measured from a 1:5 soil-water extract using a conductivity meter (DDS-307, INESA, Shanghai, China) [33]. Nitrate (NO3−-N) and ammonium (NH4+-N) were extracted with 2.0 M KCl and analyzed with a continuous flow analyzer (SAN++, Skalar Analytical B.V., Breda, the Netherlands). Total carbon (TC) and total nitrogen (TN) were determined using a C/N analyzer (Vario Max, Elementar, Langenselbold, Germany) [34]. Available phosphorus (AP) was measured by the phosphomolybdate blue method [35]. Soil organic carbon (SOC) was determined using the loss-on-ignition (LOI) method [36].
2.4. Determination of Soil Nitrogen Cycling Rates
Nitrification rates were determined by monitoring NO3− accumulation in ammonium-enriched aerobic soil suspensions, following a modified protocol based on standard potential nitrification rate (PNR) assays. Fresh soil samples were sieved (<2 mm) to remove debris. A 15 g aliquot of homogenized soil was weighed into a 150 mL conical flask, and 100 mL of 1 mM NH4Cl solution was added to provide an abundant ammonium substrate. A magnetic stir bar was used to homogenize the suspension, and the flask was loosely sealed with breathable film to allow gas exchange. Samples were incubated on a rotary shaker at 25 °C and 200 rpm in the dark to minimize photochemical interference. Subsamples (9.5 mL) were collected at 0, 4, 6, and 8 h. Prior to each sampling, the suspension was re-homogenized to ensure uniformity. Collected aliquots were centrifuged at 4000 rpm for 10 min, and the supernatants were stored at 4 °C prior to analysis. NO3− concentrations were measured spectrophotometrically at 220 nm and 275 nm, and final values were calculated using a standard calibration curve [37]. Non-amended blanks (without NH4Cl addition) were included as controls to correct for background nitrate production.
Potential denitrification and anammox rates were determined using a modified 15NO3− tracer-based slurry incubation method adapted from Xue et al. [38]. Fresh sediment was homogenized with deionized water at a 1:7 (w/v) ratio and continuously purged with helium for 30 min under magnetic stirring to establish an anaerobic environment. The slurry was then aliquoted into 12 mL Exetainer vials (Labco, High Wycombe, UK), sealed with butyl rubber septa, and pre-incubated at 25 °C in the dark for 24 h to eliminate residual NO3−, NO2−, and dissolved oxygen, following the protocol of Hou et al. [39]. After pre-incubation, each vial received 100 μL of helium-degassed 15NO3− solution (99 atom%, Cambridge Isotope Laboratories, Andover, MA, USA), yielding a final concentration of 12.5 mmol·L−1. Two sampling time points were applied: T0 samples were immediately fixed with 200 μL of 50% (w/v) ZnCl2, and T8 samples were incubated anaerobically for 8 h before quenching with the same ZnCl2 solution. Gas samples were analyzed using membrane inlet mass spectrometry (MIMS) to quantify the production of 29N2 and 30N2. Potential denitrification and anammox rates were calculated using the approach of Thamdrup and Dalsgaard [40].
Potential DNRA rates were determined using the same 15NO3− labeled anaerobic slurry incubation setup as described above for denitrification and anammox [41]. After pre-incubation, each vial received 100 μL of helium-degassed 15NO3− solution (99 atom%, Cambridge Isotope Laboratories, Andover, MA, USA) and continued to culture at 25 °C. Then, all headspace bottles were divided into two groups, one group as the starting sample and add 200 µL of 50% ZnCl2 solution inhibitor, and the other group as the terminating sample. Culturing was continued for 8 h before the inhibitor was added. After cultivation, high-purity helium gas was used to fully aerate the culture for 20 min again to eliminate nitrogen isotopes produced by denitrification and anaerobic ammonia oxidation. Subsequently, 200 µL of sodium hypobromite oxidant was added to oxidize the 15NH4+ generated by the DNRA process into 29N2 and 30N2. The resulting 15NH4+ produced via DNRA was oxidized to N2 gas using the hypobromite method, and 29N2 and 30N2 were quantified via membrane inlet mass spectrometry (MIMS, GAM200, IPI, Bremen, Germany). DNRA rates were calculated by using the equation of Li et al. [42].
2.5. Statistical Analyses
Data analyses were performed using SPSS software (v27.0, IBM Corp., Armonk, NY, USA). Prior to analysis, the normality of all variables was assessed, and no significant deviations from a normal distribution were detected. To assess the effects of the same treatment across different soil depths on soil physicochemical properties and nitrogen cycling rates (nitrification, denitrification, anammox, and DNRA), one-way analysis of variance (ANOVA) was conducted. Comparisons among treatments at the same soil depth were carried out using independent-sample t-tests. Duncan’s multiple range tests were employed to determine significant differences among treatments at the 0.05 level. All acquired data were represented by an average of three replicates and standard deviation (SD). To evaluate the relationships between nitrogen cycling rates and soil properties following DT, Pearson correlation coefficients were calculated using R software (v4.4.3; R Core Team, Vienna, Austria). Correlation plots were generated using the ggplot2 package (version 3.4.4, https://ggplot2.tidyverse.org/, accessed on 1 April 2025).
