Abstract
The green octopus, Octopus hubbsorum, is a merobenthic species that inhabits warm-temperate waters in the eastern Pacific. However, its similarity to some morphological characteristics of and its slight genetic divergence from Octopus mimus has led to the proposal that both species are conspecific. The objective of this study was the morphological and molecular identification of eggs and paralarvae of the green octopus, O. hubbsorum, to provide information contributing to clarifying its taxonomy and relationship with O. mimus. The results obtained show that although O. hubbsorum has similarities with O. mimus in terms of egg size, chromatophore pattern, number of suckers, and presence of Kölliker’s organs, the O. hubbsorum paralarvae observed in this study are smaller (1.6 mm) and have a thin layer of loose skin, not described for O. mimus. Likewise, the morphology of the beak, radula, and suckers of O. hubbsorum is described for the first time and there are no studies of these structures for O. mimus. The phylogenetic analysis (mitochondrial cytochrome C oxidase subunit I and III genes) showed that both species form a monophyletic clade but belong to separate subclades. In conclusion, although the slight genetic divergence between these two species suggests conspecificity, their disjoint geographic distribution (O. hubbsorum is found in warm-temperate waters and O. mimus in cold-temperate waters) suggests the hypothesis of being two separate species with a close phylogenetic relationship. However, further research (morphological and population analyses) is required to solve taxonomic uncertainty.
1. Introduction
The genus Octopus Cuvier, 1798 represents a significant group of cephalopods that includes numerous species of commercial relevance distributed worldwide. The correct identification of these species is essential for studying them in multiple disciplines, such as phylogenetics, biogeography, population genetics, and conservation [1,2]. Octopuses show a wide diversity in skin coloration, behavior, and life strategies but are highly similar in structural morphology. This similarity has led to the misidentification of morphologically similar species, which complicates the study of their evolutionary history, phylogenetic relationships, and distribution [3,4,5,6,7,8].
The study of paralarvae is crucial to understanding the life cycle and population dynamics of cephalopods. However, misidentification could hamper the understanding of the composition and distribution of cephalopods. The identification of paralarvae is based on various key morphological characteristics, such as mantle length, the type of arms (short or elongated), the number of suckers, eye size, and chromatophore pattern [9,10]. In addition, anatomical characteristics (internal structures) such as the beak and radula, which allow for differentiating between species [4,9,11,12], are also considered in the identification process [4,10]. However, taxonomic confusion persists due to the morphological similarity between species, underlining the need to integrate molecular techniques with descriptive morphological and anatomical studies in taxonomic and phylogenetic reviews [2,3,13,14,15,16,17].
The green octopus, Octopus hubbsorum Berry 1953, is a mid-sized merobenthic species with a planktonic phase characterized by a brown and green coloration and the absence of ocelli [18]. It inhabits shallow waters and is distributed in warm temperate waters from the Gulf of California to the coasts of Oaxaca, including the Revillagigedo Islands [19] and Magdalena Bay, on the west coast of the Baja California peninsula [20].
It has been hypothesized that O. hubbsorum and O. mimus Gould, 1852, two octopus species that inhabit the eastern Pacific, may be conspecific due to the low degree of genetic divergence (0–1.6%) between them [21]. O. mimus is a large merobenthic species that undergoes a planktonic phase. This species is characterized by an orange to purplish-red coloration and a faint ocellus on each side of the head at the base of arms 2 and 3 [18,22]. It inhabits temperate–cold waters along the western coast of South America, from southern Ecuador to northern Chile [23,24]. However, sightings have been recorded in regions of Central America and Mexico [3,25]. The uncertainty lies in whether the morphological and geographic differences between O. hubbsorum and O. mimus are sufficient to consider them separate species or whether they are variants of the same species. Pliego-Cárdenas et al. [21] in adult organisms used a mitochondrial analysis that incorporated three markers (COI, COIII, and r16S) to determine the taxonomic relationship between the two species. Their results, supported by high bootstrap values, indicated that both species belonged to a single clade, suggesting that both correspond to the same species.
The objective of this study was the morphological and molecular identification of eggs and paralarvae of the green octopus, O. hubbsorum, to contribute to elucidating the taxonomic status of O. hubbsorum and O. mimus.
2. Materials and Methods
2.1. Sample Collection
Portions of five egg clutches were collected with the assistance of local artisanal fishers in three sites in the Gulf of California: Santa Rosalia (two clutches) (27°20′21.6″ N, 112°15′49.2″ W) and San Bruno (two clutches) (27°08′29.7″ N, 112°06′06.6″ W) in June 2021, and La Paz Bay (one clutch) (24°08′38.4″ N, 110°24′02.4″ W) in May 2022 (Figure 1). There was embryo hatching during egg-mass collections in San Buno; a sample of paralarvae was kept in a 2 L container with seawater and aeration for 48 h. Before fixation, embryos and paralarvae were placed in seawater at 3 to 4 °C until relaxed. Embryo and paralarva samples were preserved using two methods, according to the type of analysis. Some samples were fixed in Davidson’s AFA for 48 h and subsequently stored in 70% ethanol to preserve their morphological integrity; the remaining samples were preserved directly in 80% ethanol for further processing in molecular analysis.
Figure 1.
Sampling sites of Octopus hubbsorum on the Gulf of California. (A) Santa Rosalia, (B) San Bruno, (C) La Paz Bay.
2.2. Molecular Identification and Phylogenetic Analyses of Embryos and Paralarvae
Since mature females were not observed during sample collection, genomic DNA was extracted from 8 embryos and 8 paralarvae of each clutch (n = 5; total 40 samples) fixed in 80% ethanol to identify the species by applying the standard salt extraction method modified from Aljanabi and Martinez [26]. Samples were incubated in 400 µL of lysis buffer (100 mM NaCl, 50 mM Tris-HCl pH 8.0, 100 mM EDTA, 1% SDS) with 10 µL of proteinase K (10 mg/mL) at 37 °C for 2 h. After complete lysis, 200 µL of 6 M NaCl was added, vortexed for 2 min, and incubated on ice for 10 min. Samples were centrifuged at 8000 rpm for 10 min at room temperature, and 500 µL of the supernatant was transferred to a clean tube. DNA was precipitated by adding two volumes of cold absolute ethanol, followed by mixing by inversion and centrifuging at 8000 rpm for 10 min. The pellet was washed with 500 µL of 70% cold ethanol, vortexed briefly, and centrifuged again. After discarding the supernatant, any residual ethanol was removed, and the DNA was resuspended in 50 µL of nuclease-free water. Amplification was performed in a segment of the mitochondrial cytochrome C oxidase subunit I and III (COI, COIII) genes using primers LCO1490 and HCO2198 [27] for COI and 5′-CAATGATGACGAGATATTATYCG-3′ [28] and 5′-TCAACAAAGTGTCAGTATCA-3′ [29] for COIII. The PCR protocol consisted of an initial denaturalization at 94 °C for 4 min, followed by 35 denaturalization cycles at 94° C for 30 s, alignment at 52 °C for 30 s (for COI) and 50 °C (for COIII), and extension for 1 min at 72 °C; a final extension at 72 °C for 10 min was completed. The PCR products were sent to and analyzed by Macrogen, Inc. (Seoul, Republic of Korea). The DNA sequences were aligned using the ClustalW (Hinxton, UK) [30] multiple alignment tool in the BioEdit program v.5.0.6 (Carlsbad, CA, USA) [31]. Following automatic alignment, each sequence was visually examined to correct possible editing errors. A BLAST analysis of the consensus sequence (forward + reverse DNA sequences) was performed with sequences from the GenBank database. Furthermore, additional sequences of various octopus species—O. mimus, Octopus insularis Leite and Haimovici 2008, Octopus bimaculatus Verrill 1883, Octopus tetricus Gould 1852 and Octopus vulgaris Cuvier 1797—were selected from GenBank for a comparative analysis of sequence divergence and the reconstruction of phylogenetic trees (Table A1).
