Biodiversity Assessment of Syrphid Flies (Diptera: Syrphidae) Within China
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Species Richness of Hoverflies Throughout China
3.2. Biodiversity Indices
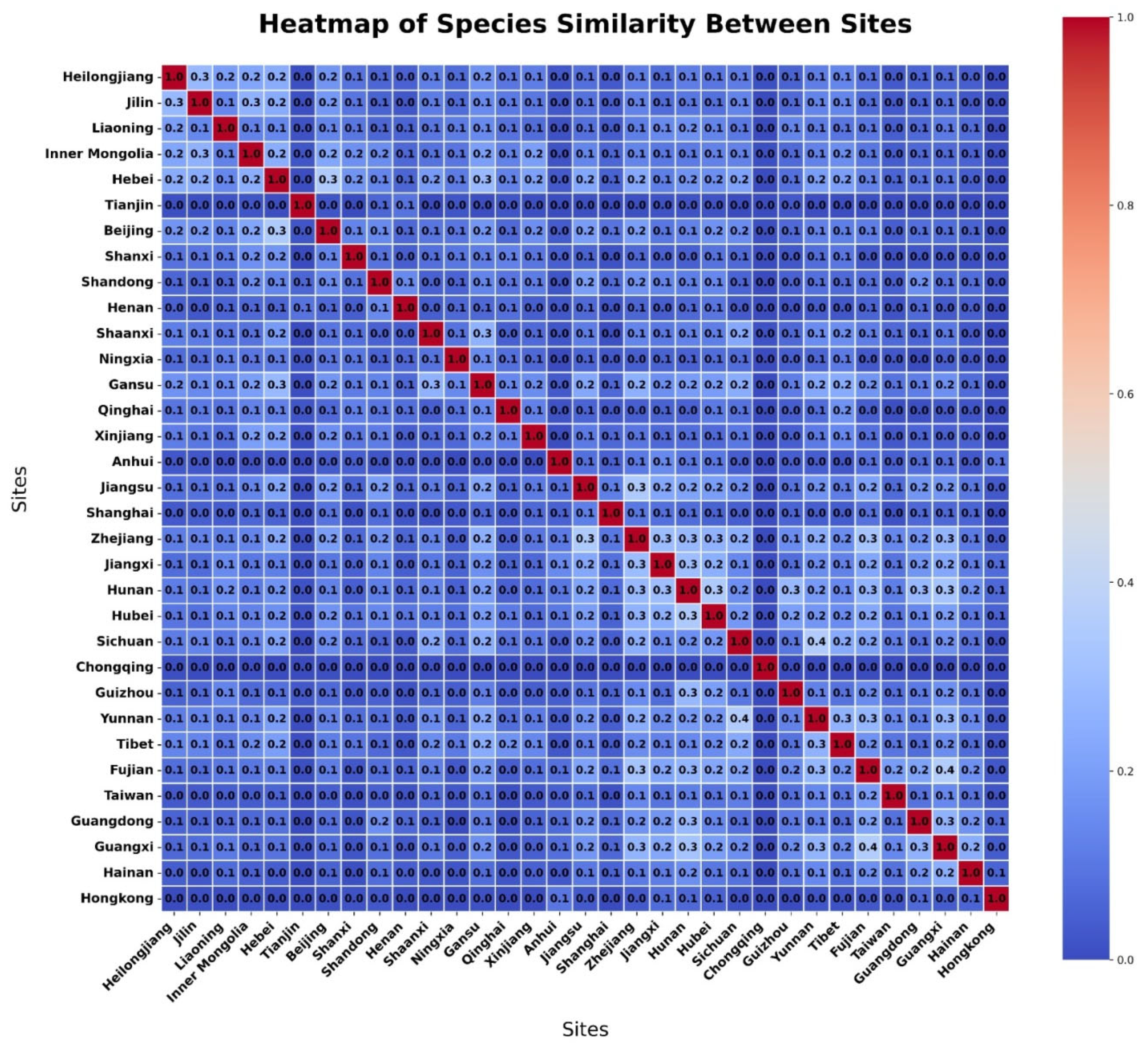
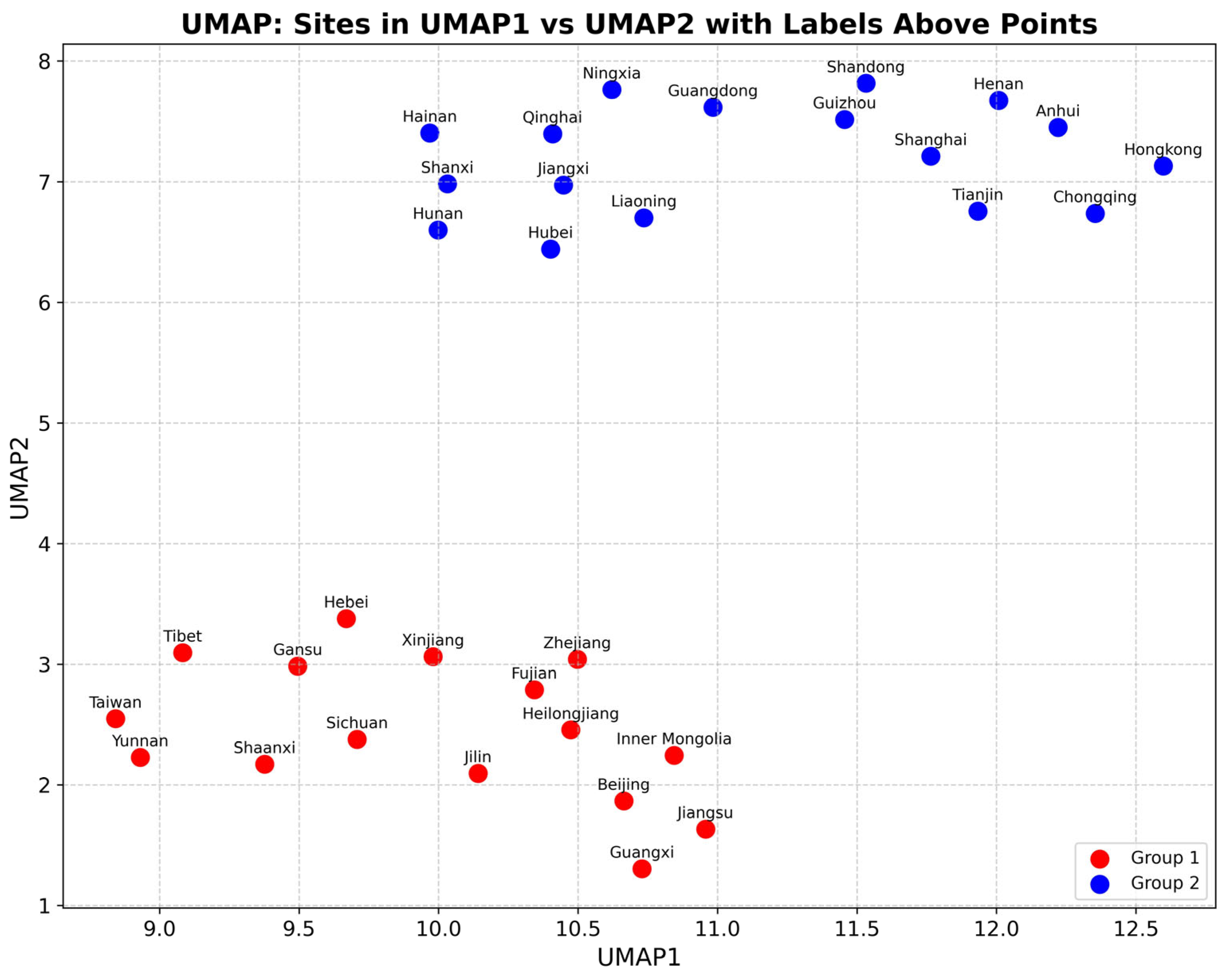
3.3. Habitat Clustering Based on UMAP Analysis
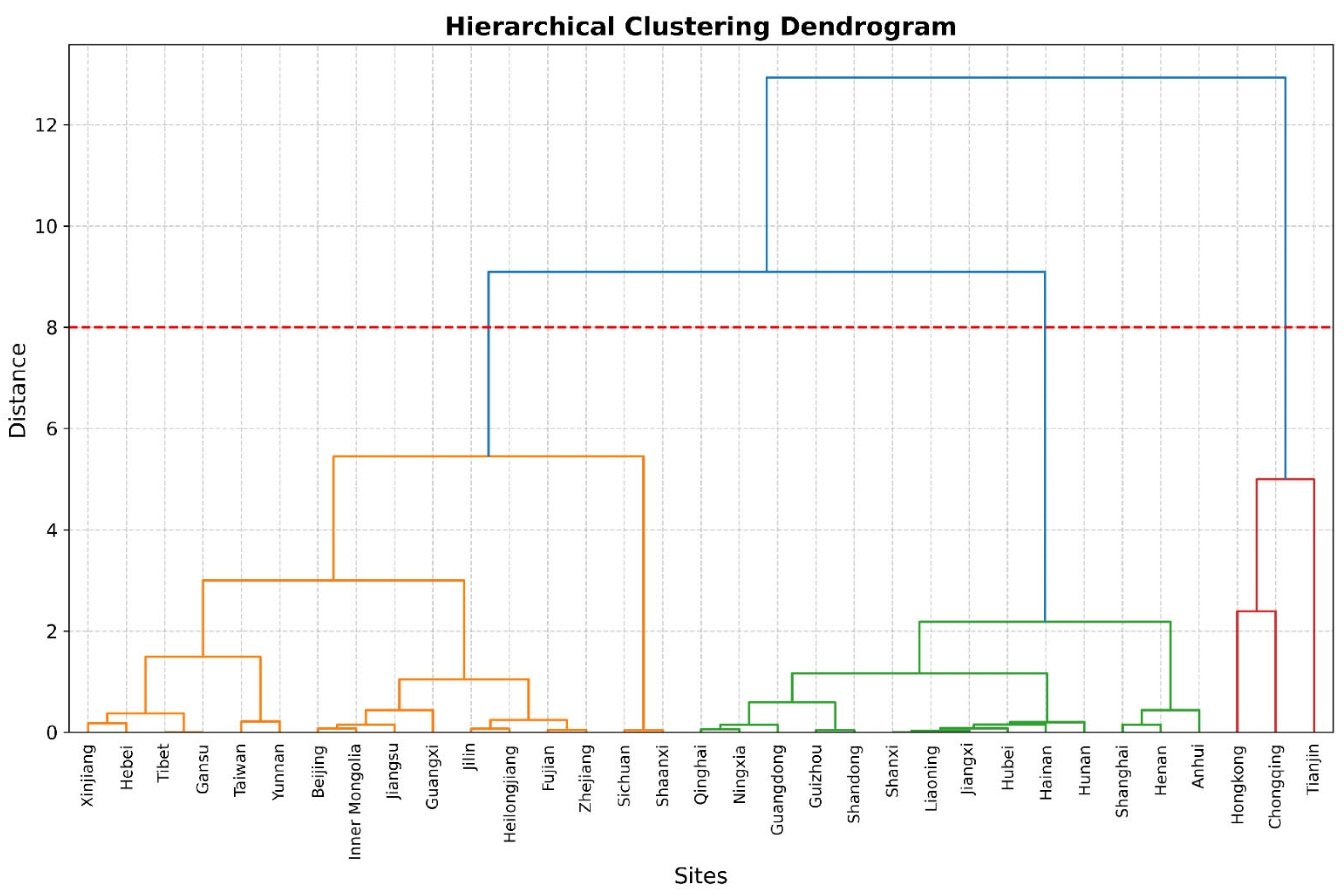
3.4. Hierarchical Habitat Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Liu, H.; Yang, Y. Uncovering the determinants of biodiversity hotspots in China: Evidence from the drivers of multiple diversity metrics on insect assemblages and implications for conservation. Sci. Total Environ. 2023, 880, 163287. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as bioindicator: A hidden gem for environmental monitoring. Front. Environ. Sci. 2023, 11, 1146052. [Google Scholar] [CrossRef]
- Aslam, S.; Naeem, M.; Hussain, S.; Riasat, M.; Rafi, M.A.; Zia, A.; Rafique, M.K.; Bashir, N.H.; Chen, H. Biodiversity of Non-Apis Bees (Hymenoptera: Apoidea) in the Potohar Region of Pakistan. Diversity 2024, 17, 4. [Google Scholar] [CrossRef]
- Rego, C.; Smit, J.; Aguiar, A.; Cravo, D.; Penado, A.; Boieiro, M. A pictorial key for identification of the hoverflies (Diptera: Syrphidae) of the Madeira Archipelago. Biodivers. Data J. 2022, 10, e78518. [Google Scholar] [CrossRef]
- Redak, R. Introduction to and Importance of Insects. In Forest Entomology and Pathology: Volume 1: Entomology; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–17. [Google Scholar]
- Du, C.; Chen, J.; Jiang, L.; Qiao, G. High correlation of species diversity patterns between specialist herbivorous insects and their specific hosts. J. Biogeogr. 2020, 47, 1232–1245. [Google Scholar] [CrossRef]
- Mi, X.; Feng, G.; Hu, Y.; Zhang, J.; Chen, L.; Corlett, R.T.; Hughes, A.C.; Pimm, S.; Schmid, B.; Shi, S. The global significance of biodiversity science in China: An overview. Natl. Sci. Rev. 2021, 8, nwab032. [Google Scholar] [CrossRef]
