Temporal Niche Partitioning as a Coexistence Mechanism Between China’s Endemic Elliot’s Pheasant (Syrmaticus ellioti) and Its Predator, the Leopard Cat (Prionailurus bengalensis)
Abstract
1. Introduction
2. Materials and Methods
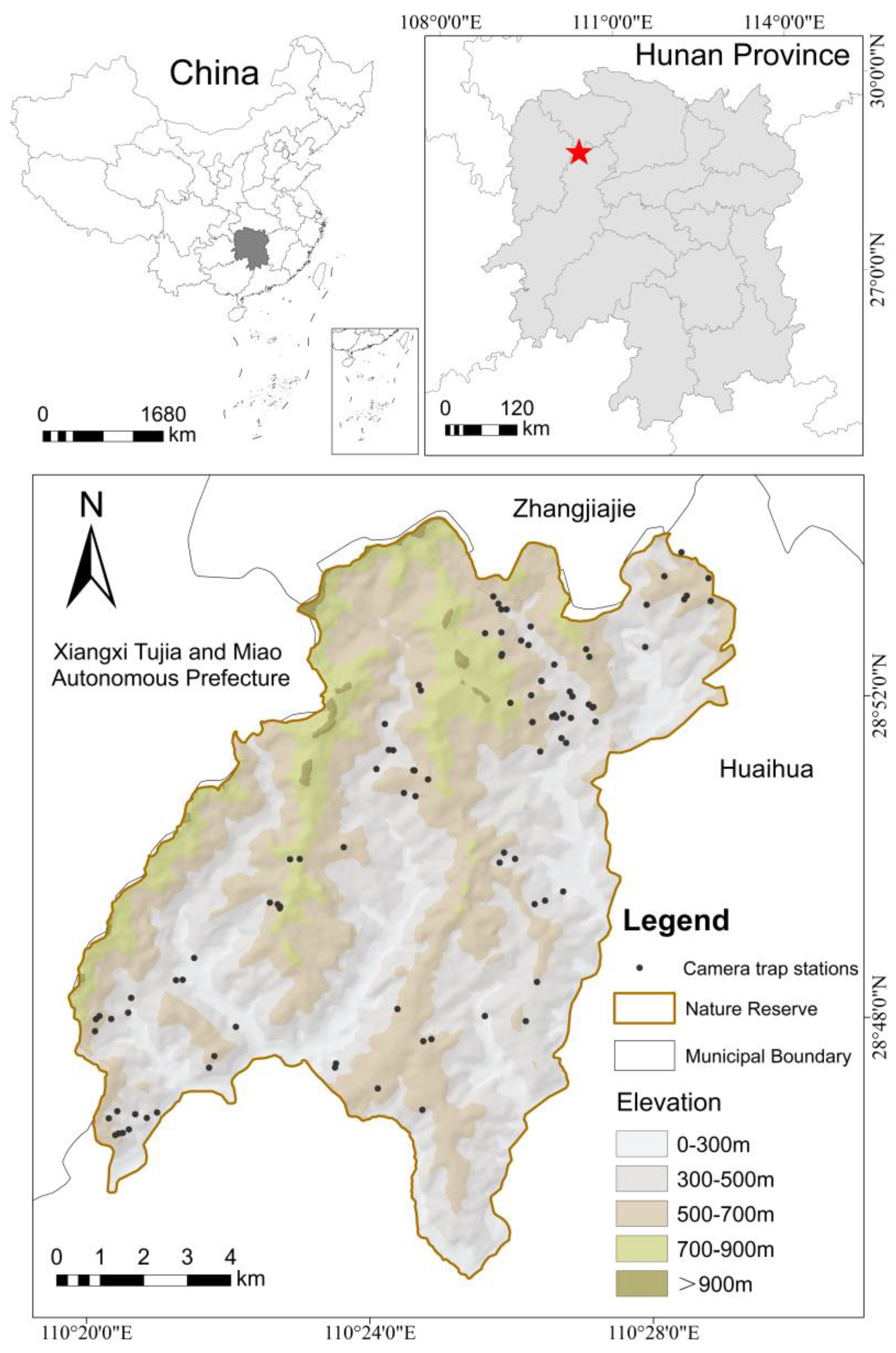
2.1. Study Area
2.2. Data Collection
2.2.1. Camera Traps Survey
2.2.2. Environment Variables
2.3. Data Analysis
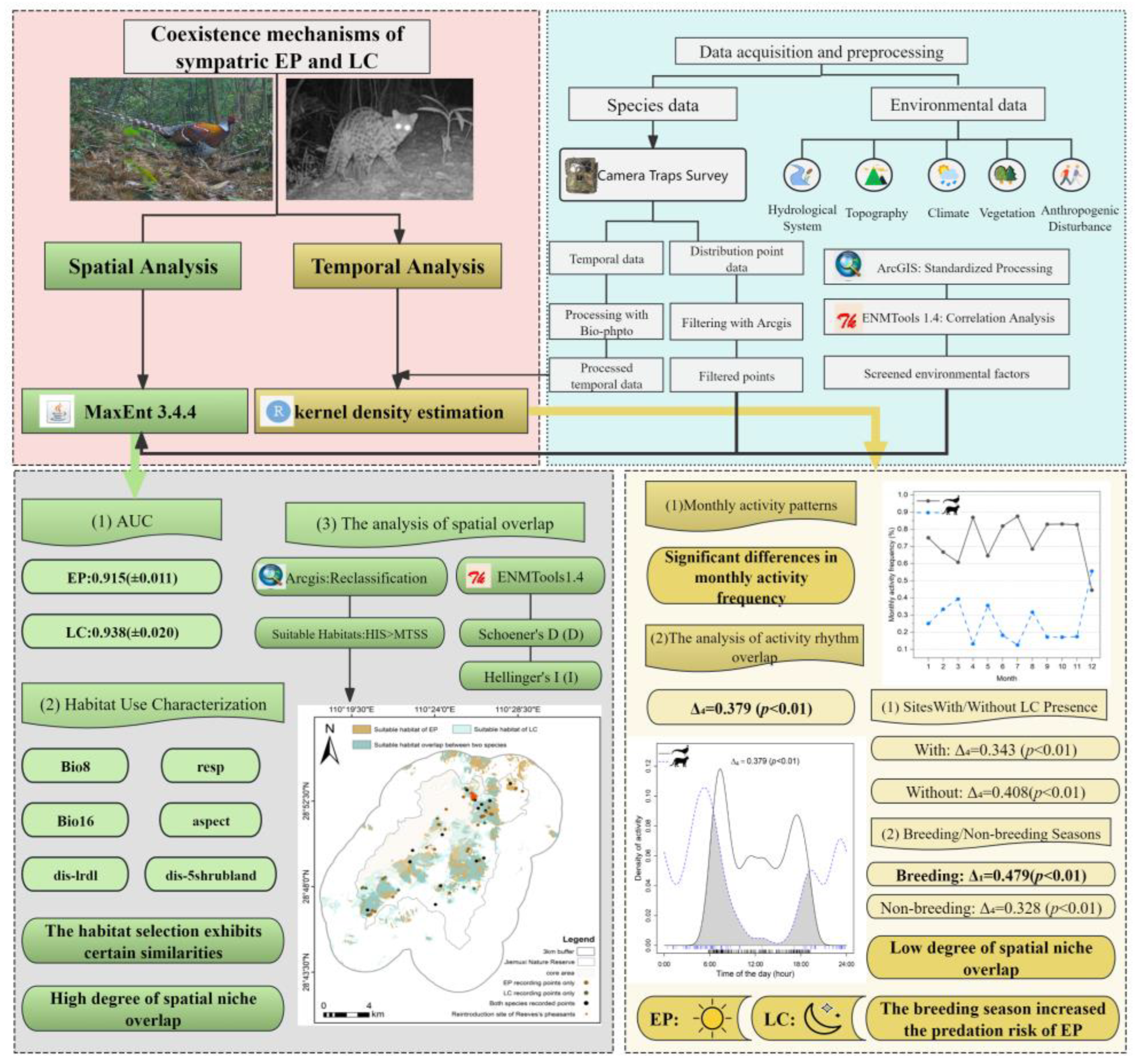
2.3.1. Spatial Scale
2.3.2. Temporal Scale
3. Results
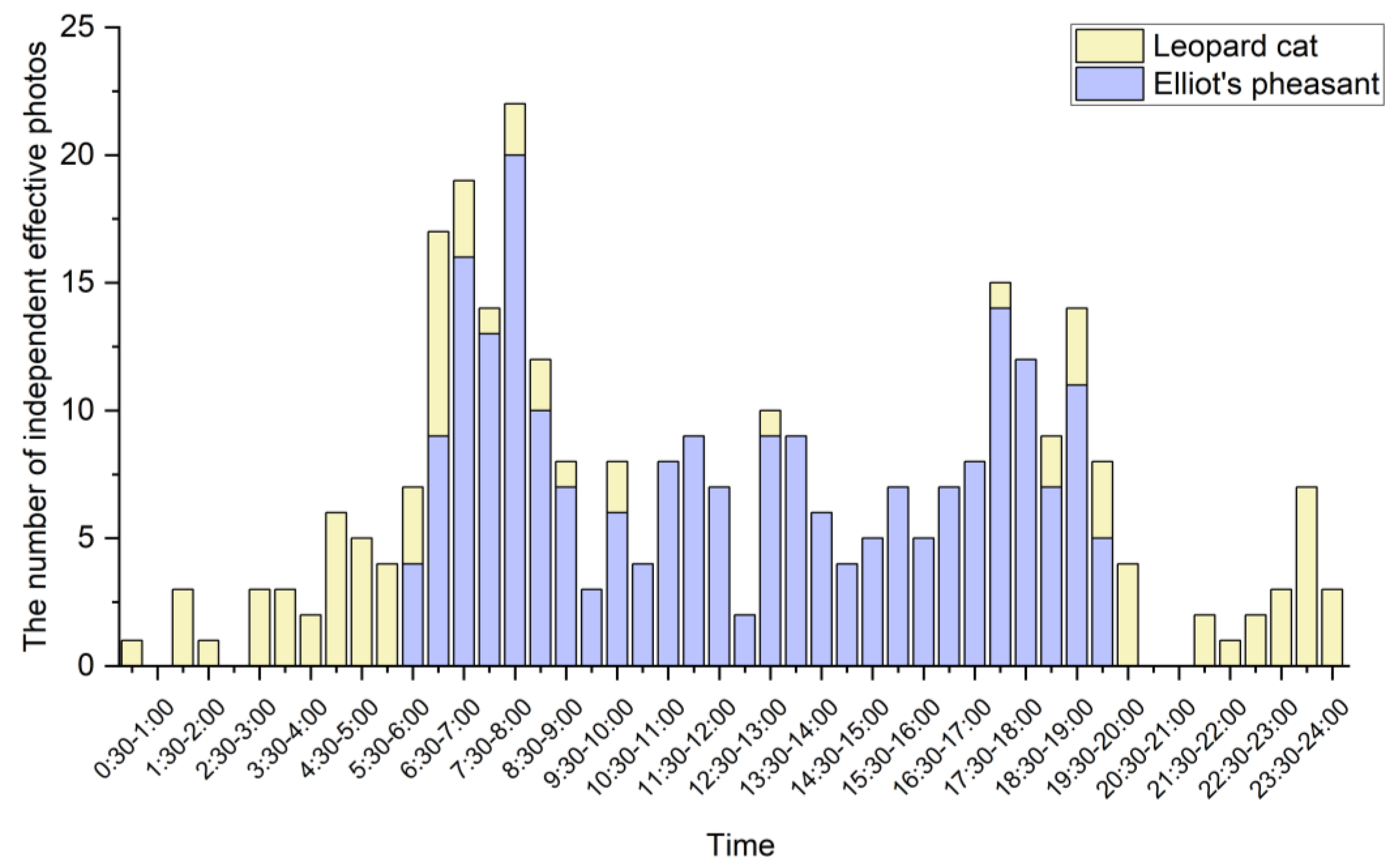
3.1. Camera-Trapping Results
3.2. Spatial Analysis
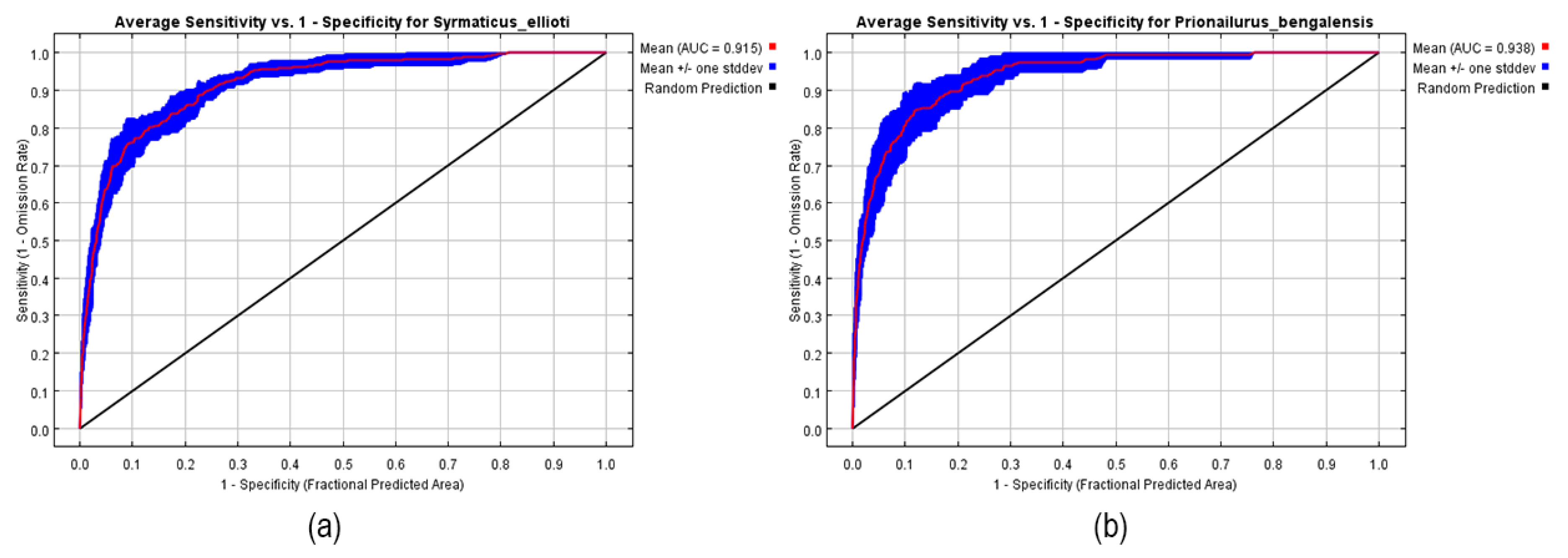
3.2.1. Model Performance
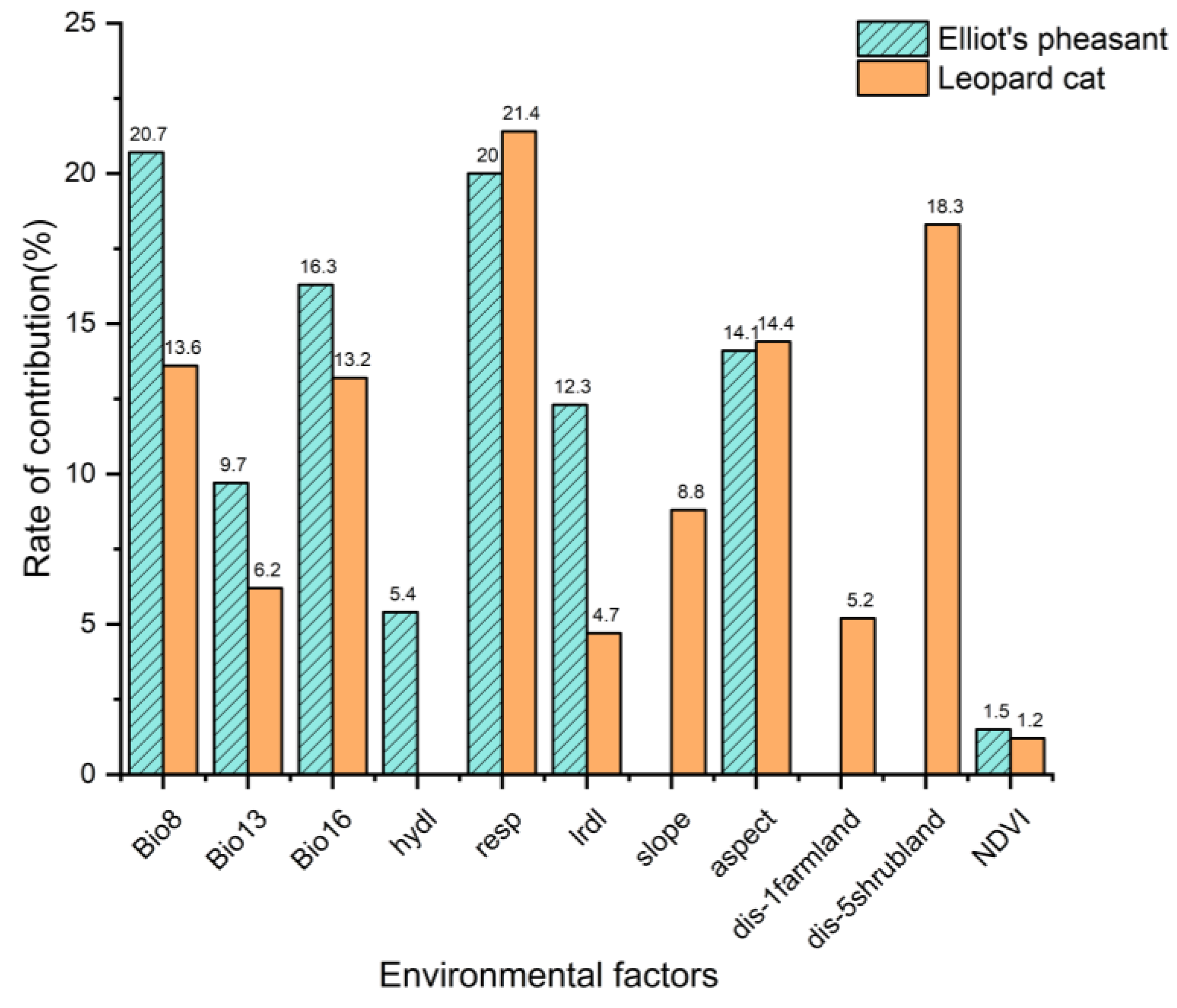
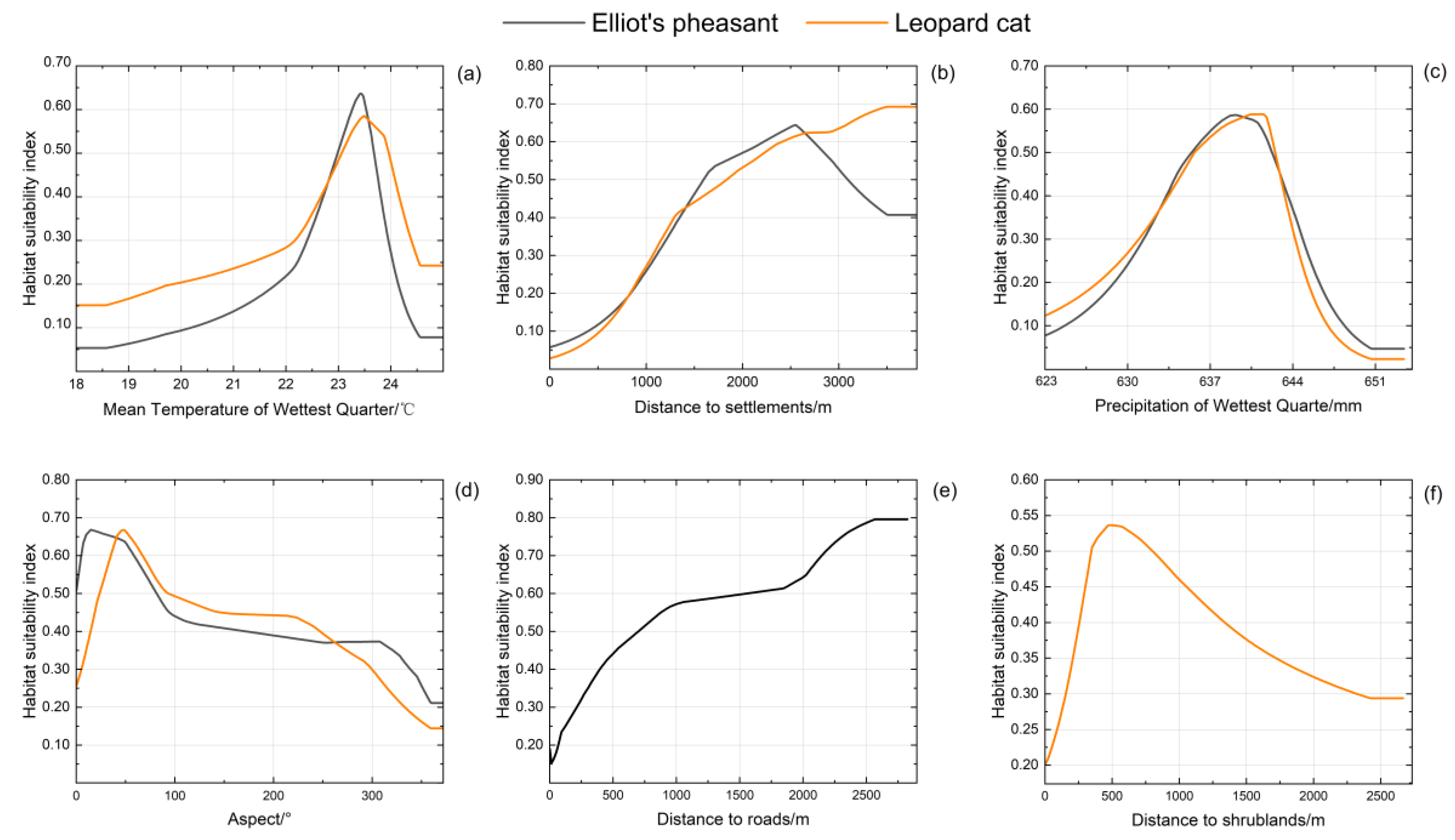
3.2.2. Habitat Use Characterization