Phylogenetic analyses were performed by sequence alignment with MEGA v. 11.0.13 (Philadelphia, PA, USA) [32], using the MUSCLE (Los Altos, CA, USA) [33] alignment algorithm. The optimum evolutionary models were selected using the jModelTest program [34], based on the Akaike information criterion [35] for maximum likelihood (MLE). These models were the General Time-Reversible model with gamma distribution (GTR + G) for COI and this same model with invariant sites (GTR + I) for COIII; both were performed with maximum likelihood (MLE) algorithms in MEGA. The trees were constructed with a bootstrap of 10,000 replicates. The genetic diversity among Octopus species was analyzed using DNASP v6.12.03 (Barcelona, Spain) [36]. The number of haplotypes (h), the number of haplotypes per sample group, haplotype diversity (Hd), nucleotide diversity (π), and the average number of nucleotide differences (k) were calculated. The genetic distances between O. hubbsorum and O. mimus were estimated using GenAlEx v6.5 (Canberra, Australia) [37]. For the COI gene, sequences were grouped as follows: O. hubbsorum_1, sequences generated in this study (OR195460, OR195458, OR195459, OR195461, OR195457); O. hubbsorum_2, reference sequences from GenBank (KF225001.1, KF225004.1, KY985006.1, KY985009.1, KF225002.1, KF225005.1, MN745318.1, MH400898.1, MH400897.1); O. mimus_1, reference sequences from GenBank (GU355923.1, MN977146.1, MH194493.1, MH194502.1, MH194545.1, GU355926.1, GU355925.1, GU355924.1); and O. mimus_2 (KT335830.1, MH194435.1). For the COIII gene, the groups included O. hubbsorum_1, sequences generated in this study (OR473046, OR473047, OR473048, OR473049), O. hubbsorum_2, reference sequences from GenBank (KF225007.1, KF225008.1, KF225009.1, KF225010.1, KF225011.1), and O. mimus (GU355927.1, GU355928.1, GU355929.1, GU355931.1, GU355933.1, KT314264.1).
2.3. Morphological Characterization of Egg Strings and Paralarvae
Morphological observations were performed using a ZEISS Stemi 508 stereo microscope (Jena, Germany) equipped with an Axioncam ERc 5s camera, in which the fixed specimens were photographed. To assess the increase in egg size during embryonic development, size data were organized into three groups: (1) Blastogenesis (stages I–VI), (2) Early Organogenesis (stages VII–XIV), and (3) Advanced Organogenesis (stages XV–XIX). Egg biometrics, including festoon length (FL), egg width (EW), and total egg length (TL) without the peduncle, were recorded from the digitalized images using the ImageJ v.1.53 software (Vigo, Spain). Mantle length (ML), mantle width (MW), total length (TL), head width (HW), eye diameter (ED), arm length (AL), and funnel length (SL) were measured in paralarvae. All measurements are reported as means ± standard deviation (SD). The terminology and measurements used to describe hatchlings are based on suggestions by Young et al. [11] and Hochberg et al. [38], wherever feasible. The chromatophore pattern was determined in terms of orientation, number, and distribution (Sweeney et al. [9]).
2.4. Microstructural Analyses of Suckers, Beaks, and Radulae of Paralarvae
The microstructure of the radulae and beaks of eight newly hatched paralarvae was obtained. To this end, a transverse incision was cut in the posterior part of the head of each paralarva, taking only the oral region (Figure A1). These samples were then placed in a 5% NaOH solution for 24 h to disintegrate the soft tissues. The technique by Geiger et al. [39] was used for sample handling, preparation, and examination. The suckers, beaks, and radulae were described through photographs under a Leica light microscope (DM4B, Leica LAS, Leica Microsystems, Wetzlar, Germany) and micrographics captured with a JEOL JSM IT300LV scanning electron microscope (SEM, JEOL USA Inc., Peabody, MA, USA). The samples were fixed for 4 h in a 2.5% glutaraldehyde solution at 4 °C. Then, paralarvae were rinsed for an hour in a Sorensen buffer solution; four rinses were performed. Afterward, samples were dehydrated by immersion in ethanol solutions of increasing concentrations and critically point-dried with CO2. Finally, the samples were sputtered with gold before observation and analysis. Suckers, jaw denticles, radulae, and radula denticles were measured. The terminology and measurements used to describe the radular apparatus are based on Kruta et al. [40].
2.5. Statistical Analyses
To evaluate the increase in egg size during embryonic development and determine differences between locations, data normality and homoscedasticity were assessed with the Shapiro–Wilk and Bartlett tests, respectively. Since the data did not meet these assumptions, a Kruskal–Wallis test was performed, followed by a Dunn post hoc test. All analyses were performed with RStudio v. 4.3.0 (Boston, MA, USA) [41].
3. Results
3.1. Morphological Characterization of Eggs and Paralarvae
Each O. hubbsorum egg has a pear-shaped chorion, which extends into a stem or peduncle with an annular widening at 0.22 ± 0.02 mm from the chorionic capsule. Peduncles are intertwined along a central axis, forming the festoons and averaging 7.12 ± 2.31 mm in total length (TL) (Figure 2). The morphometrics of eggs and paralarvae of O. hubbsorum are summarized in Table 1.

Figure 2.
Individual festoon of the Octopus hubbsorum clutch and egg structure. Abbreviations: asterisk (*), micropyle; ae, annular enlargement (or widening); ca, central axis; c, chorion; eg, egg; st, stalk; ys: yolk sac.

Table 1.
Morphometrics of the eggs and newly hatched paralarvae of Octopus hubbsorum recorded in this study. For comparison, morphometric data for O. hubbsorum and O. mimus reported by other authors are included. All measurements are in mm. Dash indicates no information. Data in bold correspond to paralarvae two days post-hatching. Ranges are in parentheses.
During embryonic development, O. hubbsorum eggs averaged 2.04 ± 0.12 mm TL, excluding the peduncle. Eggs from Bahía de La Paz were significantly smaller (in TL) than those from San Bruno and Santa Rosalía (H2,646 = 96.41; p < 0.05), between which no significant differences were found. However, at the three locations, a progressive and significant increase in egg capsule length was observed as embryonic development progressed (1) Blastogenesis, (2) Early Organogenesis, and (3) Advanced Organogenesis (H2,646 = 252.92; p < 0.05) (Figure 3).
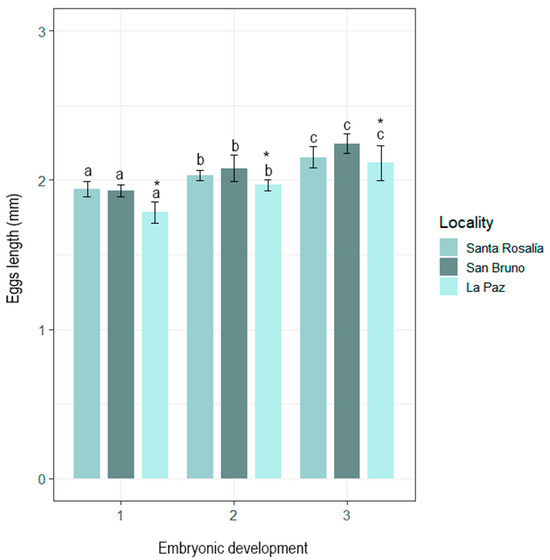
Figure 3.
Progressive elongation of the egg capsule of Octopus hubbsorum throughout embryonic development in the three locations studied. The asterisks indicate significant differences in egg length between localities, while lowercase letters represent significant differences in egg length between development stages within each locality.
Newly hatched paralarvae of O. hubbsorum had a mean size of 1.26 ± 0.08 mm ML and 0.75 ± 0.03 mm HW. The funnel extends to eye level, with the free portion representing 33% of ML. The eyes are prominent, functional, and fully developed, measuring 0.26 mm in diameter. The arms are short and subequal in length (0.34 ± 0.04 mm), with thin thread-like tips representing 26.9% of ML (Figure 4A). Each arm has three uniserialized suckers of similar size, measuring 0.07 mm in diameter (Figure 4B). Newly hatched paralarvae had between 74 and 77 predominantly brown chromatophores distributed as follows: 29 to 32 in the ventral region of the mantle, 4 in the back of the mantle, 6 (4 + 2) in the funnel, 2 in the ventral region of the head, 1 at the base of each arm, and 3 per arm. In the dorsal region, 6 to 7 chromatophores are distributed in the visceral integument, 10 in the dorsal head (2 + 4 + 4), and 1 extrategumental chromatophore on the surface of each eye (Figure 4C). Paralarvae measuring 1.68 ± 0.19 mm ML had a thin layer of non-pigmented skin that covered them completely, except in the mantle orifices, funnels, and suckers, which contained chromatophores in the ventral region of the mantle (Figure 4D). The chromatophore pattern in newly hatched paralarvae of O. hubbsorum is shown in Table 2.
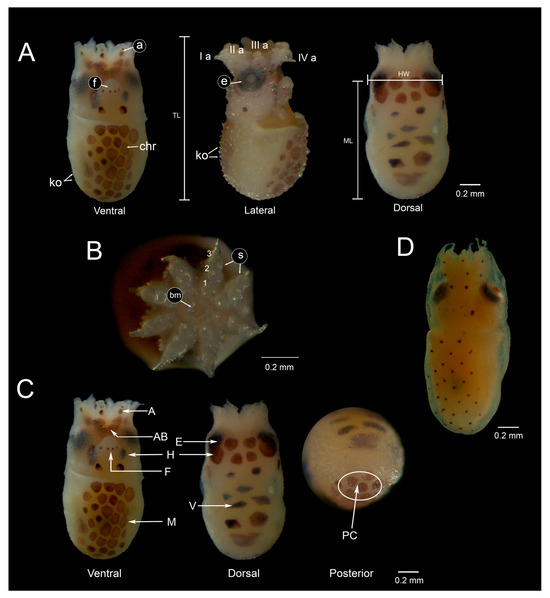
Figure 4.