- Mohammadi-Khoramabadi, A.; Dousti, A.F.; Gharaei, B. Diversity of Hoverflies (Diptera, Syrphidae) in Darab damask rose rain-fed plain, Fars province, Iran. J. Entomol. Soc. Iran 2024, 44, 279–290. [Google Scholar] [CrossRef]
- Sai Teja, K.S.; Ganiger, P. Wasp mimicking Monoceromyia eumenioides (Saunders, 1842) (Diptera: Syrphidae) visiting flowers of the tropical tree plant, Bridelia retusa (L.) A. Juss. from India. Orient. Insects 2023, 57, 421–431. [Google Scholar] [CrossRef]
- Young, A.D.; Lemmon, A.R.; Skevington, J.H.; Mengual, X.; Ståhls, G.; Reemer, M.; Jordaens, K.; Kelso, S.; Lemmon, E.M.; Hauser, M. Anchored enrichment dataset for true flies (order Diptera) reveals insights into the phylogeny of flower flies (family Syrphidae). BMC Evol. Biol. 2016, 16, 143. [Google Scholar] [CrossRef]
- Huo, K.-K.; Zhao, L.; Mengual, X.; Li, G.; Liu, X.; Zhao, L.-J.; Chen, Z.-N. Biema Huo & Zhao gen. nov., a new flower fly genus (Diptera, Syrphidae) from China. Eur. J. Taxon. 2022, 852, 98–116. [Google Scholar]
- Huo, K.K. Syrphidae. In Species Catalogue of China. Volume 2. Animals. Insecta. Diptera (3). Cyclorrhaphous Brachycera (i); Science Press: Beijing, China, 2020; pp. 30–181. [Google Scholar]
- Ball, S.; Morris, R. Britain’s Hoverflies: A Field Guide; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Babošová, M.; Ivanič Porhajašová, J. The diversity, spatial structure, and significance of the Syrphidae population in the territory of the Listové Lake Nature Reserve. J. Cent. Eur. Agric. 2024, 25, 1014–1023. [Google Scholar] [CrossRef]
- Doyle, T.; Hawkes, W.L.; Massy, R.; Powney, G.D.; Menz, M.H.; Wotton, K.R. Pollination by hoverflies in the Anthropocene. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200508. [Google Scholar] [CrossRef]
- Mitra, B. Diversity of flower-visiting flies (Insecta: Diptera) in India and their role in pollination. Rec. Zool. Surv. India 2010, 110, 95–107. [Google Scholar] [CrossRef]
- Mengual, X. New Flower Fly Records (Diptera: Syrphidae: Syrphinae) from China, Korea, and Malaysia. Proc. Entomol. Soc. Wash. 2022, 124, 302–315. [Google Scholar] [CrossRef]