Partial least squares path modeling (PLS-PM) was used to analyze the direct and indirect effects of DT treatments on nitrogen transformation processes and soil physicochemical properties. This technique is effective for revealing causal relationships between observed and latent variables [43]. Simulation studies have shown that PLS-PM performs better with small sample sizes [44] and that model evaluation is not affected by sample size [45]. Path coefficients and the coefficient of determination (R2) were estimated using the pls-pm package in R (v4.4.3) with 999 bootstrap resampling iterations. The model’s goodness-of-fit was evaluated using the GoF index, with values greater than 0.7 indicating acceptable model fit [43]. All figures were produced in Origin pro 2025 and finalized in Adobe Illustrator 2024.
3. Result
3.1. Effects of Deep Tillage on Soil Physicochemical Properties
DT had no significant effect on overall soil moisture content (p > 0.05); however, in DT plots, moisture increased significantly with depth (p < 0.05), with the 50–100 cm layer exhibiting the highest value (Figure 2a). Although pH was not significantly altered (p > 0.05), a consistent upward trend with depth was observed under DT conditions (range 8.64–8.82) (Figure 2b). EC increased significantly in the 50–100 cm layer (p < 0.05), while no differences were noted at other depths. In both treatments, the surface layer (0–10 cm) showed higher EC than deeper layers, with this disparity becoming more pronounced after tillage (1.15–1.30 times) (Figure 2c). Salinity followed a similar vertical profile (Figure 2d). TC content was significantly reduced in the 20–50 cm range (p < 0.05), while surface (0–10 cm) and deep (50–100 cm) layers maintained significantly higher values than intermediate depths (p < 0.05, Figure 2e). SOC was unaffected overall but increased significantly at the surface layer post-treatment (p < 0.05, Figure 2f). TN content decreased by 44.68% in the surface layer under DT (p < 0.05), with no significant variation observed across other depths (p > 0.05, Figure 2g). NH4+-N concentrations declined across all layers, peaking at 10–20 cm, followed by 0–10 cm (Figure 2h). Conversely, NO3−-N increased significantly in the 30–50 cm and 50–100 cm layers (p < 0.05). While surface soils originally held 0.5–1.2 times more NO3−-N than deeper layers, this trend reversed under DT conditions (p < 0.05, Figure 2i). AP rose significantly in the 0–10 cm layer but declined in the 20–30 cm layer (p < 0.05). The AP peak shifted from 30–50 cm before tillage to the surface layer following treatment (Figure 2j).
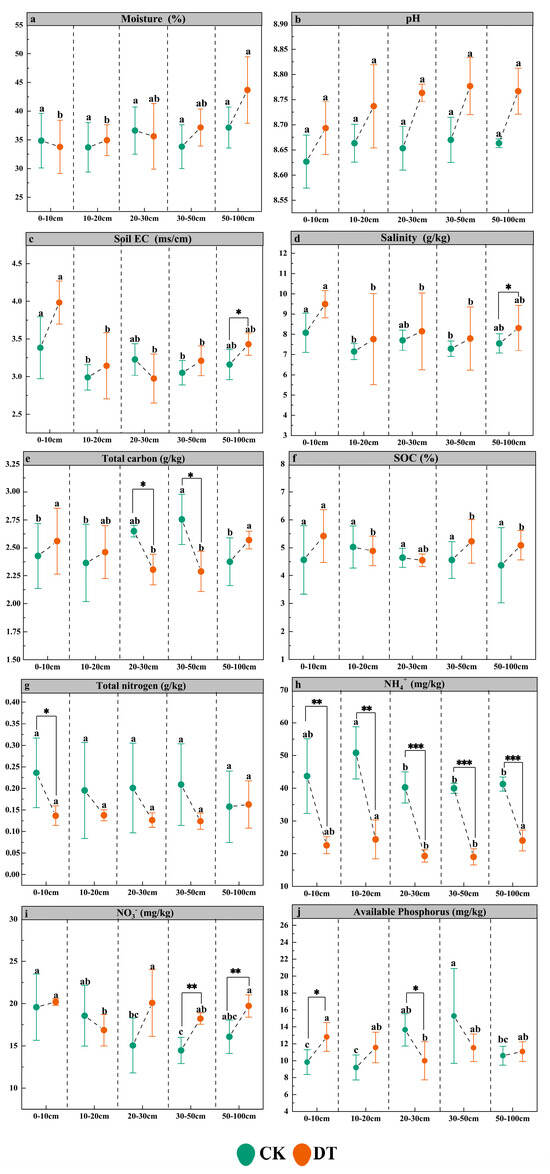
Figure 2.