Newly hatched paralarvae of Octopus hubbsorum. (A) Ventral, lateral, and dorsal views of newly hatched paralarvae. (B) Buccal mass and arms showing the arrangement of suckers. (C) Chromatophore pattern. (D) Paralarva skin film two days post-hatching. Abbreviations: a, arm; arm pairs aI-aIV; ab, arm base: bm, buccal mass; chr, chromatophores; e, eye; f, funnel; h, head; HW, head width, ko, Kölliker’s organs; m, mantle; ML, mantle length; pc, posterior cap; s, sucker; TL, total length; v, visceral mass.

Table 2.
Chromatophore pattern on the body surface of paralarvae of Octopus hubbsorum recorded in this study. For comparison, data on O. mimus reported by other authors are included. Dash indicates no information. Data in bold correspond to paralarvae two days post-hatching.
3.2. Microstructural Analyses of Suckers, Beaks, and Radulae of Paralarvae
In O. hubbsorum, the suckers of newly hatched paralarvae are similar to those of adults. The infundibulum is characterized by a smooth edge with a pronounced circumferential margin and numerous irregular, well-defined growing protrusions with tiny pores on their surface. Pores are concentrated in the growth zone and gradually recede toward the periphery. The protuberances form a “honeycomb” pattern evenly distributed around the central cavity, highlighting the typical sucker concavity. The central region shows the acetabulum with a chitinous cuticle (Figure 5).
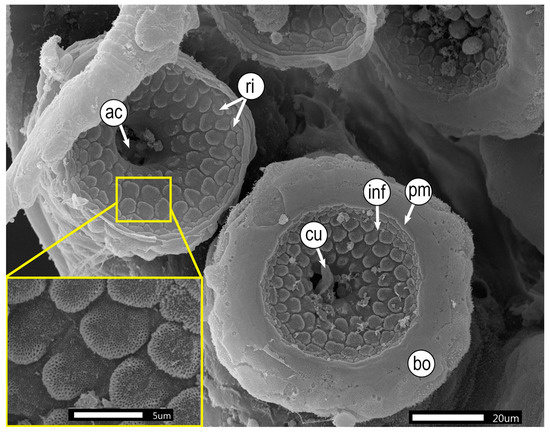
Figure 5.
Morphology of suckers in paralarvae of Octopus hubbsorum. Abbreviations: ac: acetabulum; bo, border; cu, chitinous cuticle; inf, infundibulum; pm, marginal fold; ri, ridges.
The buccal mass of newly hatched paralarvae shows well-developed buccal structures, including the beak and radular apparatus. The beak is translucent and remarkably fragile, with 30 clearly visible brown-pigmented denticles on the surface of each mandible (Figure 6A). The tip of the denticles is pointed in the upper jaw and ovular in the lower jaw (Figure 6B).
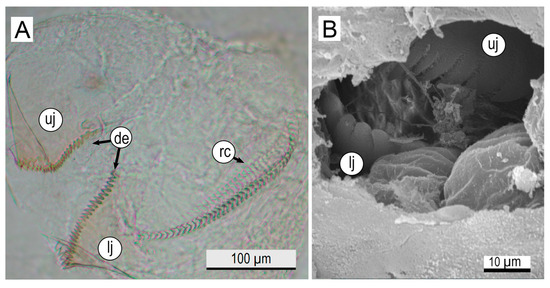
Figure 6.
Anatomy of the beak of newly hatched Octopus hubbsorum. (A) Detailed view of the upper and lower jaw, showing the denticles and radula. (B) Morphology of the upper and lower teeth of the beak (SEM image). Abbreviations: de, denticles; lj, lower jaw; rc, radular canal; uj, upper jaw.
The radula is located in the center of the buccal mass, with a total length of 401 ± 15.4 μm, comprising between 45 and 50 tooth rows (Figure 7). The arrangement of teeth in each row is repeated throughout the entire band length, from the radular sac to the beak (Figure 7A,B). Each tooth row includes 11 elements, as follows: a central tooth, called rachidian tooth (R), flanked by the lateral tooth (L); then, the first marginal tooth (M1), followed by the second marginal tooth (M2); and finally, the marginal plates (MPs). The rachidian tooth has a prominent central cusp (mesocone) and a pair of minor lateral cusps (ectocones) measuring 14.6 ± 0.7 μm long and 12.47 ± 0.9 μm wide. The lateral tooth is small and has a single cusp. On the other hand, the first marginal tooth has a wide base of 15.5 ± 1.1 μm and a small wide cusp with a small pronounced tip measuring 9.26 ± 0.6 μm TL. On the other hand, the second marginal tooth has a prominent curved tang measuring 13.6 ± 1.1 μm long and 5.5 ± 0.5 μm wide. The marginal plates are short and rectangular, measuring 7.3 ± 0.5 μm wide. Figure 8 illustrates a diagram showing the buccal complex (A) and tooth conformation of the radula (B) of O. hubbsorum newly hatched paralarvae.
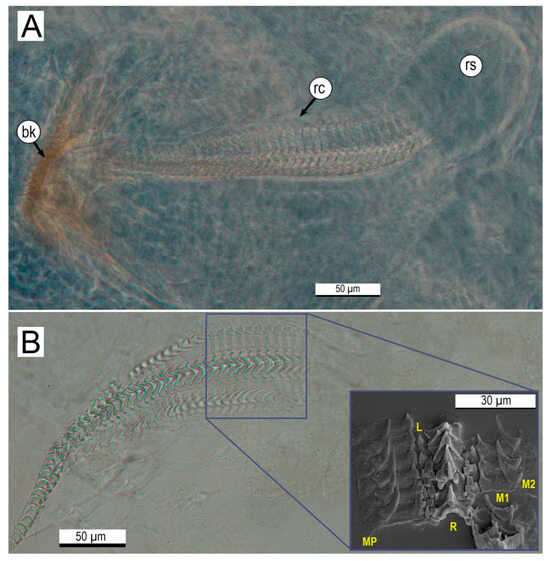
Figure 7.
Anatomy of the radular apparatus of newly hatched Octopus hubbsorum. (A) Detailed view of the upper and lower jaws, with clearly visible denticles at the extremes of the beak and radula. (B) Details of the teeth of the radial band (SEM image). Abbreviations: bk, beak; rc, radula canal; rs, radula sac; L, lateral tooth; M1, first marginal tooth; M2, second marginal tooth; MP, marginal plate; R, rachidial tooth.
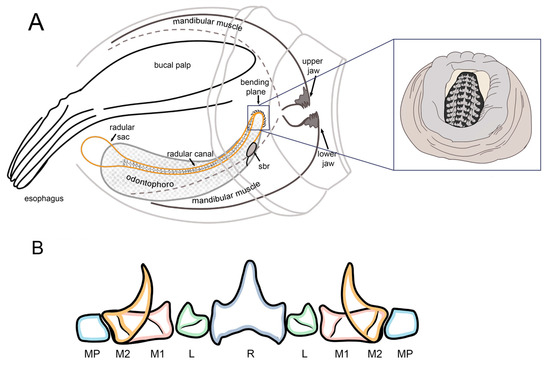
Figure 8.
Buccal complex of newly hatched Octopus hubbsorum. (A) Structure of the buccal mass and radula. (B) Radular row and tooth nomenclature. R, rachidial tooth; L, lateral tooth; M1, first marginal tooth; M2, second marginal tooth; MP, marginal plate.
3.3. Molecular Identification and Phylogenetic Analyses of Embryos and Paralarvae
Five sequences were obtained for the COI gene and four for the COIII gene. The DNA sequencing of embryos and paralarvae collected at the three study sites revealed a high homology with sequences KY985006 and KY985009 for the COI gene and KF225010 for the COIII gene, identified as O. hubbsorum and available in GenBank. The sequences obtained in this study have been deposited in GenBank with accession numbers OR195457–OR195461 for COI and OR43046-OR43049 for COIII.
The analysis of both genes (COI and COIII) shows the formation of a monophyletic clade for O. hubbsorum and O. mimus, supported by a high phylogenetic support value, but clearly separated into two well-defined subclades. This finding suggests that both species share a recent common ancestor and that their evolutionary divergence has occurred relatively recently compared to other more distant species within the genus Octopus, such as O. insularis, O. bimaculatus, O. tetricus, and O. vulgaris.
The topology obtained for both genes is consistent, grouping samples OR195457, OR195461, OR195460, OR195458, and OR195459 (COI) and OR473046, OR473049, OR473048, and OR473047 (COIII) all within the O. hubbsorum subclade, with strong phylogenetic support (Figure 9 and Figure 10). However, the analyses detected two O. mimus sequences within the O. hubbsorum group, specifically sequences KT335830.1 and MH194435.1 for the COI gene (Figure 9) and KT335842.1 for the COIII gene (Figure 10). These sequences correspond to specimens from Ecuador (KT335830.1 and KT335842.1) and the northern coast of Peru (MH194435.1).