3.2.3. Spatial Overlaps and Habitat Suitability Assessment
3.3. Temporal Analysis
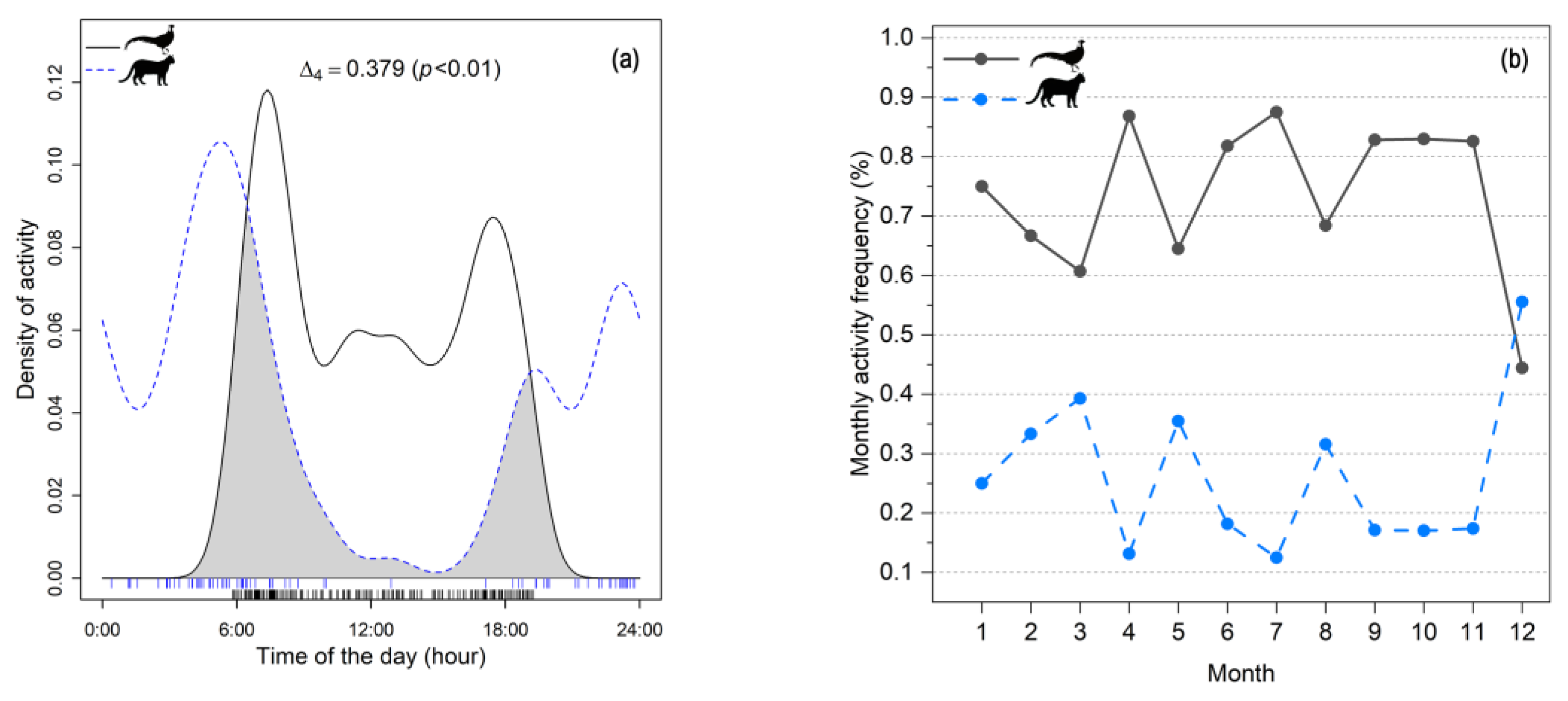
3.3.1. General Pattern of Activities
3.3.2. Activity Rhythm of Elliot’s Pheasant in Sites with and Without Leopard Cat Presence
3.3.3. Seasonal Variations in the Activity Rhythm of Elliot’s Pheasant During Breeding and Non-Breeding Seasons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalirad, A.; Sommer, R.J. The role of plasticity and stochasticity in coexistence. Ecol. Lett. 2024, 27, e14370. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wang, Y.; Liu, Y.; Jiang, L.; He, F. Advances in species coexistence theory. Biodivers. Sci. 2017, 25, 345–354, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Cresswell, W. Non-lethal