Contrasting Herbaceous Communities in South African Savannas: A Comparative Analysis of Density, Composition, and Diversity Across Three Bioregions
Abstract
1. Introduction
2. Study Area
2.1. Letlapa Pula Game Reserve: Central Bushveld
2.2. Selati Game Reserve: Mopaneveld
2.3. Kempiana Nature Reserve: Lowveld
3. Methods
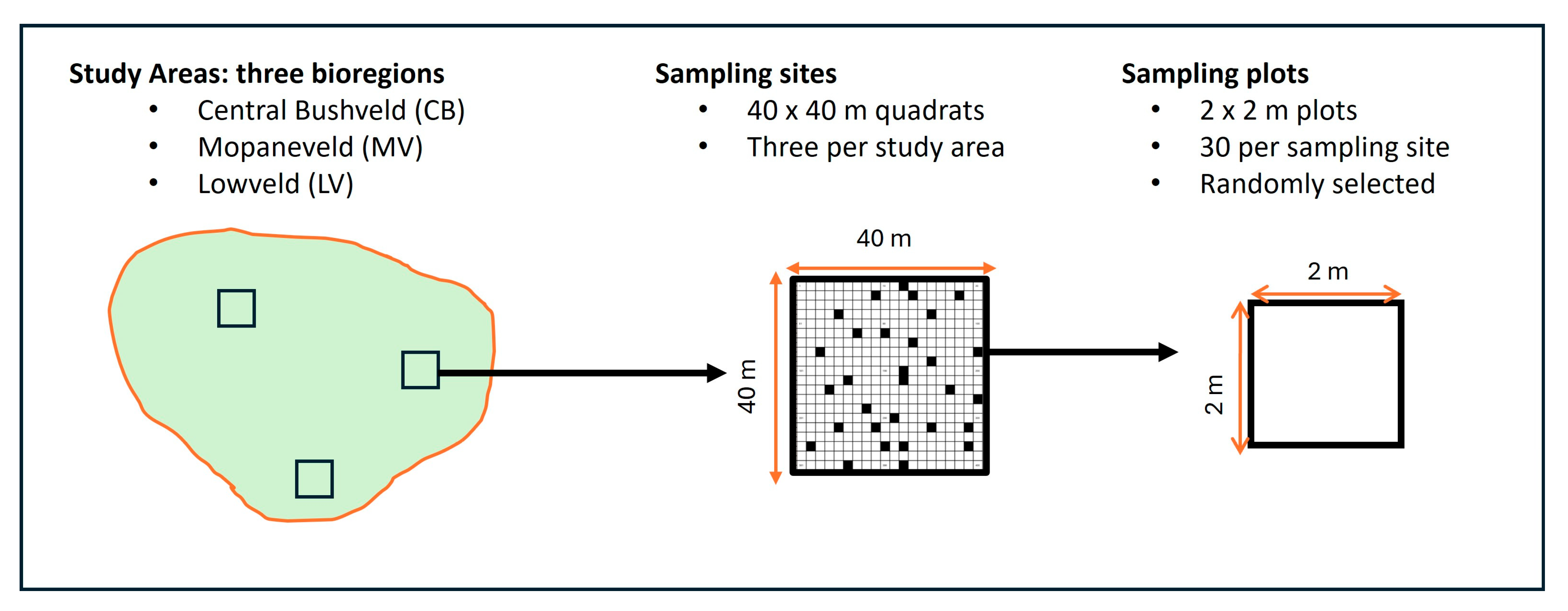
3.1. Sampling Design and Site Selection
3.2. Data Collection
3.3. Data Analysis
3.3.1. Calculation of Absolute Density and Species Richness per Quadrat
3.3.2. Comparison of Absolute Density and Species Richness Across Bioregions
3.3.3. Shannon Diversity Index (H)
3.3.4. Non-Metric Multidimensional Scaling
3.3.5. Analysis of Growth Forms
4. Results
4.1. Species Richness and Absolute Density
4.2. Shannon Diversity Index
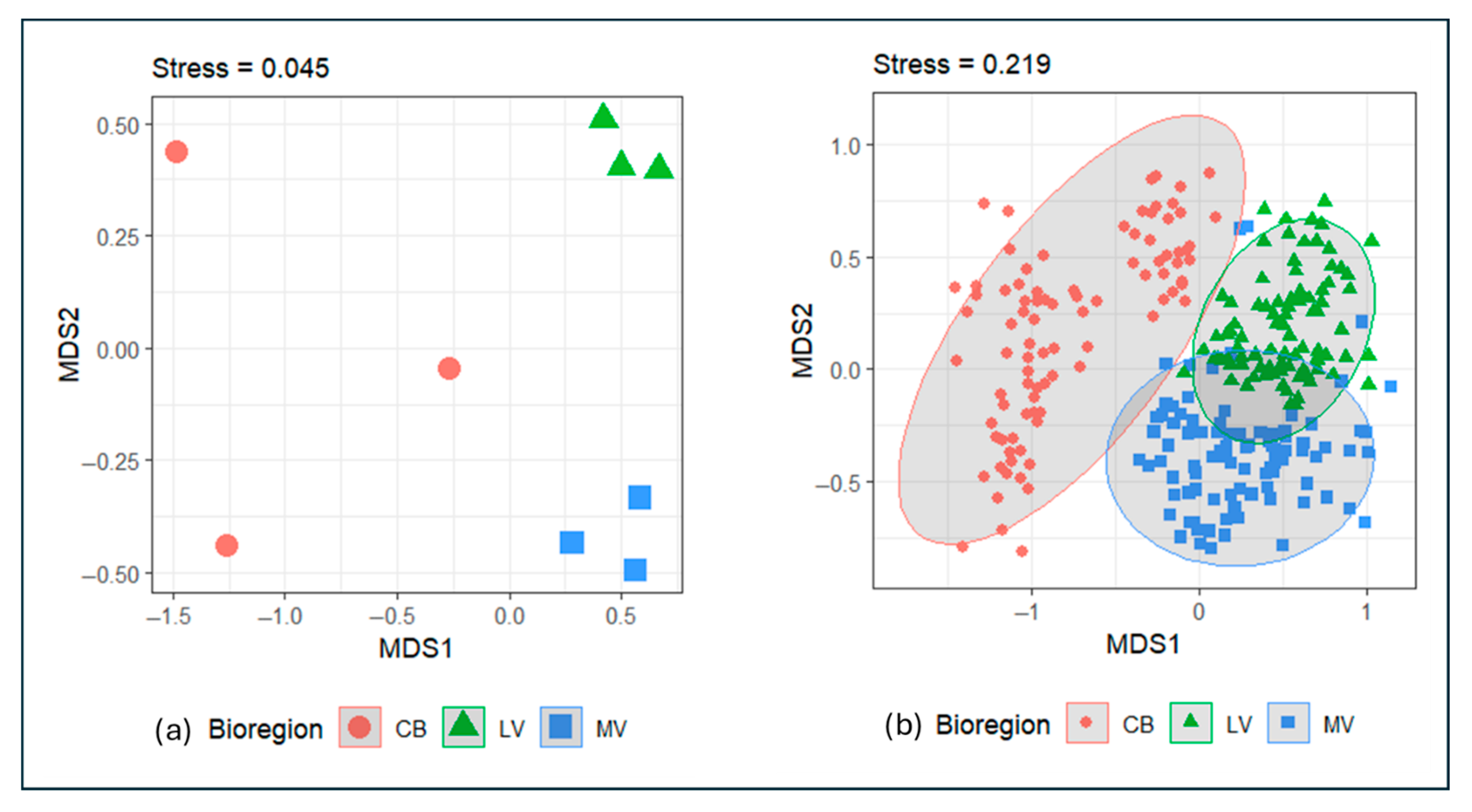
4.3. nMDS Ordination
4.4. Growth Form Analysis
5. Discussion
5.1. Herbaceous Density and Species Richness Across Bioregions
5.2. Shannon Diversity Patterns
5.3. Community Composition Differentiation
5.4. Growth Form Dynamics and the Ecological Importance of Forbs
5.5. Within-Bioregion Spatial Heterogeneity
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shorrocks, B.; Bates, W. The Biology of African Savannahs, 2nd ed.; Oxford University Press: Oxford, UK, 2014; ISBN 978-0-19-870270-2. [Google Scholar]
- The Vegetation of South Africa, Lesotho and Swaziland; Mucina, L., Rutherford, M.C., Eds.; Strelitzia; South African National Biodiversity Institute: Pretoria, South Africa, 2006; ISBN 978-1-919976-21-1. [Google Scholar]
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, D.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F.; et al. Determinants of Woody Cover in African Savannas. Nature 2005, 438, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Scholes, R.J.; Walker, B.H. An African Savanna: Synthesis of the Nylsvley Study, 1st ed.; Cambridge University Press: Cambridge, UK, 1993; ISBN 978-0-521-41971-0. [Google Scholar]