- Speight, M.C.; Lebard, T. Chrysogaster coerulea Strobl in Czerny & Strobl, 1909, espèce nouvelle pour la France (Diptera: Syrphidae). Rev. Fr. Ent. Gén. 2022, 4, 176–183. [Google Scholar]
- Niu, Y.; Ren, G. Patterns of Species Richness and Its Endemism of Beetles in the Beijing–Tianjin–Hebei Region of China. Diversity 2024, 16, 496. [Google Scholar] [CrossRef]
- Levine, J.M.; HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef]
- Sonne, J.; Rahbek, C. Idiosyncratic patterns of local species richness and turnover define global biodiversity hotspots. Proc. Natl. Acad. Sci. USA 2024, 121, e2313106121. [Google Scholar] [CrossRef]
- Arese Lucini, F.; Morone, F.; Tomassone, M.S.; Makse, H.A. Diversity increases the stability of ecosystems. PLoS ONE 2020, 15, e0228692. [Google Scholar] [CrossRef]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Shen, M.; Swengel, S.R.; Chase, J.M. Disproportionate declines of formerly abundant species underlie insect loss. Nature 2024, 628, 359–364. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Zhang, X.; Mao, B.; Yang, G.; Shi, F.; Bi, J.; Ma, Z.; Tang, G. Effects of Environmental Factors on the Diversity of Grasshopper Communities along Altitude Gradients in Xizang, China. Insects 2024, 15, 671. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dobriyal, M.; Kale, A.; Pandey, A.; Tomar, R.; Thounaojam, E. Calculating forest species diversity with information-theory based indices using sentinel-2A sensor’s of Mahavir Swami Wildlife Sanctuary. PLoS ONE 2022, 17, e0268018. [Google Scholar] [CrossRef] [PubMed]
- Leinster, T.; Cobbold, C.A. Measuring diversity: The importance of species similarity. Ecology 2012, 93, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Tomar, V.; Kumar, P.; Rani, M.; Gupta, G.; Singh, J. A satellite-based biodiversity dynamics capability in tropical forest. Electron. J. Geotech. Eng. 2013, 18, 1171–1180. [Google Scholar]
- Ahmad, Z.; Naeem, M.; Azad, R.; Hussain, I.; Bibi, R.; Zaman, M.; Akbar, R.; Zafeer, N.; Elgezouly, R.O.E.; Mustafa, G. Multivariate diversity analysis and systematics of hemipteran insects of family Reduviidae. J. King Saud Univ. Sci. 2022, 34, 101722. [Google Scholar] [CrossRef]