effects of predation in birds. Ibis 2008, 150, 3–17. [Google Scholar] [CrossRef]
- Lima, S. Stress and decision making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 1998, 27, 215–290. [Google Scholar] [CrossRef]
- Moll, R.J.; Redilla, K.M.; Mudumba, T.; Muneza, A.B.; Gray, S.M.; Abade, L.; Hayward, M.W.; Millspaugh, J.J.; Montgomery, R.A.; Prugh, L. The many faces of fear: A synthesis of the methodological variation in characterizing predation risk. J. Anim. Ecol. 2017, 86, 749–765. [Google Scholar] [CrossRef]
- Creel, S.; Winnie, J.; Maxwell, B.; Hamlin, K.; Creel, M. Elk alter habitat selection as an antipredator response to wolves. Ecology 2005, 86, 3387–3397. [Google Scholar] [CrossRef]
- Resende, P.S.; Viana-Junior, A.B.; Bovendorp, R.S. A meta-analysis of the effects of anthropogenic disturbances on tropical mammal functional diversity. Mammal Rev. 2025, 55, e12356. [Google Scholar] [CrossRef]
- Tilman, D. Competition and biodiversity in spatially structured habitats. Ecology 1994, 75, 2–16. [Google Scholar] [CrossRef]
- Guan, X.; Rao, X.; Song, G.; Wang, D. The evolution of courtship displays in Galliformes. Avian Res. 2022, 13, 11–16. [Google Scholar] [CrossRef]
- Lu, X. Summer-Autumn Habitat Use by Galliformes in a Primary Forest, Southeastern Tibet. Avian Res. 2012, 3, 113–117. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2025-1. 2025. Available online: https://www.iucnredlist.org (accessed on 22 April 2025).
