Modeling Wolf, Canis lupus, Recolonization Dynamics to Plan Conservation Actions Ahead: Will the “Big Bad Wolves” Howl Again in Slavonia, Croatia?
Abstract
1. Introduction
2. Materials and Methods
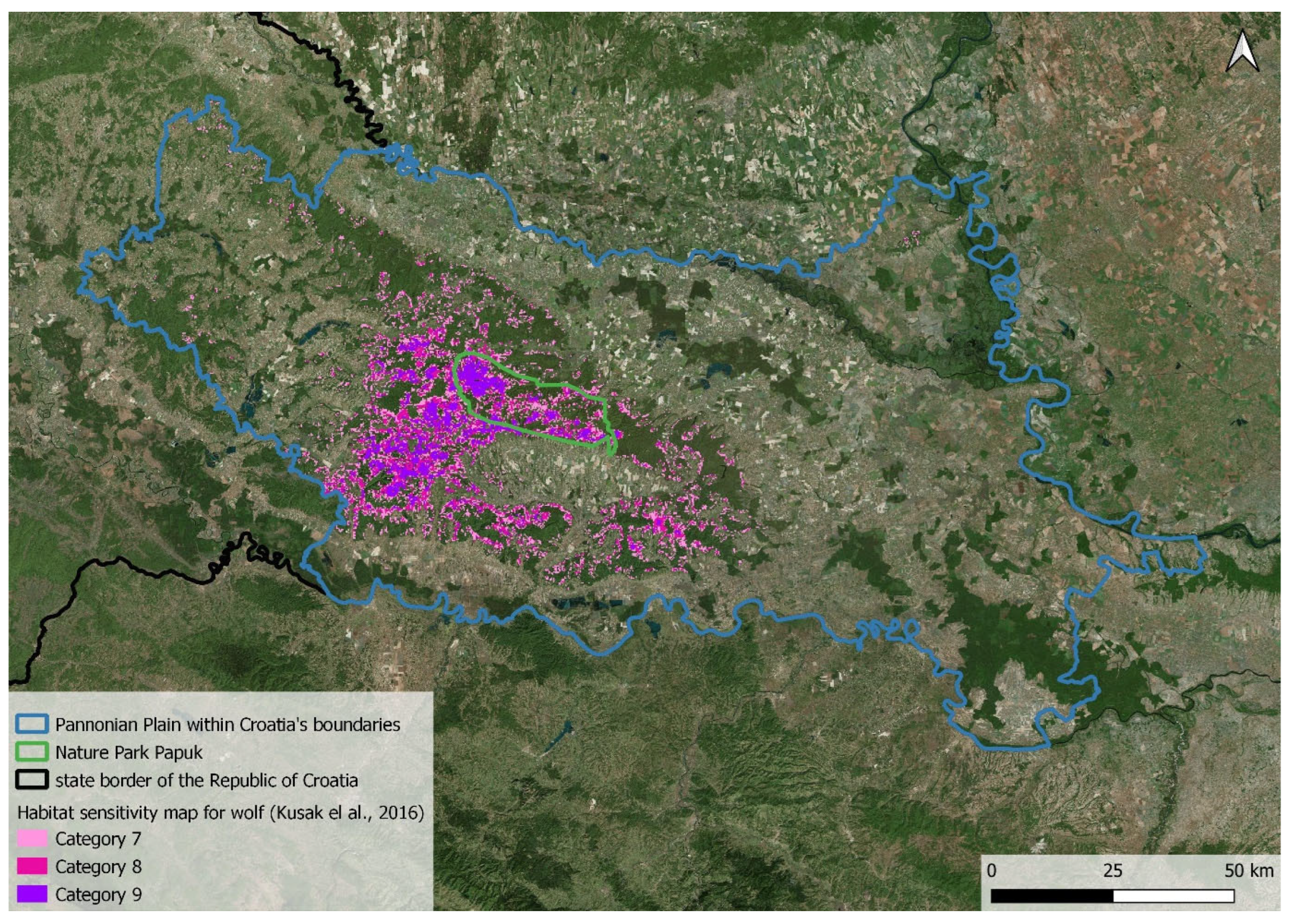
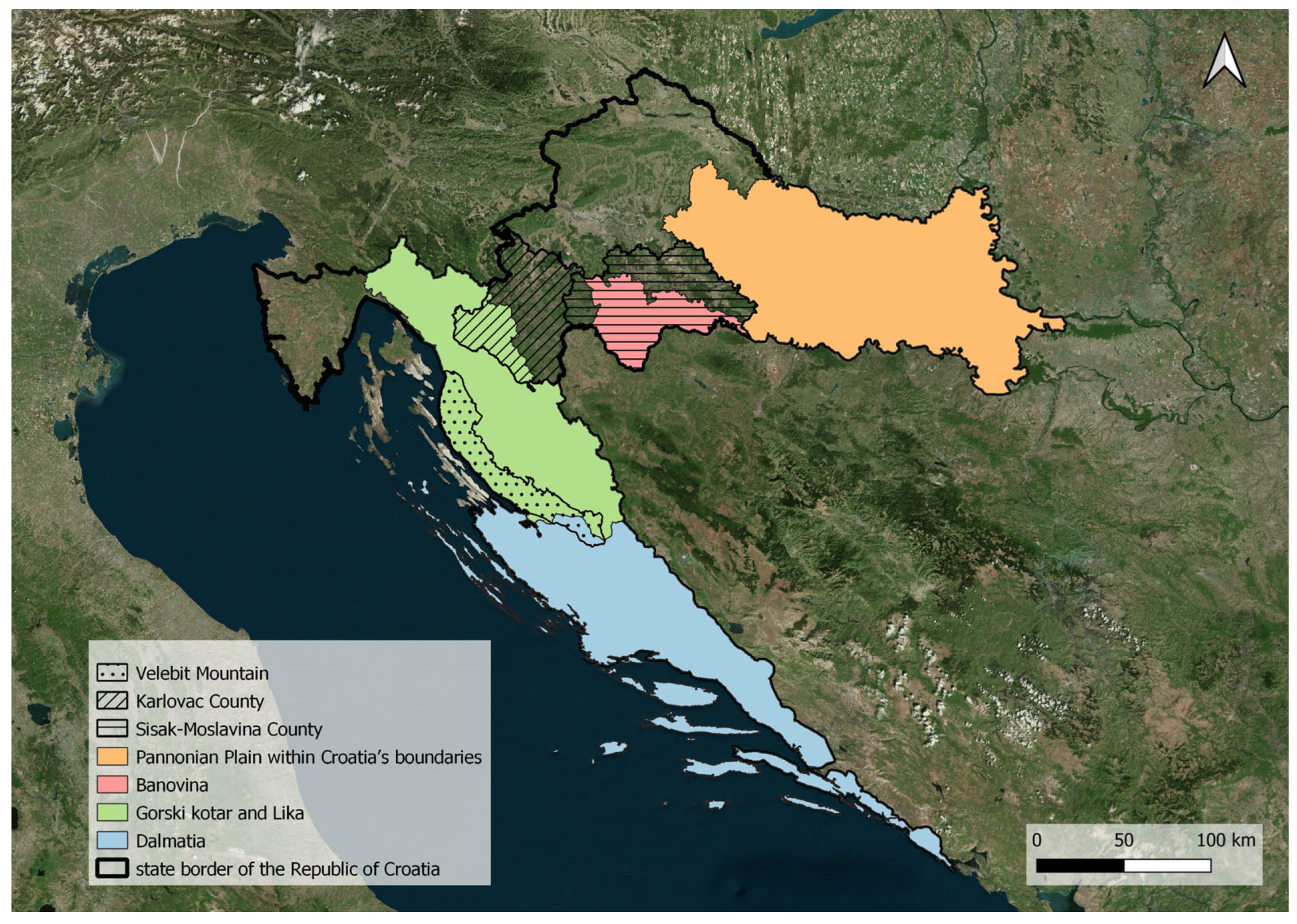
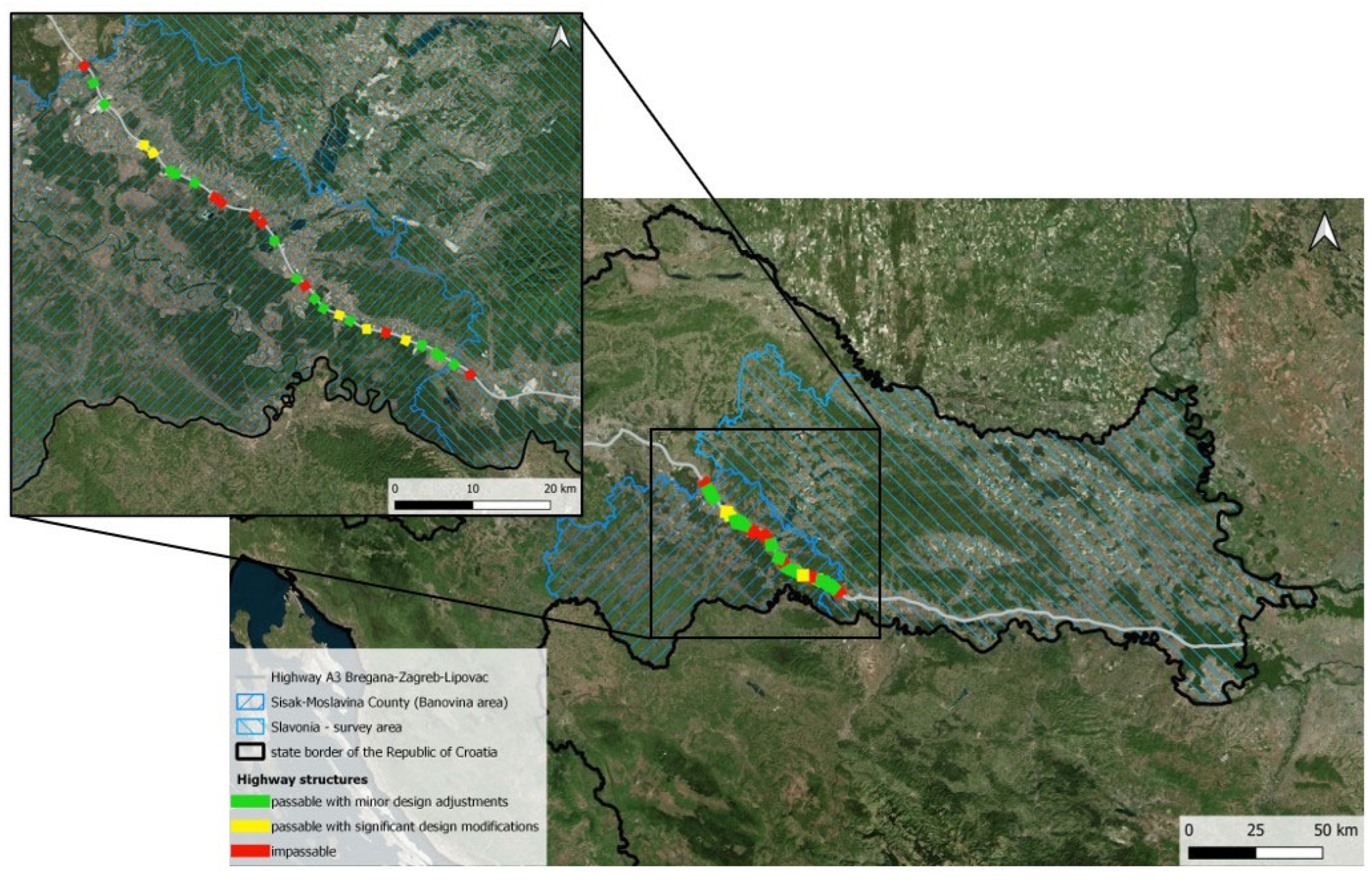
2.1. Study Area
2.2. Projection Model
2.3. Scenarios
2.4. Statistical Analysis
3. Results
3.1. The 10-Year Simulation Period
3.2. The 30-Year Simulation Period
3.3. The 100-Year Simulation Period
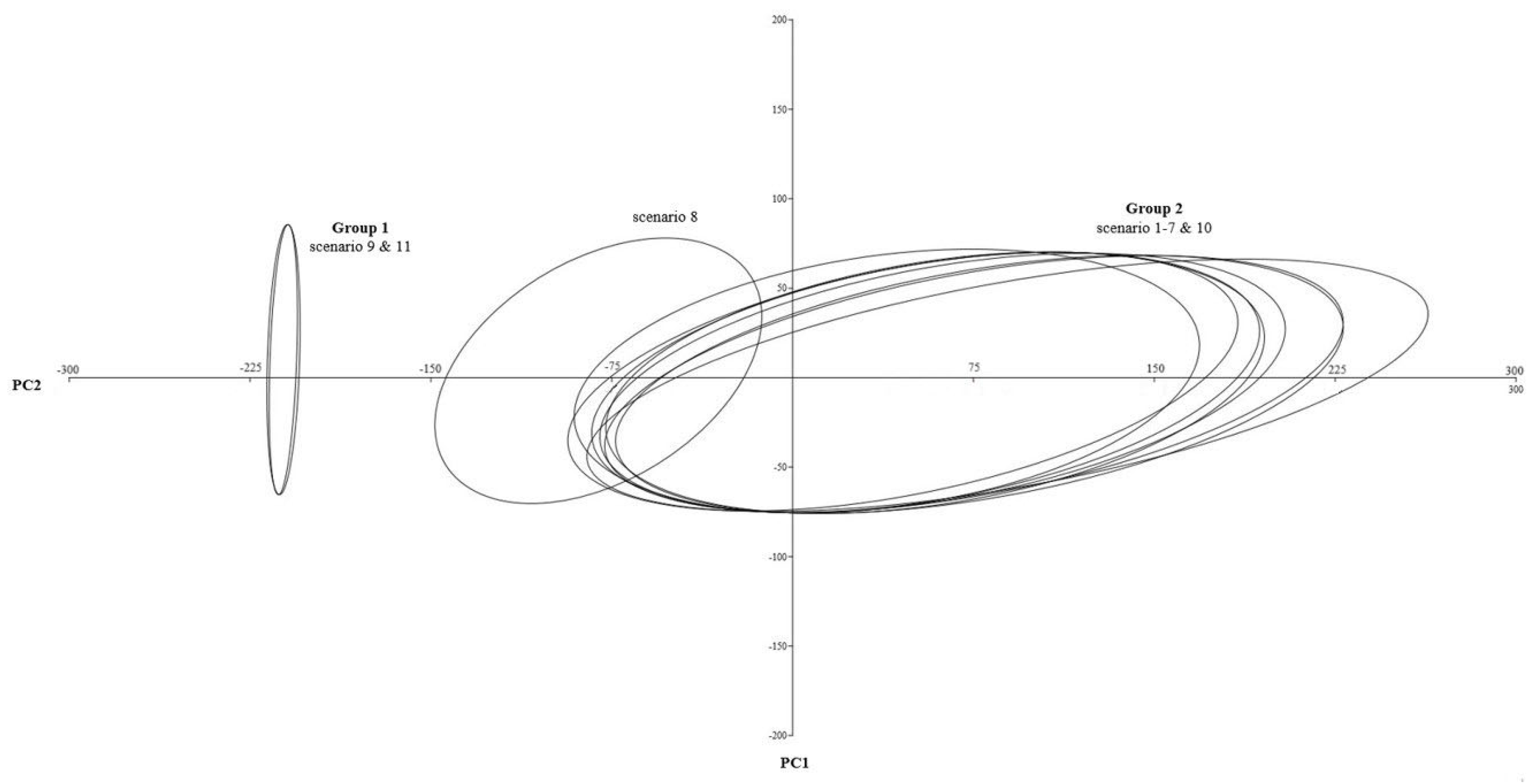
3.4. Statistics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCA | Principal Component Analysis |
| SCI | Sites of Community Importance |
| SD | Standard Deviation |
| SE | Standard Error |
| SPAs | Special Protection Areas |
Appendix A
References
- Petracca, L.S.; Converse, S.J.; Maletzke, B.T.; Gardner, B. Forecasting dynamics of a recolonizing wolf population under different management strategies. Anim. Conserv. 2023. [Google Scholar] [CrossRef]
- Coulson, T.; Mace, G.M.; Hudson, E.; Possingham, H. The Use and Abuse of Population Viability Analysis. Trends Ecol. Evol. 2001, 16, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Ellner, S.P.; Fieberg, J.; Ludwig, D.; Wilcox, C. Precision of Population Viability Analysis. Conserv. Biol. 2002, 16, 258–261. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.A.; Andelman, S.J.; Possingham, H.P. Reliability of Relative Predictions in Population Viability Analysis. Conserv. Biol. 2003, 17, 982–989. [Google Scholar] [CrossRef]
- Lawler, J.J.; Campbell, S.P.; Guerry, A.D.; Kolozsvary, M.B.; O’Connor, R.J.; Seward, L.C.N. The Scope and Treatment of Threats in Endangered Species Recovery Plans. Ecol. Appl. 2002, 12, 663–667. [Google Scholar] [CrossRef]
- Abrahms, B.; Rafiq, K.; Jordan, N.R.; McNutt, J.W. Long-term, climate-driven phenological shift in a tropical large carnivore. Proc. Natl. Acad. Sci. USA 2022, 119, e2121667119. [Google Scholar] [CrossRef]
- Elbroch, L.M.; Lendrum, P.E.; Newby, J.; Quigley, H.; Thompson, D.J. Recolonizing wolves influence the realized niche of resident cougars. Zool. Stud. 2015, 54, 41. [Google Scholar] [CrossRef]
- Rhodes, J.R.; Ng, C.F.; de Villiers, D.L.; Preece, H.J.; McAlpine, C.A.; Possingham, H.P. Using integrated population modelling to quantify the implications of multiple threatening processes for a rapidly declining population. Biol. Conserv. 2011, 144, 1081–1088. [Google Scholar] [CrossRef]
- Gordon, C.H.; Banyard, A.C.; Hussein, A.; Laurenson, M.K.; Malcolm, J.R.; Marino, J.; Regassa, F.; Stewart, A.M.E.; Fooks, A.R.; Sillero-Zubiri, C. Canine Distemper in Endangered Ethiopian Wolves. Emerg. Infect. Dis. 2015, 21, 824–832. [Google Scholar] [CrossRef]
- Lieury, N.; Besnard, A.; Ponchon, C.; Ravayrol, A.; Millon, A. Geographically isolated but demographically connected: Immigration supports efficient conservation actions in the recovery of a range-margin population of the Bonelli’s eagle in France. Biol. Conserv. 2016, 195, 272–278. [Google Scholar] [CrossRef]