- Pavoine, S.; Bonsall, M.B. Measuring biodiversity to explain community assembly: A unified approach. Biol. Rev. 2011, 86, 792–812. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Bollarapu, M.J.; Ramarao, K. Biodiversity measures-mathematical evaluation of various indices. Oecon. Copernic. 2021, 12, 46–59. [Google Scholar]
- Thukral, A.K.; Bhardwaj, R.; Kumar, V.; Sharma, A. New indices regarding the dominance and diversity of communities, derived from sample variance and standard deviation. Heliyon 2019, 5, e02606. [Google Scholar] [CrossRef]
- Barendregt, A.; Zeegers, T.; van Steenis, W.; Jongejans, E. Forest hoverfly community collapse: Abundance and species richness drop over four decades. Insect Conserv. Divers. 2022, 15, 510–521. [Google Scholar] [CrossRef]
- Klymko, J.; Schlesinger, M.D.; Skevington, J.H.; Young, B.E. Low extinction risk in the flower fly fauna of northeastern North America. J. Insect Conserv. 2023, 27, 657–668. [Google Scholar] [CrossRef]
- Hailay Gebremariam, G. A Systematic Review of Insect Decline and Discovery: Trends, Drivers, and Conservation Strategies over the past Two Decades. Psyche J. Entomol. 2024, 2024, 5998962. [Google Scholar] [CrossRef]
- Sobral-Souza, T.; Santos, J.P.; Maldaner, M.E.; Lima-Ribeiro, M.S.; Ribeiro, M.C. EcoLand: A multiscale niche modelling framework to improve predictions on biodiversity and conservation. Perspect. Ecol. Conserv. 2021, 19, 362–368. [Google Scholar] [CrossRef]
- Zarnetske, P.L.; Read, Q.D.; Record, S.; Gaddis, K.D.; Pau, S.; Hobi, M.L.; Malone, S.L.; Costanza, J.M.; Dahlin, K.; Latimer, A.M. Towards connecting biodiversity and geodiversity across scales with satellite remote sensing. Glob. Ecol. Biogeogr. 2019, 28, 548–556. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, X.; Zhang, Y.; Wang, Y.; Zhou, Z.; Liu, T. Species richness and endemism patterns of Sternorrhyncha (Insecta, Hemiptera) in China. ZooKeys 2023, 1178, 279. [Google Scholar] [CrossRef]
- Zhu, H. Biogeography of Shangri-la flora in southwestern China. Phytotaxa 2015, 203, 231–244. [Google Scholar]