- Li, H.; Wang, D.; Tian, Y. Habitat analysis of Syrmaticus Reevesil in Xiaoxi National Nature Reserve. Zhongnan Linye Keji Daxue Xuebao 2012, 32, 166–169, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Ping, D.; Shi-ren, J.; Yang, Z. The study on fragmentation of habitat used by Elliot’s pheasant in western Zhejiang. Zool. Res. 2000, 21, 65–69, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Ren, P.; Yu, J.; Chen, X.; Shen, X.; Song, X.; Zhang, T.; Yu, Y.; Ding, P. Seasonal variation in the distribution of Elliot’s pheasant (Syrmaticus ellioti) in gutianshan national nature reserve. Biodivers. Sci. 2019, 27, 13–23, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, P.; Liu, M.; Zhang, Y.; Huang, M.; Shu, H. Progress and perspective of Elliot’s pheasant in biology. Ecol. Sci. 2017, 36, 220–225, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Ding, P.; Zhuge, Y.; Zhang, C.Z. The studies on breeding ecology of Syrmaticus ellioti swinhoe. Zool. Res. 1990, 11, 139–145, (In Chinese with an English Abstract). [Google Scholar]
- Jiang, P.P.; Ge, Y.F.; Lang, Q.L.; Ding, P. Genetic structure among wild populations of Elliot’s pheasant Syrmaticus Ellioti in China from mitochondrial DNA analyses. Bird Conserv. Int. 2007, 17, 177–185. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wu, L.; Li, L.; Wang, S.Y.; Li, Z.Y.; Xu, Z.G. Havioral rhythms of Elliot’s pheasant (Syrmaticus Ellioti) during summer and autumn in captivity. Ecol. Sci. 2019, 38, 50–56. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, T.; Huang, C.; Zhao, T.; Li, H.; Li, C. Metabolism and thermoregulation between Mrs Hume’s Pheasant (Syrmaticus Humiae) and Elliot’s pheasant (S. ellioti). Zool. Res. 2011, 32, 396–402. [Google Scholar]
- Xu, Y.L.; Yang, Z.M.; Lu, S.J.; Zhou, F.; Zhou, P.C.; Wang, B. Habitat Suitability Assessment of Syrmaticus ellioti in Hunan Province Based on MaxEnt Modeling. Life Sci. Res. 2024, 28, 267–273, (in Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wu, C. Simulation and Prediction of Suitable Habitats for Rare Animals Under the Background of Climate Change: A Case Study of Syrmaticus ellioti. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, T.; Chen, D.; Ma, Y.; Wu, R. Food selection of released captive-bred Syrmaticus Ellioti in Amersham nature reserve, Guangxi, China. J. Guangxi Norm. Univ. Nat. Sci. Ed. 2019, 37, 218–222, (In Chinese with an English Abstract). [Google Scholar]
- Long, D.Z. Ecological study of Syrmaticus ellioti. Chin. Wildl. 1985, 1, 24–25, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Fan, Y.Q.; Yang, J.; Zhang, H.L.; Jiang, J.; Jiang, W.J.; Tang, S.P.; Bao, W.D. Food composition of medium sized carnivores in Beijing Songshan Nature Reserve. J. Biol. 2020, 37, 59–62. [Google Scholar] [CrossRef]
- Shao, X.N.; Song, D.Z.; Huang, Q.W.; Li, S.; Yao, M. Fast surveys and molecular diet analysis of carnivores based on fecal DNA and metabarcoding. Biodivers. Sci. 2019, 27, 543–556, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Lee, O.; Lee, S.; Nam, D.-H.; Lee, H.Y. Food habits of the Leopard cat (Prionailurus bengalensis euptilurus) in korea. Mammal Study 2014, 39, 43–46. [Google Scholar] [CrossRef]
- Shao, X.; Lu, Q.; Liu, M.; Xiong, M.; Bu, H.; Wang, D.; Liu, S.; Zhao, J.; Li, S.; Yao, M. Generalist carnivores can be effective biodiversity samplers of terrestrial vertebrates. Front. Ecol. Environ. 2021, 19, 557–563. [Google Scholar] [CrossRef]
- Macdonald, D.W. Biology and Conservation of Wild Felids; Oxford University Press: New York, NY, USA; Ipswich, UK, 2010; pp. 581–596. [Google Scholar]
- Bao, W.; Li, X.; Shi, Y. Comparative Analysis of Food Habits in Carnivores from Three Areas of Beijing. Zool. Res. 2005, 26, 118–122. [Google Scholar] [CrossRef]
- Manandhar, P.; Pereira, K.S.; Kusi, N.; Joshi, J.; Levin, N.; Chaudhary, H.K.; Wultsch, C.; Lamichhane, S.; Bhandari, S.; Guragain, L.; et al. Feeding Habits of Leopards and Leopard cats in the Fragmented Forests Surrounding the Kathmandu Valley. Ecol. Evol. 2025, 15, e70927. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Yuan, L.; Bao, W.; Yang, Y.; Baterr. Preliminary comparison of diet composition of four small sized carnivores at Saihanwula Nature Reserve, Inner Mongolia. Acta Theriol. Sin. 2011, 31, 55–61, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Luo, Y. Species Diversity and Threatened Categories Assessment of Mammals in Hunan Province, China. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2023. [Google Scholar]
- Emerson, L.D.; Wittmer, H.U.; Elbroch, L.M.; Kostoglou, K.; Bannister, K.J.; Psaila, J.J.; Whisson, D.; Ritchie, E.G. A global assessment of large terrestrial carnivore kill rates. Biol. Rev. Camb. Philos. Soc. 2025, 100, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.S.; Hearn, A.J.; Devineau, O.; Linnell, J.D.C.; Macdonald, D.W. Loss of Sunda clouded leopards and forest integrity drive potential impacts of mesopredator release on vulnerable avifauna. Heliyon 2024, 10, e32801. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.W. Molecular Diet Analysis of Leopard Cats (Prionailurus bengalensis) and Red Foxes (Vulpes vulpes) in Jiaozishan National Nature Reserve, Yunnan Province, Based on Fecal DNA and Metabarcoding. Master’s Thesis, Yunnan Normal University, Kunming, China, 2023. [Google Scholar] [CrossRef]