- Grauer, J.A.; Gilbert, J.H.; Woodford, J.E.; Eklund, D.; Anderson, S.; Pauli, J.N. Modest immigration can rescue a reintroduced carnivore population. J. Wildl. Manag. 2019, 83, 567–576. [Google Scholar] [CrossRef]
- Saunders, S.P.; Cuthbert, F.J.; Zipkin, E.F. Evaluating population viability and efficacy of conservation management using integrated population models. J. Appl. Ecol. 2018, 55, 1380–1392. [Google Scholar] [CrossRef]
- Morris, W.F.; Doak, D.F. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2002. [Google Scholar]
- Carroll, C.; Phillips, M.K.; Schumaker, N.H.; Smith, D.W. Impacts of landscape change on wolf restoration success: Planning a reintroduction program based on static and dynamic spatial models. Conserv. Biol. 2003, 17, 536–548. [Google Scholar] [CrossRef]
- Oakleaf, J.K.; Mack, C.; Murray, D.L.; Henger, C.S. Habitat selection by recolonizing wolves in the northern Rocky Mountains of the United States. J. Wildl. Manag. 2006, 70, 554–563. [Google Scholar] [CrossRef]
- Nelson, A.; Kauffman, K.; Middleton, A.; Jimenez, M.D.; McWhirter, D.E.; Barber, J.; Gerow, K. Elk migration patterns and human activity influence wolf habitat use in the Greater Yellowstone Ecosystem. Ecol. Appl. 2012, 22, 2293–2307. [Google Scholar] [CrossRef]
- Marucco, F.; McIntire, E.J.B. Predicting spatio-temporal recolonization of large carnivore populations and livestock depredation risk: Wolves in the Italian Alps. J. Appl. Ecol. 2010, 47, 789–798. [Google Scholar] [CrossRef]
- Pettersson, H.L.; Holmes, G.; Quinn, C.H.; Sait, S.M.; Blanco, J.C. Who Must Adapt to Whom? Contested Discourses on Human–Wolf Coexistence and Their Impact on Policy in Spain. People Nat. 2023, 5, 1989–2007. [Google Scholar] [CrossRef]
- Ausband, D.E.; Mech, L.D. The challenges of success: Future wolf conservation and management in the United States. BioScience 2023, 73, 587–591. [Google Scholar] [CrossRef]
- Distefano, E. Human-Wildlife Conflict Worldwide: Collection of Case Studies, Analysis of Management Strategies and Good Practices; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2005. [Google Scholar]
- Bruns, A.; Waltert, M.; Khorozyan, I. The effectiveness of livestock protection measures against wolves (Canis lupus) and implications for their co-existence with humans. Glob. Ecol. Conserv. 2020, 21, e00868. [Google Scholar] [CrossRef]
- European Commission. The situation of the wolf (Canis lupus) in the European Union. In European Commission Report; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Sonam, K.; Dorjay, R.; Lamo, S.; Bijoor, A. A community-based conservation initiative for wolves in the Ladakh Trans-Himalaya, India. Front. Ecol. Evol. 2022, 10, 809817. [Google Scholar] [CrossRef]
- Hansen, S.S.; Ejrnæs, R. Mediating human-wolves conflicts through dialogue, joint fact-finding and empowerment. Front. Environ. Sci. 2022, 10, 826351. [Google Scholar] [CrossRef]