- Van Steenis, J.; Hippa, H.; Mutin, V.A. Revision of the oriental species of the genus Sphegina Meigen, 1822 (Diptera: Syrphidae). Eur. J. Taxon. 2018, 489, 1–198. [Google Scholar] [CrossRef]
- Speight, M. StN key for the identification of the genera of European Syrphidae. Syrph Net Database Eur. Syrphidae (Diptera) 2020, 105, 1–46. [Google Scholar]
- Van Veen, M. Hoverflies of Northwest Europe: Identification Keys to the Syrphidae; KNNV Publishing: Utrecht, The Netherlands, 2004; Volume 254, p. 256. [Google Scholar]
- Zhao, L.; Liu, X.; Smit, J.T.; LI, G.; Liu, H.; Dang, L.-h.; Huo, K. A new species of the genus Psilota Meigen, 1822 (Diptera: Syrphidae) from China. Zootaxa 2022, 5154, 225–238. [Google Scholar] [CrossRef]
- Tian, J.; Huo, K.; Zhang, C.-T.; Ren, B.-Z. Microdon dentigiganteum sp. nov. and other Microdontinae species (Diptera: Syrphidae) from Northeast China. Zootaxa 2019, 4712, 065–076. [Google Scholar] [CrossRef]
- DeJong, T.M. A comparison of three diversity indices based on their components of richness and evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- Krippendorff, K. Mathematical theory of communication. In Encyclopedia of Communication Theory; Sage: Los Angeles, CA, USA, 2009; pp. 614–618. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. Information theory in ecology. Comput. Chem. 2001, 25, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Wei, J.; Niu, M.; Feng, J. Diversity and distribution patterns of scale insects in China. Ann. Entomol. Soc. Am. 2016, 109, 405–414. [Google Scholar] [CrossRef]
- Luo, Y.; Bourgoin, T.; Zhang, J.-L.; Feng, J.-N. Distribution patterns of Chinese Cixiidae (Hemiptera, Fulgoroidea), highlight their high endemic diversity. Biodivers. Data J. 2022, 10, e75303. [Google Scholar] [CrossRef]
- Mirab-balou, M. Thysanoptera (Insecta) of China: An updated checklist. J. Insect Biodivers. Syst. 2025, 11, 469–541. [Google Scholar] [CrossRef]
- Bashir, N.H.; Wang, W.; Liu, J.; Wang, W.; Chen, H. First record of the lac-producing species Kerria nepalensis Varshney (Hemiptera, Kerriidae) from China, with a key to Chinese species. ZooKeys 2021, 1061, 1–9. [Google Scholar] [CrossRef]