The effects of deep tillage on soil physicochemical properties. Different lowercase letters indicate significant differences between different soil layers under the same treatment (p < 0.05); *, ** and *** denote significant differences between the control and deep tillage treatments at the same soil depth (p < 0.05, p < 0.01 and p < 0.001, respectively). (a) Soil moisture; (b) pH; (c) soil EC; (d) salinity; (e) total carbon (TC); (f) soil organic carbon (SOC); (g) total nitrogen (TN); (h) NH4+-N; (i) NO3−-N; (j) available phosphorus (AP).
3.2. Changes in Nitrification, Denitrification, Anammox, and DNRA Rates
DT markedly enhanced denitrification rates across all depths (p < 0.05), with the strongest increases observed in surface and mid-depth layers (18.33-, 7.89-, and 11.33-fold increases) and moderate enhancement at deeper layers. The vertical pattern shifted from 50–100 cm > 30–50 cm > 10–20 cm > 20–30 cm > 0–10 cm in the control to 10–20 cm > 50–100 cm > 30–50 cm > 0–10 cm > 20–30 cm under DT (Figure 3a). Nitrification was significantly suppressed in the 0–10 cm and 10–20 cm layers by 56.9% and 68.8% (p < 0.05), respectively, while showing a slight increase in deeper layers. A reversal of the pre-treatment pattern was evident: surface soils, previously showing the highest rates, exhibited lower activity than deeper layers post-treatment (Figure 3b). Anammox rates decreased by 3–30% in the mid- and deep soils (20–100 cm) but rose by 12–20% in the top 20 cm after DT treatment. Before tillage, peak anammox activity occurred in the deepest layer (50–100 cm); however, differences among layers became nonsignificant after DT treatment (Figure 3c, p > 0.05). DNRA was significantly promoted under DT (p < 0.05), with rates increasing by 80%, 85%, and 90% in the 20–30 cm, 30–50 cm, and 50–100 cm layers, respectively (Figure 3d). Previously, DNRA was highest at 0–10 cm and decreased with depth; this trend reversed under DT, where 50–100 cm exhibited the highest rate, with no significant variation across depths (p > 0.05).
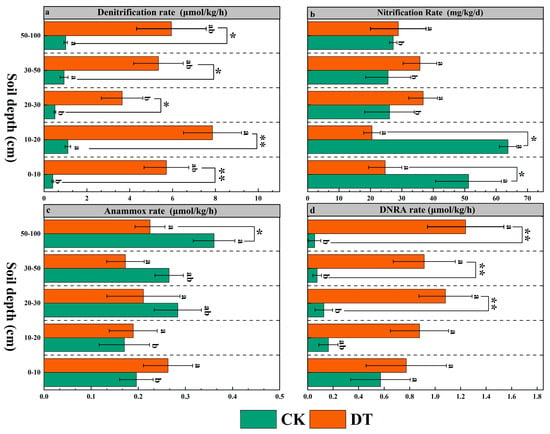
Figure 3.
The effects of deep tillage on soil nitrogen transformation rates. Different lowercase letters indicate significant differences between different soil layers under the same treatment (p < 0.05); * and ** denote significant differences between the control and deep tillage treatments at the same soil depth (p < 0.05 and p < 0.01, respectively). (a) Denitrification rate; (b) nitrification rate; (c) anammox rate; (d) dissimilatory nitrate reduction to ammonium (DNRA) rate.
3.3. Correlations Between Nitrogen Transformation Processes and Soil Physicochemical Properties
Spearman correlation analysis revealed strong linkages between nitrogen transformation processes and soil physicochemical parameters (Figure 4a). Nitrification was negatively correlated with NO3−-N and DNRA, while showing strong positive associations with TN and NH4+-N. Anammox rates were positively correlated with TN, pH, and NH4+-N and negatively correlated with DNRA. Denitrification and DNRA rates both negatively correlated with TN and NH4+-N. Denitrification also exhibited a strong positive correlation with NO3−-N and anammox, whereas DNRA showed a negative relationship with pH and a positive relationship with NO3−-N and EC. Additionally, TN was negatively correlated with NO3−-N and positively correlated with NH4+-N (p < 0.01), while pH showed a strong negative correlation with EC (p < 0.01).

Figure 4.