- Štrbenac, A.; Kusak, J.; Huber, Đ.; Jeremić, J.; Oković, P.; Majić-Skrbinšek, A.; Vukšić, I.; Katušić, L.; Desnica, S.; Gomerčić, T.; et al. Plan Upravljanja Vukom u Republici Hrvatskoj za Razdoblje od 2010. do 2015; Ministarstvo kulture, Državni Zavod za Zaštitu Prirode: Zagreb, Croatia, 2010. (In Croatian) [Google Scholar]
- Frkovic, A.; Ruff, R.; Cicnjak, L.; Huber, D. Wolf mortality during 1946–86 in Gorski kotar, Croatia. IUGB Congr. 1992, 18, 353–358. [Google Scholar]
- Kusak, J. Conditions for Life of Wolves (Canis lupus L.) in Croatia. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2002. [Google Scholar]
- Leblond, M.; St-Laurent, M.H.; Côté, S.D. Wolf habitat selection is shaped by human activities in a highly managed boreal forest. For. Ecol. Manag. 2016, 276, 125–131. [Google Scholar]
- Kusak, J.; Huber, Đ.; Trenc, N.; Desnica, S. Stručni Priručnik za Procjenu Utjecaja Zahvata na Velike Zvijeri Pojedinačno te u Sklopu Planskih Dokumenata; Agencija za zaštitu okoliša: Zagreb, Croatia, 2016. (In Croatian) [Google Scholar]
- Doboš, M.; Pavić, G.; Kukić, G.; Sikora, Č.; Jušić, K.; Jelić, D. Istraživanje Faune na Papuku Pomoću Fotozamki (Siječanj 2022.–Ožujak 2023); Javna Ustanova Park Prirode Papuk: Voćin, Croatia, 2023. (In Croatian) [Google Scholar]
- Antonić, O.; Kušan, V.; Jelaska, S.; Bukovec, D.; Križan, J.; Bakran-Petricioli, T.; Gottstein-Matočec, S.; Pernar, R.; Hećimović, Ž.; Janeković, I.; et al. Kartiranje staništa Republike Hrvatske (2000–2004). Drypis 2005, 1. (In Croatian) [Google Scholar]
- Bardi, A.; Papini, P.; Quaglino, E.; Biondi, E.; Topić, J.; Milović, M.; Pandža, M.; Kaligarič, M.; Oriolo, G.; Roland, V.; et al. Karta Prirodnih i Poluprirodnih Ne-Šumskih Kopnenih i Slatkovodnih Staništa Republike Hrvatske; Ministarstvo Zaštite Okoliša i Energetike: Zagreb, Croatia, 2016. (In Croatian) [Google Scholar]
- Merli, E.; Mattioli, L.; Bassi, E.; Bongi, P.; Berzi, D.; Ciuti, F.; Luccarini, S.; Morimando, F.; Viviani, V.; Caniglia, R.; et al. Estimating wolf population size and dynamics by field monitoring and demographic models: Implications for management and conservation. Animals 2023, 13, 1735. [Google Scholar] [CrossRef]
- Lacy, R.C.; Pollak, J.P. Vortex: A Stochastic Simulation of the Extinction Process, (Version 10.6.0); Chicago Zoological Society: Brookfield, IL, USA, 2023. [Google Scholar]
- Chapron, G.; Wikenros, C.; Liberg, O.; Wabakken, P.; Flagstad, Ø.; Milleret, C.; Månsson, J.; Svensson, L.; Zimmermann, B.; Åkesson, M.; et al. Estimating Wolf (Canis lupus) Population Size from Number of Packs and an Individual-Based Model. Ecol. Evol. 2021, 11, 11739–11748. [Google Scholar] [CrossRef]
- Kajgana, I. Utjecaj Čovjeka na Aktivnost Velikih Sisavaca na Području Zrinskog Gorja (Diplomski Rad); Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet: Zagreb, Croatia, 2015; Available online: https://urn.nsk.hr/urn:nbn:hr:217:230633 (accessed on 11 September 2023). (In Croatian)