- Bashir, N.H.; Yue, D.; Jiang, H.; Ma, L.; Li, Q. Taxonomic study of the subtribe Pemphredonina Dahlbom, 1835 (Hymenoptera: Crabronidae) with a new species and six new records from China. J. Asia-Pac. Entomol. 2021, 24, 1055–1065. [Google Scholar] [CrossRef]
- Ricarte, A.; Nedeljković, Z.; Marcos-García, M.Á. An exploratory survey and assessment of the hoverfly diversity (Diptera: Syrphidae) from the Pyrenees of Girona, Spain. Rev. Suisse Zool. 2021, 128, 381–398. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, S.; Yin, X.; Zhang, C.; Li, R.; Chen, J.; Ma, D.; Wang, Y.; Yu, Z.; Chen, Y. Patterns of tree species richness in Southwest China. Environ. Monit. Assess. 2021, 193, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, M.; Huang, Y.; Chen, K.; Sun, H.; Chen, J. Climatic change can influence species diversity patterns and potential habitats of Salicaceae plants in China. Forests 2019, 10, 220. [Google Scholar] [CrossRef]
- Schirmel, J.; Albrecht, M.; Bauer, P.M.; Sutter, L.; Pfister, S.C.; Entling, M.H. Landscape complexity promotes hoverflies across different types of semi-natural habitats in farmland. J. Appl. Ecol. 2018, 55, 1747–1758. [Google Scholar] [CrossRef]
- Lopez-Collado, J.; Jacinto-Padilla, J.; Rodríguez-Aguilar, O.; Hidalgo-Contreras, J. Bioclimatic similarity between species locations and their environment revealed by dimensionality reduction analysis. Ecol. Inform. 2024, 79, 102444. [Google Scholar] [CrossRef]
- Milošević, D.; Medeiros, A.S.; Piperac, M.S.; Cvijanović, D.; Soininen, J.; Milosavljević, A.; Predić, B. The application of Uniform Manifold Approximation and Projection (UMAP) for unconstrained ordination and classification of biological indicators in aquatic ecology. Sci. Total Environ. 2022, 815, 152365. [Google Scholar] [CrossRef]