Spearman correlation analysis between Nitrification rate, Denitrification rate (DENA), Anammox rates (ANA), DNRA rate and environmental factors (a), * indicates significant correlation (p < 0.05); ** indicates highly significant correlation (p < 0.01), the solid line represents a positive correlation, and the dashed line represents a negative correlation. Directed graph of the partial least squares path models (PLS-PM). (b) Path model output: The numbers on the arrows represent the standardized path coefficients. The value of each path coefficient is indicated by the width of the arrow. Path coefficients (i.e., direct effects) represent the strength and direction of the linear relationships between variables, while indirect effects are represented by the product of the path coefficients between the predicted and response variables, incorporating the products of all possible paths excluding direct effects. (c) Standardized total effects (direct and indirect effects) of the PLS-PM on nitrogen transformation processes.
The PLS-PM model further revealed the regulatory pathways shaping nitrogen cycling under DT (Figure 4b,c). Denitrification, DNRA, and inorganic nitrogen accumulation (NH4+-N and NO3−-N) were strongly promoted, while TN and anammox rates were suppressed. TN had a strong positive effect on nitrification. Overall, the standardized total effects on nitrogen transformation were ranked as follows: inorganic nitrogen (NH4+-N and NO3−-N) > denitrification > DNRA > TN > nitrification > anammox. The model exhibited strong predictive power (goodness-of-fit = 0.70), highlighting the central role of nitrogen forms in regulating microbially mediated nitrogen pathways under DT conditions.
4. Discussion
4.1. Effects of Deep Tillage on Soil Properties and Nitrogen Transformation
This study demonstrates that DT reshapes soil physicochemical conditions and significantly alters nitrogen transformation processes in coastal wetland sediments. While DT had limited effects on overall moisture and pH, it clearly intensified their stratification along the soil profile [46,47], indicating its role in altering soil aeration and water–salt dynamics. Such vertical differentiation implies that microbial processes become increasingly niche-specific, and microbial functional guilds may stratify in response to oxygen gradients, redox status, and substrate availability [48]. Regarding nitrogen forms, DT facilitated the downward movement of NO3−-N and reduced surface NH4+-N, suggesting enhanced mineralization and nitrification driven by improved soil oxygen availability [49,50]. Moreover, denitrification rates increased across all depths, especially at 10–20 cm, implying that anaerobic microsites conducive to denitrifier activity formed after DT [49]. These observations align with previous findings that link tillage to the enhancement of coupled nitrification–denitrification in wetland systems [51]. DT also stimulated DNRA in mid- to deep soils, highlighting microbial preference for nitrogen retention under high carbon-to-nitrogen conditions [17]. The depth-specific shift in Anammox, with activity enhanced in surface soils and reduced at depth, may be related to the redistribution of organic carbon [52]. Interestingly, the depth-specific variation in nitrogen transformation pathways deserves closer examination. For instance, DNRA was significantly stimulated in mid to deep soils, highlighting a microbial preference for nitrogen retention under high C:N conditions [53]. This may be due to higher availability of labile carbon leached from upper layers, supporting fermentative and DNRA-favoring microbes at depth [54]. Conversely, Anammox activity increased in surface soils but declined with depth. This inversion could result from changes in organic carbon availability and competitive inhibition by heterotrophic denitrifiers, which dominate in deeper, less oxic environments with higher organic inputs [55]. These depth-specific differences suggest that DT not only alters mean soil conditions but may also restructure the vertical ecological niches and metabolic hierarchies of nitrogen-transforming microbes.
4.2. Regulation of Nitrogen Transformation by Soil Properties
Pearson correlation and PLS-PM analysis identified key environmental drivers of nitrogen cycling under DT. Inorganic nitrogen (NH4+-N, NO3−-N) directly influenced nitrification, denitrification, and DNRA [56]. Nitrification was positively associated with TN and NH4+-N, while NO3−-N exerted negative feedback, consistent with NO3−-N accumulation suppressing NH4+-N oxidation in nitrogen-saturated wetlands [57]. Denitrification and DNRA were both negatively correlated with TN and NH4+-N, suggesting substrate competition and redox regulation as key determinants of pathway dominance [54]. Elevated pH promoted DNRA, while acidification favored denitrification and phosphorus mobilization [58]. Additionally, EC was positively correlated with DNRA, potentially reflecting the adaptation of salt-tolerant microbial communities [59]. Collectively, these results indicate that DT restructures microbial nitrogen processing by modulating oxygen availability, organic carbon distribution, and ionic composition across soil depths.