- Platiša, M.; Pintar, I.; Kusak, J. Tjelesne osobine sivog vuka (Canis lupus L.). Veterinar 2011, 49. (In Croatian) [Google Scholar]
- Turinski, I. Međudjelovanje Velikih Predatora Hrvatske—Eurazijskog Risa (Lynx lynx L.) i Sivog Vuka (Canis lupus L.)—Kroz Odnose Predacije i Kompeticije (Diplomski Rad); Sveučilište Josipa Jurja Strossmayera u Osijeku, Odjel za biologiju: Osijek, Croatia, 2017. (In Croatian) [Google Scholar]
- Wikenros, C.; Gicquel, M.; Zimmermann, B.; Flagstad, Ø.; Åkesson, M. Age at First Reproduction in Wolves: Different Patterns of Density Dependence for Females and Males. Proc. R. Soc. B 2021, 288, 20210207. [Google Scholar] [CrossRef]
- Constable, P.; Hinchcliff, K.; Demma, N.; Callahan, M.; Dale, B. Serum biochemistry of captive and free-ranging gray wolves (Canis lupus). J. Zoo Wildl. Med. 1998, 29, 435–440. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Vucetich, J.A.; Nelson, M.P.; Phillips, M.K. The Normative Dimension and Legal Meaning of Endangered and Recovery in the U.S. Endangered Species Act. Conserv. Biol. 2006, 20, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.; Lacy, R.C.; Fredrickson, R.J.; Rohlf, D.J.; Hendricks, S.A.; Phillips, M.K. Biological and sociopolitical sources of uncertainty in population viability analysis for endangered species recovery planning. Sci. Rep. 2019, 9, 10130. [Google Scholar] [CrossRef]
- Smith, D.W.; Peterson, R.O.; Houston, D.B. Yellowstone after wolves. BioScience 2003, 53, 330–340. [Google Scholar] [CrossRef]
- Smietana, W.; Wajda, W. Wolf number changes in Bieszczady National Park, Poland. Acta Theriol. 1997, 42, 241–252. [Google Scholar] [CrossRef]
- Bruskotter, J.T.; Vucetich, J.A.; Enzler, S.A.; Treves, A.; Nelson, M.P. Removing protections for wolves and the future of the U.S. Endangered Species Act (1973). Conserv. Lett. 2014, 7, 401–407. [Google Scholar] [CrossRef]
- Liberg, O.; Chapron, G.; Wabakken, P.; Pedersen, H.C.; Thompson Hobbs, N.; Sand, H. Shoot, shovel and shut up: Cryptic poaching slows restoration of a large carnivore in Europe. Proc. R. Soc. B Biol. Sci. 2012, 279, 910–915. [Google Scholar] [CrossRef]
- Mech, L.D.; Boitani, L. Wolves: Behavior, Ecology, and Conservation; University of Chicago Press: Chicago, IL, USA, 2003; p. 448. [Google Scholar]
- Gompper, M.E.; Ray, J.C.; Groff, C.; Berger, J. A comparison of noninvasive techniques to survey carnivore communities in northeastern North America. Wildl. Soc. Bull. 2002, 30, 962–973. [Google Scholar] [CrossRef]
- Berger, K.M.; Gese, E.M.; Berger, J. Carnivore repatriation and holarctic prey: Narrowing the deficit in ecological effectiveness. Conserv. Biol. 2007, 21, 1105–1116. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Ćirović, D.; Jurišić, A.; Kovačić, D. Golden jackal expansion in Europe: A case of mesopredator release. Ital. J. Mammal. 2017, 28, 9–15. [Google Scholar]