- Siebert, F.; Dreber, N. Forb Ecology Research in Dry African Savannas: Knowledge, Gaps, and Future Perspectives. Ecol. Evol. 2019, 9, 7875–7891. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, T.M.; Hughen, K.A.; McKay, N.P.; Overpeck, J.T.; Scholz, C.A.; Gosling, W.D.; Miller, C.S.; Peck, J.A.; King, J.W.; Heil, C.W. CO2 and Fire Influence Tropical Ecosystem Stability in Response to Climate Change. Sci. Rep. 2016, 6, 29587. [Google Scholar] [CrossRef]
- Van Langevelde, F.; Van De Vijver, C.A.D.M.; Kumar, L.; Van De Koppel, J.; De Ridder, N.; Van Andel, J.; Skidmore, A.K.; Hearne, J.W.; Stroosnijder, L.; Bond, W.J.; et al. Effects of Fire and Herbivory on the Stability of Savanna Ecosystems. Ecology 2003, 84, 337–350. [Google Scholar] [CrossRef]
- Fu, L.; Mei, X.; Xu, P.; Zhao, J.; Gao, D. The Species Richness and Community Composition of Different Growth Forms and Life Forms of Mosses Are Dominated by Different Factors along an Elevational Gradient of China. Glob. Ecol. Conserv. 2023, 47, e02646. [Google Scholar] [CrossRef]
- Archibald, S.; Bond, W.J.; Hoffmann, W.; Lehmann, C.; Staver, C.; Stevens, N. Distribution and Determinants of Savannas. In Savanna Woody Plants and Large Herbivores; Scogings, P.F., Sankaran, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–24. ISBN 978-1-119-08110-4. [Google Scholar]
- Lawal, S.; Lennard, C.; Hewitson, B. Response of Southern African Vegetation to Climate Change at 1.5 and 2.0° Global Warming above the Pre-Industrial Level. Clim. Serv. 2019, 16, 100134. [Google Scholar] [CrossRef]
- Van Staden, N.; Marquart, A.; Kellner, K. Drought Release and Post-Drought Changes in Herbaceous Composition and Diversity in Two Land Uses Subjected to Selective Bush Control in a Semi-Arid Kalahari Savanna. Afr. J. Range Forage Sci. 2024, 41, 1–14. [Google Scholar] [CrossRef]
- Klimeš, A.; Šímová, I.; Zizka, A.; Antonelli, A.; Herben, T. The Ecological Drivers of Growth Form Evolution in Flowering Plants. J. Ecol. 2022, 110, 1525–1536. [Google Scholar] [CrossRef]
- Mndela, M.; Madakadze, I.C.; Nherera-Chokuda, F.V.; Dube, S.; Ramoelo, A.; Mangwane, M.; Tjelele, J.T. Short-Term Responses of Herbaceous Vegetation to Bush Clearing in Semi-Arid Rangelands of South Africa. Pastoralism 2022, 12, 17. [Google Scholar] [CrossRef]
- Taylor, A.; Weigelt, P.; Denelle, P.; Cai, L.; Kreft, H. The Contribution of Plant Life and Growth Forms to Global Gradients of Vascular Plant Diversity. New Phytol. 2023, 240, 1548–1560. [Google Scholar] [CrossRef]