4.3. Implications for Coastal Wetland Restoration
The result indicate that DT enhances denitrification and DNRA, thereby increas-ing both nitrogen removal and retention. This reduces the risk of nutrient overloading and eutrophication in coastal wetlands [60,61]. In Spartina alterniflora-invaded zones, DT helps restore soil structure and reestablish nitrogen cycling functions, making it a promising ecological restoration strategy [60]. However, nitrogen transformation processes rarely occur in isolation and are closely interconnected with other ecosystem components. When nitrogen concentrations fluctuate, especially post-DT, interactions with other biotic factors—such as microbial guilds, plant uptake, and macrofaunal activity—can significantly influence nutrient fate [17]. For instance, elevated NH4+-N and NO3−-N levels may stimulate the growth of fast-growing opportunistic plant species or favor certain microbial taxa with high nitrogen demand, thereby affecting plant-microbe and microbe–microbe interactions [62]. Moreover, altered nitrogen availability can shift competitive dynamics between denitrifiers, DNRA-performing bacteria, and Anammox bacteria, depending on local oxygen and carbon levels [63,64]. The recovery of native plant communities post-DT may also be modulated by these interactions, particularly through root exudate feedback that influences microbial activity and nutrient availability [65]. Therefore, a systems-level view is essential for wetland restoration design. Integrating DT with adaptive revegetation and hydrological management could better harness these interactions to stabilize nutrient cycling. Although this study employed active revegetation with Scirpus mariqueter, future research should assess whether residual or regenerating invasive grasses might still limit seedling establishment despite sufficient propagule supply. In such cases, targeted post-tillage management could be essential. Further studies should also examine how tillage depth, frequency, and vegetation jointly affect carbon and nitrogen dynamics to guide more adaptive and resilient restoration strategies.
5. Conclusions
This study comprehensively assessed the effects of DT on soil physicochemical properties and nitrogen transformation processes in S. alterniflora-remediated coastal wetlands. DT effectively restructured the soil environment, intensifying vertical gradients in moisture, pH, EC, and nitrate, and driving the accumulation of available phosphorus in surface soils and nitrate in deeper layers. Nitrogen cycling pathways were significantly altered: denitrification rates increased markedly across depths; surface nitrification was suppressed; Anammox activity rose at the surface but declined in deeper soils; and DNRA was strongly enhanced in subsurface layers. Correlation and path modeling revealed that inorganic nitrogen forms (NH4+-N, NO3−-N) were primary drivers, while pH and EC served as secondary regulators of microbial nitrogen transformations. Overall, DT improves nitrogen removal and capacity in degraded wetlands, offering a viable strategy for restoring nitrogen cycling in invaded ecosystems. Our findings provide a scientific basis for degraded coastal wetland recovery and support the development of multi-scale, process-oriented management strategies. Future research should prioritize long-term monitoring of nitrogen cycling stability post-DT under varying climatic and hydrological conditions. Additional studies are needed to clarify microbial community interactions. Integrating biogeochemical modeling with socio-ecological analysis may enhance DT’s role in adaptive restoration planning. Finally, explicitly linking these findings to broader wetland goals—such as climate mitigation, eutrophication control, and policy design—could further strengthen the practical relevance of DT in integrated restoration strategies.
Author Contributions
Conceptualization, N.L., X.S. and M.W.; methodology, J.G. and M.W.; investigation, J.G., P.J. and J.L.; formal analysis, J.G. and P.J.; data curation, P.J.; writing—original draft preparation, J.G.; writing—review and editing, N.L., X.S. and M.W.; funding acquisition, N.L., X.S. and M.W.; supervision, N.L. and X.S.; project administration, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Zhejiang Province Commonwealth Projects (Grant No. LQ23C030003); Special Fund for Basic Scientific Research Operations of Central Public Welfare Research Institutes (Grant No. CAFYBB2024MA029); the Cooperation Program between Zhejiang Province and the Chinese Academy of Forestry (Grant No. 2023SY11); the Zhejiang Provincial Science and Technology Program (Grant No. 2023C03120); the Pioneer and Leading Goose R&D Program of Zhejiang Province (Grant Nos. 2025C02050 and 2025C02230); and the National Key Research and Development Program of China (Grant No. 2023YFE0101700).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author.