- Torretta, E.; Corradini, A.; Pedrotti, L.; Bani, L.; Bisi, F.; Dondina, O. Hide-and-Seek in a Highly Human-Dominated Landscape: Insights into Movement Patterns and Selection of Resting Sites of Rehabilitated Wolves (Canis lupus) in Northern Italy. Animals 2023, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, R.J.; Hedrick, P.W.; Wayne, R.K. Genetic rescue of an insular population of large mammals. Proc. R. Soc. B Biol. Sci. 2003, 270, 91–97. [Google Scholar]
- Hedrick, P.W.; Allendorf, F.W. Genetically effective population size of large mammals: An assessment of estimators. Conserv. Genet. 2004, 5, 467–483. [Google Scholar]
- Tallmon, D.A.; Luikart, G.; Waples, R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 23, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Drummer, T.D.; Murphy, K.M.; Guernsey, D.S. Survival of colonizing wolves in the Northern Rocky Mountains of the United States, 1982–2004. J. Wildl. Manag. 2010, 74, 620–634. [Google Scholar] [CrossRef]
- Vila, C.; Sundqvist, A.K.; Flagstad, O.; Seddon, J.; Björnerfeldt, S.; Kojola, I.; Casulli, A.; Sand, H.; Wabakken, P.; Ellegren, H. Rescue of a severely bottlenecked wolf (Canis lupus) population by a single immigrant. Proc. R. Soc. Lond. B 2003, 270, 91–97. [Google Scholar] [CrossRef]
- Wabakken, M.J.; Svensson, L.; Maartmann, E. The recovery, distribution, and population dynamics of wolves on the Scandinavian peninsula, 1978–1998. Can. J. Zool. 2001, 79, 710–725. [Google Scholar] [CrossRef]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 1517–1520. [Google Scholar] [CrossRef]
- Mech, L.D.; Goyal, S.M.; Paul, W.J.; Newton, W.E. Demographic effects of canine parvovirus on a free-ranging wolf population over. J. Wildl. Dis. 2008, 44, 824–836. [Google Scholar] [CrossRef]
- Almberg, E.S.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. A Serological Survey of Infectious Disease in Yellowstone National Park’s Canid Community. PLoS ONE 2009, 4, e7042. [Google Scholar] [CrossRef]
- Oliynyk, R.T. Human-caused wolf mortality persists for years after discontinuation of hunting. Sci. Rep. 2023, 13, 13853. [Google Scholar] [CrossRef]
- Liberg, O.; Suutarinen, J.; Åkesson, M.; Andrén, H.; Wabakken, P.; Wikenros, C.; Sand, H. Poaching-related disappearance rate of wolves in Sweden was positively related to population size and negatively to legal culling. Biol. Conserv. 2020, 243, 108456. [Google Scholar] [CrossRef]
- Nowak, S.; Żmihorski, M.; Figura, M.; Stachyra, P.; Mysłajek, R.W. The Illegal Shooting and Snaring of Legally Protected Wolves in Poland. Biol. Conserv. 2021, 264, 109367. [Google Scholar] [CrossRef]
- Reinhardt, I.; Kluth, G.; Nowak, C.; Szentiks, C.A.; Krone, O.; Ansorge, H.; Mueller, T. Military training areas facilitate the recolonization of wolves in Germany. Conserv. Lett. 2019, 12, e12635. [Google Scholar] [CrossRef]