- Aldworth, T.A.; Toucher, M.L.W.; Clulow, A.D. The Potential Impact of Woody Encroachment on Evapotranspiration Losses in South Africa’s Savannas: A Combined Systematic Review and Meta-Analysis Approach. Ecohydrol. Hydrobiol. 2024, 24, 25–35. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.C. Climate of South Africa. Climate Region; WS45, WS; South African Weather Service: Gauteng, South Africa, 2004; ISBN 0-9584463-3-4. [Google Scholar]
- Myburgh, H. Composition and Diversity of Mopaneveld Herbaceous Vegetation: An Exclosure Experiment. Master’s Thesis, North-West University, Potchefstroom, South Africa, 2015. [Google Scholar]
- Comley, J. Carnivore Intra-Guild Competition in Selati Game Reserve, Limpopo Province, South Africa. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2019. [Google Scholar]
- Block, S.; Moyen, J.-F.; Zeh, A.; Poujol, M.; Jaguin, J.; Paquette, J.-L. The Murchison Greenstone Belt, South Africa: Accreted Slivers with Contrasting Metamorphic Conditions. Precambr. Res. 2013, 227, 77–98. [Google Scholar] [CrossRef]
- Donaldson, J.E.; Holdo, R.; Sarakikya, J.; Anderson, T.M. Fire, Grazers, and Browsers Interact with Grass Competition to Determine Tree Establishment in an African Savanna. Ecology 2022, 103, e3715. [Google Scholar] [CrossRef]
- Van Coller, H.; Siebert, F.; Siebert, S.J. Herbaceous Species Diversity Patterns across Various Treatments of Herbivory and Fire along the Sodic Zone of the Nkuhlu Exclosures, Kruger National Park. Koedoe 2013, 55, 6. [Google Scholar] [CrossRef]
- Upton, G.J.G. Data in the wild series. In Measuring Abundance: Methods for the Estimation of Population Size and Species Richness; Pelagic Publishing: Exeter, UK, 2020; ISBN 978-1-78427-232-6. [Google Scholar][Green Version]
- Magurran, A.E.; McGill, B.J. Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-958066-8. [Google Scholar][Green Version]