Acknowledgments
We express our heartfelt thanks to Xiaohong Zhu from the Wetland Ecosystem Research Station of Hangzhou Bay for their dedicated assistance in field sampling and logistical support throughout the study. Their efforts in sample collection across challenging tidal wetland conditions were essential to the successful completion of this research. We also appreciate the support provided by the anonymous peer reviewers, whose constructive feedback significantly enhanced the quality of this manuscript.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Murray, N.J.; Worthington, T.A.; Bunting, P.; Duce, S.; Hagger, V.; Lovelock, C.E.; Lucas, R.; Saunders, M.I.; Sheaves, M.; Spalding, M. High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science 2022, 376, 744–749. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, W.; Liu, D.; Kang, H.; Freeman, C.; Xiang, J.; Lin, Y. Exotic Spartina alterniflora invasion alters ecosystem–atmosphere exchange of CH4 and N2O and carbon sequestration in a coastal salt marsh in China. Glob. Change Biol. 2015, 21, 1567–1580. [Google Scholar] [CrossRef]
- Hinshaw, S.E.; Tatariw, C.; Flournoy, N.; Kleinhuizen, A.; Taylor, C.; Sobecky, P.A.; Mortazavi, B. Vegetation loss decreases salt marsh denitrification capacity: Implications for marsh erosion. Environ. Sci. Technol. 2017, 51, 8245–8253. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Liu, L.; Tian, Y.; Yu, Z. Effects of Spartina alterniflora invasion on biogenic elements in a subtropical coastal mangrove wetland. Environ. Sci. Pollut. Res. 2015, 22, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Feagin, R.A.; Innocenti, R.A.; Hu, B.; He, M.; Li, H. Invasion and ecological effects of exotic smooth cordgrass Spartina alterniflora in China. Ecol. Eng. 2020, 143, 105670. [Google Scholar] [CrossRef]
- Fu, S.; Zheng, S.; Gao, W.; Wang, A.; Ma, X.; Sun, L.; Sun, T.; Shao, D. Effects of the water-sediment regulation scheme on the expansion of Spartina alterniflora at the Yellow River Estuary, China. Front. Environ. Sci. 2021, 9, 642442. [Google Scholar] [CrossRef]
- Yang, W.; An, S.; Zhao, H.; Xu, L.; Qiao, Y.; Cheng, X. Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China. Ecol. Eng. 2016, 86, 174–182. [Google Scholar] [CrossRef]
- Gao, D.; Li, X.; Lin, X.; Wu, D.; Jin, B.; Huang, Y.; Liu, M.; Chen, X. Soil dissimilatory nitrate reduction processes in the Spartina alterniflora invasion chronosequences of a coastal wetland of southeastern China: Dynamics and environmental implications. Plant Soil 2017, 421, 383–399. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Zeng, D.; Miao, J.; Wu, G.; Zhan, X. Nitrogen removal, microbial community and electron transport in an integrated nitrification and denitrification system for ammonium-rich wastewater treatment. Int. Biodeterior. Biodegrad. 2018, 133, 202–209. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Zhou, Y. Coupling anammox with heterotrophic denitrification for enhanced nitrogen removal: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2260–2293. [Google Scholar] [CrossRef]
- Rohe, L.; Apelt, B.; Vogel, H.-J.; Well, R.; Wu, G.-M.; Schlüter, S. Denitrification in soil as a function of oxygen availability at the microscale. Biogeosciences 2021, 18, 1185–1201. [Google Scholar] [CrossRef]
- Simek, M.; Cooper, J.E.; Picek, T.; Santrucckova, H. Denitrification in arable soils in relation to their physico-chemical properties and fertilization practice. Soil Biol. Biochem. 2000, 32, 101–110. [Google Scholar] [CrossRef]
- Anderson, C.R.; Peterson, M.E.; Frampton, R.A.; Bulman, S.R.; Keenan, S.; Curtin, D. Rapid increases in soil pH solubilise organic matter, dramatically increase denitrification potential and strongly stimulate microorganisms from the Firmicutes phylum. PeerJ 2018, 6, e6090. [Google Scholar] [CrossRef] [PubMed]
- Wendeborn, S. The chemistry, biology, and modulation of ammonium nitrification in soil. Angew. Chem. Int. Ed. 2020, 59, 2182–2202. [Google Scholar] [CrossRef]
- Kumar, M.; Lin, J.G. Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal—Strategies and issues. J. Hazard. Mater. 2010, 178, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rutting, T.; Boeckx, P.; Muller, C.; Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 2011, 8, 1779–1791. [Google Scholar] [CrossRef]
- Wang, S.; Pi, Y.; Song, Y.; Jiang, Y.; Zhou, L.; Liu, W.; Zhu, G. Hotspot of dissimilatory nitrate reduction to ammonium (DNRA) process in freshwater sediments of riparian zones. Water Res. 2020, 173, 115539. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.; Gell, P. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 1–43. [Google Scholar] [CrossRef]