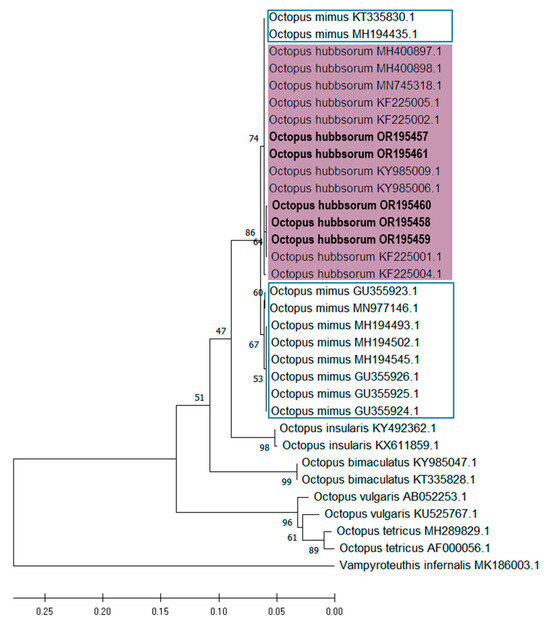
Figure 9.
Phylogenetic tree inferred by the maximum likelihood (MLE) method of mitochondrial DNA cytochrome oxidase subunit I (COI) sequences of embryo and paralarvae of Octopus hubbsorum from the Gulf of California (bold). The purple rectangle indicates the clade containing the specimens evaluated in this study; the blue rectangle marks the group of Octopus mimus sequences.
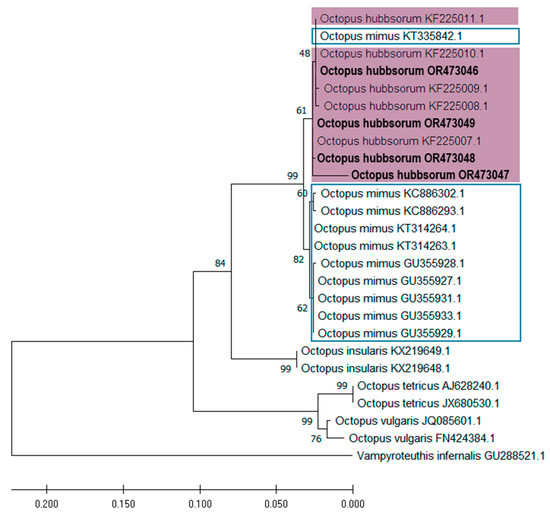
Figure 10.
Phylogenetic tree inferred by the maximum likelihood (MLE) method of mitochondrial DNA cytochrome oxidase subunit III (COIII) sequences of the embryo and paralarvae of Octopus hubbsorum from the Gulf of California (bold). The purple rectangle indicates the clade containing the specimens evaluated in this work, and the blue rectangle marks the group of Octopus mimus sequences.
A total of 12 haplotypes were identified based on the variable positions in the COI gene sequences from different Octopus species. O. tetricus exhibited two haplotypes (Hap_1 and Hap_2), and O. vulgaris also showed two haplotypes (Hap_3 and Hap_4), each represented by a single sequence. O. bimaculatus presented one haplotype (Hap_5), and O. insularis had two (Hap_6 and Hap_7). O. mimus was represented by two haplotypes (Hap_8 and Hap_9), while O. hubbsorum displayed three haplotypes (Hap_10, Hap_11, and Hap_12). Notably, Hap_12 included two sequences originally attributed to O. mimus (KT335830.1 and MH194435.1). Overall, the analysis of COI sequences revealed a haplotype diversity (Hd) of 0.8266, a nucleotide diversity (Pi) of 0.04749, and an average number of nucleotide differences (k) of 22.51.
A total of 14 haplotypes were identified based on all variable positions in the COIII gene from Octopus sequences. O. tetricus presented one haplotype (Hap_1), O. vulgaris presented two haplotypes (Hap_2 and Hap_3), with each represented by a single sequence, and O. insularis presented one haplotype (Hap_4). O. hubbsorum exhibited five haplotypes (Hap_5 to Hap_9), with Hap_8 notably including a sequence originally attributed to O. mimus (KT314263.1). O. mimus also showed five haplotypes (Hap_10 to Hap_14). The overall genetic variability for the COIII gene revealed a high haplotype diversity (Hd = 0.9233), a nucleotide diversity (Pi = 0.05458), and an average number of nucleotide differences (k = 18.77667).
Genetic distances (based on COI) between Octopus hubbsorum and O. mimus ranged from 0.008 to 0.010, indicating a slight divergence between the two species. The O. hubbsorum_1 sequences generated in this study were genetically similar to those from GenBank (O. hubbsorum_2; 0.002), supporting their conspecificity. Notably, the O. mimus_2 sequences, clustered with O. hubbsorum in the phylogenetic analysis, exhibited lower genetic distances (0.002–0.008) to O. hubbsorum than to the other O. mimus sequences (Table 3).

Table 3.
Genetic distances between mitochondrial COI sequences of Octopus hubbsorum and Octopus mimus. Sequences include those generated in this study (O. hubbsorum_1), reference sequences from GenBank (O. hubbsorum_2, O. mimus_1), and overlapping sequences used in the phylogenetic analysis (O. mimus_2).
Genetic distances based on COIII sequences showed low divergence between O. hubbsorum_1 and O. hubbsorum_2 (0.002), supporting the consistency between sequences generated in this study and those previously deposited in GenBank. Similarly, O. hubbsorum and O. mimus exhibited low divergence values ranging from 0.010 to 0.011, suggesting a close genetic relationship (Table 4).

Table 4.
Genetic distances between mitochondrial COIII sequences of Octopus hubbsorum and Octopus mimus. Sequences include those generated in this study (O. hubbsorum_1) and reference sequences from GenBank (O. hubbsorum_2, O. mimus).
4. Discussion
In octopuses, the high similarity in structural morphology leads to taxonomic misidentification. Therefore, the combined use of morphological and molecular approaches is crucial for the correct identification of species, which is important for understanding their evolutionary history, phylogenetic relationships, and distribution.
Octopus hubbsorum and O. mimus are morphologically similar species within a taxonomic complex that requires detailed reevaluation [18]. In this context, Valdez-Cibrián et al. [47] proposed that O. hubbsorum could be part of a species complex including three morphotypes: O. hubbsorum and O. mimus, which are non-ocellated, and Octopus oculifer (Hoyle 1904), which is ocellated. Furthermore, several studies based on mitochondrial DNA sequence analysis suggest that O. hubbsorum and O. mimus could be the same species due to their low genetic divergence [21,47,48,49] and their overlapping morphological characteristics [43].
In the present study, the analysis of genetic distances showed that O. hubbsorum and O. mimus have low genetic divergence based on COI sequences (0.008 and 0.010), as mentioned by Pliego-Cardenas et al. [21], and COIII sequences (0.010–0.011), suggesting a close evolutionary relationship. However, in the trees obtained in this study, both species appear in clearly separate subclades. In this analysis, two sequences corresponding to O. mimus (KT335830.1 and MH194435.1) were identified within the O. hubbsorum clade, showing low genetic distances (0.002–0.008) to the O. hubbsorum sequences obtained in this study, with evidence of haplotype overlap between both species. The first corresponds to a specimen collected in Ecuador and cataloged as O. mimus in GenBank [50]. The second sequence comes from a study with samples obtained from a seafood restaurant in the city of Trujillo on the northern coast of Peru [51]. Although the study identified the specimen as O. hubbsorum, since there is no evidence of importation of the species to Peru or concrete information on the source of the specimen, this sequence was cataloged as O. mimus [51]. In this sense, several studies have questioned the taxonomic identity of the sequences available in GenBank, suggesting that these could belong to species other than those reported in the articles where they were initially published [14,52,53]. These inconsistencies highlight the importance of supplementing molecular analyses with solid morphological data to resolve taxonomic uncertainties effectively. In this context, the genetic studies conducted to date on O. hubbsorum may still not be sufficient to support its reclassification as O. mimus. Further integrative research combining molecular and detailed morphological analyses is required to clarify its taxonomic status. Relying only on molecular sequences without morphological information can lead to misidentifications, as pointed out in several studies [4].
To help elucidate the issue in the identification of O. hubbsorum and O. mimus, this study also provides morphological information on eggs and paralarvae, such as egg size, mantle length, number of suckers, chromatophore pattern, and characteristics of the radula and beak considered of taxonomic importance [4,9,10].
The chorionic membrane of O. hubbsorum eggs has a peduncle with annular widening (described as a small vesicle of still undetermined function) and also reported by Montero-Ruiz et al. [43] as a sac filled with fluid, similar to that observed in O. mimus [46], Robsonella fontaniana (A. d’Orbigny 1834) [54], and Octopus huttoni (Benham 1943) [55].