- Holdo, R.M.; Holt, R.D.; Fryxell, J.M. Grazers, Browsers, and Fire Influence the Extent and Spatial Pattern of Tree Cover in the Serengeti. Ecol. Appl. 2009, 19, 95–109. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and Productivity in a Long-Term Grassland Experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; 9 [Nachdr.].; Blackwell: Malden, MA, USA, 2004; ISBN 978-0-632-05633-0. [Google Scholar][Green Version]
- Timis-Gansac, V.; Dinca, L.; Constandache, C.; Murariu, G.; Cheregi, G.; Timofte, C.S.C. Conservation Biodiversity in Arid Areas: A Review. Sustainability 2025, 17, 2422. [Google Scholar] [CrossRef]
- Wang, J.; Knops, J.M.H.; Brassil, C.E.; Mu, C. Increased Productivity in Wet Years Drives a Decline in Ecosystem Stability with Nitrogen Additions in Arid Grasslands. Ecology 2017, 98, 1779–1786. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Wilson, M.V.; Shmida, A. Measuring Beta Diversity with Presence-Absence Data. J. Ecol. 1984, 72, 1055. [Google Scholar] [CrossRef]
- Janecke, B.B. Vegetation Structure and Spatial Heterogeneity in the Granite Supersite, Kruger National Park. Koedoe—Afr. Prot. Area Conserv. Sci. 2020, 62, 1–12. [Google Scholar] [CrossRef]
- Shi, Z.; Bai, Z.; Guo, D.; Li, S.; Chen, M. Species Diversity and Soil Interconstraints Exert Significant Influences on Plant Survival during Ecological Restoration in Semi-Arid Mining Areas. Diversity 2023, 15, 1100. [Google Scholar] [CrossRef]
- Hossain, M.L.; Li, J. Species Richness and Dominant Functional Groups Enhance Aboveground Biomass, with No Effect on Belowground Biomass in Qinghai-Tibet Plateau’s Grasslands. Ecol. Inform. 2024, 82, 102688. [Google Scholar] [CrossRef]
- Andersen, A.N.; Lonsdale, W.M. Herbivory by Insects in Australian Tropical Savannas: A Review. J. Biogeogr. 1990, 17, 433. [Google Scholar] [CrossRef]
- Bråthen, K.A.; Pugnaire, F.I.; Bardgett, R.D. The Paradox of Forbs in Grasslands and the Legacy of the Mammoth Steppe. Front. Ecol. Environ. 2021, 19, 584–592. [Google Scholar] [CrossRef]
- Siebert, F.; Chamane, S.; Ntuli, N.; Siebert, S.J. The Functional Importance of Forbs in Grassland Ecosystems. In Proceedings of the XXIV International Grassland Congress, Virtually, 25–29 October 2021; p. 35. [Google Scholar]