- Teng, Y.; Gai, L.X.; Li, J.; Fang, X.M.; Yang, N.; Bao, W.D. A comparison of two methods of Leopard cat food content analysis. Acta Theriol. Sin. 2023, 43, 50–58, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Jin, S.L.; Qu, C.M.; Shi, X.G.; Zou, X.Y.; Liu, J.; Shao, X.N.; Yao, M.; He, T.M.; Li, S. The Species Diversity and Diet of Some Carnivores in Wolong National Nature Reserve, Sichuan Province. Chin. J. Wildl. 2021, 42, 958–964, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Shehzad, W.; Riaz, T.; Nawaz, M.A.; Miquel, C.; Poillot, C.; Shah, S.A.; Pompanon, F.; Coissac, E.; Taberlet, P. Carnivore diet analysis based on next-generation sequencing: Application to the Leopard cat (Prionailurus bengalensis) in Pakistan. Mol. Ecol. 2012, 21, 1951–1965. [Google Scholar] [CrossRef]
- Xiong, M.; Shao, X.; Long, Y.; Bu, H.; Zhang, D.; Wang, D.; Li, S.; Wang, R.; Yao, M. Molecular analysis of vertebrates and plants in scats of Leopard cats (Prionailurus bengalensis) in southwest China. J. Mammal. 2016, 97, 1054–1064. [Google Scholar] [CrossRef]
- Xiong, W.G.; Zhao, K.J. Evaluation of conservation value of Jiemuxi Nature Reserve in Hunan Province. Cent. South For. Inventory Plan. 2007, 26, 36–38, 46, (In Chinese with an English Abstract). [Google Scholar]
- Olson, D.M.; Dinerstein, E. The global 200: Priority ecoregions for global conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Ning, S.J.; He, J.; Zhou, Q.; Chen, X.Y.; Wang, X.T.; He, Y. Diversity and faunal analysis of Butterfly Species in Jiemuxi National Nature Reserve, Hunan Province. J. Nat. Sci. Hunan Norm. Univ. 2024, 47, 132–142, (In Chinese with an English Abstract). [Google Scholar]
- Nazir, S.; Kaleem, M. Object classification and visualization with edge artificial intelligence for a customized camera trap platform. Ecol. Inform. 2024, 79, 102453. [Google Scholar] [CrossRef]
- Fisher, J.T. Camera trapping in ecology: A new section for wildlife research. Ecol. Evol. 2023, 13, e9925. [Google Scholar] [CrossRef] [PubMed]
- Tudge, S.J.; Brittain, S.; Kentatchime, F.; Kamogne-Tagne, C.T.; Rowcliffe, J.M. The impacts of human activity on mammals in a community forest near the Dja biosphere reserve in Cameroon. Oryx 2022, 56, 947–955. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, G.; Zhu, P.B.D.; Liu, W.; Li, C.; Jin, K. Morphological, Phaneroptic, Habitat and Population Description of Three Muntjac Species in a Tibetan Nature Reserve. Animals 2022, 12, 2909. [Google Scholar] [CrossRef]
- Martin-Garcia, S.; Rodríguez-Recio, M.; Peragón, I.; Bueno, I.; Virgós, E. Comparing relative abundance models from different indices, a study case on the red fox. Ecol. Indic. 2022, 137, 108778. [Google Scholar] [CrossRef]
- Chen, X.; Tian, T.; Pan, H.; Jin, Y.; Zhang, X.; Long, Q.; Tang, L.; Yang, B.; Zhang, L. The minimal impact of anthropogenic disturbances on the spatial activities of Leopard cats in Xinglong, China. Animals 2023, 13, 3328. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Li, S.; Gao, H.; Cai, Z.; Lin, G.; Zhang, T. Maxent modeling for predicting the spatial distribution of three raptors in the Sanjiangyuan National Park, China. Ecol. Evol. 2019, 9, 6643–6654. [Google Scholar] [CrossRef]
- Liu, P.; Huang, X.F.; Gu, S.S.; Lu, C.H. Habitat selection of four sympatric pheasants in Ganeshan national nature reserve, Jiangxi, China. Zool. Res. 2012, 33, 170–176. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, X.; Gao, Y.; Xie, S.; Mi, J. Glc_fcs30: Global land-cover product with fine classification system at 30 m using time-series Landsat imagery. Earth Syst. Sci. Data 2021, 13, 2753–2776. [Google Scholar] [CrossRef]
- Yang, J.; Dong, J.; Xiao, X.; Dai, J.; Wu, C.; Xia, J.; Zhao, G.; Zhao, M.; Li, Z.; Zhang, Y.; et al. Divergent shifts in peak photosynthesis timing of temperate and alpine grasslands in China. Remote Sens. Environ. 2019, 233, 111395. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global potential distribution prediction of xanthium italicum based on maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tao, X.; Liao, J.; Liu, S.; Xu, L.; Yuan, S.; Zhang, Z.; Wang, F.; Deng, N.; Huang, J.; et al. Predicting potential cultivation region and paddy area for ratoon rice production in China using maxent model. Field Crops Res. 2022, 275, 108372. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Xie, G.; Wang, W. Impacts of Climate Change Conditions on the Potential Distribution of Anoplophora glabripennis and Its Host Plants, Salix Babylonia and Salix Matsuyama, in China. Ecol. Evol. 2024, 14, e70692. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Shi, X.G.; Shi, X.Y.; He, Q.; Feng, X.; Jin, S.L.; Chen, Y.H.; Zhang, J.; Yao, M.; Li, S. Spatiotemporal Relationships Between Snow Leopard (Panthera uncia) and Red Fox (Vulpes vulpes) in Qionglai Mountains, Sichuan Province. Acta Theriol. Sin. 2021, 41, 115–127. [Google Scholar] [CrossRef]
- Zhao, J.; Shao, W.; Li, Y.; Chen, H.; Lin, Z.; Wei, L. Potential impact of climate change on the distribution of Capricornis milneedwardsii, a vulnerable mammal in China. Ecol. Evol. 2024, 14, e11582. [Google Scholar] [CrossRef]
- Hammadi, A.; Kalboussi, M. Modelling and mapping the current and future potential habitats of the Algero-Tunisian endemic newt Pleurodeles nebulosus under climate change. Eur. J. Wildl. Res. 2020, 66, 61. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- You, Z.; Lu, B.; Du, B.; Liu, W.; Jiang, Y.; Ruan, G.; Yang, N. Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China. Animals 2022, 12, 2694. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Viviano, A.; Mori, E.; Fattorini, N.; Mazza, G.; Lazzeri, L.; Panichi, A.; Strianese, L.; Mohamed, W.F. Spatiotemporal Overlap between the European Brown Hare and Its Potential Predators and Competitors. Animals 2021, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns. 2021. Available online: https://CRAN.R-project.org/package=overlap (accessed on 10 October 2024).
- Rowcliffe, M. Activity: Animal Activity StatistIEs. 2014. Available online: https://CRAN.R-project.org/package=activity (accessed on 10 October 2024).