- Ghaly, A.; Ramakrishnan, V. Nitrogen sources and cycling in the ecosystem and its role in air, water and soil pollution: A critical review. J. Pollut. Eff. Control. 2015, 3, 1–26. [Google Scholar]
- Cao, M.; Cui, L.; Sun, H.; Zhang, X.; Zheng, X.; Jiang, J. Effects of Spartina alterniflora invasion on soil microbial community structure and ecological functions. Microorganisms 2021, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Luan, Z.; Yan, D.; Li, J.; Xie, S.; Liu, Y.; Chen, L.; Li, M.; Wu, C. Effects of Spartina alterniflora invasion on soil carbon, nitrogen and phosphorus in Yancheng coastal wetlands. Lands 2022, 11, 2218. [Google Scholar] [CrossRef]
- Zhang, C.B.; Liu, W.L.; Luo, B.; Guan, M.; Wang, J.; Ge, Y.; Chang, J. Spartina alterniflora invasion impacts denitrifying community diversity and functioning in marsh soils. Geoderma 2020, 375, 114456. [Google Scholar] [CrossRef]
- Wigand, C.; Brennan, P.; Stolt, M.; Holt, M.; Ryba, S. Soil respiration rates in coastal marshes subject to increasing watershed nitrogen loads in southern New England, USA. Wetlands 2009, 29, 952–963. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, R.; Zhu, H.; Zhang, F.; Huang, B.; Peng, X. Effects of Spartina alterniflora invasion on active soil organic carbon in the coastal marshes. Chin. J. Soil. Sci. 2012, 43, 102–106. [Google Scholar]
- Lopez Rosas, H.; Moreno-Casasola, P.; Espejel Gonzalez, V.E. Shade treatment affects structure and recovery of invasive C 4 African grass E chinochloa pyramidalis. Ecol. Evol. 2015, 5, 1327–1342. [Google Scholar] [CrossRef]
- Shin, W.; Kim, J.; Song, Y.; Kang, H.; Byun, C. Soil tillage effect on the control of invasive Spartina anglica in a coastal wetland. Wetl. Ecol. Manag. 2024, 32, 397–408. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Zhang, X.; Wang, W.; Li, F.; Dong, L.; Kong, F.; Xi, M. Potential ecological impacts of physical control on Spartina alterniflora in coastal wetland: Migration and transformation of nutrients and the response of bacterial community structure. J. Clean. Prod. 2023, 398, 136556. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Mei, B.; Zhang, X.; Zheng, P.; Song, W.; Tian, B.; Han, G.; Xie, B. Effects of Spartina alterniflora control on soil carbon and nitrogen in coastal wetlands. Watershed Ecol. Environ. 2024, 6, 54–62. [Google Scholar] [CrossRef]
- Wei, W.; Mei, X.; Dai, Z.; Tang, Z. Recent morphodynamic evolution of the largest uninhibited island in the Yangtze (Changjiang) estuary during 1998–2014: Influence of the anthropogenic interference. Cont. Shelf Res. 2016, 124, 83–94. [Google Scholar] [CrossRef]
- Song, Z.; Shi, W.; Zhang, J.; Hu, H.; Zhang, F.; Xu, X. Transport mechanism of suspended sediments and migration trends of sediments in the central Hangzhou Bay. Water 2020, 12, 2189. [Google Scholar] [CrossRef]
- Takamoto, A.; Takahashi, T.; Togami, K. Estimation models from soil pH with a solid-to-liquid ratio of 1: 2.5 to pH measured by other methods using soils in Japan. Soil Sci. Plant Nutr. 2023, 69, 190–198. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. Methods Assess. Soil Qual. 1997, 49, 169–185. [Google Scholar]
- Yao, X.; Yu, K.; Wang, G.; Deng, Y.; Lai, Z.; Chen, Y.; Jiang, Y.; Liu, J. Effects of soil erosion and reforestation on soil respiration, organic carbon and nitrogen stocks in an eroded area of Southern China. Sci. Total Environ. 2019, 683, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xu, J.; Tang, J.; Baig, S.A.; Xu, X. Comparison of phosphorus determination methods by ion chromatography and molybdenum blue methods. Commun. Soil Sci. Plant Anal. 2013, 44, 2535–2545. [Google Scholar] [CrossRef]
- Konen, M.E.; Jacobs, P.M.; Burras, C.L.; Talaga, B.J.; Mason, J.A. Equations for predicting soil organic carbon using loss-on-ignition for north central US soils. Soil Sci. Soc. Am. J. 2002, 66, 1878–1881. [Google Scholar] [CrossRef]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen mineralization, immobilization, and nitrification. Methods Soil Anal. Part 2 Microbiol. Biochem. Prop. 1994, 5, 985–1018. [Google Scholar]
- Xue, D.; Yu, H.; Fang, Y.; Shan, J.; Xi, D.; Wang, Y.; Kuzyakov, Y.; Wang, Z.-L. 15N-tracer approach to assess nitrogen cycling processes: Nitrate reduction, anammox and denitrification in different pH cropland soils. Catena 2020, 193, 104611. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Liu, M.; Li, X.; Lin, X.; Yin, G.; Gao, J.; Deng, F.; Chen, F.; Jiang, X. Anaerobic ammonium oxidation and its contribution to nitrogen removal in China’s coastal wetlands. Sci. Rep. 2015, 5, 15621. [Google Scholar] [CrossRef]