- Grant, R.C.; Botha, J.; Grant, T.C.; Peel, M.J.; Smit, I.P. When Less Is More: Heterogeneity in Grass Patch Height Supports Herbivores in Counter-Intuitive Ways. Afr. J. Range Forage Sci. 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Janecke, B.B.; Van Tol, J.; Smit, I.P.J.; Van Aardt, A.C.; Riddell, E.S.; Seaman, M.T.; Swart, W.J.; Du Preez, P.J.; Le Roux, P.A.L. Biotic and Abiotic Connections on a Granitic Catena: Framework for Multidisciplinary Research. Koedoe—Afr. Prot. Area Conserv. Sci. 2020, 62, 1–11. [Google Scholar] [CrossRef]
- Elahi, R.; O’Connor, M.I.; Byrnes, J.E.K.; Dunic, J.; Eriksson, B.K.; Hensel, M.J.S.; Kearns, P.J. Recent Trends in Local-Scale Marine Biodiversity Reflect Community Structure and Human Impacts. Curr. Biol. 2015, 25, 1938–1943. [Google Scholar] [CrossRef]
- Gonzalez, A.; Cardinale, B.J.; Allington, G.R.H.; Byrnes, J.; Arthur Endsley, K.; Brown, D.G.; Hooper, D.U.; Isbell, F.; O’Connor, M.I.; Loreau, M. Estimating Local Biodiversity Change: A Critique of Papers Claiming No Net Loss of Local Diversity. Ecology 2016, 97, 1949–1960. [Google Scholar] [CrossRef]
- Vellend, M.; Dornelas, M.; Baeten, L.; Beauséjour, R.; Brown, C.D.; De Frenne, P.; Elmendorf, S.C.; Gotelli, N.J.; Moyes, F.; Myers-Smith, I.H.; et al. Estimates of Local Biodiversity Change over Time Stand up to Scrutiny. Ecology 2017, 98, 583–590. [Google Scholar] [CrossRef]
- Vellend, M.; Baeten, L.; Myers-Smith, I.H.; Elmendorf, S.C.; Beauséjour, R.; Brown, C.D.; De Frenne, P.; Verheyen, K.; Wipf, S. Global Meta-Analysis Reveals No Net Change in Local-Scale Plant Biodiversity over Time. Proc. Natl. Acad. Sci. USA 2013, 110, 19456–19459. [Google Scholar] [CrossRef]
- Augustine, D.J. Spatial Heterogeneity in the Herbaceous Layer of a Semi-Arid Savanna Ecosystem. Plant Ecol. 2003, 167, 319–332. [Google Scholar] [CrossRef]
- Koerner, S.E.; Collins, S.L.; Blair, J.M.; Knapp, A.K.; Smith, M.D. Rainfall Variability Has Minimal Effects on Grassland Recovery from Repeated Grazing. J. Veg. Sci. 2014, 25, 36–44. [Google Scholar] [CrossRef]
- Wonkka, C.L.; Twidwell, D.; Allred, B.W.; Bielski, C.H.; Donovan, V.M.; Roberts, C.P.; Fuhlendorf, S.D. Rangeland Vulnerability to State Transition under Global Climate Change. Clim. Change 2019, 153, 59–78. [Google Scholar] [CrossRef]