- Cepeda-Duque, J.C.; Arango-Correa, E.; López-Velasco, V.; López-Barrera, A.; Link, A.; Lizcano, D.J.; Mazariegos, L.; Rendon-Jaramillo, U.; Oliveira, T.G.d. Avoiding the enemy while searching for dinner: Understanding the temporal niche of the threatened clouded tiger-cat in protected cloud forests of the middle Cauca, Colombia. Food Webs 2025, 42, e00385. [Google Scholar] [CrossRef]
- Allen, M.; Elbroch, L.; Wilmers, C.; Wittmer, H. The comparative effects of large carnivores on the acquisition of carrion by scavengers. Am. Nat. 2015, 185, 822–833. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Hou, L.; Su, X.; Lian, X. Spatiotemporal overlap among snow leopard, bharal, and free-ranging livestock: Suggestions on mitigating human-snow leopard conflict. Glob. Ecol. Conserv. 2024, 53, e03029. [Google Scholar] [CrossRef]
- Azevedo, F.C.; Lemos, F.G.; Freitas-Junior, M.C.; Rocha, D.G.; Azevedo, F.C.C. Puma activity patterns and temporal overlap with prey in a human-modified landscape at Southeastern Brazil. J. Zool. 2018, 305, 246–255. [Google Scholar] [CrossRef]
- Zhang, D.; An, B.; Chen, L.; Sun, Z.; Mao, R.; Zhao, C.; Zhang, L. Camera Trapping Reveals Spatiotemporal Partitioning Patterns and Conservation Implications for Two Sympatric Pheasant Species in the Qilian Mountains, Northwestern China. Animals 2022, 12, 1657. [Google Scholar] [CrossRef]
- Fokkema, R.W.; Velde, E.v.d.; Stessens, M.; Bos, D.; Belfín, O.; Jong, M.E.d.; Hooijmeijer, J.C.E.W.; Piersma, T. Mapping mammalian meadow bird nest predators in a Dutch dairy farming landscape. Eur. J. Wildl. Res. 2024, 70, 122. [Google Scholar] [CrossRef]
- Caro, T.; Girling, S. Antipredator Defenses in Birds and Mammals; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Zou, B.Y.; Luo, G.; Zhu, B.W.; Ran, J.H.; Fang, C. The spatial distribution relationship between three pheasant species and mutual predator, the red fox (Vulpes vulpes), on the Western Sichuan Plateau. Biodivers. Sci. 2021, 29, 918–926, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Tost, D.; Ludwig, T.; Strauss, E.; Jung, K.; Siebert, U. Habitat selection of black grouse in an isolated population in northern Germany-the importance of mixing dry and wet habitats. PeerJ 2022, 10, e14161. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Gordon, I.J. Habitat selection according to the ability of animals to eat, digest and detoxify foods. Proc. Nutr. Soc. 1999, 58, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Sunquist, M. Wild Cats of the World; University of Chicago Press: Chicago, IL, USA, 2002; pp. 225–229. [Google Scholar]
- Gaál, D.K.; Heltai, M.; Sándor, G.; Schally, G.; Csányi, E. Ecotones in the Spotlight-Habitat Selection of the Golden Jackal (Canis aureus Linnaeus, 1758) in the Agricultural Landscapes of Central Europe. Animals 2025, 15, 760. [Google Scholar] [CrossRef]
- Choki, K.; Dröge, E.; Sillero-Zubiri, C.; Macdonald, D.W.; Penjor, U. Anthropogenic and environmental correlates of spatial patterns of co-occurrence of small felids in a montane landscape. Glob. Ecol. Conserv. 2025, 58, e03422. [Google Scholar] [CrossRef]
- Julliard, R.; McCleery, R.H. Phenotypic adjustment of clutch size due to nest predation in the great tit. Ecology 1997, 78, 394–404. [Google Scholar] [CrossRef]
- Gehrt, J.M.; Sullins, D.S.; Haukos, D.A. Looking at the bigger picture: How abundance of nesting and brooding habitat influences lek-site selection by lesser prairie-chickens. Am. Midl. Nat. 2020, 183, 52–77. [Google Scholar]
- Lautenbach, J.M.; Haukos, D.A.; Sullins, D.S.; Hagen, C.A.; Lautenbach, J.D.; Pitman, J.C.; Plumb, R.T.; Robinson, S.G.; Kraft, J.D. Factors influencing nesting ecology of lesser prairie-chickens. J. Wildl. Manag. 2019, 83, 205–215. [Google Scholar] [CrossRef]
- Ong-in, T.; Pierce, A.J.; Gale, G.A.; Browne, S.J.; Savini, T. Nesting ecology and nest site selection of green-legged partridge. Raffles Bull. Zool. 2016, 64, 89–97. [Google Scholar]
- Macdonald, E.C.; Camfield, A.F.; Martin, M.; Wilson, S.; Martin, K. Nest-site selection and consequences for nest survival among three sympatric songbirds in an alpine environment. J. Ornithol. 2016, 157, 393–405. [Google Scholar] [CrossRef]
- Chen, X.N.; Li, Q.W.; Yu, J.P.; Yu, S.H.; Li, S.; Cao, M.C. Habitat suitability assessment of Syrmaticus ellioti in the Qiangjiangyuan National Park. Ecol. Environ. Sci. 2022, 31, 1832–1839, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Vitekere, K.; Wang, J.; Karanja, H.; Consolée, K.T.; Jiang, G.; Hua, Y. Dynamic in Species Estimates of Carnivores (Leopard cat, Red Fox, and North Chinese Leopard): A Multi-Year Assessment of Occupancy and Coexistence in the Tieqiaoshan Nature Reserve, Shanxi Province, China. Animals 2020, 10, E1333. [Google Scholar] [CrossRef] [PubMed]
- Chung, O.-S.; Lee, J.K. Association of Leopard cat Occurrence with Environmental Factors in Chungnam Province, South Korea. Animals 2022, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lu, D.; Han, Y.; Li, R.; Yang, J.; Sun, G.; Yang, L.; Qian, J.; Fang, X.; Luo, S.-J. Distribution of Leopard cats in the nearest mountains to urban Beijing and its affecting environmental factors. Biodivers. Sci. 2024, 32, 24138, (in Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, G. Study on Dispersal and Habitat Selection of Reintroduced Elliot’s Pheasant (Syrmaticus ellioti). Master’s Thesis, Guangxi Normal University, Guilin, China, 2018. [Google Scholar]
- Wang, L.; Lu, Y.; Cai, Y.; Ji, L.; Pang, D.; Zhou, M.; Cheng, Y.; Pu, F.; Zhang, B. The spatial relationship between two sympatric pheasant species and various human disturbance activities. Animals 2025, 15, 95. [Google Scholar] [CrossRef]