- Li, C.; Qiao, W.; Gao, B.; Chen, Y. Unveiling spatial heterogeneity of ecosystem services and their drivers in varied landform types: Insights from the Sichuan-Yunnan ecological barrier area. J. Clean. Prod. 2024, 442, 141158. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- González-Salazar, C.; Stephens, C.R.; Marquet, P.A. Comparing the relative contributions of biotic and abiotic factors as mediators of species’ distributions. Ecol. Modell. 2013, 248, 57–70. [Google Scholar] [CrossRef]
- Lewinsohn, T.M.; Novotny, V.; Basset, Y. Insects on plants: Diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 597–620. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]

| Index | Focus | Importance |
|---|---|---|
| Shannon’s species richness index (R) | Richness | It simply counts the number of species [45] |
| Shannon–Wiener diversity index (H′) | Richness and evenness | It gives a balanced overview of diversity [46] |
| Shannon equitability index | Evenness | Based on evenness values, it gives a dominance comparison between the sites [47] |
| Margalef index | Richness vs. size of sample | It focuses on biodiversity assessment while correcting the biasness present within the sample [48] |
| Simpson index (D) | Dominance | This is sensitive to common species present between different sites [49] |
| Simpson reciprocal index | Overall biodiversity | This assesses the overall biodiversity which is easy to interpret [3] |
| Group | Site | Species Richness | No. of Genera | Shannon–Wiener Index | Simpson’s Index | Simpson’s Reciprocal Index | Margalef’s Index | Shannon Equitability Index |
|---|---|---|---|---|---|---|---|---|
| 1 | Heilongjiang | 105 | 43 | 4.65 | 0.99 | 1.01 | 22.35 | 1 |
| Jilin | 108 | 40 | 4.68 | 0.99 | 1.01 | 22.85 | 1 | |
| Inner Mongolia | 95 | 38 | 4.55 | 0.99 | 1.01 | 20.64 | 1 | |
| Hebei | 134 | 53 | 4.90 | 0.99 | 1.01 | 27.15 | 1 | |
| Beijing | 92 | 46 | 4.52 | 0.99 | 1.01 | 20.12 | 1 | |
| Shaanxi | 256 | 68 | 5.55 | 1.00 | 1.00 | 45.99 | 1 | |
| Gansu | 142 | 55 | 4.96 | 0.99 | 1.01 | 28.45 | 1 | |
| Xinjiang | 126 | 44 | 4.84 | 0.99 | 1.01 | 25.85 | 1 | |
| Jiangsu | 88 | 42 | 4.48 | 0.99 | 1.01 | 19.43 | 1 | |
| Zhejiang | 115 | 49 | 4.74 | 0.99 | 1.01 | 24.03 | 1 | |
| Sichuan | 258 | 75 | 5.55 | 1.00 | 1.00 | 46.28 | 1 | |