- Pringle, R.M.; Abraham, J.O.; Anderson, T.M.; Coverdale, T.C.; Davies, A.B.; Dutton, C.L.; Gaylard, A.; Goheen, J.R.; Holdo, R.M.; Hutchinson, M.C.; et al. Impacts of Large Herbivores on Terrestrial Ecosystems. Curr. Biol. 2023, 33, R584–R610. [Google Scholar] [CrossRef]
- Santos, F.; Bailey, J.K.; Schweitzer, J.A. The Eco-evolutionary Role of Fire in Shaping Terrestrial Ecosystems. Funct. Ecol. 2023, 37, 2090–2095. [Google Scholar] [CrossRef]
| Growth Forms | Central Bushveld | Lowveld | Mopane | |
|---|---|---|---|---|
| Species Richness | Grasses | 36 | 20 | 28 |
| Forbs | 69 | 52 | 62 | |
| Sedges | 3 | 2 | 3 | |
| Overall | 108 | 74 | 93 | |
| Density (ind./m2) | Grasses | 11.7 ± 1.41 | 13.8 ± 0.44 | 10.1 ± 0.59 |
| Forbs | 11.5 ± 0.46 | 17.4 ± 2.02 | 19.5 ± 3.82 | |
| Sedges | 1.5 ± 0.53 | 1.6 ± 0.57 | 1.86 ± 0.50 | |
| Overall | 24.3 ± 2.31 | 32.0 ± 1.28 | 29.6 ± 4.19 | |
| Shannon Diversity Index | 3.11 ± 0.09 | 3.32 ± 0.04 | 3.39 ± 0.09 |
| Study Area | Species | Family | Ab | Mean Ab | SD | CV | Density (ind./m2) |
|---|---|---|---|---|---|---|---|
| LPGR (CB) | Melinis repens | Poaceae | 689 | 7.66 | 14.00 | 1.83 | 1.91 |
| Heteropogon contortus | Poaceae | 655 | 7.28 | 8.54 | 1.17 | 1.82 | |
| Becium obovatum | Lamiaceae | 436 | 4.84 | 7.15 | 1.48 | 1.21 | |
| Waltheria indica | Malvaceae | 390 | 4.33 | 12.85 | 2.96 | 1.08 | |
| Limeum viscosum | Limeaceae | 383 | 4.26 | 7.42 | 1.74 | 1.06 | |
| Tragus berteronianus | Poaceae | 370 | 4.11 | 7.87 | 1.91 | 1.03 | |
| Tephrosia longipes | Fabaceae | 354 | 3.93 | 6.38 | 1.62 | 0.98 | |
| Setaria sphacelata var. sphacelata | Poaceae | 314 | 3.49 | 4.55 | 1.30 | 0.87 | |
| Coleochloa setifera | Cyperaceae | 297 | 3.30 | 10.00 | 3.03 | 0.83 | |
| Cyperus obtusiflorus | Cyperaceae | 289 | 3.21 | 6.91 | 2.15 | 0.80 | |
| SGR (MV) | Hoffmannseggia burchellii | Fabaceaea | 783 | 8.70 | 8.690 | 0.999 | 2.175 |
| Indigofera oxytropis | Fabaceaea | 741 | 8.23 | 8.521 | 1.035 | 2.058 | |
| Zornia milneana | Fabaceaea | 607 | 6.74 | 13.383 | 1.984 | 1.686 | |
| Digitaria eriantha | Poaceae | 544 | 6.04 | 6.074 | 1.005 | 1.511 | |
| Waltheria indica | Malvaceae | 504 | 5.60 | 12.324 | 2.201 | 1.400 | |
| Becium obovatum | Lamiaceae | 476 | 5.29 | 13.209 | 2.497 | 1.322 | |