Egg size is an important trait from ecological and evolutionary perspectives [56]. In this study, the average egg size in O. hubbsorum during embryonic development was 2.0 mm TL, similar to the egg size reported for O. mimus from the coasts of Chile (2.03–2.2 mm TL) [45,46]. However, curiously, the size of O. hubbsorum eggs reported for the southern coast of Oaxaca, Mexico, was smaller (1.66 mm TL) [42] than previous reports. A possible explanation could be the difference in latitude between Oaxaca and the localities considered in this study. Egg size is related to the nutritional status of the female, water temperature, and latitude [57,58], as demonstrated by the sizes of eggs collected in Bahía de La Paz (24°07′ N) and San Bruno-Santa Rosalía (27°16′ N and 27°20′ N, respectively). Combined with the effect of latitude, another possible explanation could also be that the eggs from Oaxaca reported by Alejo-Plata and Herrera [42] were of the least advanced stages, which could influence the average size. As shown in this study and Castro-Fuentes et al. [46], eggs increase significantly in size during embryonic development. This hypothesis could also be the case for O. mimus (1.67 mm TL) from the coasts of Chile [24] (a latitudinally distant locality from Oaxaca), as none of these studies specify whether the measurements reported correspond to eggs in early development stages or whether they represent the average of all stages, including advanced ones. Therefore, it is evident that egg size should be considered to discriminate between species because this trait is influenced by environmental conditions. Furthermore, it is important that studies explicitly report the size of eggs, considering the embryonic development stage.
In the present study, newly hatched paralarvae of O. hubbsorum were 1.6 mm TL, similar to the size reported by Alejo-Plata and Herrera [42] for specimens collected on the coast of Oaxaca; however, they are smaller than those from the coasts of Jalisco, Mexico (2.1 mm TL) [43]. This difference could be because the size reported by Montero-Ruiz et al. [43] possibly corresponds to older paralarvae (one day post-hatching) since the present study found that two-day-old paralarvae were larger (2.17 mm TL) than newly hatched ones. However, from the above, O. hubbsorum paralarvae are smaller than those of O. mimus (2.3–3.1 mm TL) [24,45,46]. Therefore, paralarva size could be a species-specific characteristic.
The paralarvae of O. hubbsorum have a thin layer of loose skin covering the body (except in the mantle, funnel, and sucker cavities), which has been reported for O. bimaculatus, Macrotritopus defilippi (Verany 1851) [38], Argonauta argo Linnaeus 1758 [59], and Enteroctopus megalocyathus (Gould 1852) [60], and which is observed from birth to settlement [61]. However, a distinctive feature of O. hubbsorum paralarvae that has not been reported in the aforementioned species is the presence of chromatophores in this skin layer in the ventral part of the mantle. This integument has not been documented for O. mimus, so its presence in this species is unknown.
During the paralarvae stage, the most notable differences between octopus species are related to the chromatophore pattern and the number of suckers [9,10], making them effective taxonomic traits for species identification. In the present study, we did find a clear chromatophore pattern consistent with the data reported by Montero-Ruiz et al. [43] for O. hubbsorum (Table 2) and O. mimus.
O. hubbsorum and O. mimus share the characteristic of having three suckers per arm, as well as the presence of Kölliker’s organs. The SEM examination of O. hubbsorum suckers revealed that this structure has a smooth edge, as reported for O. vulgaris [62]. However, the infundibulum has flattened protrusions with tiny pores, which contrasts markedly with the rounded and concave protrusions observed in O. vulgaris paralarvae [62]. In other octopus species, the characteristics of the infundibulum vary considerably. For example, in R. fontaniana, protruding concave protrusions with an enlarged border and a double circumferential margin have been described [54], while in A. burryi (Voss, 1950), the infundibulum has a thin, smooth edge and is almost completely covered by marked protuberances [63]. Differences in sucker development and structure are even more noticeable in other cephalopod groups, such as Sepia officinalis Linnaeus 1758 and Loligo vulgaris Lamarck 1798 [62]. Therefore, it is clear that the structure of octopus suckers is species-specific and can be of taxonomic value. There are no detailed studies about the ultrastructure of suckers currently available for O. mimus.
The radula and beak are important taxonomic traits, as both are among the few hard structures in octopuses [64,65]. In the case of the radula, the number and characteristics of the spinal tooth, such as size and shape, can vary, making it a valuable element for taxonomic analysis [66,67,68].
The radula of O. hubbsorum comprises seven teeth and two marginal plates, an arrangement consistent with the one previously reported for this species [18,43] and for adult specimens of other Octopus species [9,44,47,64]. Montero-Ruiz et al. [43] pointed out that the radula of O. hubbsorum is closely similar to the radula of O. oculifer [47] and O. mimus [44] from adult organisms. However, although they all have the same number of teeth (R, L1, M1, M2, and MP), the position and morphology of M1 and M2 teeth vary markedly between these species. In O. hubbsorum, the M1 tooth has a wide base and a fine–wide cusp, while the M2 tooth is a curved–long cusp, located above the M1 in its middle part (this study). In contrast, in adults of O. mimus, M1 has a wide cusp, while M2 is curved–long–fine cusp, located at the base of M1 at approximately 90° [44]. In O. oculifer adults, M1 has a fine–wide cusp, while M2, although curved–thin, has a slightly bent cusp and is positioned above M1 [47]. These morphological differences suggest two possible interpretations: the radula may vary when compared between adult specimens, or this structure reflects inherent anatomical differences between species.
On the other hand, the beak of O. hubbsorum has dark denticles, a feature that has also been described in O. mimus [44], Eledone cirrhosa (Lamarck 1798) [69], and O. vulgaris [64,65]. However, the oral denticles of the beak of O. hubbsorum paralarvae have distinctive morphological characteristics: the denticles have a pointed cusp in the upper jaw and an oval cusp in the lower jaw. Unfortunately, there are no studies about the beak morphology in O. mimus to compare with O. hubbsorum. Differences in denticle morphology could be related to the prey consumed by each species [64,70]. For example, the pointed cusps of teeth in the upper jaw of O. hubbsorum could be adapted to scrape or tear hard food items, such as crustaceans or mollusks.
5. Conclusions
In conclusion, the low genetic divergence and the few morphological differences reported in all studies suggest that O. hubbsorum and O. mimus belong to the same species. However, it is also possible that these are two separate species with a close phylogenetic relationship in which both share a common ancestor, and that their divergence occurred relatively recently in evolutionary terms [2]. The information currently available about their distribution would support this hypothesis. In this sense, it has been reported that O. hubbsorum is distributed in temperate to tropical areas, from 24° N in Magdalena Bay to 15° N in Oaxaca, Mexico, including the Gulf of California [20,71]. In contrast, O. mimus is distributed in temperate to cold areas, from 4° S in northern Peru to 26° S in northern Chile [18,22,44,72]. This suggests that O. hubbsorum could be venturing farther south, reaching temperate waters in northern Peru, which would explain that the sequences of O. mimus from Ecuador and northern Peru reported by Pliego-Cárdenas et al. [50] and Marin et al. [51], respectively, which are assigned to the O. hubbsorum clade, could actually correspond to O. hubbsorum. However, from the above, it is necessary to carry out further morphological studies to compare the species and carry out population analyses to elucidate whether they correspond to the same species.
The integration of morphological and molecular tools is essential in taxonomic studies. While molecular techniques identify genetic lineages and solve complex taxonomic problems, traditional taxonomy describes morphological traits, diagnostic structures, and coloration patterns. Together, these two approaches provide more robust diagnoses and a more comprehensive understanding of biodiversity.
Author Contributions
Conceptualization, M.G.-F. and M.A.-M.; formal analysis, M.G.-F. and R.M.M.-C.; methodology, M.G.-F.; project administration, M.A.-M.; supervision, M.A.-M.; writing—original draft preparation, M.G.-F.; writing—review and editing, M.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto Politécnico Nacional (projects SIP 20230770, 20241674, and 20250240). Maritza García Flores received support from Beca de Estímulo Institucional de Formación de Investigadores (Instituto Politécnico Nacional [IPN]) and Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI); the results reported here are part of her PhD thesis. Marcial Arellano-Martínez received financial support from Sistema de Becas por Exclusividad (Comisión de Operación y Fomento de Actividades Académicas), Estímulos al Desempeño de los Investigadores (IPN), and Sistema Nacional de Investigadoras e Investigadores (SECIHTI).
Institutional Review Board Statement
The study did not require ethical approval due to the samples being occasional and obtained through the region’s fisheries.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors wish to acknowledge María Elena Sánchez-Salazar for her editorial contribution to the English manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
Appendix A

Table A1.
Reference octopus species used in this study with their corresponding collection locality, Phase, GenBank ID, and reference.
Table A1.