- Sunde, P.; Collet, S.; Nowak, C.; Thomsen, P.F.; Hansen, M.M.; Schulz, B.; Matzen, J.; Michler, F.-U.; Vedel-Smith, C.; Olsen, K. Where have all the young wolves gone? Traffic and cryptic mortality create a wolf population sink in Denmark and northernmost Germany. Conserv. Lett. 2021, 14, e12812. [Google Scholar] [CrossRef]
- Mech, L.D.; Adams, L.G.; Meier, T.J.; Burch, J.W.; Dale, B.W. The Wolves of Denali; University of Minnesota Press: Minneapolis, MN, USA, 1998; p. 227. [Google Scholar]
- Peterson, R. Wolves as Interspecific Competitors in Canid Ecology. In Ecology and Conservation of Wolves in a Changing World; Carbyn, L.N., Frits, S.H., Seip, D.R., Eds.; Canadian Circumpolar Institute, Occasional Publication No. 35; Canadian Circumpolar Institute: Edmonton, AB, Canada, 1995; pp. 315–323. [Google Scholar]
- Musto, C.; Gazzola, A.; Milanesi, P.; Ghidini, A.; Pizzi, R.; Apollonio, M.; Bassi, E. First Evidence of Widespread Positivity to Anticoagulant Rodenticides in Grey Wolves (Canis lupus). Sci. Total Environ. 2024, 915, 169990. [Google Scholar] [CrossRef]
- Molnar, B.; Ciucci, P.; Mastrantonio, G.; Betschart, B. Correlates of parasites and pseudoparasites in wolves (Canis lupus) across continents: A comparison among Yellowstone (USA), Abruzzo (IT) and Mercantour (FR) national parks. Int. J. Parasitol. Parasites Wildl. 2019, 10, 196–206. [Google Scholar] [CrossRef]
- Kusak, J.; Fabbri, E.; Galov, A.; Gomerčić, T.; Arbanasić, H.; Caniglia, R.; Galaverni, M.; Reljić, S.; Huber, Đ.; Randi, E. Wolf-dog hybridization in Croatia. Vet. Arh. 2018, 88, 375–395. [Google Scholar] [CrossRef]
- Cusdin, P.A.; Greenwood, A.G. The Keeping of Wolf-Hybrids in Great Britain; Department of the Environment, Transport and the Regions and Royal Society for the Prevention of Cruelty to Animals: Keighley, UK, 2000. [Google Scholar]
- Kittle, A. The Influence of Prey Availability, Habitat Attributes and Other Factors on Space Use by Social Carnivores. Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2014. Available online: https://atrium.lib.uoguelph.ca/server/api/core/bitstreams/3c9360e5-b52d-4281-8fd6-a7ab906df48d/content (accessed on 15 March 2023).
- Carricondo-Sanchez, D.; Zimmermann, B.; Wabakken, P.; Eriksen, A.; Milleret, C.; Ordiz, A.; Sanz-Pérez, A.; Wikenros, C. Wolves at the door? Factors influencing the individual behavior of wolves in relation to anthropogenic features. Biol. Conserv. 2020, 244, 108514. [Google Scholar] [CrossRef]
- Wójcicki, A.; Borowski, Z. The presence of wolves leads to spatial differentiation in deer browsing pressure on forest regeneration. Sci. Rep. 2023, 13, 17245. Available online: https://colab.ws/articles/10.1038/s41598-023-44502-y (accessed on 15 March 2024). [CrossRef]
- Ugarković, D.; Tikvić, I.; Mikac, S.; Stankić, I.; Balta, D. The influence of changing climate extremes on the ecological niche of pedunculate oak in Croatia. South-East Eur. For. 2016, 7, 143–148. [Google Scholar] [CrossRef][Green Version]