| Yunnan | 183 | 65 | 5.21 | 0.99 | 1.01 | 34.94 | 1 | |
| Tibet | 142 | 50 | 4.96 | 0.99 | 1.01 | 28.45 | 1 | |
| Fujian | 113 | 49 | 4.73 | 0.99 | 1.01 | 23.69 | 1 | |
| Taiwan | 173 | 66 | 5.15 | 0.99 | 1.01 | 33.38 | 1 | |
| Guangxi | 77 | 37 | 4.34 | 0.99 | 1.01 | 17.50 | 1 | |
| 2 | Liaoning | 55 | 32 | 4.01 | 0.98 | 1.02 | 13.48 | 1 |
| Tianjin | 2 | 2 | 0.69 | 0.50 | 2.00 | 1.44 | 1 | |
| Shanxi | 55 | 35 | 4.01 | 0.98 | 1.02 | 13.48 | 1 | |
| Shandong | 33 | 22 | 3.50 | 0.97 | 1.03 | 9.15 | 1 | |
| Henan | 22 | 16 | 3.09 | 0.95 | 1.05 | 6.79 | 1 | |
| Ningxia | 44 | 24 | 3.78 | 0.98 | 1.02 | 11.36 | 1 | |
| Qinghai | 46 | 21 | 3.83 | 0.98 | 1.02 | 11.75 | 1 | |
| Anhui | 17 | 13 | 2.83 | 0.94 | 1.06 | 5.65 | 1 | |
| Shanghai | 25 | 16 | 3.22 | 0.96 | 1.04 | 7.46 | 1 | |
| Jiangxi | 54 | 33 | 3.99 | 0.98 | 1.02 | 13.29 | 1 | |
| Hunan | 60 | 30 | 4.09 | 0.98 | 1.02 | 14.41 | 1 | |
| Hubei | 57 | 35 | 4.04 | 0.98 | 1.02 | 13.85 | 1 | |
| Chongqing | 3 | 2 | 1.10 | 0.67 | 1.50 | 1.82 | 1 | |
| Guizhou | 32 | 21 | 3.47 | 0.97 | 1.03 | 8.94 | 1 | |
| Guangdong | 41 | 25 | 3.71 | 0.98 | 1.03 | 10.77 | 1 | |
| Hainan | 51 | 23 | 3.93 | 0.98 | 1.02 | 12.72 | 1 | |
| Hongkong | 6 | 3 | 1.79 | 0.83 | 1.20 | 2.79 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, N.H.; Meng, L.; Naeem, M.; Chen, H. Biodiversity Assessment of Syrphid Flies (Diptera: Syrphidae) Within China. Diversity 2025, 17, 471. https://doi.org/10.3390/d17070471
Bashir NH, Meng L, Naeem M, Chen H. Biodiversity Assessment of Syrphid Flies (Diptera: Syrphidae) Within China. Diversity. 2025; 17(7):471. https://doi.org/10.3390/d17070471
Chicago/Turabian StyleBashir, Nawaz Haider, Licun Meng, Muhammad Naeem, and Huanhuan Chen. 2025. "Biodiversity Assessment of Syrphid Flies (Diptera: Syrphidae) Within China" Diversity 17, no. 7: 471. https://doi.org/10.3390/d17070471
APA StyleBashir, N. H., Meng, L., Naeem, M., & Chen, H. (2025). Biodiversity Assessment of Syrphid Flies (Diptera: Syrphidae) Within China. Diversity, 17(7), 471. https://doi.org/10.3390/d17070471