Reference octopus species used in this study with their corresponding collection locality, Phase, GenBank ID, and reference.
| Taxa | Developmental Phase | Sampling Region | GenBank Accession Number | Reference | |
|---|---|---|---|---|---|
| COI | COIII | ||||
| Octopus | Paralarvae | San Bruno, BCS, Mex. | OR195457 | OR473046 | This study |
| hubbsorum | Embryo | San Bruno, BCS, Mex. | OR195458 | OR473047 | This study |
| Embryo | Santa Rosalía, BCS, Mex. | OR195459 | OR473048 | This study | |
| Embryo | Santa Rosalía, BCS, Mex. | OR195460 | OR473049 | This study | |
| Embryo | Ensenada de La Paz, BCS, Mex. | OR195461 | - | This study | |
| Adult | San Juanico, BCS, Mex. | MH400897 | - | [8] | |
| Adult | Acapulco, Gro, Mex. | MH400898 | - | [8] | |
| Adult | Bahía Magdalena, BCS, Mex | MN745318 | - | [73] | |
| Adult | Michoacán, Mex. | KF225001 | KF225007 | [21] | |
| Adult | Nayarit, Mex. | KF225002 | KF225008 | [21] | |
| Adult | Nayarit, Mex. | - | KF225009 | [21] | |
| Adult | Sonora, Mexico | KF225004 | KF225010 | [21] | |
| Adult | Malpelo Island, Colombia | KF225005 | KF225011 | [21] | |
| Adult | Santa Rosalía, BCS, Mex. | KY985006 | - | [74] | |
| Adult | Santa Rosalía, BCS, Mex. | KY985009 | - | [74] | |
| Octopus mimus | Adult | Salinas, Ecuador | KT335830 | KT335842 | [50] |
| Adult | - | - | GU355931 | [14] | |
| Adult | Coloso, Chile | GU355923 | GU355928 | [14] | |
| Adult | Callao, Perú | GU355924 | GU355927 | [14] | |
| Adult | Callao, Perú | GU355925 | GU355933 | [14] | |
| Adult | Coloso, Chile | GU355926 | GU355929 | [14] | |
| Adult | - | MN977146 | - | [75] | |
| Adult | Trujillo, Perú | MN194435 | - | [51] | |
| Adult | Miraflores, Perú | MH194493 | - | [51] | |
| Adult | Huarmey, Perú | MH194502 | - | [51] | |
| Adult | San Isidro, Perú | MH194545 | - | [51] | |
| Paralarvae | Caleta Punta Arenas, Chile | - | KT314263 | [76] | |
| Paralarvae | Caleta Punta Arenas, Chile | - | KT314264 | [76] | |
| Adult | Perú | - | KC886293 | [48] | |
| Adult | Chile | - | KC886302 | [48] | |
| Octopus | Adult | Veracruz, México | KY492362 | - | [77] |
| insularis | Adult | Ceará, Brazil | KX611859 | - | [78] |
| Adult | Veracruz, México | - | KX219649 | [77] | |
| Adult | Veracruz, México | - | KX219648 | [77] | |
| Octopus s | Adult | Guerrero Negro, BCS, Mexico | KY985047 | - | [72] |
| bimaculatu | Adult | Golfo de California, Mexico | KT335828 | - | [77] |
| Octopus | Adult | Merimbula, NSW, Australia | MH289829 | - | [79] |
| tetricus | Adult | - | AF000056 | - | [80] |
| Adult | Australia | - | AJ628240 | [28] | |
| Adult | Australia: Bendalong | - | JX680530 | [81] | |
| Octopus s | Adult | - | AB052253 | - | [82] |
| vulgari | Adult | Chile: Juan Fernandez Island | KU525767 | - | [7] |
| Adult | French Southern and Antarctic Lands: Saint Paul | - | FN424384 | [83] | |
| Adult | Mediterranean Sea | - | JQ085601 | [84] | |
| Vampyroteuthis | Adult | New Zealand | MK186003 | - | [85] |
| infernalis | Adult | - | - | GU288521 | [86] |
Figure A1.
Radula extraction. Abbreviations: a, arm; bk, beak; de, denticles; e, eye; rc, radular canal.
References
- De Queiroz, K. Ernst Mayr and the Modern Concept of Species. Proc. Natl. Acad. Sci. USA 2005, 102, 6600–6607. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.D.; Strugnell, J.M.; Leite, T.S.; Lima, S.M.Q. A Biogeographic Framework of Octopod Species Diversification: The Role of the Isthmus of Panama. PeerJ 2020, 8, e8691. [Google Scholar] [CrossRef] [PubMed]
- Warnke, K.; Soeller, R.; Blohm, D.; Saint-Paul, U. Assessment of the Phylogenetic Relationship between Octopus vulgaris Cuvier, 1797 and O. mimus Gould 1852, Using Mitochondrial 16S r DNA in Combination with Morphological Characters. Abh. Geol. Bundesanst. 2002, 57, 117. [Google Scholar]
- Norman, M.D.; Hochberg, F.G. The Current State of Octopus Taxonomy. Phuket Mar. Biol. Cent. Res. Bull. 2005, 66, 127–154. [Google Scholar]
- Ortiz, N.; Ré, M.E.; Márquez, F. First Description of Eggs, Hatchlings and Hatchling Behaviour of Enteroctopus megalocyathus (Cephalopoda: Octopodidae). J. Plankton Res. 2006, 28, 881–890. [Google Scholar] [CrossRef]
- Amor, M.D.; Laptikhovsky, V.; Norman, M.D.; Strugnell, J.M. Genetic Evidence Extends the Known Distribution of Octopus insularis to the Mid-Atlantic Islands Ascension and St Helena. J. Mar. Biol. Assoc. U. K. 2015, 97, 753–758. [Google Scholar] [CrossRef]
- Amor, M.D.; Norman, M.D.; Roura, A.; Leite, T.S.; Gleadall, I.G.; Reid, A.; Perales-Raya, C.; Lu, C.C.; Silvey, C.J.; Vidal, E.A.G.; et al. Morphological Assessment of the Octopus vulgaris Species Complex Evaluated in Light of Molecular-Based Phylogenetic Inferences. Zool. Scr. 2017, 46, 275–288. [Google Scholar] [CrossRef]
- Díaz-Santana-Iturrios, M.; Salinas-Zavala, C.A.; García-Rodríguez, F.J.; Granados-Amores, J. Taxonomic Assessment of Species of the Genus Octopus from the Northeastern Pacific via Morphological, Molecular and Morphometric Analyses. PeerJ 2019, 7, e8118. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Roper, C.F.E.; Mangold, K.M.; Clark, M.R.; Boletzky, S.V. “Larval” and Juvenile Cephalopods: A Manual for Their Identification; Smithsonian Institution Press: Washington, DC, USA, 1992; p. 279. [Google Scholar]
- Messenger, J.B.; Young, J.Z. The Radular Apparatus of Cephalopods. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 161–182. [Google Scholar] [CrossRef]
- Young, R.E.; Harman, R.F.; Hochberg, F.G. Octopodid Paralarvae from Hawaiian Waters. Veliger 1989, 32, 152–165. [Google Scholar]
- Zaragoza, N.; Quetglas, A.; Moreno, A. Identification Guide for Cephalopod Paralarvae from the Mediterranean Sea; ICES Cooperative Research Reports (CRR), No. 324; ICES: Copenhagen, Denmark, 2015; p. 91. [Google Scholar]
- Warnke, K.; Söller, R.; Blohm, D.; Saint-Paul, U. Rapid Differentiation between Octopus vulgaris Cuvier (1797) and Octopus Mimus Gould (1852), Using Randomly Amplified Polymorphic DNA. J. Zool. Syst. Evol. Res. 2000, 38, 119–122. [Google Scholar] [CrossRef]
- Acosta-Jofré, M.S.; Sahade, R.; Laudien, J.; Chiappero, M.B. A Contribution to the Understanding of Phylogenetic Relationships among Species of the Genus Octopus (Octopodidae: Cephalopoda). Sci. Mar. 2012, 76, 311–318. [Google Scholar] [CrossRef]
- Santana-Cisneros, M.L.; Rodríguez-Canul, R.; Zamora-Briseño, J.A.; Améndola-Pimenta, M.; De Silva-Dávila, R.; Ordóñez-López, U.; Velázquez-Abunader, I.; Ardisson, P.L. Morphological and Molecular Identification of Octopoda (Mollusca: Cephalopoda) Paralarvae from the Southern Gulf of Mexico. Bull. Mar. Sci. 2021, 97, 281–304. [Google Scholar] [CrossRef]
- Jesus, M.D.; Sales, J.B.d.L.; Martins, R.S.; Ready, J.S.; Costa, T.A.S.; Ablett, J.D.; Schiavetti, A. Traditional Knowledge Aids Description When Resolving the Taxonomic Status of Unsettled Species Using Classical and Molecular Taxonomy: The Case of the Shallow-Water Octopus Callistoctopus furvus (Gould, 1852) From the Western Atlantic Ocean. Front. Mar. Sci. 2021, 7, 595244. [Google Scholar] [CrossRef]