- Harrington, L.A.; Harrington, A.L.; Yamaguchi, N.; Thom, M.D.; Ferreras, P.; Windham, T.R.; Macdonald, D.W. The impact of native competitors on an alien invasive: Temporal niche shifts to avoid interspecific aggression? Ecology 2009, 90, 1207–1216. [Google Scholar] [CrossRef]
- Porfirio, G.; Foster, V.C.; Fonseca, C.; Sarmento, P. Activity patterns of ocelots and their potential prey in the Brazilian Pantanal. Mamm. Biol. 2016, 81, 511–517. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Tao, B.; Peng, J.; Wang, H.; Yu, J.; Jin, L. Do domestic budgerigars perceive predation risk? Anim. Cogn. 2024, 27, 8. [Google Scholar] [CrossRef]
- Allen, M.L.; Wallace, C.F.; Wilmers, C.C. Patterns in bobcat (lynx rufus) scent marking and communication behaviors. J. Ethol. 2015, 33, 9–14. [Google Scholar] [CrossRef]
- Eichholz, M.W.; Dassow, J.A.; Stafford, J.D.; Weatherhead, P.J. Experimental evidence that nesting ducks use mammalian urine to assess predator abundance. Auk 2012, 129, 638–644. [Google Scholar] [CrossRef]
- House, P.K.; Vyas, A.; Sapolsky, R. Predator cat odors activate sexual arousal pathways in brains of toxoplasma gondii infected rats. PLoS ONE 2017, 6, e23277. [Google Scholar] [CrossRef]
- Shuai, L.; Zhengxiao, L.; Shan, T.; Kai, S.; Qian, H.; Jianqiang, L.; Jiliang, X. Sex-specific movement responses of Reeves’s pheasant to human disturbance: Importance of body characteristics and reproductive behavior. Animals 2022, 12, 1619. [Google Scholar] [CrossRef]
- Kumbhakar, R.; Jafari, S.; Pal, N. Impacts of Predation-Driven Allee Effect and Refuge on the Dynamics of a Predator–Prey Model in a Parameter Plane. Int. J. Bifurc. Chaos. 2025, 35, 2550036. [Google Scholar] [CrossRef]
- Bliard, L.; Dufour, P.; Griesser, M.; Covas, R. Family-living and cooperative breeding in birds are associated with the number of avian predators. Evolution 2024, 78, 1317–1324. [Google Scholar] [CrossRef] [PubMed]



| Type | Code | Variables Description | Unit |
|---|---|---|---|
| Climate | Bio1 | Annual Mean Temperature | °C |
| Bio2 | Mean diurnal range | °C | |
| Bio3 | Isothermality | % | |
| Bio4 | Temperature Seasonality | °C × 100 | |
| Bio5 | Max Temperature of Warmest Month | °C | |
| Bio6 | Min Temperature of Coldest Month | °C | |
| Bio7 | Temperature Annual Range | °C | |
| Bio8 | Mean Temperature of Wettest Quarter | °C | |
| Bio9 | Mean Temperature of Driest Quarter | °C | |
| Bio10 | Mean Temperature of Warmest Quarte | °C | |
| Bio11 | Mean Temperature of Coldest Quarter | °C | |
| Bio12 | Annual Precipitation | mm | |
| Bio13 | Precipitation of Wettest Month | mm | |
| Bio14 | Precipitation of Driest Month | mm | |
| Bio15 | Precipitation Seasonality | % | |
| Bio16 | Precipitation of Wettest Quarte | mm | |
| Bio17 | Precipitation of Driest Quarter | mm | |
| Bio18 | Precipitation of Warmest Quarter | mm | |
| Bio19 | Precipitation of Coldest Quarter | mm | |
| Hydrological System | dis-hydl | Distance to rivers | m |
| Anthropogenic Disturbance | dis-lrdl | Distance to roads | m |
| resp | Distance to settlements | m | |
| Vegetation | NDVI | Normalized difference vegetation index | \ |
| dis-1farmland | Distance to farmland | m | |
| dis-2CLforest | Distance to evergreen broad-leaved forests | m | |
| dis-3LYforest | Distance to deciduous broad-leaved forests | m | |
| dis-4conforest | Distance to coniferous and broad-leaved mixed forests | m | |
| dis-5shrubland | Distance to shrublands | m | |
| Topography | altitude | Altitude | m |
| slope | Slope | % | |
| aspect | Aspect | ° |
| Species | MTSS | Total Suitable Area (km2) | % of Reserve | Overlapping Area (km2) | % of Each Species’ Total Suitable Habitat |
|---|---|---|---|---|---|
| Elliot’s pheasant | 0.4113 | 32.44 | 24.88 | 21.38 | 65.91 |
| Leopard cat | 0.3445 | 36.62 | 28.08 | 58.39 |
| Analysis Context | Camera Sites (Elliot’s Pheasant) | Independent Photos (Elliot’s Pheasant) | Overlap Index | Comparative Difference vs. Overall |
|---|---|---|---|---|
| Overall activity patterns | 51 | 227 | Δ4 = 0.379 (p < 0.01) | Baseline |
| Leopard cat present sites | 22 | 78 | Δ1 = 0.343 (p < 0.01) | ↓ 0.036 |
| Leopard cat absent sites | 29 | 149 | Δ4 = 0.408 (p < 0.01) | ↑ 0.029 |
| Breeding season | \ | 71 | Δ1 = 0.479 (p < 0.01) | ↑ 0.100 |
| Non-breeding season | \ | 156 | Δ4 = 0.328 (p < 0.01) | ↓ 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Xu, Y.; Huang, C.; Li, H.; Cui, X.; Fu, Y.; Wang, B.; Mo, X. Temporal Niche Partitioning as a Coexistence Mechanism Between China’s Endemic Elliot’s Pheasant (Syrmaticus ellioti) and Its Predator, the Leopard Cat (Prionailurus bengalensis). Diversity 2025, 17, 460. https://doi.org/10.3390/d17070460
Zhou P, Xu Y, Huang C, Li H, Cui X, Fu Y, Wang B, Mo X. Temporal Niche Partitioning as a Coexistence Mechanism Between China’s Endemic Elliot’s Pheasant (Syrmaticus ellioti) and Its Predator, the Leopard Cat (Prionailurus bengalensis). Diversity. 2025; 17(7):460. https://doi.org/10.3390/d17070460
Chicago/Turabian StyleZhou, Pengchen, Yalan Xu, Chenbo Huang, Hui Li, Xinyu Cui, Ying Fu, Bin Wang, and Xiaoyang Mo. 2025. "Temporal Niche Partitioning as a Coexistence Mechanism Between China’s Endemic Elliot’s Pheasant (Syrmaticus ellioti) and Its Predator, the Leopard Cat (Prionailurus bengalensis)" Diversity 17, no. 7: 460. https://doi.org/10.3390/d17070460
APA StyleZhou, P., Xu, Y., Huang, C., Li, H., Cui, X., Fu, Y., Wang, B., & Mo, X. (2025). Temporal Niche Partitioning as a Coexistence Mechanism Between China’s Endemic Elliot’s Pheasant (Syrmaticus ellioti) and Its Predator, the Leopard Cat (Prionailurus bengalensis). Diversity, 17(7), 460. https://doi.org/10.3390/d17070460