- Thamdrup, B.; Dalsgaard, T. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbio. 2002, 68, 1312–1318. [Google Scholar] [CrossRef]
- Yin, G.; Hou, L.; Liu, M.; Liu, Z.; Gardner, W.S. A novel membrane inlet mass spectrometer method to measure 15NH4+ for isotope-enrichment experiments in aquatic ecosystems. Environ. Sci. Technol. 2014, 48, 9555–9562. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Twilley, R.R. Nitrogen dynamics of inundated sediments in an emerging coastal deltaic floodplain in mississippi river delta using isotope pairing technique to test response to nitrate enrichment and sediment organic matter. Estuar. Coasts. 2021, 44, 1899–1915. [Google Scholar] [CrossRef]
- Sanchez, G. PLS path modeling with R. Berkeley Trowchez Ed. 2013, 383, 551. [Google Scholar]
- Chin, W.W.; Marcolin, B.L.; Newsted, P.R. A partial least squares latent variable modeling approach for measuring interaction effects: Results from a Monte Carlo simulation study and an electronic-mail emotion/adoption study. Inf. Syst. Res. 2003, 14, 189–217. [Google Scholar] [CrossRef]
- Afthanorhan, A.; Awang, Z.; Aimran, N. Five common mistakes for using partial least squares path modeling (PLS-PM) in management research. Contemp. Manag. Res. 2020, 16, 255–278. [Google Scholar] [CrossRef]
- Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Wu, H.; Li, M.; Yan, L.; Zhang, K.; Wang, J.; Kang, X. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Tan, Z.; Li, Y.; Wang, X. Effects of water-table depth and soil moisture on plant biomass, diversity, and distribution at a seasonally flooded wetland of Poyang Lake, China. Chin. Geogr. Sci. 2015, 25, 739–756. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept and review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Rütting, T.; Schleusner, P.; Hink, L.; Prosser, J.I. The contribution of ammonia-oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Biol. Biochem. 2021, 160, 108353. [Google Scholar] [CrossRef]
- Afzal, M.R.; Naz, M.; Ashraf, W.; Du, D. The legacy of plant invasion: Impacts on soil nitrification and management implications. Plants 2023, 12, 2980. [Google Scholar] [CrossRef]
- Bateman, E.; Baggs, E. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Hu, B.L.; Shen, L.D.; Zheng, P.; Hu, A.H.; Chen, T.T.; Cai, C.; Liu, S.; Lou, L.P. Distribution and diversity of anaerobic ammonium-oxidizing bacteria in the sediments of the Qiantang River. Environ. Microbiol. Rep. 2012, 4, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Cuhel, J.; Saby, N.P.; Chèneby, D.; Chronakova, A.; Bru, D.; Arrouays, D.; Martin-Laurent, F.; Simek, M. Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ. Microbiol. 2009, 11, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Kartal, B.; Kuenen, J.v.; Van Loosdrecht, M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef]
- Davidson, E.A.; Hart, S.C.; Firestone, M.K. Internal cycling of nitrate in soils of a mature coniferous forest. Ecology 1992, 73, 1148–1156. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Ding, A.; Yuan, D.; Tan, Q.; Xing, Y.; Xie, E. Ecological insights into community interactions, assembly processes and function in the denitrifying phosphorus removal activated sludge driven by phosphorus sources. Front. Microbiol. 2021, 12, 779369. [Google Scholar] [CrossRef]
- Asghar, H.N.; Setia, R.; Marschner, P. Community composition and activity of microbes from saline soils and non-saline soils respond similarly to changes in salinity. Soil Biol. Biochem. 2012, 47, 175–178. [Google Scholar] [CrossRef]
- Wang, B.; Lin, X. Exotic Spartina alterniflora invasion enhances sediment N-loss while reducing N retention in mangrove wetland. Geoderma 2023, 431, 116362. [Google Scholar] [CrossRef]
- Gao, G.-F.; Li, P.-F.; Zhong, J.-X.; Shen, Z.-J.; Chen, J.; Li, Y.-T.; Isabwe, A.; Zhu, X.-Y.; Ding, Q.-S.; Zhang, S. Spartina alterniflora invasion alters soil bacterial communities and enhances soil N2O emissions by stimulating soil denitrification in mangrove wetland. Sci. Total Environ. 2019, 653, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B.; Strous, M.; Tegetmeyer, H.E. Microbial nitrate respiration–genes, enzymes and environmental distribution. J. Biotech. 2011, 155, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.M.; Rombouts, J.L.; Kuenen, J.G.; Kleerebezem, R.; van Loosdrecht, M.C. Role of nitrite in the competition between denitrification and DNRA in a chemostat enrichment culture. Amb. Express 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).