- De Luna Sales, J.B.; Haimovici, M.; Ready, J.S.; Souza, R.F.; Ferreira, Y.; de Cassia Silva Pinon, J.; Costa, L.F.C.; Asp, N.E.; Sampaio, I.; Schneider, H. Surveying Cephalopod Diversity of the Amazon Reef System Using Samples from Red Snapper Stomachs and Description of a New Genus and Species of Octopus. Sci. Rep. 2019, 9, 5956. [Google Scholar] [CrossRef]
- Jereb, P.; Roper, C.F.E.; Norman, M.; Finn, J. Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. Octopods and Vampire Squids. In FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2016; Volume 3, p. 398. [Google Scholar]
- Gotshall, D. Marine Animals of Baja California: A Guide to the Common Fish and Invertebrates; Sea Challengers: Monterey, CA, USA, 1987. [Google Scholar]
- Domínguez-Contreras, J.F.; Ceballos-Vázquez, B.P.; Hochberg, F.G.; Arellano-Martínez, M. A New Record in a Well-Established Population of Octopus hubbsorum (Cephalopoda: Octopodidae) Expands Its Known Geographic Distribution Range and Maximum Size. Am. Malacol. Bull. 2013, 31, 95–99. [Google Scholar] [CrossRef]
- Pliego-Cárdenas, R.; Hochberg, F.G.; De León, F.J.G.; Barriga-Sosa, I.D.L.A. Close Genetic Relationships between Two American Octopuses: Octopus hubbsorum Berry, 1953, and Octopus mimus Gould, 1852. J. Shellfish. Res. 2014, 33, 293–303. [Google Scholar] [CrossRef]
- Cardoso, F.; Villegas, P.; Estrella, C. Observaciones Sobre La Biología de Octopus mimus (Cephalopoda: Octopoda) En La Costa Peruana. Rev. Peru. Biol. 2004, 11, 45–50. [Google Scholar] [CrossRef]
- Sauer, W.H.H.; Gleadall, I.G.; Downey-Breedt, N.; Doubleday, Z.; Gillespie, G.; Haimovici, M.; Ibáñez, C.M.; Katugin, O.N.; Leporati, S.; Lipinski, M.R.; et al. World Octopus Fisheries. Rev. Fish. Sci. Aquac. 2021, 29, 279–429. [Google Scholar] [CrossRef]
- Ibáñez, C.M.; Carrasco, S.A.; Díaz-Santana-Iturrios, M.; Cisneros, R.; Pardo-Gandarillas, M.C. Octopus mimus, the Changos’ Octopus. In Octopus Biology and Ecology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 119–131. ISBN 9780128206393. [Google Scholar]
- Flores-Valle, A. Descripción Morfológica y Determinación De Identidades Genéticas De Pulpos De Las Costas Mexicanas. Master’s Thesis, Universidad Autónoma Metropolitana—Unidad Iztapalapa, Mexico City, Mexico, 2010; p. 119. [Google Scholar]
- Aljanabi, S.M.; Martinez, I. Universal and Rapid Salt-Extraction of High Quality Genomic DNA for PCR-Based Techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Guzik, M.T.; Norman, M.D.; Crozier, R.H. Molecular Phylogeny of the Benthic Shallow-Water Octopuses (Cephalopoda: Octopodinae). Mol. Phylogenet. Evol. 2005, 37, 235–248. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, Weighting, and Phylogenetic Utility of Mitochondrial Gene Sequences and a Compilation of Conserved Polymerase Chain Reaction Primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BIOEDIT: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/ NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle BT—Selected Papers of Hirotugu Akaike. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research-an Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Hochberg, F.G.; Nixon, M.; Toll, R.B. Order Octopoda Leach, 1818. Smithson. Contrib. Zool. 1992, 513, 213–280. [Google Scholar]
- Geiger, D.L.; Marshall, B.A.; Ponder, W.F.; Sasaki, T.; Warén, A. Techniques for Collecting, Handling, Preparing, Storing and Examining Small Molluscan Specimens. Molluscan. Res. 2007, 27, 1–50. [Google Scholar] [CrossRef]
- Kruta, I.; Landman, N.H.; Tanabe, K. Ammonoid Radula. In Ammonoid Paleobiology: From Anatomy to Ecology; Springer: Dordrecht, The Netherlands, 2015; pp. 485–505. [Google Scholar]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software: Boston, MA, USA, 2023. [Google Scholar]
- Alejo-Plata, M.D.C.; Herrera Alejo, S.N. First Description of Eggs and Paralarvae of Green Octopus Octopus hubbsorum (Cephalopoda: Octopodidae) under Laboratory Conditions. Am. Malacol. Bull. 2014, 32, 132–139. [Google Scholar] [CrossRef]
- Montero-Ruíz, R.U.; De Silva-Dávila, R.; Amezcua-Gómez, C.A.; Valdez-Cibrián, A.; Kozak, E.R. Description of the Spawning, Embryonic Development, and Paralarvae of the Green Octopus Octopus hubbsorum Berry 1953 (Cephalopoda: Octopodidae) under Laboratory Conditions. Mar. Biol. 2023, 170, 116. [Google Scholar] [CrossRef]
- Guerra, A.; Cortez, T.; Rocha, F. Redescripción Del Pulpo de Los Changos, Octopus mimus Gould, 1852, Del Litoral Chileno-Peruano (Mollusca, Cephalopoda). Iberus 1999, 17, 37–57. [Google Scholar]
- Warnke, K. Observations on the Embryonic Development of Octopus mimus (Mollusca: Cephalopoda) from Northern Chile. Veliger 1999, 42, 211–217. [Google Scholar]
- Castro-Fuentes, H.; Olivares-Paz, A.; Quintana-Fellay, A.; Zuñiga-Romero, O. Descripción Del Desarrollo Embrionario y Paralarva de Octopus mimus (Gould, 1852) (Mollusca: Cephalopoda) En Cautiverio. Estud. Oceanol. 2002, 21, 13–25. [Google Scholar]
- Valdez-Cibrián, A.; Díaz-Santana-iturrios, M.; Landa-Jaime, V.; Michel-Morfín, J.E. First Detection of an Ocellate Octopus in the Revillagigedos Ecoregion, a Biodiversity Hotspot Located in the Tropical East Pacific Province. Zookeys 2020, 2020, 81. [Google Scholar] [CrossRef]
- Pardo-Gandarillas, M.C.; Ibáñez, C.M.; Yamashiro, C.; Méndez, M.A.; Poulin, E. Demographic Inference and Genetic Diversity of Octopus mimus (Cephalopoda: Octopodidae) throughout the Humboldt Current System. Hydrobiologia 2018, 808, 124–135. [Google Scholar] [CrossRef]
- Magallón-Gayón, E.; del Río-Portilla, M.Á.; de los Angeles Barriga-Sosa, I. The Complete Mitochondrial Genomes of Two Octopods of the Eastern Pacific Ocean: Octopus mimus and ‘Octopus’ fitchi (Cephalopoda: Octopodidae) and Their Phylogenetic Position within Octopoda. Mol. Biol. Rep. 2020, 47, 943–952. [Google Scholar] [CrossRef]
- Pliego-Cárdenas, R.; Flores, L.; Markaida, U.; Barriga-Sosa, I.D.L.Á.; Mora, E.; Arias, E. Genetic Evidence of the Presence of Octopus mimus in the Artisanal Fisheries of Octopus in Santa Elena Peninsula, Ecuador. Am. Malacol. Bull. 2016, 34, 51–55. [Google Scholar] [CrossRef]
- Marín, A.; Serna, J.; Robles, C.; Ramírez, B.; Reyes-Flores, L.E.; Zelada-Mázmela, E.; Sotil, G.; Alfaro, R. A Glimpse into the Genetic Diversity of the Peruvian Seafood Sector: Unveiling Species Substitution, Mislabeling and Trade of Threatened Species. PLoS ONE 2018, 13, e0206596. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.S.; Haimovici, M.; Molina, W.; Warnke, K. Morphological and Genetic Description of Octopus insularis, a New Cryptic Species in the Octopus vulgaris Complex (Cephalopoda: Octopodidae) from the Tropical Southwestern Atlantic. J. Molluscan Stud. 2008, 74, 63–74. [Google Scholar] [CrossRef]
- Undheim, E.A.B.; Norman, J.A.; Thoen, H.H.; Fry, B.G. Genetic Identification of Southern Ocean Octopod Samples Using MtCOI. C. R. Biol. 2010, 333, 395–404. [Google Scholar] [CrossRef]
- Ortiz, N.; Ré, M.E. The Eggs and Hatchlings of the Octopus Robsonella fontaniana (Cephalopoda: Octopodidae). J. Mar. Biol. Assoc. U. K. 2011, 91, 705–713. [Google Scholar] [CrossRef]