- Niemiec, R.; Berl, R.E.W.; Gonzalez, M.; Teel, T.; Salerno, J.; Breck, S.; Camara, C.; Collins, M.; Schultz, C.; Hoag, D.; et al. Rapid changes in public perception toward a conservation initiative. Conserv. Sci. Pract. 2022, 4, e12632. [Google Scholar] [CrossRef]
- Rodríguez-Recio, M.; Wikenros, C.; Zimmermann, B.; Sand, H. Rewilding by wolf recolonization, consequences for ungulate populations and game hunting. Biology 2022, 11, 317. [Google Scholar] [CrossRef]
- Bišćan, M.; Damjanović, I. The Big Bad Wolf—The perception of media-mediated messages. In Book of Abstracts; Croatian Association of Nature and Environmental Protection Experts (HUSZPO): Vodice, Croatia, 2022; pp. 169–170. [Google Scholar]
- Bombieri, G.; Penteriani, V.; Almasieh, K.; Ambarlı, H.; Ashrafzadeh, M.R.; Das, C.S. A worldwide perspective on large carnivore attacks on humans. PLoS Biol. 2023, 21, e3001946. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Kovtun, E.; Rouart, I. Wolf Attacks on Humans: An Update for 2002–2020. NINA Report 1944; Norwegian Institute for Nature Research: Trondheim, Norway, 2021. [Google Scholar]
- Wilson, S.M. Living with predators: A 20-year case study in the Blackfoot River watershed of Montana. In Rangeland Wildlife Ecology and Conservation; McNew, L.B., Dahlgren, D.K., Beck, J.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 347–360. [Google Scholar]
- Jeremić, J.; Kusak, J.; Huber, Đ.; Štrbenac, A.; Korša, A. Report on the Status of Wolf Populations in Croatia in 2016; Croatian Agency for the Environment and Nature: Zagreb, Croatia, 2017. [Google Scholar]
- Reed, D.H.; O’Grady, J.J.; Ballou, J.D.; Frankham, R. The frequency and severity of catastrophic die-offs in vertebrates. Anim. Conserv. 2003, 6, 109–114. [Google Scholar] [CrossRef]
- Kojola, I.; Hallikainen, V.; Mikkola, K.; Gurarie, E.; Heikkinen, S.; Kaartinen, S.; Nikula, A.; Nivala, V. Wolf visitations close to human residences in Finland: The role of age, residence density, and time of day. Biol. Conserv. 2016, 198, 9–14. [Google Scholar] [CrossRef]
- Kojola, I.; Aspi, J.; Hakala, A.; Heikkinen, S.; Ilmoni, C.; Ronkainen, S. Dispersal in an expanding wolf population in Finland. J. Mammal. 2006, 87, 281–286. [Google Scholar] [CrossRef]
- Almpanidou, V.; Mazaris, A.D.; Mertzanis, Y.; Avraam, I.; Antoniou, I.; Pantis, J.D.; Sgardelis, S.P. Landscape Connectivity and Suitable Habitat Analysis for Wolves (Canis lupus L.) in the Eastern Pyrenees. Sustainability 2020, 12, 5762. [Google Scholar] [CrossRef]
- Blanco, J.C.; Cortés, Y.; Virgós, E. Wolf Response to Two Kinds of Barriers in an Agricultural Habitat in Spain. Can. J. Zool. 2005, 83, 312–323. [Google Scholar] [CrossRef]


| Scenario No. | Scenario Name | Scenario Class | Description | Variable Vortex Categories |
|---|---|---|---|---|
| 1 | Baseline | - | Population based on modeled area’s carrying capacity, determined by habitat suitability map | Carrying Capacity |