| Evolvulus alsinoides | Convolvulaceae | 474 | 5.27 | 5.623 | 1.068 | 1.317 | |
| Chamaecrista mimosoides | Fabaceaea | 458 | 5.09 | 7.457 | 1.465 | 1.272 | |
| Urochloa mosambicensis | Poaceae | 452 | 5.02 | 5.077 | 1.011 | 1.256 | |
| Ocimum americanum | Lamiaceae | 425 | 4.72 | 10.375 | 2.197 | 1.181 | |
| KNR (LV) | Indigofera oxytropis | Fabaceae | 1125 | 12.50 | 11.54 | 0.92 | 3.13 |
| Digitaria eriantha | Poaceae | 956 | 10.62 | 6.67 | 0.63 | 2.66 | |
| Panicum maximum | Poaceae | 785 | 8.72 | 6.67 | 0.76 | 2.18 | |
| Urochloa mosambicensis | Poaceae | 726 | 8.07 | 5.24 | 0.65 | 2.02 | |
| Hibiscus calyphyllus | Malvaceae | 620 | 6.89 | 12.87 | 1.87 | 1.72 | |
| Bidens bipinnata | Asteraceae | 512 | 5.69 | 12.73 | 2.24 | 1.42 | |
| Oldenlandia herbacea | Rubiaceae | 470 | 5.22 | 9.75 | 1.87 | 1.31 | |
| Perotis patens | Poaceae | 364 | 4.04 | 7.18 | 1.77 | 1.01 | |
| Aristida congesta subsp. congesta | Poaceae | 337 | 3.74 | 5.82 | 1.56 | 0.94 | |
| Pogonarthria squarrosa | Poaceae | 324 | 3.60 | 5.18 | 1.44 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biko’o, A.A.; Myburgh, W.J.; Reilly, B.K. Contrasting Herbaceous Communities in South African Savannas: A Comparative Analysis of Density, Composition, and Diversity Across Three Bioregions. Diversity 2025, 17, 475. https://doi.org/10.3390/d17070475
Biko’o AA, Myburgh WJ, Reilly BK. Contrasting Herbaceous Communities in South African Savannas: A Comparative Analysis of Density, Composition, and Diversity Across Three Bioregions. Diversity. 2025; 17(7):475. https://doi.org/10.3390/d17070475
Chicago/Turabian StyleBiko’o, Armand Arthur, Willem Johannes Myburgh, and Brian Kevin Reilly. 2025. "Contrasting Herbaceous Communities in South African Savannas: A Comparative Analysis of Density, Composition, and Diversity Across Three Bioregions" Diversity 17, no. 7: 475. https://doi.org/10.3390/d17070475
APA StyleBiko’o, A. A., Myburgh, W. J., & Reilly, B. K. (2025). Contrasting Herbaceous Communities in South African Savannas: A Comparative Analysis of Density, Composition, and Diversity Across Three Bioregions. Diversity, 17(7), 475. https://doi.org/10.3390/d17070475