- Carrasco, S.A. The Early Life History of Two Sympatric New Zealand Octopuses: Eggs and Paralarvae of Octopus huttoni and Pinnoctopus cordiformis. N. Z. J. Zool. 2014, 41, 32–45. [Google Scholar] [CrossRef]
- Lango-Reynoso, F.; Chávez-Villalba, J.; Cochard, J.C.; Le Pennec, M. Oocyte Size, a Means to Evaluate the Gametogenic Development of the Pacific Oyster, Crassostrea gigas (Thunberg 1793). Aquaculture 2000, 190, 183–199. [Google Scholar] [CrossRef]
- McEdward, L.; Carson, S. Variation in Egg Organic Content and Its Relationship with Egg Size in the Starfish Solaster stimpsoni. Mar. Ecol. Prog. Ser. 1987, 37, 159–169. [Google Scholar] [CrossRef]
- Moran, A.L.; McAlister, J.S. Egg Size as a Life History Character of Marine Invertebrates: Is It All It’s Cracked up to Be? Biol. Bull. 2009, 216, 226–242. [Google Scholar] [CrossRef]
- Diekmann, R.; Piatkowski, U.; Schneider, M. Early Life and Juvenile Cephalopods Around Seamounts of the Subtropical Eastern North Atlantic: Illustrations and a Key for Their Identification; Institut für Meereskunde: Kiel, Germany, 2002; p. 42. [Google Scholar]
- Norman, M.D.; Kubodera, T. Taxonomy and Biogeography of an Australian Subtropical Octopus with Japanese Affinities. In Proceedings of the 7th and 8th Symposia on Collection Building and Natural History Studies in Asia and the Pacific Rim; National Museum of Nature and Science: Tsukuba, Japan, 2006; pp. 171–189. [Google Scholar]
- Kubodera, T. Distribution and Abundance of the Early Life Stages of Octopus, Octopus dofleini Wülker, 1910 in the North Pacific. Bull. Mar. Sci. 1991, 49, 235–244. [Google Scholar]
- Schmidtberg, H. The Structure of Suckers of Newly Hatched Sepia officinalis, Loligo vulgaris, and Octopus vulgaris. Vie Milieu 1997, 47, 155–159. [Google Scholar]
- Forsythe, J.W.; Hanlon, R.T. Aspects of Egg Development, Post-Hatching Behavior, Growth and Reproductive Biology of Octopus burryi Voss, 1950 (Mollusca: Cephalopoda). Vie Milieu 1985, 35, 273–282. [Google Scholar]
- Nixon, M.; Mangold, K. The Early Life of Octopus vulgaris (Cephalopoda: Octopodidae) in the Plankton and at Settlement: A Change in Lifestyle. J. Zool. 1996, 239, 301–327. [Google Scholar] [CrossRef]
- Villanueva, R.; Norman, M.D. Biology of the Planktonic Stages of Benthic Octopuses. Oceanogr. Mar. Biol. 2008, 46, 111–208. [Google Scholar] [CrossRef]
- Nixon, M. The Radulae of Cephalopoda. Smithson. Contrib. Zool. 1998, 586, 39–53. [Google Scholar]
- Roper, C.F.E.; Voss, G.L. Guidelines for Taxonomic Descriptions of Cephalopod Species. Mem. Mus. Vic. 1983, 44, 48–63. [Google Scholar] [CrossRef]
- Samuel, D.V.; Patterson, J. A Comparative Study on the Radula of Three Coleoid Cephalopods. S. Pac. Study 2003, 24, 33–38. [Google Scholar]
- Boletzky, S.V. The Larvae of Cephalopoda: A Review. Thalass. Jugosl. 1974, 10, 45–76. [Google Scholar]
- Wells, M.J. Octopus: Physiology and Behaviour of an Advanced Invertebrate, 1st ed.; Springer: Dordrecht, The Netherlands, 1978. [Google Scholar]
- López-Uriarte, E.; Ríos-Jara, E.; Pérez-Peña, M. Range Extension for Octopus hubbsorum (Mollusca: Octopodidae) in the Mexican Pacific. Bull. Mar. Sci. 2005, 77, 171–176. [Google Scholar]
- Defeo, O.; Carlos Castilla, J. Harvesting and Economic Patterns in the Artisanal Octopus mimus (Cephalopoda) Fishery in a Northern Chile Cove. Fish. Res. 1998, 38, 121–130. [Google Scholar] [CrossRef]
- Dueñas-Romero, J.d.J.; Granados-Amores, J.; Palacios-Salgado, D.S.; Domínguez-Contreras, J.F.; Flores-Ortega, J.R.; García-Rodríguez, F.J. Diversity and Population Genetic Structure of Octopus hubbsorum in the Mexican Pacific Inferred from Mitochondrial DNA Sequences. Mar. Freshw. Res. 2020, 72, 35–43. [Google Scholar] [CrossRef]
- Domínguez-Contreras, J.F.; Munguia-Vega, A.; Ceballos-Vázquez, B.P.; Arellano-Martínez, M.; García-Rodríguez, F.J.; Culver, M.; Reyes-Bonilla, H. Life Histories Predict Genetic Diversity and Population Structure within Three Species of Octopus Targeted by Small-Scale Fisheries in Northwest Mexico. PeerJ 2018, 6, e4295. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Ramilo-Fernández, G.; Sotelo, C.G. A Real-Time PCR Method for the Authentication of Common Cuttlefish (Sepia officinalis) in Food Products. Foods 2020, 9, 286. [Google Scholar] [CrossRef]
- Pardo-Gandarillas, M.C.; Ibáñez, C.M.; Ruiz, J.F.; Bustos, C.A.; Peña, F.A.; Landaeta, M.F. Paralarvae of Cephalopods in Channels and Fjords of the Southern Tip of Chile (46–53°S). Fish. Res. 2016, 173, 175–182. [Google Scholar] [CrossRef]
- Flores-Valle, A.; Pliego-Cárdenas, R.; De Lourdes Jimenéz-Badillo, M.; Arredondo-Figueroa, J.L.; De Los Ángeles Barriga-Sosa, I. First Record of Octopus insularis Leite and Haimovici, 2008 in the Octopus Fishery of a Marine Protected Area in the Gulf of Mexico. J. Shellfish. Res. 2018, 37, 221–227. [Google Scholar] [CrossRef]
- Lima, F.D.; Berbel-Filho, W.M.; Leite, T.S.; Rosas, C.; Lima, S.M.Q. Occurrence of Octopus insularis Leite and Haimovici, 2008 in the Tropical Northwestern Atlantic and Implications of Species Misidentification to Octopus Fisheries Management. Mar. Biodivers. 2017, 47, 723–734. [Google Scholar] [CrossRef]
- Ramos, J.E.; Pecl, G.T.; Moltschaniwskyj, N.A.; Semmens, J.M.; Souza, C.A.; Strugnell, J.M. Population Genetic Signatures of a Climate Change Driven Marine Range Extension. Sci. Rep. 2018, 8, 9558. [Google Scholar] [CrossRef]
- Carlini, D.B.; Graves, J.E. Phylogenetic Analysis of Cytochrome c Oxidase I Sequences to Determine Higher-Level Relationships within the Coleoid Cephalopods. Bull. Mar. Sci. 1999, 64, 57–76. [Google Scholar]
- Reid, A.; Wilson, N. Reid y Wilson, Octopuses of the Kermadec Islands_ Discovery and Description of New Member of the Octopus. Bull. Auckl. Mus. 2015, 20. [Google Scholar]
- Minakata, H.; Iwakoshi, E.; Takuwa, K. Octopus vulgaris MRNA for Cytochrome c Oxidase Subunit I. 2016, Unpublished work.
- Guerra, Á.; Roura, Á.; González, Á.F.; Pascual, S.; Cherel, Y.; Pérez-Losada, M. Morphological and Genetic Evidence That Octopus vulgaris Cuvier, 1797 Inhabits Amsterdam and Saint Paul Islands (Southern Indian Ocean). ICES J. Mar. Sci. 2010, 67, 1401–1407. [Google Scholar] [CrossRef]
- Fadhlaoui-Zid, K.; Knittweis, L.; Aurelle, D.; Nafkha, C.; Ezzeddine, S.; Fiorentino, F.; Ghmati, H.; Ceriola, L.; Jarboui, O.; Maltagliati, F. Genetic Structure of Octopus vulgaris (Cephalopoda, Octopodidae) in the Central Mediterranean Sea Inferred from the Mitochondrial COIII Gene. C. R. Biol. 2012, 335, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Braid, H.E.; Bolstad, K.S.R. Cephalopod Biodiversity of the Kermadec Islands: Implications for Conservation and Some Future Taxonomic Priorities. Invertebr. Syst. 2019, 33, 402–425. [Google Scholar] [CrossRef]
- Strugnell, J.; Allcock, A.L. Co-Estimation of Phylogeny and Divergence Times of Argonautoidea Using Relaxed Phylogenetics. Mol. Phylogenet. Evol. 2010, 54, 701–708. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).