| 2 | Disease | Uncertainty | 50% population reduction over two six-month periods due to disease | Carrying Capacity and Catastrophes |
| 3 | Removals | Management | Annual removal of an animal due to human–wildlife conflicts | Carrying Capacity and Harvest |
| 4 | Illegal hunting | Uncertainty | Annual removal of two animals due to illegal hunting | Carrying Capacity and Harvest |
| 5 | Succession: Carrying capacity boost | Uncertainty | Increase in suitable habitat due to natural succession | Carrying Capacity |
| 6 | Forest Management: Carrying capacity boost | Management | Increase in suitable habitat due to alterations in forest management practices | Carrying Capacity |
| 7 | Hunting Management: Carrying capacity boost | Management | Increase in carrying capacity by modifying hunting management | Carrying Capacity |
| 8 | One-Region Corridor Enhancement: Immigration by existing corridors (Banovina) | Management | Supplementation with one male wolf from Banovina—enhancing existing corridors | Carrying Capacity and Supplementation |
| 9 | Corridor construction: Immigration by new corridors (Banovina) + succession | Management | Supplementation with one pair of wolves (male and female) from Banovina—creating new corridors with an increase in suitable habitats due to natural succession | Carrying Capacity and Supplementation |
| 10 | Two-Region Corridor Enhancement: Immigration by existing corridors (Banovina and Bosnia and Herzegovina) | Management | Supplementation with one male wolf from Banovina and one male wolf from Bosnia and Herzegovina—enhancing existing corridors from both regions | Carrying Capacity and Supplementation |
| 11 | Translocation: Translocation from Croatia | Management | Translocation of one male and one female wolf from another Croatian wolf population | Carrying Capacity and Supplementation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bišćan, M.; Jelić, D.; Maguire, I.; Massolo, A. Modeling Wolf, Canis lupus, Recolonization Dynamics to Plan Conservation Actions Ahead: Will the “Big Bad Wolves” Howl Again in Slavonia, Croatia? Diversity 2025, 17, 461. https://doi.org/10.3390/d17070461
Bišćan M, Jelić D, Maguire I, Massolo A. Modeling Wolf, Canis lupus, Recolonization Dynamics to Plan Conservation Actions Ahead: Will the “Big Bad Wolves” Howl Again in Slavonia, Croatia? Diversity. 2025; 17(7):461. https://doi.org/10.3390/d17070461
Chicago/Turabian StyleBišćan, Matko, Dušan Jelić, Ivana Maguire, and Alessandro Massolo. 2025. "Modeling Wolf, Canis lupus, Recolonization Dynamics to Plan Conservation Actions Ahead: Will the “Big Bad Wolves” Howl Again in Slavonia, Croatia?" Diversity 17, no. 7: 461. https://doi.org/10.3390/d17070461
APA StyleBišćan, M., Jelić, D., Maguire, I., & Massolo, A. (2025). Modeling Wolf, Canis lupus, Recolonization Dynamics to Plan Conservation Actions Ahead: Will the “Big Bad Wolves” Howl Again in Slavonia, Croatia? Diversity, 17(7), 461. https://doi.org/10.3390/d17070461






